Abstract
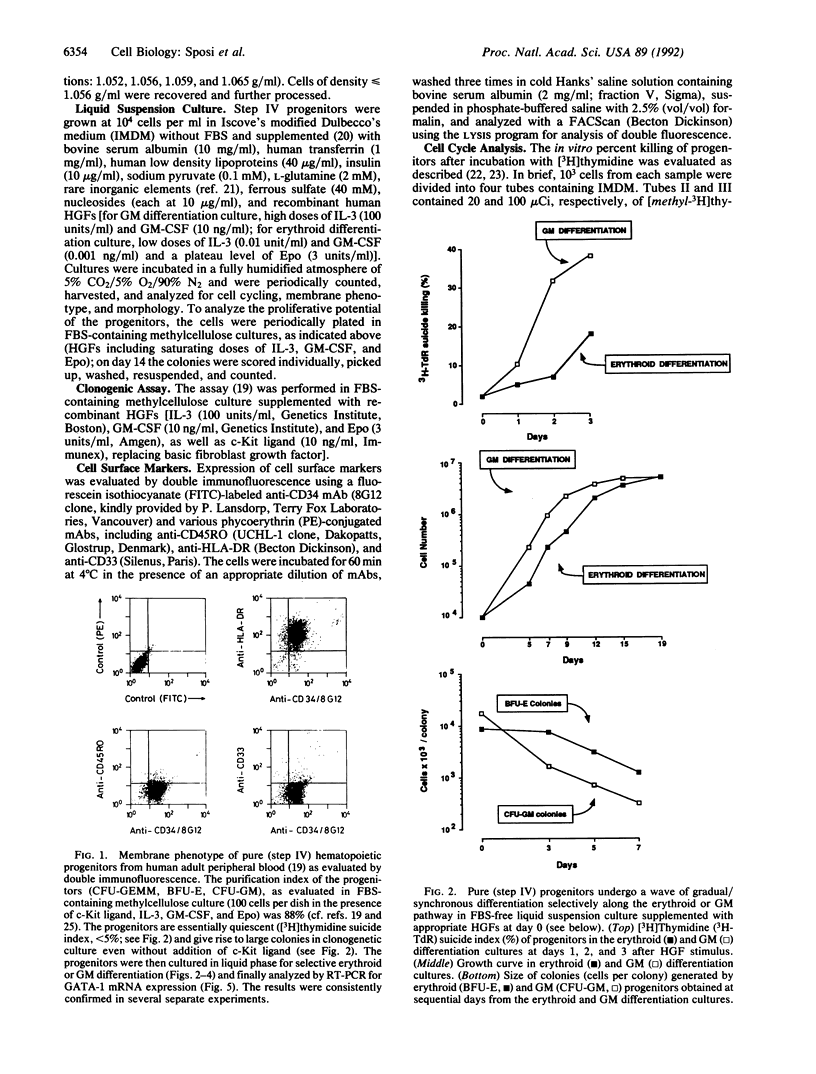
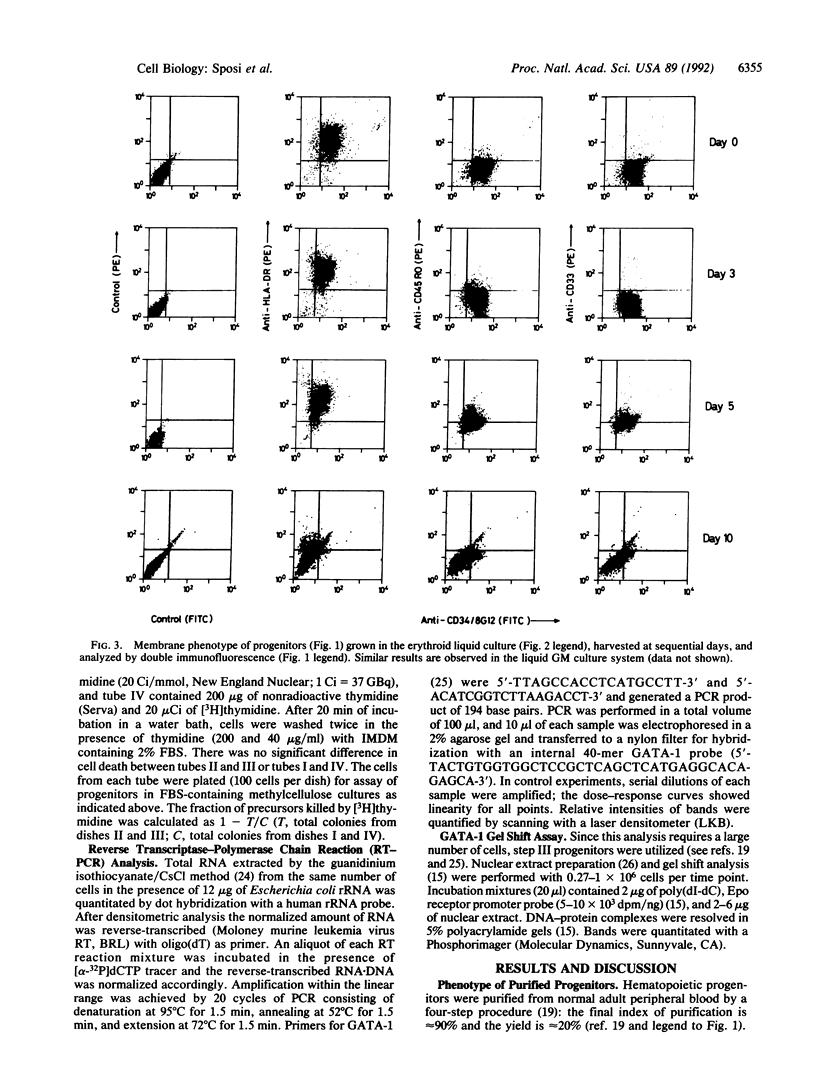
The programmed activation/repression of transcription factors in early hematopoietic differentiation has not yet been explored. The DNA-binding protein GATA-1 is required for normal erythroid development and regulates erythroid-expressed genes in maturing erythroblasts. We analyzed GATA-1 expression in early human adult hematopoiesis by using an in vitro system in which "pure" early hematopoietic progenitors are induced to gradual and synchronized differentiation selectively along the erythroid or granulocyte-macrophage pathway by differential treatment with hematopoietic growth factors. The GATA-1 gene, though virtually silent in quiescent progenitors, is activated after entrance into the cell cycle upon stimulation with hematopoietic growth factors. Subsequently, increasing expression along the erythroid pathway contrasts with an abrupt downregulation in the granulocyte-macrophage lineage. These results suggest a microenvironment-directed, two-step model for GATA-1 expression in differentiating hematopoietic progenitors that involves (i) cycle-dependent initiation and (ii) lineage-dependent maintenance or suppression. Hypothetically, on/off switches of lineage-restricted transactivators may underlie the binary fate decisions of hematopoietic progenitors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989 May 1;169(5):1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger W. I., Berenson R. J., Andrews R. G., Kalamasz D. F., Hill R. S., Bernstein I. D., Lopez J. G., Buckner C. D., Thomas E. D. Positive selection of hematopoietic progenitors from marrow and peripheral blood for transplantation. J Clin Apher. 1990;5(2):74–76. [PubMed] [Google Scholar]
- Berenson R. J., Andrews R. G., Bensinger W. I., Kalamasz D., Knitter G., Buckner C. D., Bernstein I. D. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988 Mar;81(3):951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Crotta S., Nicolis S., Ronchi A., Ottolenghi S., Ruzzi L., Shimada Y., Migliaccio A. R., Migliaccio G. Progressive inactivation of the expression of an erythroid transcriptional factor in GM- and G-CSF-dependent myeloid cell lines. Nucleic Acids Res. 1990 Dec 11;18(23):6863–6869. doi: 10.1093/nar/18.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason J. F. Granulocyte-macrophage colony formation in serum-free culture: effects of purified colony-stimulating factors and modulation by hydrocortisone. J Cell Physiol. 1986 Aug;128(2):231–238. doi: 10.1002/jcp.1041280214. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Identification of megakaryocytes, macrophages, and eosinophils in colonies of human bone marrow containing neurtophilic granulocytes and erythroblasts. Blood. 1979 May;53(5):1023–1027. [PubMed] [Google Scholar]
- Gabbianelli M., Sargiacomo M., Pelosi E., Testa U., Isacchi G., Peschle C. "Pure" human hematopoietic progenitors: permissive action of basic fibroblast growth factor. Science. 1990 Sep 28;249(4976):1561–1564. doi: 10.1126/science.2218497. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Siena S., Bregni M., Tarella C., Stern A. C., Pileri A., Bonadonna G. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet. 1989 Sep 9;2(8663):580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991 Jun 14;65(6):927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Valtieri M., Gabbianelli M., Pelosi E., Bassano E., Petti S., Russo G., Testa U., Peschle C. Erythropoietin alone induces erythroid burst formation by human embryonic but not adult BFU-E in unicellular serum-free culture. Blood. 1989 Jul;74(1):460–470. [PubMed] [Google Scholar]
- Valtieri M., Venturelli D., Caré A., Fossati C., Pelosi E., Labbaye C., Mattia G., Gewirtz A. M., Calabretta B., Peschle C. Antisense myb inhibition of purified erythroid progenitors in development and differentiation is linked to cycling activity and expression of DNA polymerase alpha. Blood. 1991 Mar 15;77(6):1181–1190. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]