Abstract
Objectives:
To evaluate the presence of antibodies to conformation-intact myelin oligodendrocyte glycoprotein (MOG) in a subgroup of adult patients with clinically definite multiple sclerosis (MS) preselected for a specific clinical phenotype including severe spinal cord, optic nerve, and brainstem involvement.
Methods:
Antibodies to MOG were investigated using a cell-based assay in 3 groups of patients: 104 preselected patients with MS (group 1), 55 age- and sex-matched, otherwise unselected patients with MS (group 2), and in 22 brain-biopsied patients with demyelinating diseases of the CNS (n = 19 with MS), 4 of whom classified as MS type II (group 3). Recognized epitopes were identified with mutated variants of MOG.
Results:
Antibodies to MOG were found in about 5% (5/104) of preselected adult patients with MS. In contrast, in groups 2 and 3, none of the patients tested positive for MOG antibodies. Patients with MS with antibodies to MOG predominantly manifested with concomitant severe brainstem and spinal cord involvement and had a severe disease course with high relapse rates and failure to several disease-modifying therapies. Three of them had been treated with plasma exchange with a favorable response. All anti-MOG–positive patients with MS showed typical MS lesions on brain MRI. Longitudinal analysis up to 9 years revealed fluctuations and reappearance of anti-MOG reactivity. Epitope mapping indicated interindividual heterogeneity, yet intraindividual stability of the antibody response.
Conclusions:
Antibodies to MOG can be found in a distinct subgroup of adult MS with a specific clinical phenotype and may indicate disease heterogeneity.
Identification of biomarkers of disease heterogeneity has been a challenge in multiple sclerosis (MS) research. A subgroup of patients with MS shows neuropathologic features of antibody (Ab)-mediated demyelination (pattern II according to reference 1). Observations in animal models have identified myelin oligodendrocyte glycoprotein (MOG), a molecule exposed on the myelin surface and therefore accessible to Abs, as a prime candidate antigen of demyelinating Abs,2 reviewed in references 3–5.
Abs against conformation-intact MOG were initially detected in children with demyelinating syndromes,6–9 more recently also in a subset of adult patients with aquaporin-4 Ab–negative neuromyelitis optica spectrum disorders (NMOSD),10–14 in adult patients with inflammatory demyelination distinct from NMOSD and MS,15 bilateral optic neuritis (ON),16 and rarely in anti-NMDA-receptor Ab–associated demyelinating syndromes.17 In contrast, little is known about Abs to MOG in adults with clinically definite MS according to the McDonald criteria.3,4
Recently, we reported a patient with Abs to MOG with recurrent severe myelitis as well as brainstem involvement, who showed on biopsy a typical neuropathologic pattern II MS.18 This led us to hypothesize that there may exist a subgroup of patients with MS with specific clinical features who have Abs to MOG.19 To test this, we investigated selected patients with MS who had experienced episodes of severe ON, myelitis, or brainstem syndrome and patients with biopsy-proven CNS inflammatory demyelinating disease including MS type II pathology for Abs to MOG. In the selected MS patient cohort, we identified 5 patients who had serum Abs to MOG and assessed their anti-MOG reactivity longitudinally.
METHODS
Patients.
Three groups of patients with MS were investigated (table 1). Group 1 consisted of 104 selected patients from our outpatient clinic with a definite diagnosis of MS according to the McDonald criteria.20 Clinical selection criteria were as follows: relapses with severe or recurrent myelitis and/or one or more episodes of severe (visual acuity <0.5) ON, and/or brainstem syndrome. Two neurologists (T.K. and L.A.G.) independently assessed the clinical inclusion criteria for each patient. All patients had to be negative for Abs to aquaporin-4. Group 2 consisted of 55 age- and sex-matched, otherwise unselected patients with MS. Group 3 consisted of 22 patients with biopsy-proven CNS inflammatory demyelinating disease. Four of them had MS type I pathology, 4 had MS type II pathology, 3 had MS type III pathology, while in 11 patients, MS-type classification was not possible (table 1). Brain biopsy was performed because of tumefactive lesions on cerebral MRI (cMRI) to rule out a tumor, and serum was taken close to this acute episode. None of the serum samples had been pretested for Abs to MOG before stratification.
Table 1.
Clinical characteristics of patient groups 1–3

Clinical characteristics including age, disease course, disease duration, Expanded Disability Status Scale score, MS Severity Score,21 first clinical manifestation, number of relapses, relapses with severe myelitis, severe ON or brainstem syndrome, MRI features, presence of oligoclonal bands (OCBs) in the CSF, presence of other autoimmune diseases, disease-modifying therapies (DMTs), and response to plasma exchange in the past (if applicable) were collected retrospectively from all patients of group 1 (tables 1 and 2). In patients with Abs to MOG, additional stored serum samples were tested for MOG Abs longitudinally and patients were invited for follow-up visits. MRI characteristics were evaluated in more detail in all MOG Ab–positive patients. Serum samples from all patients were collected during regular visits in our outpatient clinic, immediately centrifuged, and frozen at −80°C.
Table 2.
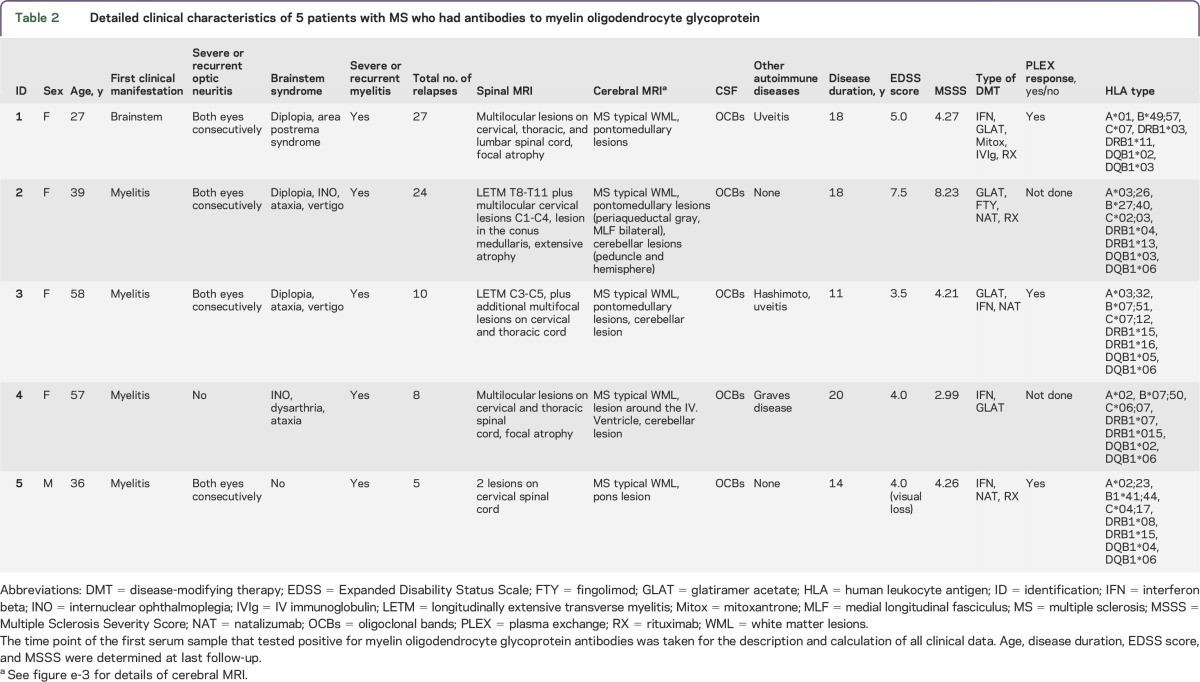
Detailed clinical characteristics of 5 patients with MS who had antibodies to myelin oligodendrocyte glycoprotein
Standard protocol approvals, registrations, and patient consents.
All patients gave written informed consent. The study was approved by the local ethics committee (project 159-03).
Detection of Abs to MOG.
For detection of Abs to MOG and for epitope mapping, serum samples from all patients were analyzed by a cell-based flow cytometric assay as previously described.18,22,23 HeLa cells were transiently transfected with human full-length MOG fused C-terminally to enhanced green fluorescent protein (EGFP)-N1 (CLONTECH Laboratories, Mountain View, CA) or with EGFP alone (control cells). For the determination of anti-MOG immunoglobulin G (IgG), 50,000 unfixed live cells were incubated 24 hours after transfection with a 1:50 dilution of the serum sample for 45 minutes at 4°C. As secondary reagents, a 1:500 dilution of a biotin-SP–conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) and a 1:2,000 dilution of Alexa Fluor 647–conjugated streptavidin (Jackson ImmunoResearch) were applied. For the determination of anti-MOG reactivity, we gated on cells with a fluorescein isothiocyanate fluorescence intensity above 500 (details and picture in reference 23) and determined their mean channel fluorescence intensity (MFI) in the allophycocyanin channel. Then we calculated the MFI ratio between MOG-EGFP–transfected cells and cells transfected with EGFP alone. Sera that scored positive for anti-MOG IgG were also analyzed for the presence of anti-MOG immunoglobulin M (IgM) using allophycocyanin-labeled anti-human IgM (clone: SA-DA4; eBioscience, San Diego, CA). Diagnosis and clinical data were unknown to the testing person. The threshold for MOG reactivity was set to the mean plus 3 SDs (MFI ratio 2.27) of 39 adult healthy controls as described before.18 In addition, sera that tested anti-MOG IgG–positive in cells transiently transfected with MOG-EGFP were also analyzed with a cell line stably transfected with full-length human MOG not fused to GFP, which we had used in a previous study.8 This assay was performed with the same secondary reagents as described above.
Human leukocytes antigen type determination.
In all MOG Ab–positive patients, human leukocytes antigen (HLA) type determination was performed by standard molecular genotyping techniques. HLA 2-digit typing of the loci HLA-A, -B, -C, -DRB1, and -DQB1 was performed using sequence-specific oligonucleotide technique.
RESULTS
Abs to MOG in adult patients with MS.
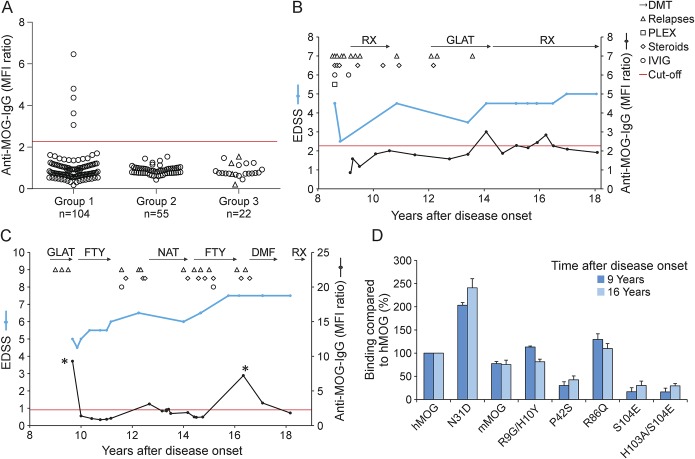
Five of 104 patients with MS from group 1 had Abs to MOG (figure 1A). In contrast, none of the unselected patients with MS from group 2 and none of the patients with CNS demyelinating disease who had undergone brain biopsy from group 3 had Abs to MOG (figure 1A). Figure 1A shows the anti-MOG response of all analyzed patients and figure e-1 at Neurology.org/nn shows the histograms of the flow cytometric analysis of the 5 patients with MOG Abs. The intensity of anti-MOG reactivity in these cases was similar to our previously published case with MOG Abs and MS type II pathology.18 These 5 patients tested positive for anti-MOG IgG (figure e-1A) but negative for anti-MOG IgM (data not shown). We could analyze the anti-MOG response longitudinally in these 5 patients with a mean observation period of 7 years (figures 1, B and C; e-2). In 3 of these patients, we noted intermittent disappearance and reappearance of the anti-MOG response (figures 1, B and C; e-2C). This was particularly striking in patient 2 in whom a strong anti-MOG response transiently disappeared and then reappeared 7 years later at a similar intensity (figure 1C). In this patient, we observed exactly the same epitope specificity in the anti-MOG response that reappeared after 7 years (figure 1D). Each of the 5 patients with Abs to MOG showed a different reactivity to our panel of MOG mutants (figures 1D; e-1, B–E) indicating interindividual heterogeneity of epitope specificity. Specifically, mutations of amino acids 42, 86, and 103/104 of MOG revealed heterogeneity between different patients. Four of these 5 patients showed a higher binding to deglycosylated MOG (N31D) than to wild-type MOG (figures 1D; e-1, B, D, and E). In addition to the measurements with transiently transfected cells, we used a stably transfected cell line with full-length human MOG not fused to EGFP8 for the 5 anti-MOG–positive patients and noted an anti-MOG response only in 2 of these 5 patients. Thus, the assay with the stably transfected cells8 is less sensitive than the method used in this study with a transient transfection of MOG-EGFP and gating on the cells with the highest EGFP expression.
Figure 1. Antibodies to MOG in a proportion of adult patients with MS.
(A) Sera from 3 groups of patients with MS were analyzed for anti-MOG reactivity. Group 1: patients with MS with a specific clinical phenotype, namely, with severe or recurrent myelitis and/or severe (visual acuity <0.5) or recurrent optic neuritis, and/or brainstem syndrome (n = 104). Group 2: unselected patients with MS matched for age and sex (n = 55). Group 3: biopsied patients (n = 22), 4 with MS type II pathology (empty triangles). The red line indicates the cutoff for anti-MOG positivity. Values shown for each anti-MOG IgG–positive patient represent the mean of 3 independent measurements using the first serum sample analyzed, which was 14, 16, 3, 18, and 9 years after disease onset for patients 1 through 5. (B, C) Clinical features, therapies, and longitudinal analysis of antibodies to MOG in patients 1 and 2. (D) Analysis of the epitope specificity of the MOG reactivity of the samples indicated with asterisks (*) in C. Reactivity of the serum samples diluted 1:50 to the indicated variants of hMOG and to mMOG is shown. Values obtained with wild-type hMOG were set as 100% and the other reactivities were calculated as described.22 Depicted are the mean values of 3 independent experiments ± SEM. DMF = dimethyl fumarate; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; FTY = fingolimod; GLAT = glatiramer acetate; hMOG = human myelin oligodendrocyte glycoprotein; IgG = immunoglobulin G; IVIG = IV immunoglobulins; MFI = mean fluorescence intensity; mMOG = mouse myelin oligodendrocyte glycoprotein; MOG = myelin oligodendrocyte glycoprotein; MS = multiple sclerosis; NAT = natalizumab; PLEX = plasma exchange; RX = rituximab.
Clinical phenotype of anti-MOG–positive patients with MS.
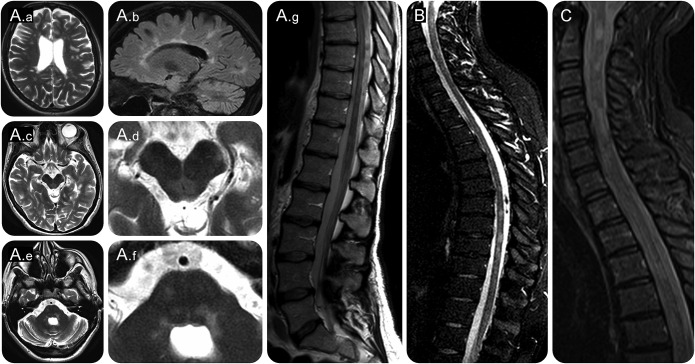
Detailed clinical characteristics of anti-MOG–positive patients with MS are shown in table 2. All 5 patients with MS had a typical relapsing-remitting MS with several relapses over time, typical CSF findings with presence of OCBs, and MS-typical cMRI features from the beginning of the disease (figures 2, A.a and A.b; e-3). In addition, patients 1–4 had recurrent and severe myelitis with evidence of severe spinal cord involvement on spinal MRI including longitudinally extensive transverse myelitis in patients 2 and 3 (table 2; figure 2, A.g, B, and C). They also showed severe brainstem involvement on cMRI (figure 2, A.c–A.f). When we compared the clinical features of the 5 anti-MOG–positive patients with MS to anti-MOG–negative patients of group 1, we noted that 4 patients with Abs to MOG had concomitant severe spinal cord and brainstem involvement, while this combination was seen in only 7 of 99 patients without Abs to MOG. A combination of all 3 specific clinical characteristics (severe/recurrent ON, severe/recurrent myelitis, and brainstem syndrome) was observed in 3 of 5 patients with Abs to MOG and in 3 of 99 patients without Abs to MOG (table e-1). Three of 5 patients with MS who had Abs to MOG carried the HLA-DRB1*15 allele suggesting that the well-established HLA-DR2 linkage is also present within this subgroup of patients with MS; patient 1 carried an HLA-DRB1*03 allele that has been associated with NMOSD. None of these 5 patients tested positive for anti-NMDAR Abs.
Figure 2. MRI features of patients with MS who had antibodies to myelin oligodendrocyte glycoprotein.
(A) Patient 2: cerebral MRI showing typical MS deep white matter lesions (A.a, T2w, axial plane) and partially confluent callosal lesions (A.b, FLAIR, sagittal plane). Furthermore, bilateral lesions in the periaqueductal gray (A.c, T2w, A.d: magnified view) and bilateral pontomedullary lesions were observed (A.e, T2w, A.f: magnified view). sMRI displaying LETM and segmental atrophy in the lower thoracic and lumbar spinal cord (A.g, level T8-T11). (B) Patient 1: sMRI showing multiple partially confluent lesions in the cervical cord from C1-C6 (FLAIR, sagittal) and in the thoracic cord with focal atrophy. (C) Patient 3: sMRI demonstrating LETM from C2-C5 (FLAIR, sagittal). FLAIR = fluid-attenuated inversion recovery; LETM = longitudinally extensive transverse myelitis; MS = multiple sclerosis; sMRI = spinal MRI; T2w = T2-weighted.
All 5 patients with MS who had MOG-specific Abs showed an active disease course as indicated by high relapse rates and therapy failure to several DMTs (tables 2 and e-1). Severe relapses at the beginning of MS led to Expanded Disability Status Scale scores ≥4.0 in 4 patients early on. In 2 patients, natalizumab had a sustained treatment effect on clinical disease activity. Two patients stabilized with rituximab therapy. Three of the 5 patients with Abs to MOG were treated with plasma exchange for steroid-resistant exacerbations; all 3 responded favorably to this intervention.
Serum samples from MOG Ab–positive patients were not available from disease onset; however, we could analyze the anti-MOG response longitudinally in all 5 patients with a mean observation period of 7 years (figures 1, B and C; e-2). The anti-MOG reactivity fluctuated over time and only partially correlated with clinical disease activity. In patients 1 and 2, anti-MOG reactivity reappeared during episodes of clinical and/or radiologic disease activity during long-term observation (figure 1, B and C). Patient 3 tested positive for MOG Abs during a severe brainstem and ON relapse, but tested negative 7 and 8 years later during a phase of clinical stabilization while under natalizumab therapy (figure e-2A). Patient 4 could be tested only twice during a time interval of 2 years and showed ongoing clinical and paraclinical disease activity accompanied by positive anti-MOG reactivity at both time points (figure e-2B). In patient 5 with a stable disease course, Abs to MOG fluctuated during continuous natalizumab treatment (figure e-2C).
DISCUSSION
We identified 5 adult patients who fulfilled the McDonald criteria for MS and who tested positive for serum anti-MOG IgG. These patients with MS had clinical features seen in patients with NMOSD,19 namely, relapses with severe ON, myelitis, and/or brainstem syndrome. On spinal MRI, 4 of these 5 patients showed severe spinal cord involvement with multisegmental lesions as well as extensive cord atrophy including longitudinally extensive transverse myelitis in 2 patients. Otherwise, all 5 patients had typical MS lesions on cranial MRI, positive OCBs in the CSF, and a typical relapsing-remitting MS disease course. It has already been assumed that some patients with Abs to MOG represent a subgroup of opticospinal MS,24 a notion supported by our observation but also expanded by the fact that our patients had otherwise rather typical MS.
Because we had stored longitudinal serum samples from these patients, we could follow their anti-MOG responses for up to 9 years. Abs to MOG tended to fluctuate over time and intermittently fell below cutoff. This indicates that cross-sectional studies may underestimate the prevalence of anti-MOG IgG, arguing for repeated testing. Using our panel of MOG mutants,22 we mapped the epitopes of conformation-intact MOG recognized by the patients' Abs. Interindividual comparison showed that all 5 patients recognized different epitopes of MOG, in line with the epitope heterogeneity observed in pediatric demyelination.22 In one patient, the anti-MOG reactivity reappeared after 7 years, and remarkably showed the same epitope specificity. This is compatible with the view that reappearance of MOG Abs after years of seronegativity reflects reactivation of previously differentiated B cells and is similar to our previously described case of MOG Ab–positive encephalomyelitis.18 The intensity of the anti-MOG reactivity in these 5 patients is similar to the reactivity of a previous case sharing features of NMOSD where anti-MOG reactivity was associated with activated complement at the site of active demyelination18 but was lower than the reactivity we have observed in some patients with typical NMOSD (supplement in reference 18).
The presence of Abs to MOG in adult MS has been a contentious debate for more than a decade (reviewed in references 3−5). While earlier studies using cell-based assays reported a high percentage of Abs to MOG in adult patients with MS,25,26 several studies that included childhood demyelinating disorders7,8 or adults with NMOSD12,16 found little or no reactivity to MOG in adult MS.12,27–29 However, in most of these studies, patients with MS were not selected for a specific phenotype. Only in a very recent report, it is stated that 4 of 26 patients with MS who all tested negative for Abs to MOG had clinical features of NMOSD.15 In our observation, especially patients with concomitant spinal cord and brainstem involvement tested positive for Abs to MOG, indicating that such patients with MS should be screened for the presence of MOG Abs. Patients with MS who have Abs to MOG may be at higher risk of progressive disease and need escalating therapies, as indicated by high relapse rates and lack of satisfactory response to several DMTs in the past. Two patients showed a favorable response to rituximab, which is observed in MS as well as in patients with NMOSD. Two other patients stabilized with natalizumab therapy supporting the diagnosis of MS rather than NMOSD.
It is difficult to estimate the frequency of Abs to MOG in adult MS. Our present observations indicate that it may range between 5% (in a selected cohort with specific clinical features) and less than 1% in unselected MS cohorts. Furthermore, it is unknown whether and how the presence of serum Abs to MOG is related to the neuropathologically defined MS type II.1 Our previous observation regarding a patient with anti-MOG–associated encephalomyelitis and neuropathologic MS type II,18 and 2 recent cases with fulminant encephalomyelitis and Abs to MOG,30,31 indicate that at least a subgroup of MS type II might be associated with Abs to MOG. However, our present observation that none of 22 biopsied cases, including 4 cases of MS type II, tested positive for anti-MOG Abs indicates that MS type II in itself may be heterogeneous, and is possibly associated with Abs against different antigens. This conclusion is in harmony with the recent observation that only 1 of 13 patients with an MS type II pathology have Abs to MOG.31 However, as discussed above, it should be noted that MOG Ab reactivity may escape detection if MOG Abs transiently fall below cutoff.
One limitation of our study is the relatively small number of anti-MOG–positive patients with MS, although it is still the largest and best-characterized cohort of anti-MOG–positive adult MS described to date. Furthermore, no serum samples were available from the time of clinical onset and thus the role of Abs to MOG in early MS is still not known. We do not know whether Abs to MOG were present from the beginning or developed later during the course of MS. Previous pediatric studies detected anti-MOG Abs at the first demyelinating event arguing for a primary event in these patients.7,8 Recent animal experiments, however, showed that an autoimmune reaction against MOG can follow a toxic oligodendrocyte death.32 Thus, Abs to MOG could be primary or secondary; therefore, we propose that also adult patients with MS who have the clinical phenotype we describe here should be tested at disease onset and also later for Abs to MOG.
These Abs to conformationally intact MOG as displayed in a cell-based assay are expected to contribute to the pathogenicity in these patients, because (1) in rodent and primate models, such MOG Abs induce demyelination (reviewed in reference 5), (2) the breached blood–brain barrier in MS allows entry of Abs, (3) sera from anti-MOG–positive patients induced damage in oligodendroglioma cells in vitro,33 (4) intracerebral injection of pooled anti-MOG–positive sera from patients with NMOSD induced pathology,34 and (5) intrathecal injection of IgG from an anti-MOG–positive patient enhanced encephalitis in rodents.35
Taken together, Abs to MOG can be found in a small proportion of adult MS. Especially MS patients with concomitant severe spinal cord and brainstem involvement should be tested for Abs to MOG. Our longitudinal analysis showed that anti-MOG reactivity may fluctuate over time, indicating that repeated analysis may be helpful if clinical features are compatible with anti-MOG–associated MS. It remains to be seen whether patients with MS who have Abs to MOG benefit from specific therapies and whether Abs to MOG will be a useful biomarker.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. N. Kawakami and K. Dornmair for comments on the manuscript. They also thank EUROIMMUN (Labor Dr. Stöcker, Lübeck, Germany) for anti-NMDAR Ab testing.
GLOSSARY
- Ab
antibody
- cMRI
cerebral MRI
- DMT
disease-modifying therapy
- EGFP
enhanced green fluorescent protein
- HLA
human leucocyte antigen
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- MFI
mean fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NMOSD
neuromyelitis optica spectrum disorders
- OCBs
oligoclonal bands
- ON
optic neuritis
Footnotes
Funding information and disclosures are provided at the end of the article. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by Institute of Clinical Neuroimmunology, LMU-Munich.
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
M. Spadaro: acquisition, analysis and interpretation of data, manuscript writing. Dr. Gerdes, Dr. Kümpfel: study design, patient evaluation and care, acquisition and interpretation of clinical data, manuscript writing. Dr. Krumbholz: statistical analysis, patient evaluation and care, interpretation of data, manuscript writing. Dr. Ertl-Wagner: MRI data analysis and interpretation, manuscript writing. Dr. Thaler, Dr. Schuh: patient evaluation and care, interpretation of data, manuscript editing. Dr. Metz: neuropathology of biopsy cases, manuscript editing. Dr. Blaschek: patient evaluation and care, interpretation of data, manuscript editing. Dr. Dick: HLA analysis, manuscript editing. Prof. Brück: neuropathology of biopsy cases, manuscript editing. Prof. Hohlfeld: supervision of patient care, interpretation of the data, manuscript writing and editing. Prof. Meinl: study design, interpretation of data, manuscript writing and editing, fund-raising.
STUDY FUNDING
This study was supported by the Deutsche Forschungsgemeinschaft (SFB-TR 128; B8, SyNergy), the Klinische Kompetenznetz Multiple Sklerose, Verein zur Therapieforschung für MS Kranke, Werner Reichenberger Stiftung, and Gemeinnützige Hertie-Stiftung.
DISCLOSURE
M. Spadaro reports no disclosures. L.A. Gerdes received travel funding and/or speaker honoraria from Biogen, Bayer Schering, received research support from Gemeinnützige Hertie-Stiftung. M. Krumbholz received travel funding from Novartis, Niogen, consulted for Genzyme, received research support from Novartis. B. Ertl-Wagner served on the scientific advisory boards for Philips, Bracco, Springer Medical Publisher, received travel funding and/or speaker honoraria from Siemens, Philips, Bracco, Bayer Schering, is an associate international editor for Journal of American College of Radiology, is on the editorial board for European Radiology, received publishing royalties from Springer Medical Publisher, Thieme Medical Publisher, has consulted for Munich Medical International, Philips Healthcare, received research support from Guerbet, Eli Lilly, Bracco, Merck Serono, Novartis, Bayer Schering institutional support, Bundesministerium fuer Forschung und Technik, Deutsche Forschungsgemeinschaft, NIH, Bayerische Forschungsstiftung, spouse holds stock or stock options for Siemens Healthcare. F.S. Thaler received travel support from Novartis. E. Schuh reports no disclosures. I. Metz received travel funding and/or speaker honoraria from Biogen Idec, Bayer Healthcare, TEVA, Serono, Novartis, Genzyme, received research support from Biogen Idec, German Ministry for Education and Research. A. Blaschek served on the scientific advisory board for Novartis, received travel funding from Novartis, Merck Serono, is on the editorial board for European Journal of Pediatric Neurology. A. Dick reports no disclosures. W. Brück serves on the scientific advisory board for Genzyme, Novartis, Biogen, Teva, received speaker honoraria from Teva, Sanofi, Genzyme, Novartis, Merck Serono, Biogen, Bayer, is on the editorial board for Acta Neuropathologica, Therapeutic Advances in Neurological Disorders, Multiple Sclerosis International, Neuropathology and Applied Neurobiology, received research support from Teva Pharma, Novartis, Biogen, Genzyme, German Research Foundation, Tschira Foundation, served as an expert witness for Teva Pharma. R. Hohlfeld served on the scientific advisory board for Novartis, Biogen Idec, Bayer Schering, Merck Serono, Sanofi-Aventis, Teva, CSL Behring, MedDay, Actelion, received travel funding from Novartis, Biogen Idec, Bayer Schering, Merck Serono, Sanofi-Aventis, Teva, Genzyme, is on the editorial board for Neurology®, Brain, Clinical and Experimental Immunology, Deutsche Medizinische Wochenschrift, Expert Opinion on Biological Therapy, Journal of Neuroimmunology, Multiple Sclerosis, Nervenarzt, Practical Neurology, Seminars in Immunopathology, Therapeutic Advances in Neurological Disorders, has consulted for Novartis, Biogen Idec, Bayer Schering, Merck Serono, Sanofi-Aventis, Teva, Genzyme, MedDay, Actelion, received research support from Novartis, Biogen Idec, Bayer Schering, Teva. E. Meinl received honorarium from Roche, served on the editorial board for Journal of Pathology, Clinical Experimental Immunology, PLoS One, Journal of Biological Chemistry, received research support from Novartis, DFG, SFB TRR 128, BMBF, KKNMS. T. Kümpfel received travel funding and/or speaker honoraria from Teva, Merck Serono, Genzyme, Novartis, Biogen Idec. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717. [DOI] [PubMed] [Google Scholar]
- 2.Lebar R, Lubetzki C, Vincent C, Lombrail P, Boutry JM. The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin Exp Immunol 1986;66:423–434. [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer MC, Meinl E. Glycoproteins as targets of autoantibodies in CNS inflammation: MOG and more. Ther Adv Neurol Disord 2012;5:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reindl M, Di Pauli F, Rostasy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 2013;9:455–461. [DOI] [PubMed] [Google Scholar]
- 5.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol 2016;15:317–331. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 2007;13:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol 2009;66:833–842. [DOI] [PubMed] [Google Scholar]
- 8.Pröbstel AK, Dornmair K, Bittner R, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 2011;77:580–588. [DOI] [PubMed] [Google Scholar]
- 9.Ketelslegers IA, Van Pelt DE, Bryde S, et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler 2015;21:1513–1520. [DOI] [PubMed] [Google Scholar]
- 10.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012;79:1273–1277. [DOI] [PubMed] [Google Scholar]
- 11.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 2011;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waters P, Woodhall M, O'Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89. doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobo-Calvo A, Sepulveda M, Bernard-Valnet R, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler 2015;22:312–319. [DOI] [PubMed] [Google Scholar]
- 14.Pröbstel AK, Rudolf G, Dornmair K, et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflammation 2015;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SM, Woodhall MR, Kim JS, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm 2015;2:e163. doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan S, Reddel SW, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 2014;1:e40. doi: 10.1212/NXI.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spadaro M, Gerdes LA, Mayer MC, et al. Histopathology and clinical course of MOG-antibody associated encephalomyelitis. Ann Clin Transl Neurol 2015;2:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005;64:1144–1151. [DOI] [PubMed] [Google Scholar]
- 22.Mayer MC, Breithaupt C, Reindl M, et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J Immunol 2013;191:3594–3604. [DOI] [PubMed] [Google Scholar]
- 23.Spadaro M, Meinl E. Detection of autoantibodies against myelin oligodendrocyte glycoprotein in multiple sclerosis and related diseases. Methods Mol Biol 2016;1304:99–104. [DOI] [PubMed] [Google Scholar]
- 24.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015;2:e62. doi: 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou D, Srivastava R, Nessler S, et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci USA 2006;103:19057–19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalive PH, Menge T, Delarasse C, et al. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci USA 2006;103:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pauli F, Mader S, Rostasy K, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol 2011;138:247–254. [DOI] [PubMed] [Google Scholar]
- 28.Höftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 2015;21:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Tanaka K. Anti-MOG antibodies in adult patients with demyelinating disorders of the central nervous system. J Neuroimmunol 2014;270:98–99. [DOI] [PubMed] [Google Scholar]
- 30.Di Pauli F, Höftberger R, Reindl M, et al. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm 2015;2:e175. doi: 10.1212/NXI.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarius S, Metz I, König FB, et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in 'pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case. Mult Scler Epub 2016 Feb 11. [DOI] [PubMed]
- 32.Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci 2016;19:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale RC, Tantsis EM, Merheb V, et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol Neuroimmunol Neuroinflamm 2014;1:e12. doi: 10.1212/NXI.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saadoun S, Waters P, Owens GP, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun 2014;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flach AC, Litke T, Strauss J, et al. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc Natl Acad Sci USA 2016;113:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.