Abstract
IgG4 purified from patients undergoing specific allergen immunotherapy inhibits the activities of the serum IgE in in vitro assays and is thought to reduce the symptoms of the disease. However, it is not known whether this is related to an intrinsic property of this subclass or only the allergen specificity. We tested the hypothesis that allergen specificity is the critical determinant for this activity using a panel of antibodies with identical specificity but different subclasses. The different antibodies were all able to inhibit the activity of IgE to the same extent. We demonstrate that specificity is the dominant factor determining the ability of an antibody to block allergen‐dependent IgE activity.
Keywords: allergen, antibody, immunotherapy, isotype, specificity
Abbreviations
- BAT
basophil activation test
- BSA
bovine serum albumin
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme‐linked immunosorbent assay
- FAP
facilitated antigen presentation
- HBS
Hepes‐buffered saline
- HPLC
high‐performance liquid chromotography
- PBS
phosphate‐buffered saline
- PIPE
polymerase incomplete primer extension
- SCIT
subcutaneous immunotherapy
- SPR
surface plasmon resonance
- TMB
tetramethybenzidine
Allergy is associated with the excessive production of allergen‐specific IgE. However, allergen‐specific antibodies of other isotypes are produced both in allergic disease and in states of tolerance, for example in hyperimmune beekeepers and in patients treated by allergen immunotherapy 1, 2. The four human IgG subclasses, IgG1, IgG2, IgG3 and IgG4, differ mainly in the length and rigidity of the hinge region. These differences impart different functional roles, based on their ability to activate the immune system. Similarly, IgA is represented by two subclasses: IgA1, which predominates within the serum, and IgA2, with a shorter hinge region and more compact structure, which predominates at mucosal surfaces 3 (Fig. 1A).
Figure 1.
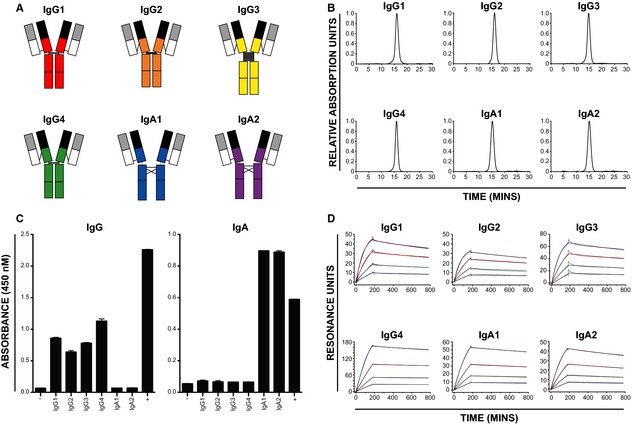
(A) Immunoglobulin domain structures of human IgG1‐4 and IgA1‐2. Schematic representations of the polypeptide and domain structures of human IgG1, IgG2, IgG3, IgG4, IgA1 and IgA2 showing the intrachain disulphide bridges 3, 13. Variable domains are shown in black and grey. (B) Size exclusion purification profiles. Constant domain‐exchanged Phl p 7‐specific monoclonal antibodies were purified by size exclusion chromatography (Superdex™ 200 10/300, flow rate 0.75 ml/min in PBS, pH 7.0). (C) Isotype exchange retains the antibody specificity. ELISA plates were coated with Phl p 7, and antibody binding was confirmed using isotype‐specific detection antibodies. Assay buffer and mixed patient serum were included as negative (−) and positive (+) controls as indicated. (D) Binding kinetics and affinity for Phl p 7 are comparable between antibody isotypes. Recombinant Phl p 7‐specific antibodies were captured on a CM5 sensor chip with a covalently immobilized antilambda antibody. Binding experiments were carried out with twofold serial dilutions of Phl p 7 from a starting concentration of 10 nm. Curves were fit (black lines) to derive on‐ and off‐rates.
Serum from patients receiving allergen immunotherapy blocks the activity of IgE, and this inhibitory activity co‐elutes with the IgG4 fraction 4. Furthermore, depletion of IgG4 from this serum correlates with a decrease in IgE inhibitory activity 5. However, it is unknown whether blocking activity is restricted to the IgG4 subclasses or whether other subclasses that recognize the same epitope are equally effective in blocking IgE‐mediated activity. To test this, we generated a set of recombinant monoclonal antibodies of the same specificity for the grass pollen allergen Phl p 7, with different constant region domains, representing all of the IgG and IgA subclasses. We then measured the affinity of antigen binding and ability of these antibodies to inhibit IgE‐mediated activities in in vitro assays.
Methods
Detailed methods are available in the supporting information.
Antibody cloning and expression
Matched heavy‐ and light‐chain variable antibody sequences specific to Phl p 7 allergen were previously isolated from a single B cell derived from a patient undergoing grass pollen immunotherapy 6. These sequences were subcloned into the dual antibody expression vector pVITRO1‐102.1F10‐IgG4/λ 7. Phl p 7‐specific human IgG1, IgG2, IgG3, and IgA1 and IgA2 expression vectors were subsequently cloned and expressed using the PIPE method 7.
Characterization of recombinant antibodies
Human 102.1F10 IgG1 and IgG2, and IgG3 and IgG4 were purified by affinity chromatography with a 5‐ml HiTrap Protein‐G HP column (GE Healthcare Life Sciences, Amersham, UK). Human 102.1F10 IgA1 and IgA2 were purified by affinity chromatography with immobilized SSL7/Agarose (InvivoGen, Toulouse, France). The purified antibodies were analysed by size exclusion chromatography 14, and specificity was confirmed by Phl p 7 allergen ELISA using biotin‐labelled isotype‐specific antibodies. SPR was performed using a Biacore T200 instrument; antibodies were captured using an immobilized antilambda antibody (Life Technologies Ltd., Paisley, UK), and binding of Phl p 7 (kindly provided by Dr. Rebecca Beavil) was measured using a 3‐min association phase followed by 10‐min dissociation.
IgE‐facilitated allergen binding (FAB) assay
IgE‐facilitated allergen binding to B cells was performed as previously described 5 using serum from a grass pollen‐sensitized donor (12 ISU Phl p 7‐IgE), recombinant Phl p 7 (kindly provided by Dr. Rebecca Beavil) and purified Phl p 7‐specific antibodies (10 μg/ml) 6, postimmunotherapy serum (SCIT) (patient 102) or assay media (RPMI‐1640).
Basophil activation assay
Basophil (CD3−, CD303−, CD294+) activation (upregulation of CD63) was measured by flow cytometry following incubation of blood from a Phl p 7‐sensitized donor with recombinant Phl p 7 in the additional presence of Phl p 7‐specific antibodies (10 μg/ml), postsubcutaneous immunotherapy serum (SCIT) (patient 102) or control serum (human AB sera, Lonza, Verviers, Belgium).
Results
Antibody characterization
Size exclusion chromatography and ELISA confirmed the purified Phl p 7‐specific IgG1, IgG2, IgG3, IgG4, IgA1 and IgA2 consisted of monodisperse antibodies of the expected size (Fig. 1B) and specificity (Fig. 1C). Changing the constant region had negligible effects on antibody affinities for Phl p 7 (Fig. 1D), with similar KD values obtained for all antibody subclasses tested (Table S1).
IgE blocking activity
As IgG4 has been previously shown to be an effective blocking antibody for IgE‐mediated activity, we wished to determine whether this blocking activity was specific to the IgG4 subclass. We therefore tested the IgG and IgA subclasses in two independent in vitro assays of IgE activity. Similar to IgG4, IgG1, IgG2, IgG3, IgA1 and IgA2 were able to inhibit binding of IgE‐Phl p 7 complexes to the IgE receptor CD23 (FcεRII) on the surface of B cells (Fig. 2A). In a separate assay, all the monoclonal antibodies tested were able to inhibit IgE‐dependent Phl p 7‐mediated basophil activation to a similar degree (Fig. 2B and 2C).
Figure 2.
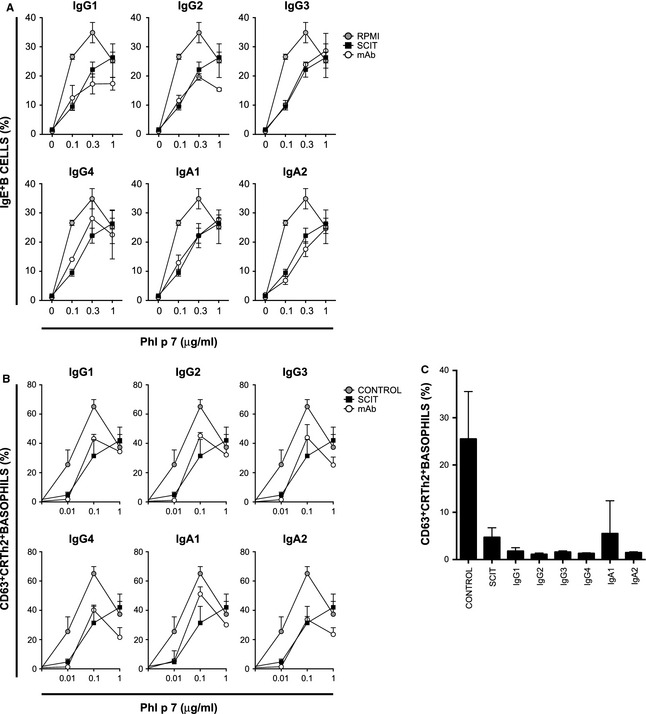
Inhibition of IgE‐facilitated allergen presentation and basophil activation is comparable between different isotypes. Serum containing Phl p 7‐specific IgE was incubated with Phl p 7 in the presence of 10 μg/ml monoclonal antibodies specific to Phl p 7 (open circles). Undiluted immunotherapy serum (SCIT, closed squares) and assay media (RPMI, grey circles) were included as positive and negative controls for IgE blocking, respectively. Binding of IgE‐Phl p 7 complexes was detected by flow cytometry; data are shown as mean ± SEM. B. Basophil activation was detected by flow cytometry following incubation of whole blood from two Phl p 7‐sensitized individuals with increasing concentrations of Phl p 7 and 10 μg/ml monoclonal antibodies specific to Phl p 7 (open circles). Undiluted immunotherapy serum (SCIT, closed squares) and undiluted healthy control serum (control, grey circles) were included as positive and negative controls, respectively. C. Basophil activation following incubation at 10 ng/mL Phl p 7 in the presence of 10 μg/ml monoclonal antibodies specific to Phl p 7. Undiluted immunotherapy (SCIT) serum and healthy control serum were included as positive and negative controls, respectively.
Discussion
The inhibitory activity of non‐IgE antibodies in an allergic reaction is thought to be due to their competition with IgE, by masking the epitopes on the allergen. These so‐called blocking antibodies represent a potentially valuable but as yet untested therapeutic commodity for use in passive allergen immunotherapy 8. We previously isolated and cloned a monoclonal IgG4 antibody, specific to the grass pollen allergen Phl p 7, from a single B cell isolated from the peripheral blood of a patient treated by specific allergen immunotherapy 6. This single antibody was able to inhibit Phl p 7‐induced IgE activity by up to 60%. This was comparable to the blocking activity of the polyclonal postimmunotherapy serum from the same patient. To test whether this blocking activity was related solely to the specificity or to the (IgG4) subclass of the antibody, we compared the ability of other subclasses to inhibit IgE. Although the affinities for Phl p 7 were similar in all of the recombinant antibodies we generated, subtle but significant differences in binding rates were observed (about threefold differences in both on‐ and off‐rate constants, in the most extreme cases), which tended to cancel out to give very similar overall affinities (ranging from 250–570 pm). These differences may reflect subtle conformational changes that constant region domains impart on the variable region, which have been reported to influence the fine specificity and affinity of isotype‐swapped antibodies 9. Nevertheless, we found that the specificity for Phl p 7 was retained and, importantly, isotype exchange had no effect on IgE blocking activity; all subclasses were able to inhibit IgE to nearly the same degree in our in vitro assays. Of course, we cannot exclude the possibility that more subtle effects might be observed by titration of the different antibodies. However, under the conditions used here, blocking activities were dependent only on the ability to bind allergen and not on the constant region effector function.
It is almost certain that the blocking activity of an antibody is dependent on several factors such as epitope specificity, concentration and affinity for antigen 10. The affinity of IgE for allergen is an important determinant of its effector function 11, and a blocking antibody must be of approximately equal or higher affinity to prevent IgE binding. Antibody affinity will therefore be a critical factor for selecting blocking antibodies for passive immunotherapy. It is well established that IgG4‐expressing B cells secrete the most efficacious blocking antibodies in vivo after specific allergen immunotherapy 2. It follows that they would be the best source of heavy‐ and light‐chain genes from which to derive recombinant blocking antibodies for passive immunotherapy. Indeed, IgG4 has properties that may favour its use over other IgG subclasses, such as its inability to bind complement, and also the unique property of exchanging one heavy‐/light‐chain pair with an IgG4 antibody of a different specificity to generate a bispecific antibody unable to form immune complexes 12.
In summary, our results demonstrate that all IgG and IgA subclasses are capable of inhibiting the activity of IgE in an allergen‐specific manner. Further experiments will be required to examine whether antibodies with the same specificity but different isotype are similarly inhibitory in vivo or whether other mechanisms as mentioned above come into play. Phl p 7 is a relatively small allergen, and while our study provides a proof‐of‐concept for passive immunotherapy, it is likely that combinations of allergen‐specific monoclonal antibodies directed against multiple allergens, and possibly multiple epitopes, will be required to ameliorate symptoms depending on the sensitization pattern of each individual 8.
Author contributions
TSD, HB and HJB produced and characterized antibodies; MHS performed functional assays; JMM helped to design experiments and analyse data; LKJ, AJB, SRD and BJS helped to design experiments and write the manuscript; and LKJ performed and coordinated experiments. HJG proposed the study and helped to write the manuscript.
Conflict of interest statements
None of the authors have any conflict of interest to declare.
Supporting information
Figure S1. ELISA plates were coated with Phl p 7 and antibody binding was confirmed using subclass‐specific monoclonal detection antibodies.
Appendix S1. Methods.
Table SI. SPR analysis of the interaction between Phl p 7 and recombinant antibodies (±SE).
Acknowledgments
We thank Rachel Yan for patient recruitment, and Rebecca Parkin and Orla McMahon for technical assistance with functional assays and Rebecca Beavil for producing recombinant Phl p 7. This research was supported by the MRC (HJG, BJS, AJB, JMM), the London Law Trust (LKJ) and the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London (HB & TSD). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, Durham SR, Sutton BJ, Gould HJ, James LK. Inhibition of allergen‐dependent IgE activity by antibodies of the same specificity but different class. Allergy 2015; 70: 720–724.
Edited by: Reto Crameri
Tihomir S. Dodev and Holly Bowen contributed equally to this work.
References
- 1. Sin BA, Akdis M, Zumkehr J, Bezzine S, Bekpen C, Lambeau G et al. T‐cell and antibody responses to phospholipase A2 from different species show distinct cross‐reactivity patterns. Allergy 2011;66:1513–1521. [DOI] [PubMed] [Google Scholar]
- 2. Shamji MH, Ljorring C, Francis JN, Calderon MA, Larche M, Kimber I et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012;67:217–226. [DOI] [PubMed] [Google Scholar]
- 3. Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol 2011;4:590–597. [DOI] [PubMed] [Google Scholar]
- 4. Nouri‐Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK et al. Grass pollen immunotherapy induces mucosal and peripheral IL‐10 responses and blocking IgG activity. J Immunol 2004;172:3252–3259. [DOI] [PubMed] [Google Scholar]
- 5. James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol 2011;127:509–516. [DOI] [PubMed] [Google Scholar]
- 6. James LK, Bowen H, Calvert RA, Dodev TS, Shamji MH, Beavil AJ et al. Allergen specificity of IgG(4)‐expressing B cells in patients with grass pollen allergy undergoing immunotherapy. J Allergy Clin Immunol 2012;130:663–670. [DOI] [PubMed] [Google Scholar]
- 7. Dodev TS, Karagiannis P, Gilbert AE, Josephs DH, Bowen H, James LK et al. A tool kit for rapid cloning and expression of recombinant antibodies. Sci Rep 2014;4:5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flicker S, Linhart B, Wild C, Wiedermann U, Valenta R. Passive immunization with allergen‐specific IgG antibodies for treatment and prevention of allergy. Immunobiology 2013;218:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem 2012;287:35409–35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holm J, Willumsen N, Wurtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol 2011;127:1029–1037. [DOI] [PubMed] [Google Scholar]
- 11. Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol 2008;122:298–304. [DOI] [PubMed] [Google Scholar]
- 12. Rispens T, den Bleker TH, Aalberse RC. Hybrid IgG4/IgG4 Fc antibodies form upon ‘Fab‐arm’ exchange as demonstrated by SDS‐PAGE or size‐exclusion chromatography. Mol Immunol 2010;47:1592–1594. [DOI] [PubMed] [Google Scholar]
- 13. Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012;4:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunt J, Beavil RL, Calvert RA, Gould HJ, Sutton BJ, Beavil AJ. Disulfide linkage controls the affinity and stoichiometry of IgE Fcepsilon3‐4 binding to FcepsilonRI. J Biol Chem 2005;280:16808–16814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ELISA plates were coated with Phl p 7 and antibody binding was confirmed using subclass‐specific monoclonal detection antibodies.
Appendix S1. Methods.
Table SI. SPR analysis of the interaction between Phl p 7 and recombinant antibodies (±SE).


