Abstract
To investigate whether a positive transition into retirement may be associated with later cognitive ageing, we included a subset of 4,926 Nurses’ Health Study participants who retired from work at ages 60–69, then provided a subjective assessment of the change in overall quality of life (QOL) with retirement. Subsequently (range: 1 month to 4.7 years later), when all were aged 70+ years, they completed a baseline telephone cognitive battery evaluating global cognition, episodic memory and executive function. They had up to three follow-up cognitive assessments. Controlling for various occupational factors before retirement and socioeconomic, lifestyle, and health-related factors as of the baseline cognitive assessment, we used generalized linear models for repeated measures to estimate mean differences in rates of cognitive decline across categories of QOL transition at retirement: “worse”, “same” or “better”. Over a median 6 years of follow-up, the global cognitive score change was −0.123 on average. Compared with women who reported no change in QOL at retirement (31%), women who reported improvement (61%) showed a significantly slower rate of cognitive decline (difference= +0.011 95% CI =0.004, 0.019). This mean difference was equivalent to that observed between women who were 2 years apart in age. No significant differences in cognitive decline rates were observed for the women who reported worsened QOL (8%). Secondary analyses to address possible reverse causation showed robust associations. A positive transition into retirement was associated with better maintenance of cognitive function over time in aging women. These findings need to be replicated in other populations.
Keywords: Cognition, Aging, Quality of life, Retirement, Cohort studies, Epidemiology
INTRODUCTION
Over the last century, longevity in the USA has increased from 47.3 years in 1900 to 78.7 years in 2010 [1]. This increase in life expectancy has expanded the length of time people spend in retirement and into older ages. Of substantial importance in aging is maintaining cognitive health [2]. As the transition from employment to retirement may be accompanied with both favorable and unfavorable life and lifestyle changes that may impact later health [3–5], the quality of the transition to retirement may influence cognitive health. Furthermore, this period may offer a timely window of opportunity for interventions that may positively impact cognitive health at an older age. However, data are scarce on this topic. Although the influence of retirement on quality of life (QOL) and health has been greatly debated [6], with studies finding adverse [7–9], no [8, 10, 11], or beneficial [11–16] associations, any putative effects may depend on pre-retirement work conditions (e.g., whether satisfactory or not), type of retirement (e.g., whether voluntary or not), time since retirement and the health outcome studied. Overall, studies have focused on physical or mental health as outcomes, with no direct evaluations of associations with cognitive function. In the few studies that have evaluated retirement and cognitive function, they have examined the association with some pre-retirement job characteristics [17–22] (e.g., job strain and occupational complexity) or retirement status itself [23–27], but studies have not explored the potential associations with how retirees experienced their transition to retirement. Our objective was to assess to what extent the transition from working to retired life could impact subsequent cognitive ageing. We hypothesized that a retirement which was positively experienced would contribute to the maintenance of high levels of cognition into old age. Using longitudinal data from 4,926 participants from the Nurse’s Health Study, we examined how self-rated change in QOL at retirement was subsequently associated with change in cognitive function over 6-years of follow-up.
MATERIALS AND METHODS
The Nurses’ Health Study cohort and the cognitive subcohort
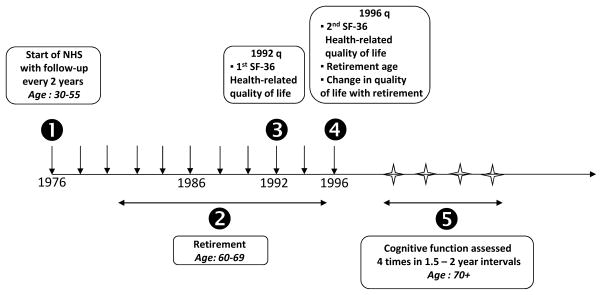
The Nurses’ Health Study (NHS) began in 1976, when 121,700 US registered nurses, aged 30–55 years, completed a mailed questionnaire about their health and lifestyle. Follow-up questionnaires were mailed biannually. From 1996 to 2000, participants who were ≥70 years old and free of stroke were invited to participate in a telephone-based study of cognitive function (Figure 1). For the first interview, 93% of all eligible women participated. We conducted three follow-up interviews at 1.5 year intervals (median cognitive follow-up time: 6 years) (Figure1). Participation in the follow-up interviews was > 85% in living women. The study was approved by the Institutional Review Board of Brigham and Women’s Hospital (Boston, MA).
Figure 1.
Study timeline.
Cognitive assessment
The telephone interview consisted of a battery of six cognitive tests, which have been validated and previously described in detail [28–30]. Overall cognitive status was assessed with Telephone Interview of Cognitive Status (TICS, range 0 to 41 points) [31], which is a telephone adaptation of the Mini-Mental State Examination. Verbal memory, a strong predictor of Alzheimer’s Disease [32], was assessed with the TICS 10-word list (immediate and delayed recalls) and the East Boston Memory Test (immediate and delayed recalls) [33]. A test of category fluency [34], which partially assesses executive function, and the digit span backwards [35], a measure of attention, were also administered.
Our primary outcome was the global composite score, computed as the mean of the z-scores from all cognitive tests. As secondary outcomes, we considered the TICS score, the verbal memory composite score (mean of the z-scores from the immediate and delayed recalls of both TICS-10 word list and the East Boston Memory Test), the category fluency score and the digit span backwards score.
Composite scores (i.e., global score and verbal memory score) were constructed only for women who completed all contributing tests Participation rates were identical across all cognitive assessments and remained stable over time.
Assessment of change in QOL at retirement
To be eligible for the present study, participants needed to have answered the 1996 biennial NHS questionnaire and retired before it (Figure 1). In this questionnaire, along with retirement status and age at retirement, participants were asked to rate their satisfaction regarding retired life: “Overall, how would you say the quality of retired life compares with life when you were working?”. Possible responses were “much worse”, “somewhat worse”, “about the same”, “somewhat better” and “much better”. Due to the skewed distribution of the responses, the categories of “much worse” and “somewhat worse” were combined into one category and the “somewhat better” and “much better” categories were combined into another.
Population for analysis
Of the 19,415 participants who completed the initial cognitive interview, we excluded women who did not complete any follow-up cognitive interviews (n=2,274). We then excluded those whose baseline cognitive function evaluation occurred before the 1996 questionnaire or more than 5 years after the 1996 questionnaire response (n=2,609). We further excluded those who were not fully retired or those whose age at retirement was unknown (n=5,268 women) and those whose age at retirement was below 60 or above 70 years (n=2,631) as it is possible that early or late retirement compared to the most common retirement ages (60–69 years) may be associated with cognitive outcomes [36]. We then excluded those who did not complete the retirement QOL assessment (n=200) and those with missing data on important work-related factors (from the 1982 NHS questionnaire) such as occupation type before retirement and history of shift work (n=1,507). After these exclusions, 4,926 women were included in the analysis. At the time of the initial cognitive interview, these women were similar in age and in cognition when compared with the larger population of 19,415 women (mean age (standard deviation) = 74.4 (2.2) years vs. 74.3 (2.3) years in the larger population, mean TICS score (standard deviation) = 33.9 (2.5) vs. 33.7 (2.8) points in the larger population).
In our analysis sample, the time elapsed between the 1996 questionnaire and the first cognitive interview ranged from 1 month to 4.7 years, with a median time of 3.6 years.
Correlation between post-hoc assessment of change in QOL and prospective pre-and post-retirement health-related QOL
Because the 1996 question on change in QOL with retirement was retrospectively assessed at a single timepoint, we aimed to test its validity in a subset of participants in whom prospective data was available on a QOL proxy both before retirement and after retirement. The QOL proxy we used was the health-related QOL as measured by the Short Form (36 items) Health Survey (SF-36) [37, 38]. General QOL is broader in concept than just health-related QOL because it encompasses non-health related life features, such as environment or social relationships, whereas health-related QOL is directly connected to an individual’s health or disease status. However, they both aim to capture a respondent’s subjective perception of well-being, so a correlation is likely. Therefore, in the subset of 777 women of our analysis sample in whom the SF-36 information was available in 1992 and in 1996, and who retired between 1992 and 1996, we considered whether the changes in physical and mental health component summary measures [39] from 1992 (pre-retirement) and 1996 (post-retirement) were consistent with participants’ responses in 1996 of the question on how the quality of retired life compared with working life, using the Jonckheere-Terpstra test, a nonparametric test for ordered differences among classes [40].
Covariates
We also obtained information on multiple potential confounders plausibly linked with both cognition and QOL at retirement, including demographic, socioeconomic, occupational, lifestyle and health-related factors. As adjustment variables, basic models included age (continuous, in years), education (registered nurse, Bachelor’s degree, Master’s or Doctorate), age at retirement (60–64, 65–69 years), as well as the delay between retirement and post-hoc assessment of change in QOL at retirement (<5, 5-<10, ≥10 years). In multivariable-adjusted models, we further adjusted for 1) factors assessed using the most updated available data as of the first cognitive assessment: a) lifestyle and health factors (smoking status, alcohol intake, body mass index, physical activity, diet quality indicator based on the Alternate Healthy Eating Index [41, 42], age at menopause, postmenopausal hormone, vitamin E, ibuprofen, aspirin, diabetes, hypertension, hyperlipidemia, myocardial infarction, SF-36 Physical Functioning score), b) psychosocial factors (SF-36 Vitality score, SF-36 Mental Health score, antidepressant use, Berkman-Syme social network index [43], stress from caregiving) and c) socioeconomic factors (husband’s education level, median income of US census tract, whether participants ever had to forego medical treatment for financial reasons); 2) pre-retirement occupational factors (1982 questionnaire responses to current type of work and any history of shift work ≥20 years as assessed in 1988), 3) pre-retirement stress (1982 questionnaire response to stress at home or at work in daily life) and 4) early life socioeconomic factors (birth below 37° N latitude, father's occupation at 16 years of age).
Statistical analysis
To evaluate the association between QOL at retirement and cognitive decline in later life, we used generalized mixed models for repeated measures. For each outcome, we evaluated basic and multivariable-adjusted models with covariates previously described. We estimated mean differences in rates of cognitive decline across categories of QOL at retirement: worse, same, and improved QOL; in all analyses, the “same” category was the reference group. Such models assumed that a participant’s change in cognitive function followed that of the population mean except for random effects for initial cognitive levels (i.e., random intercepts) and rates of change (i.e., random slopes). They also allowed for missing values in cognitive scores during follow-up, optimizing use of available cognitive data. We calculated 95% confidence intervals (95% CIs) for all models and performed linear tests of trend.
Given the possibility that various factors could modify the association between change in the QOL with retirement and subsequent cognitive function, we evaluated, in separate multivariable-adjusted models, the potential effect modification by seven factors: 1) age at retirement (60–64 vs. 65–69 years), 2) delay between retirement and post-hoc assessment of change in QOL at retirement (<5 years vs. ≥5 years), 3) work status as assessed in 1982 (working vs. homemaker), 4) vitality (SF-36 Vitality score ≥50 vs. <50), 5) mental health (SF-36 Mental Health score ≥53 vs. <53), 6) physical functioning (SF-36 Physical Functioning score >30 vs. ≤30) and 7) subjective memory complaint at first cognitive interview (which was appraised as the number of positive response(s) to seven items: change in memory, difficulties in remembering a short list of items, difficulties in remembering things from one second to the next, difficulties in remembering recent events, difficulties in understanding instructions, difficulties in following a conversation, difficulties in finding the way around familiar streets) that has been shown to be a risk factor for long-term cognitive decline [44, 45].
Finally, to reduce any potential bias due to reverse causation [16] where women may have first had impaired cognition that led to early retirement and / or a poorer subjective assessment of QOL after retirement, we conducted several restricted analyses excluding women who had the worst cognitive function at the initial assessment (defined as those in the worst 10% of the distribution or alternatively as those whose TICS were below 34), women who completed less than all four of the cognitive telephone interviews, women with >1 subjective memory complaints or women whose retirement age was below 65. To evaluate if QOL change with retirement and cognition may be mediated by depression, we also restricted the analysis to those without evidence of depression or severe depression symptoms as of baseline cognitive assessment (no antidepressant use and SF-36 Vitality score ≥50 and SF-36 Mental Health score >=53).
All models were fitted by maximum likelihood method using the SAS software (SAS release 9.3, SAS Institute Inc., Cary, NC).
RESULTS
At first cognitive assessment, the mean score in our sample of 4,926 retired nurses aged 70–80 years was 33.9 on TICS and 17.3 on category fluency test. 61% of these women had reported in 1996 that their quality of life had improved after retirement, 31% that it remained the same, and 8% that it worsened. At that time, 27 % had retired for less than 5 years, whereas 20% for more than 10 years. The median (min, max) estimated number of years since retirement was 7 years (0, 14).
Correlation between post-hoc assessment of change in QOL and prospective pre-and post-retirement health-related QOL in a subsample
Post-hoc self-reported change in QOL at retirement was associated with prospectively-observed change in SF-36 score before and after retirement (Table 1). Whereas differences in change in SF-36 between the “worse” and the “same” groups were clear for both physical and mental health components (with less favorable SF-36 change in the worse group as expected), the difference in change in SF-36 between the “same” and “improved” groups were only in the mental health component. Across the QOL change categories, the relation with the physical component was not significant (p-value for the Jonckheere-Terpstra test=0.22) while the relation with the mental component was significant (p <0.001).
Table 1.
Prospective changes in SF-36 physical and mental health component summary measures between 1992 (pre-retirement) and 1996 (post-retirement) and their relation to a subjective assessment of change in quality of life at retirement asked in 1996 among a subset of 777 participants who retired between 1992 and 1996.
| Self-reported change in quality of life at retirement | ||||
|---|---|---|---|---|
|
| ||||
| Mean change (SD) in SF-36 measure (=1996 value – 1992 value) | Worse (n= 76; 10%) | Same (n= 251; 32%) | Improved (n=450; 58%) | p-valuea |
| Physical health component | −4.2 (8.4) | −2.4 (8.2) | −2.7 (8.2) | 0.22 |
| Mental health component | −0.7 (9.1) | 1.1 (6.5) | 2.4 (7.0) | <0.001 |
Jonckheere-Terpstra test (test for an ordered alternative hypothesis)
Factors associated with change in QOL at retirement
Compared to women reporting no change in QOL at retirement, those who reported worsened QOL showed clear differences in socioeconomic and health profile, with less favorable circumstances overall (Table 2): they showed less healthy behaviors (higher rates of smoking and obesity, lower levels of physical activity and diet quality), were more likely to have cardiovascular conditions or risk factors (myocardial infarction, hyperlipidemia, etc.), as well as mental health risk factors (caregiving burden, low social network, stress in daily life). On average, their SF-36 scores for vitality, mental health and physical functioning were lower. In contrast, the same and improved groups were very similar as far as socioeconomic, behavioral and health-related factors were concerned. Some slight differences occurred at the occupational/retirement level; in particular, women who reported an improved QOL at retirement were more likely to have been working as a nurse in 1982 (vs. other work/homemaker) and had on average retired at an earlier age than the “same” group.
Table 2.
Characteristicsa of participants by 1996 response to change in quality of life (QOL) at retirement assessment (n=4,926)
| Self-reported change in QOL at retirement | |||
|---|---|---|---|
| Worse (n=419; 8%) | Same (n=1514; 31%) | Improved (n=2993; 61%) | |
| Characteristics updated as of baseline cognitive interview | |||
|
| |||
| Mean age at initial cognitive interview, in years (SD) | 74.8 (2.1) | 74.5 (2.2) | 74.3 (2.3) |
| Mean delay between retirement and post-hoc assessment of change in quality of life at retirement, in years (SD) | 6.8 (3.3) | 6.8 (3.3) | 7.1 (3.2) |
| Age at retirement: 65–69 years (vs. 60–64 years), % | 37 | 36 | 29 |
| Highest education of Master’s or Doctorate, % | 10 | 6 | 8 |
| Husband’s highest education of Master’s or Doctorate, % | 18 | 16 | 18 |
| Median income of US census tract, in $ | 61,557 | 60,179 | 59,936 |
| Ever had to forego medical treatment for financial reasons, % | 12 | 6 | 7 |
| Current cigarette smoking, % | 13 | 7 | 7 |
| Current alcohol use, % | 43 | 47 | 52 |
| Obesity (≥30 kg/m2), % | 26 | 18 | 17 |
| Mean physical activity level, in MET-hours/weekb(SD) | 10.6 (15.0) | 15.3 (18.2) | 16.9 (20.5) |
| Mean AHEI score (SD) | 45.9 (10.6) | 46.9 (10.1) | 47.6 (10.4) |
| History of myocardial infarction, % | 9 | 6 | 6 |
| History of hypertension, % | 60 | 54 | 53 |
| History of diabetes, % | 12 | 10 | 9 |
| History of hyperlipidemia, % | 73 | 66 | 68 |
| SF-36 Vitality score <50, % | 43 | 14 | 13 |
| SF-36 Mental Health score <53, % | 17 | 3 | 3 |
| Mean SF-36 Physical Functioning score, (SD) | 59.3 (26.1) | 75.3 (21.3) | 76.3 (20.3) |
| Antidepressant use, % | 13 | 5 | 5 |
| Current post-menopausal hormone use, % | 30 | 34 | 35 |
| Vitamin E use, % | 47 | 50 | 52 |
| Ibuprofen use, % | 25 | 19 | 19 |
| Currently providing caregiving to family, % | 33 | 27 | 22 |
| High Berkman-Syme Social Network Index, % | 38 | 52 | 53 |
|
| |||
| Pre-retirement work characteristics | |||
|
| |||
| Was working as a nurse in 1982 (vs. other work/ homemaker), % | 78 | 76 | 81 |
| Reported severe stress at home or work in 1982, % | 29 | 13 | 16 |
| Shift work ≥20 years (as assessed in 1988), % | 10 | 8 | 7 |
|
| |||
| Early life socioeconomic characteristics | |||
|
| |||
| Father's occupation as a professional / manager at 16 years old, % | 22 | 23 | 25 |
| Birth below 37° N latitude, % | 4 | 6 | 7 |
Statistics are computed among non-missing values and are age-adjusted (except for the age-variable) AHEI: Alternative Healthy Eating Index score; SD: standard-deviation; SF-36: Short Form (36 items) Health Survey
Weekly calories expended from exercise and climbing the stairs; 1 metabolic-equivalent-hour (MET-hour) is equal to the amount of energy expended while sitting for one hour
Age at retirement and cognitive decline
In our study sample (restricted to women whose retirement age was 60–69 years), no significant association between retirement age and cognitive decline was observed (p=0.36), but in the larger sample of all women who retired before 1996 (n=7,557), those who retired at age 65–69 experienced slower cognitive decline overall as compared to those who had retired earlier (at ages <50–64 years) (data not shown). Thus, in all analyses for this study, we adjusted for age at retirement (65–69 years vs. 60–64 years).
Change in QOL at retirement and cognitive decline in later life
Among 4,926 women followed for a median of 6 years, the overall cognitive change during follow-up in the global composite score was −0.123 on average.
Basic and multivariable-adjusted models of differences in rate of cognitive decline showed very similar results. Based on model fit statistics, the multivariable-adjusted models significantly improved the fit to the data (Supplementary Table 1). In multivariable-adjusted models (Table 3), when compared with women who reported no change in QOL at retirement, women who reported improvement showed a significantly slower rate of decline in the global composite score (+0.011 standard unit / year; 95% confidence interval [0.004, 0.019]), the TICS (+0.041 point [0.007, 0.075]) and the verbal memory score (+0.012 standard unit [0.003, 0.021]). Fixed effects for intercept, time from baseline, QOL change at retirement and time by QOL change at retirement from the multivariable-adjusted model of global composite score are provided in Supplementary Table 2. Because mean differences in rate of cognitive decline can be difficult to interpret, particularly when z-scores were used, we compared these effect estimates to that for age and cognitive decline, thus using the effect of age on cognitive decline as a “benchmark” for interpreting the mean differences. The mean difference in cognitive rates was equivalent to that observed between women who were ~2 years apart in age.
Table 3.
Change in quality of life (QOL) at retirement and rate of cognitive decline over 6-year follow-up period: adjusted mean differences (95% confidence intervals) in rates of annual change (n=4,926)
| Self-reported change in QOL at retirement | ||||
|---|---|---|---|---|
| Worse (n=419; 8%) | Same (n=1514; 31%) | Improved (n=2993; 61%) | p for trende | |
| Global composite scorea | ||||
| Basic-adjusted modelb | 0.002 (−0.012, 0.015) | 0 (Ref) | 0.011d (0.004, 0.019)** | 0.005 |
| Multivariable-adjusted modelc | 0.006 (−0.007, 0.020) | 0 (Ref) | 0.011d (0.004, 0.019)** | 0.021 |
| TICS | ||||
| Basic-adjusted modelb | 0.019 (−0.043, 0.081) | 0 (Ref) | 0.041d (0.007, 0.076)* | 0.059 |
| Multivariable-adjusted modelc | 0.042 (−0.023, 0.107) | 0 (Ref) | 0.041d (0.007, 0.075)* | 0.17 |
| Verbal memorya | ||||
| Basic-adjusted modelb | −0.003 (−.019, 0.013) | 0 (Ref) | 0.011d (0.003, 0.020)** | 0.004 |
| Multivariable-adjusted modelc | −0.001 (−0.017, 0.016) | 0 (Ref) | 0.012d (0.003, 0.021)** | 0.007 |
| Category fluency | ||||
| Basic-adjusted modelb | 0.022 (−0.074, 0.119) | 0 (Ref) | 0.042 (−0.011, 0.095) | 0.25 |
| Multivariable-adjusted modelc | 0.066 (−0.035, 0.167) | 0 (Ref) | 0.044 (−0.010, 0.097) | 0.53 |
| Digit span backward | ||||
| Basic-adjusted modelb | 0.015 (−0.031, 0.061) | 0 (Ref) | 0.010 (−0.015, 0.036) | 0.76 |
| Multivariable-adjusted modelc | 0.016 (−0.032, 0.064) | 0 (Ref) | 0.011 (−0.014, 0.037) | 0.69 |
TICS: Telephone Interview of Cognitive Status
p<0.05;
p<0.01;
p<0.001
Global composite score is the average of the z-scores of the TICS, delayed recall of the TICS 10-word list, immediate and delayed recalls of the East Boston Memory Test, category fluency and digit span backward; verbal memory composite score is the average of the z-scores of the immediate and delayed recalls of both the TICS 10-word and the East Boston Memory Test
Adjusted for age at initial cognitive interview (continuous), education (registered nurse, BA, MA/DR), age at retirement (60–64, 65–69) and delay between retirement and post-hoc assessment of change in quality of life at retirement (<5, 5-<10, ≥10 years)
Additionally adjusted for smoking status (never, past, current), alcohol intake (none, 1–14 grams/day, ≥15 grams/day), body mass index (<22, 22–24, 25–29, ≥30 kg/m2), physical activity (quartiles of MET-hours), AHEI score (<50, ≥50), age at menopause (<50, 50–52, >52 years), use of postmenopausal hormone (yes, no), vitamin E use (no, yes), ibuprofen use (no, yes), aspirin use (no, 1–2, >2 per week), history of diabetes (no, yes), history of hypertension (no, yes), history of hyperlipidemia (no, yes), history of myocardial infarction (no, yes), SF-36 Physical Functioning score (≤30, 31–64, 65–85, ≥86), type of work before retirement (in-patient nursing, other nursing, non-nursing work, homemaker), shift work ≥20 years (yes, no), husband’s education (<high school, some high school, high school degree, BA, MA/DR), median income of US census tract (quartiles), ever had to forego medical treatment for financial reasons (no, yes), birth below 37° N latitude (no, yes), father's occupation at 16 years (professional/manager, other, n/a), SF-36 Vitality score (<50, ≥50), SF-36 Mental Health score (<53, ≥53), antidepressant use (yes, no), Berkman social network index score (4 categories), reported severe stress at home or at work in 1982 (no, yes), caregiving (0, 1–8, ≥9 hours per week).
Equivalent to ~2 years (of age) difference in rates of cognitive aging (2.1 years difference for the global composite score, 1.9 for the TICS, 2.5 for the verbal memory score)
Test for linear trend using the ordinal score on categories of QOL change at retirement
In contrast, no significant differences in cognitive decline rates were observed for the women who reported worsened QOL (Table 3) when compared to those who reported no change. While the “worse” group was small, we observed significant trends of slower cognitive decline across the three categories of change in QOL, going from worse to same to better QOL with retirement for the global composite score and the verbal memory score.
Effect modification and restricted analyses
We observed no significant interactions with age at retirement, delay between retirement and post-hoc assessment of change in QOL at retirement, work status as assessed in 1982, vitality, mental health, physical functioning and subjective memory complaint at first cognitive interview. Models limited to women with preserved cognitive function at baseline (in the top 90th percentile of global score or alternatively with TICS ≥34), to women without evidence of severe depression symptoms, as well as to women with full cognitive follow-up yielded similar results as the primary analyses (Table 4). Among those who had no or little (≤1) subjective memory complaints and among those who retired aged ≥65 years, results were no longer significant, but the magnitude and direction of the estimates were virtually unchanged.
Table 4.
Restricted analyses of change in quality of life (QOL) at retirement and later cognitive decline
| Decline in global composite scoreab | |||||
|---|---|---|---|---|---|
|
| |||||
| Selection criteria | N | Worse | Same | Improved | p-trendd |
| Subjective memory complaint scorec ≤1 | 2,473 | 0.004 (−0.016, 0.024) | 0 (Ref) | 0.006 (−0.004, 0.015) | 0.40 |
| Baseline global composite score > p10 | 3,887 | 0.003 (−0.012, 0.018) | 0 (Ref) | 0.011 (0.003, 0.019)** | 0.016 |
| Baseline TICS ≥34 | 2,966 | 0.003 (−0.014, 0.020) | 0 (Ref) | 0.014 (0.005, 0.022)** | 0.005 |
| Underwent all 4 interviews | 3,537 | 0.007 (−0.007, 0.022) | 0 (Ref) | 0.010 (0.002, 0.017)** | 0.07 |
| Age at retirement ≥65 | 1,587 | −0.001 (−0.023, 0.021) | 0 (Ref) | 0.008 (−0.004, 0.021) | 0.17 |
| No antidepressant use and SF-36 Vitality score ≥50 and SF-36 Mental Health score >=53 |
3,906 | 0.013 (−0.006, 0.031) | 0 (Ref) | 0.011 (0.003, 0.019)** | 0.09 |
p<0.05;
p<0.01;
p<0.001
Global composite score is the average of the z-scores of the TICS, delayed recall of the TICS 10-word list, immediate and delayed recalls of the East Boston Memory Test, category fluency and digit span backward
All models are multivariable-adjusted as indicated in footnote c of Table 3
Score defined as the number of positive response(s) among seven items: change in memory, difficulties in remembering a short list of items, difficulties in remembering things from one second to the next, difficulties in remembering recent events, difficulties in understanding instructions, difficulties in following a conversation, difficulties in finding the way around familiar streets; in our sample, ‘1’ is the median score
Test for linear trend using the ordinal score on categories of QOL change at retirement
DISCUSSION
In this large prospective study of retired women, most of whom have been nurses, self-reported improvement in QOL at retirement (61%), when asked an estimated median 7 years after retirement, was associated with better cognitive maintenance in later life. The mean difference in cognitive change was equivalent to that observed between women who were 2 years apart in age. Various restricted analyses to address potential reverse causation (i.e., where low cognitive function may have led to retirement or to a negative assessment of change in the QOL with retirement) showed robust associations. No significant differences in cognitive decline rates were observed for the women who reported worsened QOL (8%), possibly due to a lack of power in this group.
The literature on the link between retirement and cognition have been mixed: some longitudinal studies from the Health and Retirement Study (HRS) [23, 25], the English Longitudinal Study of Ageing (ELSA) [23], the Survey of Health, Ageing and Retirement in Europe (SHARE) [23, 25] or the Whitehall II study [24], suggested that the state of being retired was associated with less favorable cognitive outcomes [26]. In contrast, a HRS study restricted to men [27], found no clear association between retirement duration and later life cognition for white collar workers, and if anything, a positive association for blue-collar workers. However, to our knowledge, no study to date has explored the potential association of cognitive aging with how retirees had experienced their transition to retirement. Yet, this aspect may be more etiologically important as it uniquely captures the individual experience of the transition and whether the benefits provided by paid work (e.g., social contact, common goals, activity, etc. [46]) have been maintained or replaced by other benefits into the retired life. Determinants of such successful transition have been amply documented [47, 48]; however, the relation of each of these determinants and later health depends on circumstances surrounding retirement. For example, one important determinant of well-being in later life post-retirement has been the “voluntariness” of entering retirement, but the health effects of voluntariness of retirement may be modified by factors, such as pension amount and retirement savings, pre-retirement work conditions (whether retirement from a stimulating job versus an unfulfilling stressful job), substitution of the work role with other forms of civic engagement and social participation, the availability of social support and marital satisfaction, as well as health status prior to retirement. Another determinant of positive QOL transition at retirement may be the overall satisfaction with the new post-retirement schedule (where more voluntary social engagement and/or various physical, manual, intellectual activities could be undertaken). However, this would also depend on the pre-retirement level of these factors as well as the other aforementioned retirement factors; for example, personal goal pursuits in retirement have been associated with pre-retirement work characteristics, particularly one’s work ability and job involvement [49, 50]. Finally, the time elapsed since retirement and personality traits could also influence post-hoc assessment of QOL change at retirement. A unique advantage of our study was that the study population was a cohort of mostly retired nurses with overall similar education and socioeconomic status, and that we were able to adjust for some of the major pre-retirement work characteristics.
In our subset of elderly participants of the NHS, we observed that women who reported an improved QOL at retirement showed slower rate of cognitive decline, supporting the hypothesis that a successful retirement would have an overall positive effect on later cognition. Underlying mechanisms could involve several changes in lifestyle at retirement, which had been associated with better cognitive outcome: enhancement of social interactions, strengthening of the private ties, increase of physical activity, diet quality improvement, continued involvement in intellectual activities, etc. Indeed, current evidence, based mostly on cohort studies [19, 51–53], suggests that greater social stimulation, physical exercise, diet quality and cognitive activity may improve cognitive health. In our sample, when comparing 1986 (likely pre-retirement for most participants) to 1996 (post retirement) lifestyle factors, we observed tendencies for greater increases in physical activity (and to a lesser extent, in diet quality) in those who reported QOL improvement with retirement. We further observed some evidence that change in both physical activity and diet may mediate the association between QOL change at retirement with cognitive ageing, supporting greater behavioral counseling by health care providers and the implementation of exercise/nutrition programs upon retirement as a component of cognitive preventive strategy. Another possible mechanism is that those who are more optimistic would be protected against cognitive decline [54], directly, or indirectly through proactive strategies [55]. Incidentally, we observed in our sample a direct association between self-report of a positive QOL change at retirement and higher optimism level as measured by the Life Orientation Test Revised (LOT-R) in 2004. We did not find evidence that the association between QOL at retirement and cognitive decline was primarily driven by poor mental health, as we observed similar associations in our restricted analysis among women without current antidepressant use or severe depressive symptoms; however, we cannot exclude a mediating role of milder or subsyndromal forms of depression, which can be impairing in older adults [56]. Overall, our results support the hypothesis that a positive transition to retirement and individuals’ efforts to enhance the QOL post-retirement [55] may be associated with better cognitive maintenance.
Our study had several strengths. Four repeated cognitive assessments with high response rates were completed, maximizing information and minimizing biases due to death/loss to follow-up. Moreover, our exposure assessment, as an indicator of satisfaction toward retirement transition, although straightforward, showed close relationship with parallel independent SF-36 evolution, supporting the meaningfulness of such information at the individual level. Indeed, this indicator would capture the balance between social/financial disadvantage of retirement and personal benefits of ceasing to work. Another advantage of our study was the availability of extensive information on health status as well as socioeconomic context and lifestyle, which allowed us to address confounding by baseline status.
Some methodological limitations should also be considered, and most importantly, the possibility of reverse causation bias, where a less positive opinion of the retirement transition itself may reflect subtle cognitive impairment that was pre-existing before retirement and that may have influenced the decision to retire. In fact, the difficulty in disentangling the retirement process prompted by health-related reasons from the health consequences is challenging in any observational study of retirement, especially where retirement is voluntary (versus in other countries where retirement is mandated after a certain age), and the decision to quit work is often influenced by the health status prior to retirement. Indeed, we observed that the group who reported a deterioration in their QOL at retirement had also worse mental health indicators, more disease, and more behavioral risk factors at first cognitive test, but the reference group (QOL unchanged at retirement) and improved group seemed similar on most characteristics, suggesting that the improved vs. same QOL group comparison would be less affected by reverse causation bias. Furthermore, in the various restricted analyses in subsets of women in whom reverse causation would be less probable, results were consistent with the main analyses, making it unlikely that reverse causation totally accounts for the observed associations. Another limitation of our study is that cognitive function was assessed by telephone, possibly leading to misclassification of cognitive function compared to in-person assessments. However, in a validation study, the telephone-based cognitive battery performed well compared with detailed, in-person interviews (ρ = 0.81 comparing the two models of assessment [29]). We also observed no differences in results when we stratified analyses by presence of any hearing loss. In fact, errors in assessing by telephone interview compared to the in-person assessment are unlikely to be related to responses to the change in QOL question and thus, results may be biased to the null. Of course, our results are based on observational data and interpretation requires appropriate caution, particularly as the association was rather modest (equivalent to being 2 years younger in age), and there is little data on this topic. Nonetheless, residual confounding, if present, would be limited when compared to other studies of retirement in the general population given the relative homogeneity in occupational history, educational level, access to health care and health knowledge of our sample of female health professionals and given our adjustment on a wide array of potential confounders. Also, the age-education adjusted results and the multivariable-adjusted results were similar, so the degree of confounding even in the age-education adjusted results were not substantial. On the other hand, generalizability of our results is restricted as the NHS was an occupational cohort of registered nurses. Therefore, it will be important to replicate our study in other population samples to test whether the association between change in QOL at retirement and cognitive aging would still hold in those with different work environments, different skill sets and possibly different mental processing skills.
CONCLUSION
The present study is the first to investigate in a cohort sample cognitive decline in relation to change in QOL at retirement. Our results suggest that QOL improvement at retirement may contribute to maintaining cognitive function into old age. Thus, our study highlights the period of retirement, a very common life transition, as a critical window of opportunity to promote healthy ageing. Because all our participants were female and most worked as nurses, these findings should be replicated in other populations. If confirmed, they would support greater behavioral counseling (e.g., to improve diet or increase physical activity [57]) by health care providers or the implementation of programs (e.g., general wellness or exercise programs) for helping to maintain or enhance QOL throughout the retirement transition.
Supplementary Material
Acknowledgments
Financial disclosure: This work was supported by the National Institutes of Health (UM1 CA186107, P01 CA87969, R29 AG013482, R01 AG015424, R01 AG036755).
Role of the Sponsor: The National Institutes of Health financially supported the study but was not involved in any of the following: design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Additional contribution: We are grateful to the participants, staff and investigators of the NHS.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Marie-Noël Vercambre, Email: mvercambre@mgen.fr.
Olivia I. Okereke, Email: ookereke@partners.org.
Ichiro Kawachi, Email: ikawachi@hsph.harvard.edu.
Francine Grodstein, Email: phfrg@channing.harvard.edu.
Jae H. Kang, Email: nhjhk@channing.harvard.edu.
References
- 1.Arias E. National Vital Statistics Reports. National Center for Health Statistics; Hyattsville, MD: 2014. [Google Scholar]
- 2.Blazer DG, Yaffe K, Karlawish J. Cognitive aging: A report from the institute of medicine. JAMA. 2015 doi: 10.1001/jama.2015.4380. [DOI] [PubMed] [Google Scholar]
- 3.Zins M, Gueguen A, Kivimaki M, Singh-Manoux A, Leclerc A, Vahtera J, Westerlund H, Ferrie JE, Goldberg M. Effect of retirement on alcohol consumption: longitudinal evidence from the French Gazel cohort study. PLoS One. 2011;6:e26531. doi: 10.1371/journal.pone.0026531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjosten N, Kivimaki M, Singh-Manoux A, Ferrie JE, Goldberg M, Zins M, Pentti J, Westerlund H, Vahtera J. Change in physical activity and weight in relation to retirement: the French GAZEL Cohort Study. BMJ Open. 2012;2:e000522. doi: 10.1136/bmjopen-2011-000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zantinge EM, van den Berg M, Smit HA, Picavet HSJ. Retirement and a healthy lifestyle: opportunity or pitfall? A narrative review of the literature. The European Journal of Public Health. 2014;24:433–439. doi: 10.1093/eurpub/ckt157. [DOI] [PubMed] [Google Scholar]
- 6.van der Heide I, van Rijn RM, Robroek SJ, Burdorf A, Proper KI. Is retirement good for your health? A systematic review of longitudinal studies. BMC Public Health. 2013;13:1180. doi: 10.1186/1471-2458-13-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolzenberg RM. Do not go gentle into that good night: the effect of retirement on subsequent mortality of U.S. Supreme Court justices, 1801–2006. Demography. 2011;48:1317–1346. doi: 10.1007/s13524-011-0065-9. [DOI] [PubMed] [Google Scholar]
- 8.Laaksonen M, Metsa-Simola N, Martikainen P, Pietilainen O, Rahkonen O, Gould R, Partonen T, Lahelma E. Trajectories of mental health before and after old-age and disability retirement: a register-based study on purchases of psychotropic drugs. Scand J Work Environ Health. 2012;38:409–417. doi: 10.5271/sjweh.3290. [DOI] [PubMed] [Google Scholar]
- 9.Leinonen T, Martikainen P, Laaksonen M, Lahelma E. Excess mortality after disability retirement due to mental disorders: variations by socio-demographic factors and causes of death. Soc Psychiatry Psychiatr Epidemiol. 2014;49:639–649. doi: 10.1007/s00127-013-0747-2. [DOI] [PubMed] [Google Scholar]
- 10.Ekerdt DJ, Baden L, Bosse R, Dibbs E. The effect of retirement on physical health. Am J Public Health. 1983;73:779–783. doi: 10.2105/ajph.73.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerlund H, Vahtera J, Ferrie JE, Singh-Manoux A, Pentti J, Melchior M, Leineweber C, Jokela M, Siegrist J, Goldberg M, Zins M, Kivimaki M. Effect of retirement on major chronic conditions and fatigue: French GAZEL occupational cohort study. BMJ. 2010;341:c6149. doi: 10.1136/bmj.c6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahtera J, Westerlund H, Hall M, Sjosten N, Kivimaki M, Sal OP, Ferrie JE, Jokela M, Pentti J, Singh-Manoux A, Goldberg M, Zins M. Effect of retirement on sleep disturbances: the GAZEL prospective cohort study. Sleep. 2009;32:1459–1466. doi: 10.1093/sleep/32.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coe NB, Zamarro G. Retirement effects on health in Europe. Journal of Health Economics. 2011;30:77–86. doi: 10.1016/j.jhealeco.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjosten N, Nabi H, Westerlund H, Singh-Manoux A, Dartigues JF, Goldberg M, Zins M, Oksanen T, Salo P, Pentti J, Kivimaki M, Vahtera J. Influence of retirement and work stress on headache prevalence: a longitudinal modelling study from the GAZEL Cohort Study. Cephalalgia. 2011;31:696–705. doi: 10.1177/0333102410394677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oksanen T, Vahtera J, Westerlund H, Pentti J, Sjosten N, Virtanen M, Kawachi I, Kivimaki M. Is retirement beneficial for mental health?: antidepressant use before and after retirement. Epidemiology. 2011;22:553–559. doi: 10.1097/EDE.0b013e31821c41bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca R, Kapteyn A, Lee J, Zamarro G, Feeney K. A Longitudinal Study of Well-being of Older Europeans: Does Retirement Matter? J Popul Ageing. 2014;7:21–41. doi: 10.1007/s12062-014-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging. 2009;24:563–573. doi: 10.1037/a0015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher GG, Stachowski A, Infurna FJ, Faul JD, Grosch J, Tetrick LE. Mental work demands, retirement, and longitudinal trajectories of cognitive functioning. J Occup Health Psychol. 2014;19:231–242. doi: 10.1037/a0035724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marioni RE, Proust-Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, Jacqmin-Gadda H. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. Eur J Epidemiol. 2014;29:211–219. doi: 10.1007/s10654-014-9881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gow AJ, Avlund K, Mortensen EL. Occupational characteristics and cognitive aging in the Glostrup 1914 Cohort. J Gerontol B Psychol Sci Soc Sci. 2014;69:228–236. doi: 10.1093/geronb/gbs115. [DOI] [PubMed] [Google Scholar]
- 21.Smart EL, Gow AJ, Deary IJ. Occupational complexity and lifetime cognitive abilities. Neurology. 2014;83:2285–2291. doi: 10.1212/WNL.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andel R, Infurna FJ, Hahn Rickenbach EA, Crowe M, Marchiondo L, Fisher GG. Job strain and trajectories of change in episodic memory before and after retirement: results from the Health and Retirement Study. Journal of Epidemiology and Community Health. 2015 doi: 10.1136/jech-2014-204754. [DOI] [PubMed] [Google Scholar]
- 23.Rohwedder S, Willis RJ. Mental Retirement. The journal of economic perspectives : a journal of the American Economic Association. 2010;24:119–138. doi: 10.1257/jep.24.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts BA, Fuhrer R, Marmot M, Richards M. Does retirement influence cognitive performance? The Whitehall II Study. J Epidemiol Community Health. 2011;65:958–963. doi: 10.1136/jech.2010.111849. [DOI] [PubMed] [Google Scholar]
- 25.Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? J Health Econ. 2012;31:490–501. doi: 10.1016/j.jhealeco.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Mazzonna F, Peracchi F. Ageing, cognitive abilities and retirement. European Economic Review. 2012;56:691–710. [Google Scholar]
- 27.Coe NB, von Gaudecker HM, Lindeboom M, Maurer J. The effect of retirement on cognitive functioning. Health Econ. 2012;21:913–927. doi: 10.1002/hec.1771. [DOI] [PubMed] [Google Scholar]
- 28.Devore EE, Kang JH, Stampfer MJ, Grodstein F. The Association of Antioxidants and Cognition in the Nurses' Health Study. American Journal of Epidemiology. 2013;177:33–41. doi: 10.1093/aje/kws202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devore EE, Grodstein F, van Rooij FJA, Hofman A, Stampfer MJ, Witteman JCM, Breteler MMB. Dietary Antioxidants and Long-term Risk of Dementia. Arch Neurol. 2010;67:819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt J, Spencer M, Folstein M. The telephone Interview for Cognitive Status. Neuropsych Neuropsychol and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- 32.Small BJ, Fratiglioni L, Backman L. Canaries in a coal mine: cognitive markers of preclinical Alzheimer disease. Arch Gen Psychiatry. 2001;58:859–860. doi: 10.1001/archpsyc.58.9.859. [DOI] [PubMed] [Google Scholar]
- 33.Scherr PA, Albert MS, Funkenstein HH, Cook NR, Hennekens CH, Branch LG, White LR, Taylor JO, Evans DA. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 35.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer's disease. A longitudinal study. Brain. 1991;114( Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 36.Rennemark M, Berglund J. Decreased cognitive functions at the age of 66, as measured by the MMSE, associated with having left working life before the age of 60: results from the SNAC study. Scand J Public Health. 2014;42:304–309. doi: 10.1177/1403494813520357. [DOI] [PubMed] [Google Scholar]
- 37.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 39.Ware JE, Kosinski M, Kelle SK. SF-36® Physical and Mental Health Summary Scales: A User’s Manual. The Health Institute; Boston, MA: 1994. [Google Scholar]
- 40.Bewick V, Cheek L, Ball J. Statistics review 10: Further nonparametric methods. Critical Care. 2004;8:196–199. doi: 10.1186/cc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. The American Journal of Clinical Nutrition. 2015;101:587–597. doi: 10.3945/ajcn.114.090688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 44.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA. Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samieri C, Proust-Lima C, MMG, Okereke OI, Amariglio RE, Sperling RA, Rentz DM, Grodstein F. Subjective cognitive concerns, episodic memory, and the APOE epsilon4 allele. Alzheimers Dement. 2014;10:752–759. e751. doi: 10.1016/j.jalz.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahoda M. Employment and Unemployment. A Social-Psychological Analysis. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- 47.Bender KA. An analysis of well-being in retirement: The role of pensions, health, and ‘voluntariness’ of retirement. The Journal of Socio-Economics. 2012;41:424–433. [Google Scholar]
- 48.Dingemans E, Henkens K. How do retirement dynamics influence mental well-being in later life? A 10-year panel study. Scand J Work Environ Health. 2015;41:16–23. doi: 10.5271/sjweh.3464. [DOI] [PubMed] [Google Scholar]
- 49.Feldt T, Hyvonen K, Oja-Lipasti T, Kinnunen U, Salmela-Aro K. Do work ability and job involvement channel later personal goals in retirement? An 11-year follow-up study. Int Arch Occup Environ Health. 2012;85:547–558. doi: 10.1007/s00420-011-0705-9. [DOI] [PubMed] [Google Scholar]
- 50.Platts LG, Webb E, Zins M, Goldberg M, Netuveli G. Mid-life occupational grade and quality of life following retirement: a 16-year follow-up of the French GAZEL study. Aging Ment Health. 2014:1–13. doi: 10.1080/13607863.2014.955458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovden M, Xu W, Wang HX. Lifestyle change and the prevention of cognitive decline and dementia: what is the evidence? Curr Opin Psychiatry. 2013;26:239–243. doi: 10.1097/YCO.0b013e32835f4135. [DOI] [PubMed] [Google Scholar]
- 53.Roberts RO, Cha RH, Mielke MM, Geda YE, Boeve BF, Machulda MM, Knopman DS, Petersen RC. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84:1854–1861. doi: 10.1212/WNL.0000000000001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dar-Nimrod I, Chapman BP, Robbins JA, Porsteinsson A, Mapstone M, Duberstein PR. Gene by neuroticism interaction and cognitive function among older adults. Int J Geriatr Psychiatry. 2012;27:1147–1154. doi: 10.1002/gps.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelm K, Geerligs L, Peisah C. Successful transition to later life: strategies used by baby boomers. Australas J Ageing. 2014;33:81–85. doi: 10.1111/ajag.12025. [DOI] [PubMed] [Google Scholar]
- 56.Lyness JM, Heo M, Datto CJ, Ten Have TR, Katz IR, Drayer R, Reynolds CF, 3rd, Alexopoulos GS, Bruce ML. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144:496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 57.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: The time for action is now. JAMA. 2015:1–2. doi: 10.1001/jama.2015.16244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.