Abstract
Purpose
The aim of this study was to assess the advantages of robotic surgery, comparing perioperative and oncological outcomes between robot-assisted radical cystectomy (RARC) and open radical cystectomy (ORC).
Materials and Methods
Between August 2008 and May 2014, 112 radical cystectomies (42 RARCs and 70 ORCs) were performed at a single academic institution following Institutional Review Board approval. Patient demographics, perioperative variables (e.g., complications), and oncologic outcomes including metastasis-free survival (MFS), cancer-specific survival (CSS), and overall survival (OS) were reported using the Kaplan-Meier analyses.
Results
The median follow-up period was 40 months (range, 0–70 months) vs. 42 months (range, 0–74 months) in RARC and ORC, respectively. Baseline characteristics of both groups were balanced. Blood loss (median, [range]; 300 mL [125–925 mL] vs. 598 mL [150–2,000 mL], p=0.001) and perioperative transfusion rates (23.8% vs. 45.7%, p=0.020) were significantly lower in the RARC group than in the ORC group. The overall complication rates were greater in the ORC group, but this was not statistically significant (65.7% vs. 64.3%, p=0.878). However, there were significantly higher major complication rates in the ORC group (45.7% vs. 26.2%, p=0.040). No significant differences were found with regards to MFS, CSS, and OS.
Conclusions
While histopathological findings, overall complications, and survival rates do not reveal definite differences, RARC has more advantages compared to ORC in terms of estimated blood loss, perioperative transfusion rates and fewer perioperative major complications. We propose that RARC is a safer treatment modality with equivalent oncological outcomes compared to ORC.
Keywords: Complications, Cystectomy, Robotic surgical procedures, Urinary bladder neoplasms
INTRODUCTION
Radical cystectomy (RC) and pelvic lymph node dissection are being used widely for the treatment of localized muscle invasive bladder cancer and high risk, recurrent nonmuscle invasive disease, including carcinoma in situ (CIS) [1]. Open RC (ORC) is still thought to be the standard treatment, but perioperative complications and high morbidity remained despite improved surgical techniques [2]. Minimally invasive surgeries have been established to overcome these challenges [3].
Menon et al. [4] reported robot assisted radical cystectomy (RARC) for the first time in 2003. Since then, many studies have compared ORC and RARC [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. RARC has shown not only lower rates of complications and faster recovery but also equivalent oncologic outcomes compared to ORC. Galich et al. [5] reported that RARC showed significantly lower blood loss and shorter hospital stay, but longer operation time compared with ORC. Similarly, Wang et al. [6], while comparing 21 ORC and 33 RARC cases, reported longer operation time but lower blood loss, lower transfusion rate, and shorter hospital stay for RARC. Furthermore, their findings suggest that the robotic approach can yield pathological measures of early oncological efficacy equivalent to that of a concurrent series of patients undergoing ORC. However, large, randomized studies with long-term functional and oncological outcomes are still awaited to better define the role of RARC in the surgical treatment of bladder cancer.
Our hospital started performing RARC since 2008. We reported our operative techniques and initial experiences regarding RARC in March 2010 [22]. Between October 2009 and November 2009, 17 consecutive patients underwent RARC (13 ileal conduits, 4 orthotopic neobladders). Since then, we have concluded that RARC was a safe and feasible operative procedure, with minimal blood loss and rapid recovery. However, the sample population in this previous study was small. Furthermore, the study design was descriptive in nature and not comparative. As that study period was also short, longer-term data was needed for further evaluation of RARC. To expand upon our previous findings, we compared our RARC and ORC cohorts with regards to perioperative complications and analyzed their long-term oncologic outcomes.
MATERIALS AND METHODS
1. Patients
Between August 2008 and May 2014, 112 patients underwent either RARC (n=42) or ORC (n=70) at our hospital following Institutional Review Board approval (approval number: KNUMC 201302012). Bef ore RC, transurethral resections of bladder tumor were performed in all patients. Following histopathological examination and imaging work-up, RC was performed. The indications for RC included muscle-invasive bladder cancer without evidence of distant metastasis (clinical T2-4, Nx, M0), recurrent multifocal superficial tumor refractory to repeated transurethral resection, and bacille Calmette-Guerin-resistant CIS. Exclusion criteria included previous pelvic radiation, clinical stage M1, and patients who underwent combination surgery. The clinical T stage was based on the 2010 American Joint Committee on Cancer TNM staging system for bladder cancer [23]. Patients with pT3, pT4, or node-positive disease based on the analysis of RC specimens but with good performance status, received at least 4 cycles of cisplatin-based chemotherapy. Each patient was followed-up and managed according to standard practice.
Parameters examined included sex, American Society for Anesthesiologists (ASA) classification score, operation time, age, body mass index, type of urinary diversion, operator, estimated blood loss, transfusion rate, complications, time to diet and discharge, and oncological outcomes (tumor stage, grade, lymph node state, metastasis-free survival [MFS], overall survival [OS], and cancer specific survival [CSS]). Surgery-related complications were classified using the Clavien-Dindo classification as reported previously [24,25].
2. Surgical technique
ORC was performed through a midline incision in the traditional manner [26,27]. RARC was performed by same surgical procedure as reported by Menon et al. [4]. Patients were placed in the extended lithotomy with a 30° Trendelenburg position. A 6-port transperitoneal approach was used. A 12-mm camera port was inserted 5 cm above the upper umbilical margin and two 8-mm robotic ports were placed 8 cm away from the umbilicus, along with the line from the umbilicus to the anterior spine of the iliac crest bilaterally. An additional 8-mm robotic port for the fourth arm was placed 8 cm directly lateral from the right-sided robotic port. A 12-mm assistant port for retraction and stapling was placed 8 cm directly lateral from the left-side robotic port. A further 5-mm assistant port for suction and irrigation was placed on the left side between the camera port and the left robotic port. Following docking of the robotic system, RC was performed by the same process as standard laparoscopic RC. Standard pelvic lymphadenectomy (both obturator- and external iliac nodes) was performed in all patients, except for 2 patients undergoing RARC and 7 patients undergoing ORC because of severe adhesions. All patients underwent extracorporeal urinary diversions. A 5- to 7-cm midline incision below the umbilicus was made for specimen removal and urinary diversion. In case of an ileal conduit, uretero-ileal anastomosis was performed over 6-Fr double J stents using a 4–0 polydioxanone suture, and the distal end of the conduit was fashioned as a stoma at the right port site of the robot arm. All orthotopic neobladders were performed using the Studer method and ureteral stents. Urethro-enteric anastomosis was then performed intracorporeally after redocking the robotic system. A Jackson-Pratt drain was placed in the pelvic cavity and around the uretero-enteric anastomosis site. The nasogastric tube was removed 4 days after surgery and oral liquids were started as tolerated. The drain and ureteral stents were removed 2–3 weeks postsurgery. Patients were reviewed at 4 weeks and assessed by renal ultrasonography (2 weeks poststent removal) and computed tomography scans (3 and 6 months postoperatively, and then at 6-month intervals). Every visit comprised a clinical examination with assessment of hemoglobin, creatinine, chloride, bicarbonate, and urethral washing cytology [22].
3. Statistical analysis
Several perioperative parameters and oncologic outcomes including MFS, CSS, and OS of RARC and ORC were compared using Student t-test, chi-square test, and Kaplan-Meier analysis. Statistical analysis was performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA), with p-values <0.05 considered statistically significant.
RESULTS
1. Patient demographic findings
Patient demographics are compared in Table 1 for those who underwent RARC (n=42) and ORC (n=70). There were no significant differences in age, sex, ASA score, body mass index, neoadjuvant chemotherapy, and lymph node dissection between RARC and ORC. In the RARC group, 29 patients underwent ileal conduit, 11 Studer neobladder, 1 ureterocutaneostomy, and 1 percutaneous nephrostomy. In the ORC group, 56 patients underwent ileal conduit, 1 Studer neobladder, 12 ureterocutaneostomy, and 1 percutaneous nephrostomy (Table 1). Neobladder procedures were performed more frequently in the RARC group than in the ORC group, while ileal conduit was more common among patients in the ORC group than among those in the RARC group (p<0.001).
Table 1. Patient demographics.
| Variable | RARC (n=42) | ORC (n=70) | p-value |
|---|---|---|---|
| Age (y) | 70 (31??6) | 70 (44??4) | 0.739 |
| Sex | 0.948 | ||
| Male | 35 (83.3) | 58 (82.9) | |
| Female | 7 (16.7) | 12 (17.1) | |
| ASA score | 2 (1??) | 2 (1??) | 0.216 |
| Body mass index (kg/m2) | 22.2 (18.4??9.4) | 22.3 (14.9??1.6) | 0.936 |
| Urinary diversion | <0.001 | ||
| Ileal conduit | 29 (69.0) | 56 (80.0) | |
| Studer neobladder | 11 (26.2) | 1 (1.4) | |
| Ureterocutaneostomy | 1 (2.4) | 12 (17.1) | |
| Both PCN | 1 (2.4) | 1 (1.4) | |
| Neoadjuvant chemotherapy | 0.799 | ||
| No | 34 (81.0) | 58 (82.9) | |
| Yes | 8 (19.0) | 12 (17.1) | |
| Lymph node dissection | 0.550 | ||
| None | 2 (4.8) | 7 (10.0) | |
| Standard | 38 (90.5) | 61 (87.1) | |
| Extended | 2 (4.8) | 2 (2.9) |
Values are presented as median (range) or number (%).
RARC, robot-assisted radical cystectomy; ORC, open radical cystectomy; ASA, American Society for Anesthesiologists; PCN, percutaneous nephrostomy.
2. Perioperative variables
There were no differences in median time to diet, time to discharge, overall complication rates, and perioperative mortality between RARC and ORC. However, the median operation time was 75 minutes longer in the RARC group than in the ORC group (RARC 480 minutes vs. ORC 405 minutes, p=0.004). Blood loss was significantly less with RARC (median: RARC 330 mL vs. ORC 598 mL, p=0.001). The proportion of patients receiving peri- and intraoperative blood transfusion was 23.8% in the RARC group and 45.7% in the ORC group (p=0.020). Using the Clavien-Dindo classification system to classify perioperative morbidity and mortality, the RARC and ORC groups did not differ significantly. However, the RARC group showed lower major complication rate (RARC 26.2% vs. ORC 45.7%, p=0.040) (Table 2) and zero perioperative mortality.
Table 2. Perioperative variables.
| Variable | RARC (n=42) | ORC (n=70) | p-value |
|---|---|---|---|
| Operation time (min) | 480 (287–760) | 405 (205–665) | 0.004 |
| Blood loss (mL) | 300 (125–925) | 598 (150–2,000) | 0.001 |
| Transfusion rate | 0.020 | ||
| No | 32 (76.2) | 38 (54.3) | |
| Yes | 10 (23.8) | 32 (45.7) | |
| Time to diet (d) | 5 (3–11) | 6 (2–29) | 0.300 |
| Time to discharge (d) | 19 (10–56) | 21 (9–96) | 0.507 |
| Overall complication rate | 27/42 (64.3) | 46/70 (65.7) | 0.878 |
| Major complication (Clavien-Dindo grade ≥ IIIA) | 11/42 (26.2) | 32/70 (45.7) | 0.040 |
| Perioperative mortality | 0/42 (0) | 2/70 (2.9) | 0.269 |
Values are presented as median (range) or number (%).
RARC, robot-assisted radical cystectomy; ORC, open radical cystectomy.
3. Complications
Regarding complications, 15 patients (35.7%) undergoing RARC and 33 patients (47.1%) undergoing ORC had 1 or more complications throughout hospitalization. Postoperative ileus was the most common complication during hospitalization, occurring in 7 of 42 patients (16.7%) and 11 of 70 patients (15.7%) for the RARC and ORC groups, respectively (p=0.894). The second most common complication was urinary tract infection during hospitalization (RARC 9.5% vs. ORC 8.6%, p=0.864).
Hydronephrosis (ureteral stricture) was the most common post-hospital discharge complication, occurring in 12 of 42 patients (28.6%) and 12 of 70 patients (17.1%) for the RARC and ORC groups, respectively (p=0.154). The second most common complication following discharge was also urinary tract infection (RARC 11.5% vs. ORC 11.4%, p=0.939). There were no statistically significant differences between RARC and ORC in the overall postoperative complications (RARC 64.3% vs. ORC 65.7%, p=0.878) (Tables 3, 4).
Table 3. Overall complications.
| Complication | RARC (n=42) | ORC (n=70) | p-value |
|---|---|---|---|
| Early complicationa | 15 (35.7) | 33 (47.1) | 0.237 |
| Febrile UTI | 4 (9.5) | 6 (8.6) | 0.864 |
| Ileus | 7 (16.7) | 11(15.7) | 0.894 |
| Ascites | 1 (2.4) | 2 (2.9) | 0.880 |
| Wound disruption | 1 (2.4) | 4 (5.7) | 0.408 |
| Atelectasis | 1 (2.4) | 1 (1.4) | 0.713 |
| Pulmonary edema | 0 (0) | 2 (2.9) | 0.269 |
| Cardiogenic event | 0 (0) | 4 (5.7) | 0.115 |
| Hydronephrosis | 1 (2.4) | 1 (1.4) | 0.713 |
| Hernia | 0 (0) | 1 (1.4) | 0.437 |
| Rectal perforation | 0 (0) | 1 (1.4) | 0.437 |
| Late complicationa | 21 (50) | 34 (48.5) | 0.884 |
| Febrile UTI | 5 (11.5) | 8 (11.4) | 0.939 |
| Ileus | 2 (4.8) | 5 (7.1) | 0.599 |
| Ascites | 0 (0.0) | 2 (2.9) | 0.269 |
| Wound disruption | 0 (0.0) | 1 (1.4) | 0.437 |
| Hydronephrosis | 12 (28.6) | 12 (17.1) | 0.154 |
| Stone formation | 2 (4.8) | 0 (0) | 0.065 |
| Pancytopenia | 0 (0) | 1 (1.4) | 0.437 |
| Cerebrovascular accident | 0 (0) | 1 (1.4) | 0.437 |
| Hernia | 0 (0) | 3 (4.3) | 0.174 |
| Perineal abscess | 0 (0) | 1 (1.4) | 0.437 |
Values are presented as number (%).
RARC, robot-assisted radical cystectomy; ORC, open radical cystectomy; UTI, urinary tract infection.
a:We defined early complications as those developed during hospitalization and late as those developed postdischarge.
Table 4. Major complications.
| Major complication (Clavien-Dindo grade≥IIIA) | RARC (n=42) | ORC (n=70) | p-value |
|---|---|---|---|
| Hydronephrosis | 9 (21.4) | 11 (15.7) | 0.445 |
| Sepsis | 0 (0) | 7 (10.0) | 0.034 |
| Ascites | 0 (0) | 3 (4.3) | 0.174 |
| Hernia | 0 (0) | 3 (4.3) | 0.174 |
| Wound disruption | 0 (0) | 3 (4.3) | 0.174 |
| Stone formation | 1 (2.4) | 0 (0) | 0.195 |
| Ileus (Bowel obstruction) | 1 (2.4) | 0 (0) | 0.195 |
| Perineal abscess | 0 (0) | 1 (1.4) | 0.437 |
| Pancytopenia | 0 (0) | 1 (1.4) | 0.437 |
| Cardiogenic event | 0 (0) | 1 (1.4) | 0.437 |
| Pneumonia | 0 (0) | 1 (1.4) | 0.437 |
| Rectal perforation | 0 (0) | 1 (1.4) | 0.437 |
Values are presented as number (%).
RARC, robot-assisted radical cystectomy; ORC, open radical cystectomy.
As shown in the Table 4, hydronephrosis (ureteral stricture) was the most common major complication (Clavien-Dindo classification grade≥IIIA) in both groups. There were more patients with sepsis in the ORC compared to the RARC group (10.0% vs. 0%, p=0.034). We did not observe any significant differences in the type of major complications between the 2 groups, with the exception of low incidence of sepsis in the RARC group.
4. Histopathological outcomes
Histopathological data are shown in Table 5. Nonmuscle invasive bladder tumor (Ta, T1, CIS) was present on in 11 of 42 patients (26.2%) and 21 of 70 patients (30.0%) in the RARC and ORC groups, respectively. Meanwhile, 18 of 42 patients (42.9%) in the RARC group and 32 of 70 patients (45.7%) in the ORC group had pT3 or pT4 (p=0.887). High-grade urothelial carcinoma was the most common histopathological grade in both groups (90.5% vs. 94.3%, p=0.565). Pathologic N stage, lymphovascular invasion, and adjuvant chemotherapy showed no significant differences between RARC and ORC (Table 5).
Table 5. Histopathological outcomes.
| Variable | RARC (n=42) | ORC (n=70) | p-value |
|---|---|---|---|
| Histopathological T stage | 0.887 | ||
| Ta, T1, CIS | 11 (26.2) | 21 (30.0) | |
| T2 | 13 (31.0) | 17 (24.3) | |
| T3 | 12 (28.6) | 22 (31.4) | |
| T4 | 6 (14.3) | 10 (14.3) | |
| Pathologic grade | 0.565 | ||
| High grade | 38 (90.5) | 66 (94.3) | |
| Low grade | 1 (2.4) | 2 (2.9) | |
| Other | 3 (7.1) | 2 (2.9) | |
| Pathologic N stage | 0.614 | ||
| N0 | 31 (73.8) | 49 (70.0) | |
| N+ | 9 (21.4) | 14 (20.0) | |
| Nx | 2 (4.8) | 7 (10.0) | |
| Lymphovascular invasion | 0.969 | ||
| No | 33 (78.6) | 55 (78.3) | |
| Yes | 9 (21.4) | 15 (21.7) | |
| Adjuvant chemotherapy | 0.124 | ||
| No | 31 (73.8) | 41 (59.4) | |
| Yes | 11 (26.2) | 28 (40.6) |
Values are presented as number (%).
RARC, robot-assisted radical cystectomy; ORC, open radical cystectomy; CIS, carcinoma in situ.
5. Survival analysis: MFS, CSS, and OS
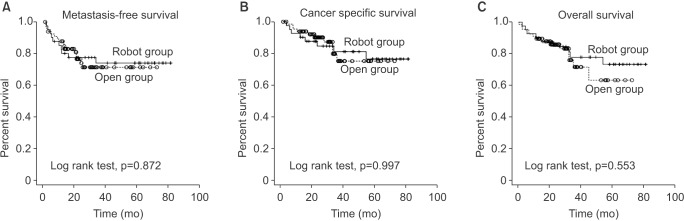
The median follow-up period was 40 months (range, 0–70 months) vs. 42 months (range, 0–74 months) in RARC and ORC, respectively. Metastases in RARC and ORC was 23.8% (10 of 42) and 23.2% (16 of 70), respectively during follow-up periods. There were 22 deaths; 18 of these were related to cancer. According to the Kaplan-Meier survival curve analysis, RARC and ORC outcomes were similar with respect to MFS, CSS and OS (log-rank test, p-values; 0.872, 0.997, 0.553, respectively) (Fig. 1).
Fig. 1. Metastasis-free survival (A), cancer specific survival (B), overall survival (C) between robot-assisted radical cystectomy and open radical cystectomy.
DISCUSSION
Ever since RARC was introduced by Menon et al. [4], many comparative studies between RARC and ORC have been done [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. These studies showed that RARC is associated with lower median blood loss, lower need for perioperative transfusion and shorter postoperative hospitalization. These results indicate that RARC might be safer compared to ORC. RARC has been performed at our institute since 2008. We collected and analyzed data from patients who underwent RARC between 2008 and 2014, and compared the perioperative and oncological outcomes between RARC and ORC at this institute.
Our study demonstrated significantly lower median blood loss in the RARC group than in the ORC group (p=0.001), with a consequent decrease in transfusion rates (p=0.020). These results were in accordance with previous published data [7,8,11,18]. This may have been secondary to the excellent visualization provided by the da Vinci Surgical Robotic System, which in turn facilitated meticulous dissection and bleeding control. Furthermore, robot-assisted surgery is usually performed with an intra-abdominal pressure of 12–15 mmHg, which prevents oozing of small veins [3]. These may have contributed to lower median blood loss. As reported recently, perioperative transfusion is associated with adverse prognosis, including OS and CSS in patients with RC [28]. Therefore, by decreasing intraoperative blood loss, RARC might provide a significant advantage over ORC.
Operation time for RARC is longer than that for ORC (p=0.004). The longer time can be attributed to the extraoperative steps associated with trocar placement as well as the docking and un-docking of the robot arm. Additionally, neobladder procedures were performed more frequently in the RARC cohort than in the ORC group. Sung et al. [12] showed that the RARC group had more cases of ileal neobladder urinary diversion. Musch et al. [19] also reported longer operation time in RARC, similar to our study.
Overall perioperative complication rates were similar between RARC and ORC (p=0.878), while major complication (Clavien-Dindo classification grade≥IIIA) rates were comparable between the 2 groups; 11 (26.2%) and 32 (45.7%) in RARC and ORC, respectively (p=0.040). These results were also consistent with those reported by Sung et al. [12]. Those authors reported that the ORC group had significantly more grade II (or greater) complications, wound problems, and multiple complications than RARC group.
In case of ureteral stricture with hydronephrosis, there were no statistically difference between RARC and ORC. But there were a fewer sepsis in RARC group. We think that the reason why RARC has a fewer sepsis was that. A little manipulation of intra-abdominal organs, especially ureter. Because of relatively little manipulation, ureteral stricture in RARC could be less likely to occur. Also as we can see that more postoperative complications occur in ORC (e.g., bowel perforation, hernia, wound disruption). It is considered that broad manipulation and long-term traction of skin and muscles to get a clear operation view have a influence on postoperative complications
Additionally we needed to consider for learning curve. The data we had selected have almost same beginning of period between ORC and RARC. RARC initial date: 2008.08.19. ORC initial date: 2008.4.24. At that time we started a research, surgeon already had experienced performing over 50 ORC procedures.
Nepple et al. [13]. reported early oncologic outcomes of RARC vs. ORC. There were no differences in surgical cystectomy pathology between the 2 groups. In addition, the 2-year outcomes for recurrence-free, disease-specific-and overall survival were similar between RARC and ORC. However, the median follow-up period of the above study was just 12.2 months. In contrast, the median follow-up period was 40 months (range, 0–70 months) vs. 42 months (range, 0–74 months) in RARC and ORC, respectively. In these patients, the RARC and ORC outcomes were similar with respect to MFS, CSS, and OS. This factor was the strongest point in our study compared to previous studies.
Cost analysis, although not one of our study outcomes, deserved to be discussed because of its importance to a patient. RARC is costlier than ORC [14]; in Korea, it is approximately 5 times the cost of ORC. However, given the indirect costs of complications, lower blood loss, and lower transfusion rates, RARC is likely worthy of its price.
Although RARC has its advantages, ORC is still an important and indispensable procedure every urologist should learn. Even in the United States, which is equipped with the largest number of robotic surgical system in the world, RARC comprised only a small portion of total RC cases. Data from the Nationwide Inpatient Sample in 2009 to 2011 showed that RARC accounted for about 12% of all RC cases [29]. ORC is the gold standard for muscle-invasive bladder cancer, and its training can familiarize younger surgeons with the anatomical structures. Besides, surgical skills and experience on open cases are essential, as all surgeons must be prepared for intraoperative conversion during any minimally invasive procedure. However, we believe that refining surgical equipment and improving the knowledge of younger urologists will help in making RARC the treatment of choice for bladder cancer in future.
CONCLUSIONS
Our results revealed that RARC is more advantageous compared to ORC in terms of median blood loss, perioperative transfusion rates, and lower major complications (Clavien-Dindo classification grade ≥ IIIA), whereas histopathologic findings, overall complications and survival rates did not demonstrate significant differences. We propose that RARC is a safer treatment modality with equivalent oncological outcomes compared to ORC.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (2014R1A1A3049460); (NRF-2014M3A9D3033887), and supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI14C1642).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Chang SS, Wells N, Parekh DJ, Smith JA., Jr Complications of radical cystectomy for nonmuscle invasive disease: comparison with muscle invasive disease. J Urol. 2003;169:101–104. doi: 10.1016/S0022-5347(05)64045-1. [DOI] [PubMed] [Google Scholar]
- 3.Sterrett S, Mammen T, Nazemi T, Galich A, Peters G, Smith L, et al. Major urological oncological surgeries can be performed using minimally invasive robotic or laparoscopic methods with similar early perioperative outcomes compared to conventional open methods. World J Urol. 2007;25:193–198. doi: 10.1007/s00345-006-0140-9. [DOI] [PubMed] [Google Scholar]
- 4.Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232–236. doi: 10.1046/j.1464-410x.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 5.Galich A, Sterrett S, Nazemi T, Pohlman G, Smith L, Balaji KC. Comparative analysis of early perioperative outcomes following radical cystectomy by either the robotic or open method. JSLS. 2006;10:145–150. [PMC free article] [PubMed] [Google Scholar]
- 6.Wang GJ, Barocas DA, Raman JD, Scherr DS. Robotic vs open radical cystectomy: prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy. BJU Int. 2008;101:89–93. doi: 10.1111/j.1464-410X.2007.07212.x. [DOI] [PubMed] [Google Scholar]
- 7.Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010;57:274–281. doi: 10.1016/j.eururo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Gondo T, Yoshioka K, Nakagami Y, Okubo H, Hashimoto T, Satake N, et al. Robotic versus open radical cystectomy: prospective comparison of perioperative and pathologic outcomes in Japan. Jpn J Clin Oncol. 2012;42:625–631. doi: 10.1093/jjco/hys062. [DOI] [PubMed] [Google Scholar]
- 10.Khan MS, Challacombe B, Elhage O, Rimington P, Coker B, Murphy D, et al. A dual-centre, cohort comparison of open, laparoscopic and robotic-assisted radical cystectomy. Int J Clin Pract. 2012;66:656–662. doi: 10.1111/j.1742-1241.2011.02888.x. [DOI] [PubMed] [Google Scholar]
- 11.Styn NR, Montgomery JS, Wood DP, Hafez KS, Lee CT, Tallman C, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology. 2012;79:1303–1308. doi: 10.1016/j.urology.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Sung HH, Ahn JS, Seo SI, Jeon SS, Choi HY, Lee HM, et al. A comparison of early complications between open and robotassisted radical cystectomy. J Endourol. 2012;26:670–675. doi: 10.1089/end.2011.0372. [DOI] [PubMed] [Google Scholar]
- 13.Nepple KG, Strope SA, Grubb RL, 3rd, Kibel AS. Early oncologic outcomes of robotic vs. open radical cystectomy for urothelial cancer. Urol Oncol. 2013;31:894–898. doi: 10.1016/j.urolonc.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin AD, Nunez RN, Castle EP. Robot-assisted radical cystectomy versus open radical cystectomy: a complete cost analysis. Urology. 2011;77:621–625. doi: 10.1016/j.urology.2010.07.502. [DOI] [PubMed] [Google Scholar]
- 15.Abaza R, Dangle PP, Gong MC, Bahnson RR, Pohar KS. Quality of lymphadenectomy is equivalent with robotic and open cystectomy using an extended template. J Urol. 2012;187:1200–1204. doi: 10.1016/j.juro.2011.11.092. [DOI] [PubMed] [Google Scholar]
- 16.Ahdoot M, Almario L, Araya H, Busch J, Conti S, Gonzalgo ML. Oncologic outcomes between open and robotic-assisted radical cystectomy: a propensity score matched analysis. World J Urol. 2014;32:1441–1446. doi: 10.1007/s00345-014-1242-4. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Lin T, Fan X, Xu K, Bi L, Duan Y, et al. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev. 2013;39:551–560. doi: 10.1016/j.ctrv.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013;189:474–479. doi: 10.1016/j.juro.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 19.Musch M, Janowski M, Steves A, Roggenbuck U, Boergers A, Davoudi Y, et al. Comparison of early postoperative morbidity after robot-assisted and open radical cystectomy: results of a prospective observational study. BJU Int. 2014;113:458–467. doi: 10.1111/bju.12374. [DOI] [PubMed] [Google Scholar]
- 20.Knox ML, El-Galley R, Busby JE. Robotic versus open radical cystectomy: identification of patients who benefit from the robotic approach. J Endourol. 2013;27:40–44. doi: 10.1089/end.2012.0168. [DOI] [PubMed] [Google Scholar]
- 21.Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK. Robot-assisted laparoscopic vs open radical cystectomy: comparison of complications and perioperative oncological outcomes in 200 patients. BJU Int. 2013;112:E290–E294. doi: 10.1111/bju.12167. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SY, Kim BS, Kim TH, Yoo ES, Kwon TG. Initial experiences with robot-assisted laparoscopic radical cystectomy. Korean J Urol. 2010;51:178–182. doi: 10.4111/kju.2010.51.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osunkoya AO, Grignon DJ. Practical issues and pitfalls in staging tumors of the genitourinary tract. Semin Diagn Pathol. 2012;29:154–166. doi: 10.1053/j.semdp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyun JH, Kim HK, Kim JY, Kim SB, Cho S, Kang SG, et al. Standardized analysis of complications after robot-assisted radical cystectomy: Korea University Hospital experience. Korean J Urol. 2015;56:48–55. doi: 10.4111/kju.2015.56.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein JP, Quek ML, Skinner DG. Lymphadenectomy for invasive bladder cancer. II. technical aspects and prognostic factors. BJU Int. 2006;97:232–237. doi: 10.1111/j.1464-410X.2006.05901.x. [DOI] [PubMed] [Google Scholar]
- 27.Stein JP, Skinner DG. Surgical atlas: radical cystectomy. BJU Int. 2004;94:197–221. doi: 10.1111/j.1464-410X.2004.04981.x. [DOI] [PubMed] [Google Scholar]
- 28.Linder BJ, Frank I, Cheville JC, Tollefson MK, Thompson RH, Tarrell RF, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013;63:839–845. doi: 10.1016/j.eururo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Monn MF, Cary KC, Kaimakliotis HZ, Flack CK, Koch MO. National trends in the utilization of robotic-assisted radical cystectomy: an analysis using the Nationwide Inpatient Sample. Urol Oncol. 2014;32:785–790. doi: 10.1016/j.urolonc.2014.04.007. [DOI] [PubMed] [Google Scholar]