Abstract
Aim
To analyse and predict early response 3 months post definitive chemoradiation (CCRT) utilising tumour volume (TV) measurement in locally advanced head and neck cancers (LAHNC).
Background
LAHNC are 3-dimentional lesions. The largest diameter of these tumours measured for T-classification may not necessarily reflect the true tumour dimensions. TV accurately reflects the tumour burden because it is a measurement of tumour burden in all three dimensions.
Materials and methods
It is a single institutional prospective study including 101 patients with LAHNC treated with definitive CCRT. TV data noted were primary tumour volume (PTV), total nodal volume (TNV) and total tumour volume (TTV). Response evaluation was done at 3 months after the completion of definitive CCRT and patients were categorised either having achieved complete response (CR) or residual disease.
Results
Patients who had not achieved CR were found to have larger TV compared with those who had achieved CR. There were significant inverse correlations between PTV and response (median 16.37 cm3 vs. 45.2 cm3; p = 0.001), and between TTV and response (median 36.14 cm3 vs. 66.06 cm3; p < 0.001). Receiver operating characteristic (ROC) analysis identified an “optimal cut-off” value of 41 cm3 for PTV and 42 cm3 for TTV above and below which the magnitude of difference in response was the greatest.
Conclusions
If response evaluation 3 months post CCRT is to be predicted it is simply not enough to measure the largest single dimension of the tumour. TV seems to be a better and more accurate reflection of the true total tumour burden or extent of the disease.
Keywords: Chemoradiation, Early response, Head neck, Prognostic factors, Tumour volume
1. Background
Head and neck cancers (HNC), particularly locally advanced, are a group of heterogeneous tumours and are diverse in their natural history and behaviour. To understand and bring this diversity into clinical use, the American Joint Committee on Cancer (AJCC) uses a TNM staging system. Amongst many others, the purpose of this staging system is to predict prognosis.
The TNM system is an expression of the anatomic extent of disease. T classification in TNM staging system depends mainly on the measurement of maximum single dimension of the primary tumour. Although this system of staging is user-friendly, universally applicable and provides the basis for most cancer care and research, it is felt that this system has certain limitations. Firstly, HNC are 3-dimensional lesions, not only spreading into different planes and directions within the head and neck region but also with unequal rate of spread/invasion/infiltration into the surrounding tissues. Hence, the largest diameter of tumour measured for T classification may not necessarily reflect the true tumour dimensions. Indeed, studies have shown that there is a significant variation in the tumour volumes within the same T-classification.1, 2 This reflects the poor ability of T-classification to describe true dimensions of locally advanced head and neck cancers (LAHNC). Secondly, for certain sites, T-classification takes into account tumour features which are mainly important for surgeons to decide on operability or resectability, such as invasion and infiltration of primary tumour into surrounding important structures. Although this information is of paramount significance to surgeons, it is not so for a non-surgical treatment modality such as definitive radiotherapy (RT) or concurrent chemoradiation (CCRT).
Thus, there is a need to take into account certain other feature(s) of these heterogeneous tumours, besides maximum single dimension, which can reflect the total tumour burden more accurately. While doing so we must also ensure that we do not lose out the very vital information about the anatomical extent of disease provided by the TNM staging system.
Tumour develops from a single transformed cell. In order to completely sterilise a tumour by RT or, in other words, achieve complete response at the end of RT, every single clonogenic cell capable of tumour growth has to be killed. Many authors have shown that the number of clonogenic cells increases almost linearly with increase in tumour load and thus tumour volume (TV).3, 4 But it was Fletcher who first proved that there does exist a direct relationship between clonogen numbers and TV.5 Hence, it seems that there exists a relationship between the probability of tumour response to RT and the TV. There is growing evidence recognising positive correlation between TV and prognosis.6, 7, 8 Knegjens et al.9 have shown TV to be a good predictor of early response to RT, there being larger TV in those with residual disease.
2. Aim
This study aims at analysing and predicting early response (3 months post definitive CCRT) utilising TV data in patients with LAHNC in the era of intensity modulated RT (IMRT). While doing so we will also discuss various ways in which TV data can be incorporated in day to day clinical practice to predict early response and few related issues.
3. Materials and methods
It was a single institutional prospective study conducted from June 2013 to April 2015. In the study, 108 newly diagnosed patients with LAHNC treated with definitive CCRT were recruited. All patients provided informed consent for the proposed treatment. Clearance from the institute's review and scientific committee board was obtained. Patient inclusion criteria were histologically proven squamous cell carcinoma of any grade, oropharyngeal, hypopharyngeal or laryngeal primary sites, accurately TNM staged locally advanced disease – stage III or IV non-metastatic disease, clear and well visualised primary and nodal disease on diagnostic imaging, age >18 years, no co-existing or prior malignancy in the head and neck region, no prior RT to the head and neck region, and eastern cooperative oncology group status ≤2. All underwent staging as per AJCC recommendations. The pre-treatment work up included detailed history, physical examination, and endoscopic assessment for primary tumour extent and to rule out synchronous malignancy. 18F-fluorodeoxy-d-glucose (18F-FDG) positron emission tomography–computed tomography (PET CT) was done to assess for loco-regional tumour extent and distant metastasis. All patients were planned for definitive CCRT.
3.1. Diagnostic imaging, RT planning and simulation, and tumour volume measurement
PET-CT scans were performed using full ring dedicated PET scanner (Siemens Biograph scanner, Lutetium Oxyorthosilicate crystal based 40 slice scanner) in 3D mode. Non-contrast CT scans (120 kV, 80 mA) were performed for attenuation correction and anatomical localisation. Standard whole body PET-CT scans were acquired from skull base to mid-thigh. The acquisition time was 120–180 s/bed position. An appropriate head rest was used during PET-CT. After the diagnostic scan was done, the table couch was changed to a flat table couch. The patients were immobilised with ordinary head, neck and shoulder thermoplastic orfit cast along with the same head rest as used during PET-CT. Alignment was ensured with the help of lasers. Planning CT scans were done with slice thickness of 3 mm from the vertex of skull to carina. The regional scan was taken with the same set up criteria as that of planning CT scan. All data sets were sent by way of Digital Imaging and Communications in Medicine to SomaVision v10 (Varian Medical System, Palo Alto, CA) radiation planning workstation. The contrast-enhanced CT simulation scans were fused with PET-CT scans using ECLIPSE v10 (Varian Medical System, Palo Alto, CA) using automatic image registration algorithm. Fine manual adjustments were required in most cases for more precise fusion. Target volumes and organs at risk (OARs) were contoured.
For the purpose of this research, various TV delineated were as follows: primary tumour volume (PTV) – volume occupied by macroscopic visible primary tumour, yielding the gross demonstrable extent and location of the primary malignant growth; total nodal volume (TNV) – the sum total volume of all macroscopic nodal disease visible; and total tumour volume (TTV) – sum total of PTV and TNV. All TVs were verified by experienced radiologist and appropriate modifications in target delineation were made if required. Volumetric three-dimensional measurements of the contoured PTV and TNV were noted from SomaVision v10 (Varian Medical System, Palo Alto, CA) radiation planning workstation. The TTV was the sum total of PTV and TNV. All the three TV were expressed in cubic centimetres (cm3).
3.2. Treatment protocol
As per institutional protocol all patients were treated with 3 dose target levels simultaneous integrated boost (SIB) IMRT. The 3 clinical target volumes (CTV) delineated were prescribed following doses: CTV1 = 70 Gy, CTV2 = 63 Gy and CTV3 = 50 Gy in 35 fractions, daily once and 5 days a week. All patients received concurrent weekly cisplatin chemotherapy 40 mg/m2.
3.3. Disease response evaluation
Disease response evaluation was done at 12 weeks after the completion of definitive CCRT by clinical examination, PET-CT and endoscopic assessment. PET-CT done at this time gap after the completion of CCRT provides the best specificity, sensitivity and very high negative predictive value (approximately 95%). Complete response (CR) by PET was defined as complete disappearance of FDG activity attributable to tumour, without regard to the degree of CT response, as assessed on fused PET-CT. Those found to have CR were planned to follow up regularly. Those found to have residual disease (non-complete responders) of any amount were discussed in tumour board and if found operable underwent salvage surgery, and if inoperable underwent palliative chemotherapy/targeted therapy/supportive care. Further follow up was continued as per institutional protocol.
3.4. Statistical analysis
Statistical analysis was conducted with the statistical package for the social science system (SPSS) version 20.0. Continuous variables were presented as mean or median and interquartile range (IQR). The Spearman's rank-order correlation test was run to measure the strength and direction of the association between either two continuous variables, two ordinal variables and one ordinal and one continuous variable. The Kruskal–Wallis H test was used to determine if there was a statistically significant difference between two or more groups of an independent variable on a continuous dependent variable. A receiver operating characteristics (ROC) analysis was performed to determine an “optimal threshold cut-off value” for PTV, TNV and TTV above and below whichthe difference in response at 3 months of completion of CCRT was the greatest. The area under the curve, sensitivity and specificity were also calculated to analyse the diagnostic value of this “optimal threshold cut-off value”. For all statistical tests, a p value less than 0.05 was taken to be significant. All p values reported represent two-sided tests.
4. Results
Of 108 patients included in the study, 106 completed planned CCRT, 1 defaulted and 1 died of disease progression while on treatment. Of 106 patients who completed CCRT, 101 were available for disease response evaluation at 3 months of completion of CCRT, the remaining 5 were lost to follow up and did not return for 3 months post CCRT evaluation, and these 5 patients were excluded from the analysis. Patient and disease characteristics of these 101 patients available for analysis are summarised in Table 1. Tumour volume data is summarised in Fig. 1.
Table 1.
Patient and disease related characteristics.
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| Median (range) | 57 (38–64) |
| Gender | |
| Male | 91 (90.1) |
| Female | 10 (9.9) |
| Sites | |
| Oropharynx | 61 (60.4) |
| Hypopharynx | 24 (23.8) |
| Larynx | 16 (15.8) |
| Histological grade | |
| Grade I | 7 (6.9) |
| Grade II | 82 (81.2) |
| Grade III | 12 (1.9) |
| Clinical T-stage | |
| T1 | 9 (8.9) |
| T2 | 10 (9.9) |
| T3 | 36 (35.6) |
| T4 | 46 (45.5) |
| Clinical N-stage | |
| N0 | 16 (15.8) |
| N1 | 18 (17.8) |
| N2a | 3 (3) |
| N2b | 18 (17.8) |
| N2c | 40 (39.6) |
| N3 | 6 (5.9) |
| Clinical stage | |
| III | 18 (17.8) |
| IVa | 75 (74.3) |
| IVb | 8 (7.9) |
|
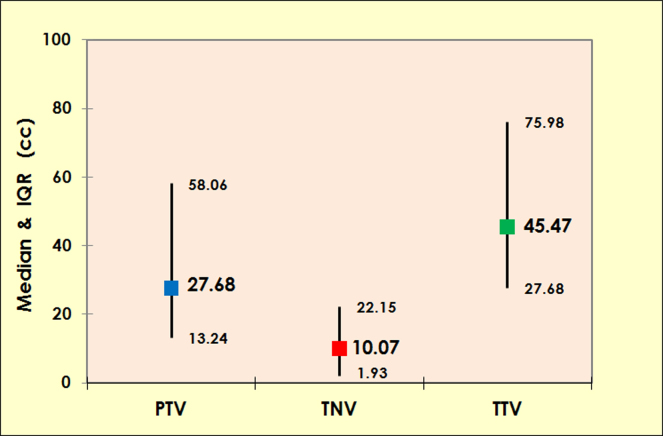
Tumour volumes (cc) (all sites combined) Median (IQRd) | |
| PTVa | 27.68 cc (13.24–58.06) |
| TNVb | 10.07 cc (1.93–22.15) |
| TTVc | 45.47 cc (27.68–75.98) |
Primary tumour volume.
Total nodal volume.
Total tumour volume.
Interquartile range.
Fig. 1.
Graph showing median and interquartile range (in cc) for primary tumour volume (PTV), total nodal volume (TNV) and total tumour volume (TTV) (all sites combined).
Of 101 patients available for analysis, 56 (55.4%) were found to have CR and remaining 45 (44.6%) were found to have residual disease on response evaluation. The 5 patients who were found to have resectable residual disease underwent salvage surgery, and surgical histopathological examinations for all of them were positive for disease.
4.1. Relationship between T stage, N stage, and clinical stage and corresponding TV
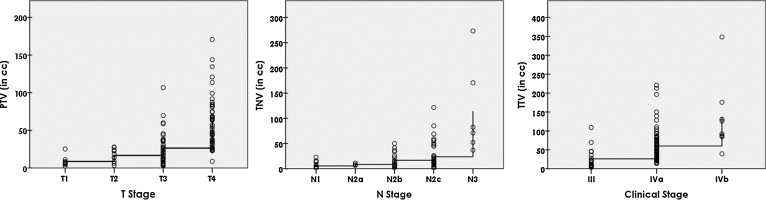
A Spearman's rank-order correlation was run to observe the relationship between T-stage and PTV, N-stage and TNV, and clinical stage and TTV. Preliminary analysis showed the relationship between the variables to be correlated as monotonic, as assessed by visual inspection of scatter plots in Fig. 2. There was a strong positive correlation between T-stage and PTV, rs(99) = 0.686, p < 0.001. That is, PTV increased as T-stage increased. Similarly, there was a strong positive correlation found between N-stage and TNV, rs(83) = 0.540, p < 0.001 and clinical stage and TTV, rs(99) = 0.540, p < 0.001.
Fig. 2.
Scatter plots showing correlations between primary tumour volume (PTV) and T-stage (Left), total nodal volume (TNV) and N-stage (middle) and total tumour volume (TTV) and clinical stage (Right).
4.2. Variability of TV within similar stage
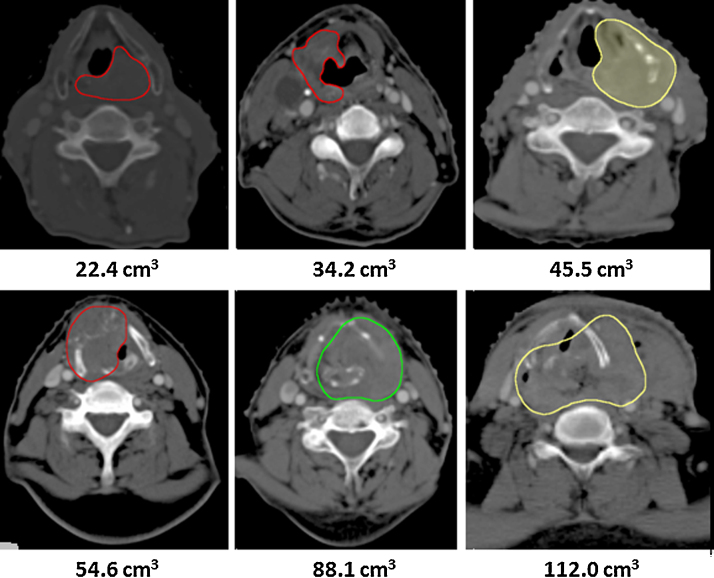
Distributions of PTVs were similar for all four T-stages, T1 (n = 9), T2 (n = 10), T3 (n = 36) and T4 (n = 46), as assessed by visual inspection of a boxplot. A Kruskal–Wallis H test showed median PTVs to be statistically significantly different between four groups of T-stages, χ2(3) = 46.563, p = 0.000. Subsequently, pairwise comparisons were performed using Dunn's (1964) procedure with a Bonferroni correction for multiple comparisons. Adjusted p-values are presented. This post hoc analysis revealed statistically significant differences in median PTV between the T1 (7.29 cm3) and T4 (54.88 cm3) (p = 0.000); T2 (16.08 cm3) and T4 (p = 0.000); and T3 (20.92 cm3) and T4 (p = 0.000); but not between the T1 and T2; T1 and T3; and T2 and T3. One example of variability of PTV within similar T-staged tumours of LAHNC of 6 patients from the current study is shown in Fig. 3.
Fig. 3.
Primary tumours delineated on computed tomography of 6 patients having similar T-stages but different primary tumour volumes (PTV).
Distributions of TNV were similar for all five N-stages, N1 (n = 18), N2a (n = 3), N2b (n = 18), N2c (n = 41) and N3 (n = 6), as assessed by visual inspection of a boxplot. Median TNV were statistically significantly different between five N-stages, χ2(4) = 29.60, p = 0.000. The post hoc analysis revealed statistically significant differences in median TNV between the N1 (2.12 cm3) and N2c (13.62 cm3) (p = 0.001); N1 and N3 (76.60 cm3) (p = 0.000); N2b (11.77 cm3) and N3 (p = 0.016); and N2c (13.62 cm3) and N3 (p = 0.048) but not between other groups.
Similarly, distributions of TTV were similar for all three clinical stages, III (n = 18), IVa (n = 75) and IVb (n = 8), as assessed by visual inspection of a boxplot. Median TTV were statistically significantly different between the three clinical stages, χ2(3) = 27.18, p = 0.000. The post hoc analysis revealed statistically significant differences in median TTV between the stage III (17.81 cm3) and IVa (46.45 cm3) (p = 0.000); III and IVb (p = 0.000); and IVa and IVb (p = 0.000). Thus, these results show that there is a great variability in TV within the same T-stage, N-stage and clinical stage.
4.3. Prediction of early response utilising TV data
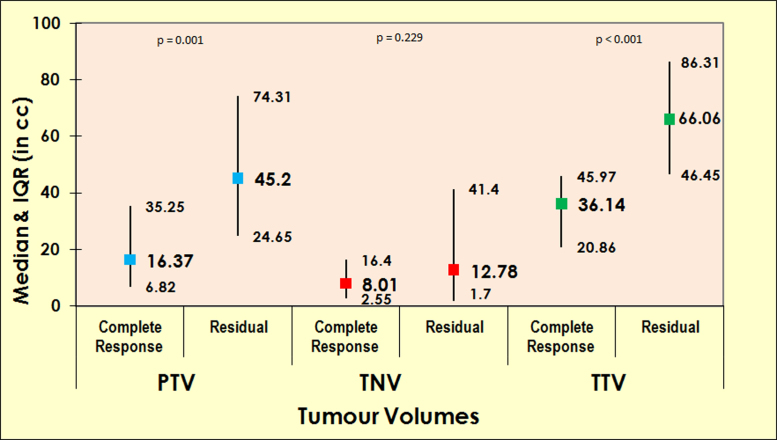
There were significant inverse correlations between PTV and response at 3 months post CCRT (median 16.37 cm3 vs. 45.2 cm3; p = 0.001), and TTV and response at 3 months post CCRT (median 36.14 cm3 vs. 66.06 cm3; p < 0.001). However, there was no significant correlation found between TNV and response (median 8.01 cm3 vs. 12.78 cm3; p = 0.229) (Fig. 4).
Fig. 4.
Graph showing association between tumour volumes and early tumour response.
4.4. Determination of “optimal threshold cut-off” value for each TV to predict early response
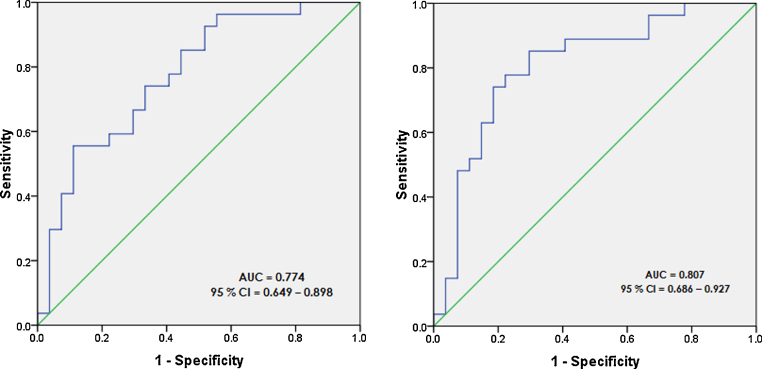
ROC analysis was done to determine an “optimal threshold cut-off” value for PTV and TTV (Fig. 5). PTV of <41 cm3 predicted the CR with a sensitivity of 55.8%, specificity of 88.9%, positive predictive value of 83.3% and negative predictive value of 66.7%. The greatest magnitude of difference in outcome was found in patients having PTV above and below this cut-off of 41 cm3 (p = 0.001). Similarly, ROC analysis for TTV identified an optimal threshold cut-off of <42 cm3, which predicted the CR with a sensitivity of 85.2%, specificity of 70.4%, positive predictive value of 74.2% and negative predictive value of 82.6%. The greatest magnitude of difference in outcome was found in patients having TTV above and below this cut-off of 42 cm3 (p < 0.001). This implies that patients having a PTV <41 cm3 and/or TTV <42 cm3 have the greatest chances of achieving CR.
Fig. 5.
ROC curve for predictive value of primary tumour volume (PTV) in detecting complete response (CR) (left), and total tumour volume (TTV) in detecting CR (right).
5. Discussion
Patients with LAHNC have poor prognosis, approximately half of them achieving CR at 3 months of completion of definitive CCRT. It is therefore important to identify those who will benefit most from this morbid treatment modality. Although AJCC-TNM staging system has been successful to a great extent for prognostication purposes, it does not take into account the true three-dimensional load or burden of this class of heterogeneous and diverse disease. TV data easily calculated from today's RT planning systems seems to be an answer to this limitation of the TNM staging system.
Similar staged HNC do not necessarily have similar TV.1, 2 There is indeed a great variability in TV within the same T-stage, N-stage and clinical stage, as seen in our study too. Considering the growing evidence that TV are associated with prognosis, it seems unjustified to give similar probability of achieving CR with CCRT to two or more patients having similar staged HNC but with different TV. Physician may often face this dilemma in their day to day clinical practice.
It should be the goal of any radiation oncologist to predict residual disease shortly after CCRT and identify early those to whom salvage surgery for cure could be offered as soon as possible before it becomes surgically unresectable or radiation induced fibrosis sets in making surgery difficult. However, the early disease response evaluation post CCRT has proved to be a difficult task due to the post-treatment effects which hinder clinical and imaging (CT and MRI) findings such as delayed anatomical response in the tumour, distortions as a result of early mucositis, late fibrosis, etc. PET-CT has also been found to have poor positive predictive value to detect failure post CCRT owing to radiation-induced acute inflammation.10, 11 Pre-treatment TV measurement can help in the prediction of early response to RT, thereby identifying upfront those who are likely to have residual disease and may require salvage surgery. Knegjens et al.9 in a large multi-institutional study of 361 patients with LAHNC treated with definitive CCRT showed that patients having residual disease post CCRT had larger pre-treatment TV compared with those who had achieved CR (mean 51.9 cm3 vs. 33.8 cm3; p = 0.0001). In our study, too, we found a significant inverse correlation between tumour volumes (PTV and TTV) and response at 3 months. Patients who did not achieve CR at 3 months post CCRT had larger tumour volume (PTV and TTV) compared with those who achieved CR. However TNV was found to be not correlated with response at 3 months in our study. A similar observation was noted by Bhatia et al. in a study of 69 HNC patients for whom MRI was performed at diagnosis (pre-treatment), 2 weeks during the CCRT and 6 weeks after CCRT, and early treatment outcome was correlated with TV. Those who were found to have local failure had higher TV compared to those with local remission, at diagnosis (p = 0.01), 2 weeks during CCRT (p = 0.009) and 6 weeks after CCRT (p = 0.0001), which led to the conclusion that TV based on MRI is predictive of response, however, the correlation is the strongest at 6 weeks post CCRT.12
Having established and concluded that TV affect response at 3 months post CCRT, the next more important relevant question is how to incorporate TV data objectively in day to day clinical practice in predicting early treatment response to CCRT. The most accurate way studied so far is to identify an “optimal threshold cut-off” value (using ROC analysis as explained above) for various TVs, above and below which the magnitude of difference in outcome is the greatest. Various studies with long term tumour control and survival as end points have identified such cut-off values for PTV ranging from 19.6 cm3 to 35 cm3 and for TTV, from 22.8 cm3 to 110 cm3 depending upon the places where these were conducted.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 In our study, for early response evaluation at 3 months of CCRT “optimal threshold cut-off” for PTV and TTV were higher (41 cm3 and 42 cm3, respectively), primarily owing to a large tumour burden in our country. This method may categorise patients into either a favourable group (TV less than cut-off) or unfavourable group (TV more than cut-off) at a pre-treatment stage. For example, a patient with carcinoma oropharynx (base tongue) cT3N2cM0 with TTV 62 cm3 may even be more accurately staged as cT3N2cM0 (unfavourable). Another method of utilising TV data in prediction of early response is to stratify TV in serially increasing categories, for example 1–10 cm3, 11–15 cm3, 16–20 cm3, 21–25 cm3, etc. in which one individual patient's TV can fit in. Yet another method, although least accurate one, could be to dichotomise TV at median or mean value set on the basis of individual or multi-institutional data.
With the advances in radiologic imaging and conformal RT techniques, tumour volume (PTV, TNV and TTV) measurements are easy to obtain. Since all patients undergo pre-treatment imaging, little extra cost is needed to measure TV.
There is a need for newer methods of staging by which response to treatment can be determined more accurately but without losing the very vital anatomical extent of disease information given by TNM system. One such method shown in this study is incorporation of TV data into already existing TNM staging. Tumour volume staging system or derived prognostic cut-off values of tumour volumes may be brought into routine clinical practice for staging and prognostication purposes. If TVs are to be used clinically for prognostication purposes, it is vital that the methods of TV measurement be more standardised and reliable across the world, so as to have accurate exchange and comparison of results.
One limitation of the study is that the whole premise of this research, as outlined in the introduction, is based on the assumption that TV is a reflection of the number of tumour cells. This assumption may not hold true for cystic tumours, those with central necrosis, those that may have sustained bleeding or inflammation due to diagnostic biopsy or concurrent reactive inflammation (likely to be an important factor at nodal sites). Another limitation of this study is that Human papilloma virus (HPV) status was not checked in participating cohorts primarily due to cost factors. The prognosis and treatment response to HPV positive tumours is markedly different to HPV negative tumours, and if this variable was known it could have been factored into the analysis.
6. Conclusions
If response evaluation 3 months post CCRT is to be predicted it is simply not enough to measure the largest single dimension of the tumour. TV seems to be a better and more accurate reflection of the true total tumour burden or extent of the disease. TVs, particularly PTV and TTV may be utilised as predictors for 3 months post CCRT response. However, in order to incorporate TV staging system or derived prognostic cut off values into routine clinical practice for staging and prognostication, the study's findings on an independent sample and then undertaking further prospective validation studies are needed.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Pameijer F.A., Balm A.J., Hilgers F.J., Muller S.H. Variability of tumor volumes in T3-staged head and neck tumors. Head Neck. 1997;19(1):6–13. doi: 10.1002/(sici)1097-0347(199701)19:1<6::aid-hed2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Chong V.F., Zhou J.Y., Khoo J.B., Chan K.L., Huang J. Correlation between MR imaging-derived nasopharyngeal carcinoma tumor volume and TNM system. Int J Radiat Oncol Biol Phys. 2006;64(1):72–76. doi: 10.1016/j.ijrobp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Johnson C.R., Thames H.D., Huang D.T., Schmidt-Ullrich R.K. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys. 1995;33(2):281–287. doi: 10.1016/0360-3016(95)00119-j. [DOI] [PubMed] [Google Scholar]
- 4.Brenner D.J. Dose, volume, and tumor-control predictions in radiotherapy. Int J Radiat Oncol Biol Phys. 1993;26(1):171–179. doi: 10.1016/0360-3016(93)90189-3. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher G.H. Basic clinical parameters. In: Fletcher G.H., editor. Textbook of radiotherapy. Lea and Febinger; Philadelphia, PA: 1980. pp. 180–219. [Google Scholar]
- 6.Johnson C.R., Khandelwal S.R., Schmidt-Ullrich R.K., Ravalese J., III, Wazer D.E. The influence of quantitative tumour volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995;32:635–641. doi: 10.1016/0360-3016(95)00031-S. [DOI] [PubMed] [Google Scholar]
- 7.Lee R.W., Mancuso A.A., Saleh E.M., Mendenhall W.M., Parsons J.T., Million R.R. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottis larynx treated with radiotherapy alone? Int J Radiat Oncol Biol Phys. 1993;25:683–687. doi: 10.1016/0360-3016(93)90016-o. [DOI] [PubMed] [Google Scholar]
- 8.Hermans R. Head and neck cancer: how imaging predicts treatment outcome. Cancer Imaging. 2006;65:S145–S153. doi: 10.1102/1470-7330.2006.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knegjens J.L., Hauptmann M., Pameijer F.A. Tumour volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck. 2011;33:375–382. doi: 10.1002/hed.21459. [DOI] [PubMed] [Google Scholar]
- 10.Porceddu S.V., Jarmolowski E., Hicks R.J. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175–181. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]
- 11.Yao M., Graham M.M., Hoffman H.T. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001–1010. doi: 10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia K.S., King A.D., Yu K.H. Does primary tumour volumetry performed early in the course of definitive concomitant chemoradiotherapy for head and neck squamous cell carcinoma improve prediction of primary site outcome? Br J Radiol. 2010;83:964–970. doi: 10.1259/bjr/27631720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doweck I., Denys D., Robbins K.T. Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope. 2002;112:1742–1749. doi: 10.1097/00005537-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Millan J., Toledo M.D., Lupianez Y. Competing causes of death in patients with loco regionally advanced head and neck cancer treated with concomitant boost radiation plus concurrent weekly cisplatin. Clin Transl Oncol. 2013;15:321–326. doi: 10.1007/s12094-012-0925-9. [DOI] [PubMed] [Google Scholar]
- 15.Plataniotis G.A., Theofanopoulou M.E., Kalogera-Fountzila A. Prognostic impact of tumor volumetry in patients with locally advanced head-and-neck carcinoma (non-nasopharyngeal) treated by radiotherapy alone or combined radiochemotherapy in a randomized trial. Int J Radiat Oncol Biol Phys. 2004;59:1018–1026. doi: 10.1016/j.ijrobp.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Tsou Y.A., Hua J.H., Lin M.H., Tsai M.H. Analysis of prognostic factors of chemoradiation therapy for advanced hypopharyngeal cancer – does tumor volume correlate with central necrosis and tumor pathology? ORL J Otorhinolaryngol Relat Spec. 2006;68:206–212. doi: 10.1159/000091803. [DOI] [PubMed] [Google Scholar]
- 17.Chen S.W., Yang S.N., Liang J.A., Lin F.J., Tsai M.H. Prognostic impact of tumor volume in patients with stage III–IVA hypopharyngeal cancer without bulky lymph nodes treated with definitive concurrent chemoradiotherapy. Head Neck. 2009;31:709–716. doi: 10.1002/hed.21011. [DOI] [PubMed] [Google Scholar]
- 18.Strongin A., Yovino S., Taylor R. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1823–1830. doi: 10.1016/j.ijrobp.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Johnson C.R., Khandelwal S.R., Schmidt-Ullrich R.K., Ravalese J., Wazer D.E. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995;32:635–641. doi: 10.1016/0360-3016(95)00031-S. [DOI] [PubMed] [Google Scholar]
- 20.Grabenbauer G.G., Steininger H., Meyer M. Nodal CT density and total tumor volume as prognostic factors after radiation therapy of stage III/IV head and neck cancer. Radiother Oncol. 1998;47:175–183. doi: 10.1016/s0167-8140(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 21.Morris M.M., Schmidt-Ullrich R.K., DiNardo L. Accelerated superfractionated radiotherapy with concomitant boost for locally advanced head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2002;52:918–928. doi: 10.1016/s0360-3016(01)02715-8. [DOI] [PubMed] [Google Scholar]
- 22.Dunst J., Stadler P., Becker A. Tumor volume and tumor hypoxia in head and neck cancers. The amount of the hypoxic volume is important. Strahlenther Onkol. 2003;179:521–526. doi: 10.1007/s00066-003-1066-4. [DOI] [PubMed] [Google Scholar]
- 23.Chufal K.S., Rastogi M., Srivastava M., Pant M.C., Bhatt M.L., Srivastava K. Analysis of prognostic variables among patients with locally advanced head and neck cancer treated with late chemo-intensification protocol: impact of nodal density and total tumor volume. Jpn J Clin Oncol. 2006;36:537–546. doi: 10.1093/jjco/hyl081. [DOI] [PubMed] [Google Scholar]