Abstract
Sickle cell anaemia (SCA) is one of the commonest chronic hemolytic anaemias in the Sudan; it is a disease with high mortality and morbidity. This study was conducted aiming to observe the clinical pattern of cardiac abnormalities in children with sickle cell anaemia, and to assess the relationship between the cardiac abnormalities and the severity of the disease. The study was conducted in sickle cell disease clinic at Khartoum Children Emergency Hospital. The study group consisted of 289 patients with sickle cell anaemia, age range from 6 months to 18 years. Data were collected using a questionnaire which include full history, clinical examination findings, chest x-rays, and Electro-cardiography. Tachycardia, systolic murmurs, and cardiomegaly were detected in 28%, 61%, and 54% of patients with SCA respectively. Left ventricular dilatation was observed in 51% of the study group, while right ventricular dilatation was observed in 22% of the patients. Left and right atrial dilatations were observed in 16% and 6% of the patients respectively. Contractility, ejection fraction (EF) were found almost always normal in all study subjects. Chamber dilatations were not associated with any abnormality in Left ventricular functions. Hemglobin (Hb) levels correlated negatively with cardiomegaly. Left Ventricular End Diastolic Dimension (LVEDD) correlates negatively with Hb levels and positively with the severity index. Only four patients (1%) had abnormal valves. In conclusion, cardiac abnormalities in patients with SCA correlate with the age of the patients and the severity of the disease.
Keywords: Cardiac manifestations, sickle cell anaemia, Sudan
Introduction
Sickle cell anemia (SCA) is a life-long debilitating disease often requiring multiple and frequent hospital admissions. Multiple organ system become damaged, but the heart, although demonstrating abnormalities, is relatively spared. With increasing life span of these patients, cardiac dysfunctions may become more prominent [1].
Sporadic case reports of pathologic findings in patients dying of heart disease have suggested the presence of a specific cardiomyopathy [2], whereas other studies have failed to document this [3]. With the continued development of better supportive care for patients with sickle cell anemia, life span is increasing, and thus cardiovascular abnormalities may have time to develop. Chronic anemia of any cause leads to hemodynamic changes to maintain tissue perfusion.
Several mechanisms may compensate for reduced tissue oxygenation. Increased RBC 2, 3 diphosphoglycerate shifts hemoglobin dissociation curve to the right and increase release of oxygen at lower tissue oxygen tension. Viscosity is reduced in many anemias, decreasing peripheral resistance and increasing cardiac output [4]. Unlike most chronic anemias the plasma viscosity in SCD is not reduced [5], probably due to abnormal shape of RBCs. In addition to hypoxia, aggravated by the effect of recurrent insults to tissue causing infection, ventilation perfusion mismatching and decreased cardiac capacity [6]. Despite the normal plasma viscosity, it was proved that for a given level of anemia, patients with SCD have a higher cardiac output than patients with chronic anemias of other causes [7]. This may be due to tissue hypoxia, which may activate reflux mechanism to increase cardiac output.
The majority of patients with SCA demonstrate some abnormalities on cardiovascular examination, although in many cases this appears to reflect circulatory adjustment to chronic anemia rather than cardiac abnormality. On physical examination signs reflecting hyperdynamic circulatory state such as tachycardia, widened pulse pressure, active pericardium impulse and laterally displaced prominent apex are usually found [8]. Systolic murmurs are very common and may be ejection in type, commonly pansystolic [1, 8, 9, 10, 11]. Congestive heart failure may supervene in a few patients with SCA. In autopsy series, between 10% and 30% patients die primarily of congestive cardiac failure [3, 12].
There are no specific ECG changes in sickle cell anemia, although ECGs are commonly abnormal [13]. The cardiac size in patients with SCD is almost always enlarged [12,13]. This primarily represents the compensatory dilatation and hypertrophy from the increased venous return. There are no specific features and all chambers tend to be enlarged giving globular appearance [1, 14].
Echocardiography is a simple and effective way of assessing left ventricular function. Lindasy et al [6] quoted a small personal series characterized by vigorous posterior wall and septal motion. They noted that left ventricular dilatation was frequent and right ventricular dilatation was rather less frequent.
The frequency with which Rheumatic heart disease (RHD) occurs in patients with SCA is unknown. Clinical features of SCA such as intermittent fever, joint pain, cardiomegaly and heart murmur may mimic acute Rheumatic fever, and lead to diagnostic difficulties. Prolongation of PR interval may contribute to the confusion. RHD was the initial erroneous diagnosis in over one third of patients in two series of cases with SCA [1]. Congenital heart diseases may be diagnosed in error in young children with SCD but may also be overlooked.
Ischemic heart disease (IHD) is excessively rare in SCD, perhaps because few patients demonstrate the risk factors commonly observed in the general population. Obesity is uncommon in SCD, serum cholesterol levels are low, atheroma is uncommon, blood pressure is usually significantly lower than in age matched controls, and until recently a relatively small proportion of patients with SCD reached the age at which myocardial infarction is common in the general population [11,15].
The objectives of this study was to determine the clinical pattern of cardiac abnormalities in children with sickle cell anemia and to assess the relationship between the cardiac abnormalities and the severity of the disease.
Patients and Methods
The study populations were 289 children presented to the sickle cell clinic, their age range was 6 months to 18 years. The diagnosis of sickle cell anemia was confirmed by electrophoresis. All patients included in this study were in the steady state that was defined as subjects who were crisis free for at least two weeks prior to the study and had not received blood transfusion in the preceding four months [9].
Sicklers who were diagnosed previously as having congenital heart disease or rheumatic heart disease were excluded from the study.
Parents were inquired about number of crisis, hospitalization, and numbers of blood transfusions. The severity of sickle cell anemia was assessed using the severity index [9,16, 7] which was defined as the total number of attacks, hospitalizations, crisis, transfusions, symptoms and signs per year. A severity index was calculated for patient according to the following parameters (Table 1).
Parameter |
Score of severity index |
|||
|---|---|---|---|---|
|
0 |
1 |
2 |
3 |
Crisis/year |
<1 |
1–2 |
3–6 |
>6 |
Tx/year |
0 |
1 |
2–3 |
>3 |
ASH + |
>10 |
8–10 |
6–7.9 |
<6 |
+ ASH = average steady-state hemoglobin g/dl
Tx/year = number of blood transfusion/year
Any child who scored >6 ASH (average steady-state hemoglobin g/dl) was considered as a severe case, those with scores of 4–5 were considered as moderate cases, and those with scores of 0–3 were considered as mild cases [17].
Every child in the study was subjected to routine clinical examination, with especial emphasis on cardiovascular examination.
Tachycardia was defined as heart rate more than 140 beat/min for the age group 0–2 years, and more than 120 beats/min for the age group > 2–6years and more than 110 beat/min in the age group >6–12 years and more than 90 beats/min for the age group >12years [18].
Hemoglobin was documented with reference to normal levels of Sudanese children [18]. ECG, radiographical investigations and 2-dimensional echocardiography were done for all patients.
Results
A total number of 289 patients who were diagnosed as having sickle cell anaemia were studied. Children in the age group 6 months to 6 years were 119 children, and constituted (41%) of the total number. Children in the age group >6–12 years were 97 children and constituted (34%). While children in the age group >12–18 years were 73 children (25%).
Regarding sex characteristics of the study population, males included in the study were 161 (56%), while females were 128 (44%). The male to female ratio was approximately 1.25:1.
The majority of the patients (63%) were from Misseria tribe. Parents of one hundred and fifty seven (54%) patients were first degree cousins, and the majority of the families 248 (86%) had a low income. It was observed that 259 patients (90%) of the study group had past history of hospitalization. Among patients who had history of hospitalization, 43 of them (16%) had been admitted once during the last year, 84 (32%) of them have been admitted twice, 53 patients (21%) were admitted three-times and 79 (31%) were admitted more than three-times within the last year.
Almost half of the patients had history of blood transfusions. Fifty three patients (38%) were transfused once within the last year, 27 patients (19%) were transfused twice, 19 patients (14%) were transfused three-times, and 40 patients (29%) were transfused more than three times within the last year. Tachycardia was observed in 82 (28%) of patients with sickle cell anaemia (figure 1). Twenty patients (16%) in the age group 6 months-6 years were found to have tachycardia, 28 patients (28%) in the age group >6–12 years had tachycardia, while 34 patients (46%) of the patients in the age group >12–18 years were found to have tachycardia. The differences between the age groups is statistically significant (p = 0.002).
Figure 1.
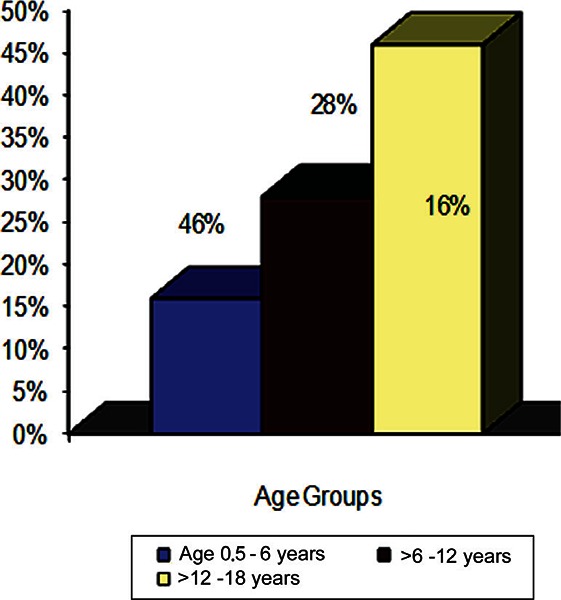
Tachycardia in children with sickle cell anemia.
Table 2.
Number of hospital admissions for children with sickle cell anaemia in last year
Number of hospital admissions |
No. |
% |
|---|---|---|
Once |
43 |
16 |
Twice The study group consisted of 289 patients of whom |
84 |
32 |
Three times |
53 |
21 |
Four times and more |
79 |
31 |
Total |
259 |
100 |
Table 3.
Number of blood transfusions for children with sickle cell anaemia within the last year
Number of blood transfusions |
No. |
% |
|---|---|---|
Once |
53 |
38 |
Twice |
27 |
19 |
Three times |
19 |
14 |
More than three times |
40 |
29 |
Total |
139 |
100 |
The majority of the patients 175 (61%), were found to have systolic murmurs (haemic murmurs). These murmurs were detected in 43 patients (36%) in the age group 6 months to 6 years as shown in fgure 2. Among the age groups >6–12 years and >12–18 years, systolic murmurs were detected in 73 patients (75%) and 59 patients (81%) respectively. The differences between age groups concerning systolic murmurs were found to be statistically significant (p = 0.03).
In patients with SCA, 144 patients (50%) were found to have Hb concentrations below the mean, 92 patients (32%) were found to have Hb concentration at the mean, while 53 patients (18%) were found to have Hb concentrations above the mean for age.
The chest x-rays of 133 patients (46%) were found to be normal, while cardiomegaly was detected in 156 patients (54%). Thirty-two patients (27%) in the age group 6 months to 6 years, were found to have cardiomegaly, 61 patients (63%) in the age group >6–12 years and 63 patients (86%) in the age group>12–18 years were found to have cardiomegaly.
Prolonged PR interval was found in 24 patients (8%) in the study group. Moreover, left axis deviation was found in 24 cases (8%). Left ventricular hypertrophy was found in 70 patients (24%), while right ventricular hypertrophy was observed only in 4 patients (1%).
Normal valves were found in 285 (99%) of patients with sickle cell anaemia, while 4 (1%) of patients had abnormal valves. These patients showed evidence of mitral stenosis most probably due to RHD. Left ventricular dilatation was found in 147 (51%) of patients of SCA, while right ventricular dilatation was found in 64 (22%) patients. Left atrial dilatation was observed in 46 patients (16%), while right atrial dilatation was observed only in 16 patients (6%). Left ventricular hypertrophy was found in 21 patients (7%), while right ventricular hypertrophy was found in 4 patients (1%). Left Ventricular End Diastolic Dimension (LVEDD) was normal in 196 (67%) of patients with SCA, while it was increased in 93 (33%) patients. Contractility was normal in all patients with SCA. Ejection fraction (EF) was found to be normal in all patients with SCA.
Figure 2.
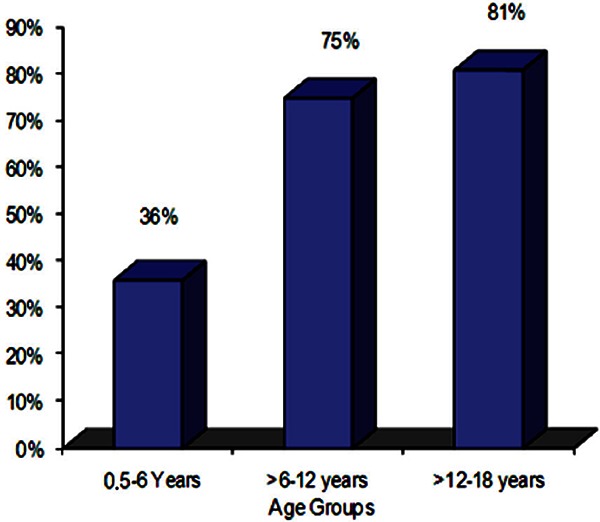
Systolic murmur in children with sickle cell anemia.
Figure 3.
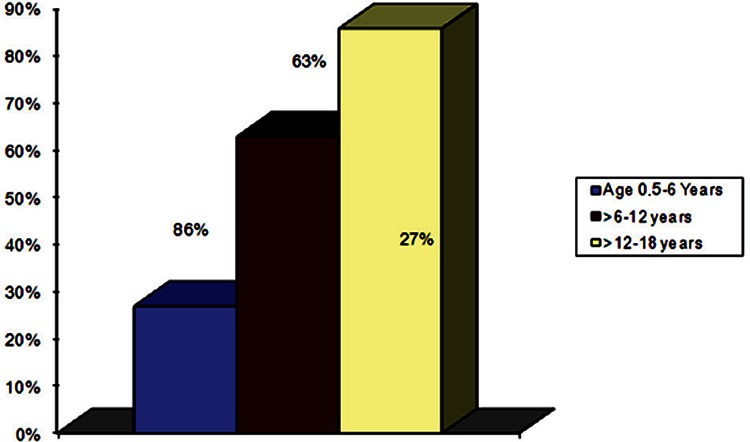
Age distribution of cardiomegaly in children with sickle cell anemia.
Figure 4.
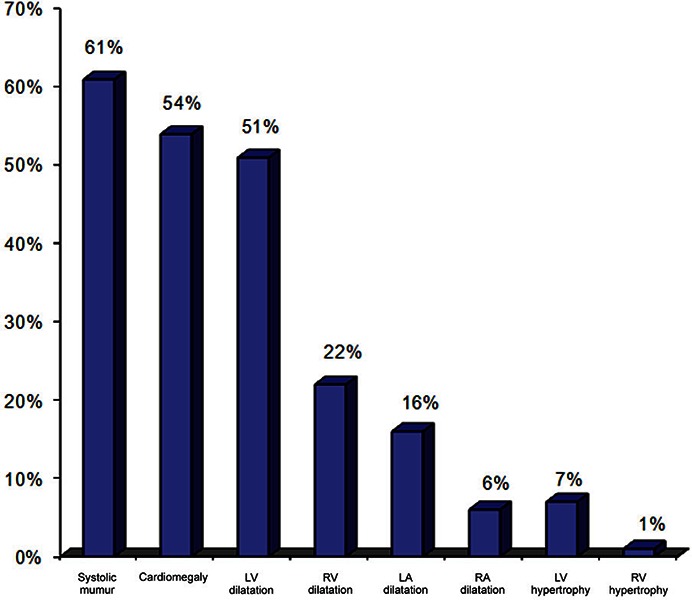
Cardiac abnormalities in children with sickle cell anemia.
Severity index was calculated for all patients and it was found to be severe in 90 patients (31%), moderate in 61 patients (21%), and mild in 138 cases (48%). In the age group 6 months to 6 years, 19 (21%) were found to be severe cases, 31 (51%) were moderate, and 69 (50%) were mild cases. In the age group >6–12 years, 35 (39%) of cases were found to be severe cases, 21 (34%) were moderate, and 41 (30%) were mild cases. Meanwhile, 36 (40%) cases were found to be severe, 9 (15%) were moderate and 20 (20%) were mild cases among the age group >12–18 years. These differences were statistically significant (p=0.001).
Twenty-two patients (88%) with displaced apex beat were found to be severe cases, and only 2 cases (8%) were mild cases. The difference between the groups was statistically significant (p = 0.001). All the patients who were found to have left parasternal heave, were found to be severe cases. This result was statistically significant (p = 0.001).
The majority of severe cases 84 cases (94%), were found to have systolic murmurs. The majority of the patients with cardiomegaly 87 patients (56%) were found to be severe cases, 31 (19%) cases were moderate and 38 cases (25%) were mild cases. The differences were statistically significant (p = 0.001). The majority of the patients with left ventricular hypertrophy (76%) were found to be severe cases, 11 (14%) cases were moderate and 7 (9%) cases were mild. The result was statistically significant (p = 0.002). All patients with right ventricular hypertrophy, 4 cases (100%), were found to be severe cases. Regarding the findings in the Echo cardiology, the majority of patients with left ventricular dilatation, 76 (52%), were severe cases, 32 cases (22%) were moderate cases, and 39 (26%) cases were mild cases. This result was statistically significant (p=0.001). Twenty-seven (62%) patients with left atrial dilatation were found to be severe cases. The result is statistically significant (p=0.002). Fifty patients (78%) with right ventricular dilatation were severe cases, 5 cases (8%) were moderate cases, while 9 (14%) cases were mild cases. This result is also statistically significant (p = 0.002). The majority of patients, 14 (87%), with right atrial dilatation were severe cases. This result was statistically significant (p = 0.006). The majority of patients with left ventricular hypertrophy, 13 cases (62%), were severe cases, while all cases with right ventricular hypertrophy 4(100%) were found to be severe cases. These results were statistically significant (p = 0.011). Hb level correlated negatively with cardiomegaly (p < 0.0001). LVEDD correlated negatively with the Hb level and positively with the severity index (p < 0.0001).
Discussion
The study group consisted of 289 patients of whom 75% were in the age group 6 months to 12 years, while only 25% of them were in the age group 12–18 years. This may indicate decrease life span with age due to different complications of SCD. This is similar to what was reported in other studies [1, 19].
The majority of the patients in the study group were of low socioeconomic status. This is a true reflection of our country’s social status. Poverty is predominant feature in the Sudanese social context.
It was found that 90% of patients of the study group had past history of hospitalization and 48% of the patients had history of blood transfusions. The pattern of sickle cell anaemia observed in this study group is comparable to the sever type described in Africans. It was observed that there is high incidence of aneamia (98%), infections (86%), hand and foot syndrome (66%) and jaundice (55%). A great deal of similarity was observed when the pattern of sickle cell anemia in this group was compared with the African pattern [20, 21].
Tachycardia was observed in 82 patients (28%) with SCA, 46% of whom were in the age group >12–18 years, and the majority of them (68%) were found to be severe cases. Laterally displaced apex and third heart sound were detected in 9% and 2% of the patients with sickle anaemia respectively. This is similar to findings in previous studies [1, 6].
Tachycardia, laterally displaced apex, and third heart sound reflect hyperdynamic circulatory state as a response to chronic anaemia rather than failing myocardium, since all children in this study were in the steady state and there were no other signs of heart failure. The majority of the patients with sickle cell anaemia were found to have systolic murmur (61%). Moreover, 50% of them were found to be severe cases. This is also similar to what was reported in other studies [1,8,9,10,11]. These results showed that tachycardia and systolic murmur were directly correlated with the age of the patient and the severity of sickle cell anemia. Regarding Hb level in patients with SCA, 50% of the patients had Hb levels below the mean, 32% had Hb concentrations at the mean and only 18% had Hb level above the mean. This result is similar to the finding in African children reported by Sergeant G. R. and Powars D. R. [20,21].
Cardiomegaly was observed in 54% of patients. The majority of these patients had severe cases. This was similar to data obtained from other studies [1,12,13]. The percentage of cardiomegaly was higher in a similar previous study [8]. This difference can be explained by the fact that all children in our study group were in the steady state.
Left ventricular hypertrophy was observed in 25% of patients, while only one per cent of patients showed right ventricular hypertrophy. The majority of patients with left ventricular hypertrophy (76%) had severe disease, while all patients with right ventricular hypertrophy had severe disease. This was in agreement with reports of other studies [12, 13].
Although the ECG changes in our study group were not specific but, they are directly correlated with the severity of sickle cell anemia.
Contractility and Ejection fraction (EF) were found to be normal in all patients with sickle cell anaemia. These results were comparable to data obtained by other studies [15,22,23,24].
Normal valves were observed in 99% of patients with SCA, while only 1% of patients showed mitral valve affection in form of mitral regurritation (MR) most probably due to RHD. This finding is in consistent with findings in anther study [6].
Left ventricular dilatation was observed in 51% of patients, the majority of these patients (52%) had severe disease. While right ventricular dilatation was observed only in 22% of our patients, 78% of this group had severe disease. These findings were similar to what was reported by Gery and Ayuo in previous studies [22, 24], and lower than that was found in other studies [15,23]. Left atrial dilatation was observed in 16% of patients, 62% were severe cases, while right atrial dilatation was observed in only 6% of patients. These results are similar to other studies [7,24].
LVEDD was increased in 33% of patients. Left ventricular hypertrophy was observed in 7% of the study group, 62% of these patients were severe cases. These results are comparable to data obtained from other studies [14,22,23,24]. These results showed that, the main cardiac abnormality in patients with sickle cell anemia was champers dilatations which were directly correlated with the severity of sickle cell anemia. Since contractility and EF were normal in almost all patients in the study group, we can conclude that chambers dilatations were not associated with any abnormality in the left ventricular function. Hb levels were correlated negatively with cardiomegaly, and LVEDD was correlating negatively with Hb levels, while it was correlating positively with severity index (p < 0.0001).
Conclusion
The peak of occurrence of cardiac abnormalities in patients with SCA and the majority of severe cases were found in the older age group. Systolic murmur was the main presenting cardiac abnormality. The dilated cardiac chambers in patients with SCA were not associated with any abnormality in left ventricular function, and there was no evidence for distinct sickle cell cardiomyopathy. Cardiac abnormalities in patients with SCA correlated directly with the age of the patients and the severity of the disease.
Recommendations
Increase the awareness of the population that SCA is an inherited disease so, avoid the consanguineous marriages. Hb level should be maintained in higher values to decrease the severity of the disease and to decrease the cardiovascular abnormalities. Further studies should be done to assess the effect of periodic hyper-transfusion therapy in normalization of the heart chamber and decreasing the severity of the disease.
References
- 1.Falk H F, Hood W B. The heart in sickle cell anaemia. Arch Intern Med 1982; 142: 1680–1684. [PubMed] [Google Scholar]
- 2.Olivera E, Gomez PN. Falcemic cardiopathy. Report of a case. Am J Cardiol 1963; 11: 686–688. [Google Scholar]
- 3.Gerry J L, Bulkley B H, Hutchins G M. Clinicopathologic analysis of cardiac dysfunction in 52 patients with sickle cell anaemia. Am J Cardiol 1987; 42,211–216. [DOI] [PubMed] [Google Scholar]
- 4.Duke M, Abelmann W H. The haemodynamic response to chronic anaemia. Circulation 1969; 39: 503–515. [DOI] [PubMed] [Google Scholar]
- 5.Horne M K. Sickle cell anaemia as archeologic disease. Mm J Med 1981; 70: 288–298 [DOI] [PubMed] [Google Scholar]
- 6.Lindsay J, Meshel J C, Patterson R H. The cardio-vascular manifestations of sickle cell disease. Arch Intern Med 1974; 133: 643–651. [PubMed] [Google Scholar]
- 7.Varta A M, Adolph R J, Fowler N O. Cardio-vascular effects of anaemia. Am Heart J 1972; 85: 415–426. [DOI] [PubMed] [Google Scholar]
- 8.Karayalcin G, Rosnerf, Kim K Y, et al. Sickle cell anaemia. Clinical manifestations in 100 patients and review of literature. Am J Med Sci 1975; 269: 51–68. [DOI] [PubMed] [Google Scholar]
- 9.Wali V A, Uenugopalan P, Rivera E, and Al-Lamki Z. Cardio-vascular function in Omani children with sickle cell anaemia. Ann Trop Paediatr 2000; 20: 243–246. [DOI] [PubMed] [Google Scholar]
- 10.Gacon P H, Dona Lien Y. Cardiac manifestation of sickle cell anaemia. Press-med 2001; 30: 841–845. [PubMed] [Google Scholar]
- 11.Bruce S, Alpert M D, et al. Haemo-dynamic and ECG responses to exercise in children with sickle cell anaemia. Am J Dis Child 1981; 135: 362–366. [DOI] [PubMed] [Google Scholar]
- 12.Uzsoy N G. Cardio-vascular findings in patients with sickle cell anaemia. Am J Cardiol 1964; 13: 320–328. [DOI] [PubMed] [Google Scholar]
- 13.Winsor T, Burch G E. The electrocardiogram and cardiac state in active sickle disease. Am Heart J 1945; 29: 685–696. [Google Scholar]
- 14.Haywood LJ. Cardiovascular function and dysfunction in sickle cell anemia. J Natl Med Assoc. 2009. January; 101(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 15.Lester A L, Sodt P C, Hutcheon N, Arcilla R A. Cardiac abnormalities in children with sickle cell anaemia. Chest 1990. November; 98 (5): 1169–74. [DOI] [PubMed] [Google Scholar]
- 16.El-Hazmi M A F. The features of sickle cell disease in Saudi children. J Trop Paediatr 1990. August; 36: 148–155. [DOI] [PubMed] [Google Scholar]
- 17.Emeribe A O, Udoh A E, Etukudoh M H, Okary C C, and Catty D. Hypofibronectinaemia and severity of sickle cell anaemia in Nigeria. Br J Haematol 2000. December; 111 (4): 1194–1197. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla FD. Haemoglobin, packed cell volume with Blood Count in school children in Khartoum city. Thesis 1987. clinical MD in paediatrics and child health, Board for postgraduate medical studies, university of Khartoum.
- 19.Platt O, Nathan D G. Sickle cell disease in: Nathan D G, Oski F A. Haematology of Infancy and Childhood. 2nd ed W B Saunders company; Philadelphia, 1989; 617–625. [Google Scholar]
- 20.Powars D R. Natural history of sickle cell disease. The first ten years. Semin Hematol 1975; 12: 267. [PubMed] [Google Scholar]
- 21.Serjeant G R. The emerging understanding of sickle cell disease. Br J Haematol 2001; 112: 3–18. [DOI] [PubMed] [Google Scholar]
- 22.Gerry J L, Baird M G, Fortuin N J. Evaluation of left ventricular function in patients with sickle cell anaemia. Am J Med 1976; 60: 968–972. [DOI] [PubMed] [Google Scholar]
- 23.Rees A M, Stefadouros M A, Strong W B, et al. Left ventricular performance in children with homozygous sickle cell anaemia. Br Heart J 1978; 40: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayuo P O, Ablnya N A, Joshi M D, Lore W. Cardio-vascular features in adolescents and adults with sickle cell anaemia. East Afr Med J 1993. May; 70 (5): 270–276. [PubMed] [Google Scholar]


