Abstract
Titanium dioxide engineered nanoparticles (nano-TiO2) are widely used in the manufacturing of a number of products. Due to their size (<100 nm), when inhaled they may be deposited in the distal lung regions and damage Clara cells. We investigated the mechanisms by which short-term (one-hour) incubation of human airway Clara-like (H441) cells to nano-TiO2 (6 nm in diameter) alters the ability of H441 cells to adhere to extracellular matrix. Our results show that one h post incubation, there was a three fold increase of extracellular H2O2, increased intracellular oxidative stress as demonstrated by 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) oxidation, and a five-fold increase of phosphor-ERK1/2 as measured by Western blotting. These changes were accompanied by a 25% decrease of H441 adherence to fibronectin (p<0.05 compared to vehicle incubated H441 cells). Pretreatment with the ERK1/2 inhibitor U0126 for three h, partially prevented this effect. In conclusion, short-term exposure of H441 cells to nano-TiO2 appears to reduce adherence to fibronectin due to oxidative stress and activation of ERK1/2.
Keywords: Airway epithelial cells, Clara Cells, ERK1/2, Fibronectin, Titanium nanoparticles, Reactive oxygen species
1. Intoduction
Engineered nanoparticles are particles of matter purposely sized to less than 100 nm at least in one of their dimensions. Their physicochemical properties are unique, compared to particles of larger dimensions but of similar material composition (Auffan et al., 2009). The nanoparticle industry is developing rapidly and by the year 2015, it is estimated that two million workers would be required to satisfy the demands of nanoparticle-based usage worldwide (Buzea et al., 2007; Lanone and Boczkowski, 2011). As human exposure to nanoparticles on a occupational and consumer basis is inevitable, it is important to investigate their biological effects.
Titanium dioxide (TiO2) nanoparticles are used in sunscreens, cosmetics, toothpastes, milk industry, pigments, toner, coating material as well as drug delivery vehicles (Institute of Occupational Medicine, HSE, 2004). In 2004, worldwide production of titanium dioxide was 4.4 million tons (Vainio, 2006). Humans are highly likely to be exposed to nano-TiO2 through inhalation, digestion, and uptake through the skin (Oberdörster et al., 2005). Although TiO2 has been considered inert and used as a negative control in many toxicological studies investigating animal exposure to nanoparticles, several lines of investigation support the notion that this is not the case. For example, exposure of cells to anatase (a crystalline form of TiO2) for periods ranging from 4–48 h, leads to production of reactive oxygen species (ROS), cytotoxicity and genotoxicity (Bhattacharya et al., 2009; Falck et al., 2009; Jin et al., 2010; Msiska et al., 2010; Sohaebuddin et al., 2010). Moreover, intratracheal instillation of nano-TiO2 can induce severe emphysema-like lung injury in mice, while intra-peritoneal injection was shown to lead to interstitial pneumonia, thrombosis of the pulmonary vascular system and lesion formation in the lung, kidney, spleen and liver (Chen et al., 2006; 2009). Thus, there is increasing evidence that nano-TiO2 exert toxic effects at the cellular and organism level. Additionally, the International Agency for Research on Cancer recently classified TiO2 as a Group 2B carcinogen (Msiska et al., 2010). Moreover, various inhaled nanoparticles (including TiO2) of approximately 20 nm in size or less have been shown to enter the systemic circulation rapidly (within an hour), therefore studying their effects on distal lung cells for exposure times of this range is very relevant (Buzea et al., 2007).
Clara cells (non-ciliated bronchiolar cells) are located in the distal airways and play a pivotal role in the maintenance of the epithelium in the distal conducting airways as well as in the injury repair of the bronchi and the alveolus (Atkinson et al., 2008; Dombu et al., 2010). Therefore, their ability to migrate towards the site of injury and to adhere to the extracellular matrix is essential for effective injury repair. Clara cells predominantly adhere to fibronectin and express alpha-5 integrin which along with beta-1 integrin form the fibronectin receptor (Atkinson et al., 2008). Human H441 cells, that possess properties of Clara cells and bronchial epithelial cells, are a well-established model system for studying the pathophysiology of Clara cells (Althaus et al., 2011).
In this study we assessed the short-term effects (1h of exposure) of ultrafine nano-TiO2 on H441 cells on their adhesion to fibronectin. We found that nano-TiO2 reduced the adhesive properties of H441 cells by the induction of extracellular and intracellular oxidative stress and activation of ERK1/2 pathway. Our results suggest that even transient exposures to nano-TiO2 may affect airway epithelial physiology.
2. Materials and methods
2.1 Titanium dioxide nanoparticles
Anatase TiO2 nanoparticles were prepared using 3:1 mixture of titanium isobutanol, Ti(Obu)4, and iso-propanol, as precursor as previously described (Ao et al., 2008). This mixture was slowly added to ultrapure water under stirring; the final molar ratio of water to Ti(Obu)4 was 150. Then, the solution was kept under reflux condition at approximately 75°C for 24 h. The resulting milky suspension was centrifuged, the supernatant was removed and replaced with ultrapure bacteria-free water at least five times; the suspension was then ultrasonicated for 1 h in a Branson 2510 sonicator operating at 100 W. Just prior to each experiment, TiO2 nanoparticles in ultrapure sterile water were dispersed by sonication at room temperature for 30 s and re-suspended at final concentrations of 10 or 100 μg/mL in RPMI 1640 cell culture medium [supplemented with 1% L-glutamine, 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml)] leading to a transparent stable colloid of primary anatase particles. This suspension was then added onto cells.
2.2 X-Ray Diffractometry and Fourier Transform Infrared Spectrometry for TiO2 nanoparticle characterization
A Philips X’pert thin film X-ray diffractometer with CuK radiation (Philips Analytical, Netherlands); (operating parameters were 45 kV and 40 mA) was used to determine the structure, phase composition, and mean size of anatase nanoparticles. The average grain was estimated from the major X-ray diffraction (101) peak for the anatase phase using the Scherrer formula (Suryanarayana, 1998). A high-resolution scan of (101) peak was utilized for the latter. In addition, Fourier-transform infrared spectroscopy (Bruker Optics Vertex 70 FT-IR spectrometer) was used in transmission regime in the range from 4000 cm−1 to 400 cm−1 for the analysis of anatase nanoparticles.
Transmission electron microscopy was conducted using a FEI Tecnai 12 transmission electron microscope at 80 kV acceleration voltage, and images were captured using a AMT 2048×2048 pixel CCD camera. To prepare the samples of TiO2 nanoparticles for TEM studies, a drop of dilute aqueous solution was deposited and dried on TEM nickel grid first covered with a thin film of Formvar-supported carbon.
2.3 Cell culture
H441 cells were obtained from American Type Culture Collection (passage 50; Manassas, VA, USA) and grown in RPMI 1640 medium (Mediatech, Manassas, VA, USA) as described before (Lazrak and Matalon, 2003; Lazrak et al., 2009).
2.4 Cytotoxicity assay
H441 cells were seeded at a density of 15×103 cells/well and grown for 24 h in 96-well plates. Subsequently they were exposed for 1 h to 100 μL of 0, 10, or 100 μg/mL nano-TiO2 media suspensions. Cell membrane impairment was studied using the lactate dehydrogenase (LDH) release assay (LDH Cytotoxicity Assay Kit II, BioVision, USA) and cytotoxicity was calculated according the manufacturer’s protocol. The principle of the assay is that the amount of LDH (an intracellular enzyme) detected in the cell supernatant is directly proportional to the magnitude of cell membrane impairment and thus to the number of dead cells.
2.5 Measurement of Extracellular Hydrogen Peroxide (H2O2) concentrations as an index of oxidative stress
We measured H2O2 as an index of extracellular level generation of reactive intermediates generated by H441 cells incubated with nano-TiO2. H441 cells were seeded at a density of 15×103 cells/well and grown for 24 h in 96-well plates. Concentrations of H2O2 in the cell supernatant after incubation for 1 h with 100 μL of 0, 10 or 100 μg/mL nano-TiO2 suspensions, with the OxiSelect™ Hydrogen Peroxide Assay Kit (Cell Biolabs, USA) according to the manufacturer’s instruction. This method is based on the ability of sorbitol to convert peroxide to a peroxyl radical, which oxidizes Fe+2 into Fe+3. Then Fe+3 reacts with an equal molar amount of xylenol orange in the presence of acid to create a purple product that absorbs maximally at 540 nm. H2O2 concentrations in the samples were calculated using a standard curve of known concentrations of H2O2 in RPMI media (range from 0–25 μM).
2.6 Intracellular reactive oxygen species (ROS) imaging
Intracellular formation of ROS was assessed by the oxidation of 2′,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA). When the reduced fluorescein moiety of DCFH-DA is oxidized by cellular ROS, it emits a bright green light (Ex. 488nm/Em525 nm). H441 cells were seeded at a density of 15×104 and grown for 24h in Lab-Tek II Chamber Slides for imaging. Cells were then incubated for one h with TiO2, washed twice with PBS and incubated with 1 ml/well of H2DCF-DA (Invitrogen, USA) in cell media for 30 min at 37°C. Subsequently, cell nuclei were counterstained with Hoechst 33342 (Invitrogen, USA) for 4 min. Cells were then washed twice with PBS/Ca2+/Mg2+ and imaged immediately using fluorescence microscopy with the appropriate filters (Leica DM RXA2 Fluorescent Microscope with Simple PCI imaging software). All images were taken with a dry objective at a magnification of 400x.
2.7 Cell Adhesion assay
The assessment of H441 cell adhesion properties during incubation with TiO2 nanoparticles was done using the colorimetric Fibronectin CytoSelect™ 48-Well Cell Adhesion Assay (Cell Biolabs, USA), according to the manufacturer’s protocol. 150 μL containing 3×105 cells/mL with or without TiO2 nanoparticles were added in wells coated with Fibronectin or BSA (controls) and allowed to adhere for 1 h. Wells were then washed to remove unattached cells, and the adherent cells were fixed and stained with Crystal Violet. Finally, the stain was extracted by 30 min incubation with 10% Acetic Acid water solution and its optical density mas measured at 540 nm.
2.8 Immunoblot analysis
For western blot analysis, cells were incubated with TiO2 nanoparticles at concentrations of 0, 10, or 100 μg/ml for 1 h. The harvested cell pellets were lysed in Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Thermo Scientific, Rockford, IL, USA) containing protease inhibitors (Roche Applied Bioscience, Indianapolis, USA). The cell lysates were then analyzed for protein content with BCA protein assay (Pierce, Rockford, IL, USA). Equal amounts of protein (30 μg) were loaded in 10% Tris-HCl Criterion precast gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA); proteins were transferred to Polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and immunostained with integrin α5 (Santa Cruz), phosphor-ERK1/2, phospho-p38 and α-tubulin (Cell Signalling Technology, Danvers, MA, USA) antibodies. Appropriate secondary antibodies conjugated to HRP were used. Protein bands were revealed by enhanced chemiluminescence using HRP substrate (Pierce Biotechnology, Rockford, IL) and X-ray films. Values were corrected for α-tubulin signal intensity. Lipid peroxidation was assessed in the cell lysates treated as above and quantified with the OxiSelectTM Malondialdehyde (MDA) Immunoblot Kit (Cell Biolabs, Inc., San Diego, CA) according the manufacturer’s instructions as previously described (Zarogiannis et al., 2011).
2.9 Statistical Analysis
All data are expressed as means ± SEM of the percentages of normalized data. Statistical significance was determined with One-Way ANOVA for comparison among three groups. In case of non-parametric data the analysis was performed by Kruskal-Wallis test for multiple comparisons. Results were considered significant if p<0.05.
3. Results and Discussion
3.1 X-Ray Diffractometry (XRD), Fourier Transforms Infrared Spectrometry (FTIR) and Transmission Electron Microscopy (TEM) for TiO2 characterization
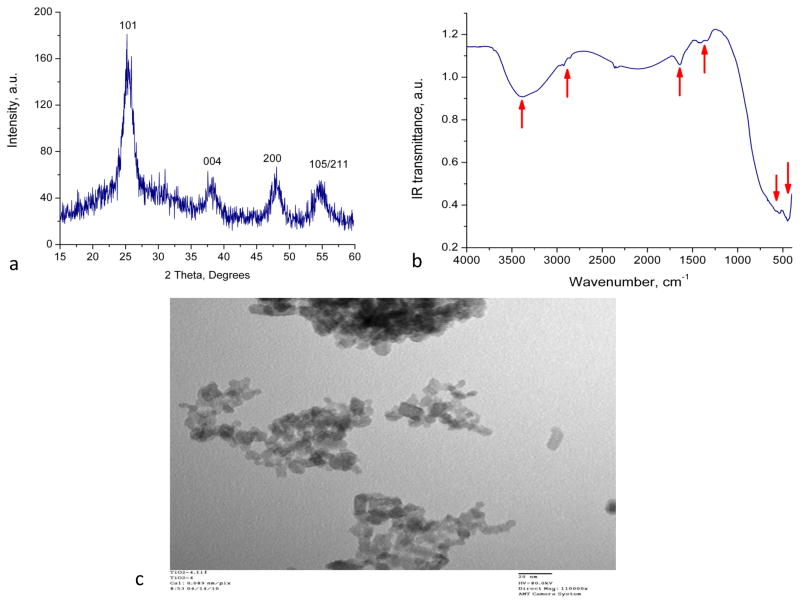
X-Ray Diffraction spectra of TiO2 nanoparticles are shown in Figure 1a. Characteristic diffraction peaks for the anatase phase are present and labeled in the spectrum. The existence of a weak peak at approximately 2θ = 30.4° may indicate the presence of a very small amount of the brookite phase. The average size of anatase crystallites was estimated around 6 nm. These results are in good agreement with previously published data (Hamilton et al., 2009).
Figure 1. Nano-TiO2 characterization.
(a) X-Ray diffractometry, (b) Fourier Transform Infrared Spectroscopy and (c) Transmission Electron Microscopy of 6 nm diameter TiO2 anatase nanoparticles in distilled water (scale 20nm).
The FT-IR spectra of the prepared and cleaned anatase nanoparticles show strong absorption band around at 435 cm−1 that has been assigned Ti-O vibrations in anatase phase (Tong et al., 2008) (Figure 1b). The broad band centered at 3390 cm−1 and another band around 1640 cm−1 can been attributed to the surface-adsorbed H2O and –OH on TiO2 particles. Other low-intensity absorption bands observed 2920 cm−1, 2870 cm−1, 1423 cm−1 and 1342 cm−1 are associated with small amounts of organic residue of the precursor material.
The TEM provided information on the size and shape of nanoparticles. As shown in Figure 1c the nanoparticles are in the range of 6 nm that corroborates the findings the XRD spectra and their shape is spherical. However, nanoparticles agglomerate when dried on the microscopic observation slide.
3.2 Short-term exposure of H441 cells to nano-TiO2 induces cell death
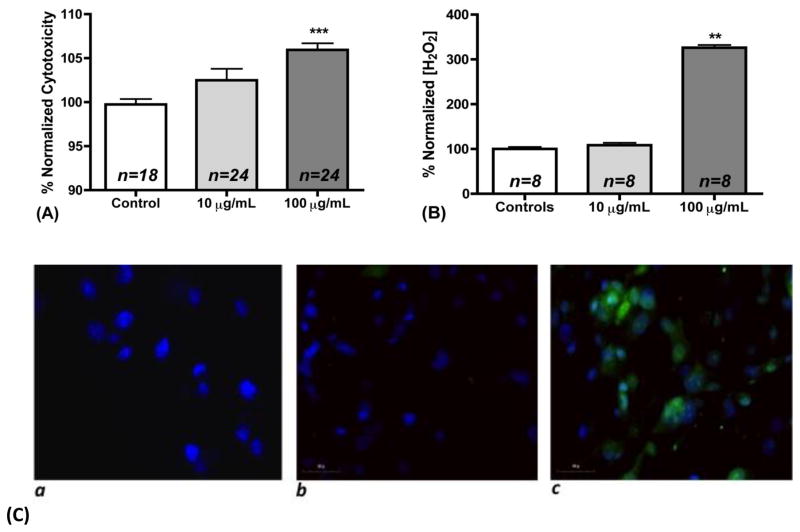
Injury to H441 cells by nano-TiO2 was assessed by measuring lactate dehydrogenase (LDH) concentrations in the supernatant. As shown in Figure 2a there was a 2.8% increase in the LDH in the 10 μg/mL group and a 6.2% increase in the 100 μg/mL group (p<0.001) compared with baseline levels (n=24). Nanomaterial cytotoxicity is composition, size and cell type specific (Sohaebuddin et al., 2010). In this study anatase TiO2 was used, which is estimated to be 100 times more cytotoxic than rutile TiO2 (Madl and Pinkerton, 2009). Exposure of human telomere-immortalized bronchiolar epithelial cells to 10 and 100 μg/mL of similar size nano-TiO2 for 24 h resulted in significant cytotoxicity (15 and 10% respectively) (Sohaebuddin et al., 2010). In addition, exposure of HaCaT cells to 7nm size and around 40 mg/mL TiO2 suspension for 6 h resulted in 20% cell death (Horie et al., 2010). In contrast, the cytotoxicity levels observed in our study were modest.
Figure 2. Cytotoxicity and extracellular and intracellular oxidative stress after nano-TiO2 exposure.
(a) Normalized % cytotoxicity after Lactate Dehydrogenase measurement in cell supernatants of H441 cells incubated with 0, 10, and 100 μg/mL TiO2 for 1 h, (b) Normalized concentration of H2O2 in cell supernatant of H441 cells incubated with 0, 10, and 100 μg/mL TiO2 for 1 h, (c) Imaging of DCF in (a) untreated H441 cells without DCF loading, (b) H441 cells loaded with DCF and incubation with 10 μg/mL TiO2 for 1 h and (c) H441 cells loaded with DCF and incubation with 100 μg/mL TiO2 for 1 h.
3.3 Short-term exposure of H441 cells to nano-TiO2 increases extracellular Hydrogen Peroxide (H2O2) and intracellular reactive oxygen species (ROS) levels but does not induce lipid peroxidation
Nano-TiO2 are known to produce ROS spontaneously (in cell free media) (Veranth et al., 2007). Moreover, H2O2 has been shown to be produced in the cell supernatant of a rat alveolar type II cell line after incubation with nano-TiO2 for 18h (Kim et al., 2003). H2O2 is a non-radical ROS that is also produced in low concentrations by cells for signaling purposes (Casanova et al., 2009). Therefore, we hypothesized that incubation of H441 cells with nano-TiO2 would increase the extracellular steady state levels of H2O2. To this end, we incubated H441 cells with 0,10, and 100 μg/mL of nano-TiO2 for 1 h, and assessed the concentration of H2O2 in their supernatants. Consistent with previous findings (Kim et al., 2003), significantly increased levels of H2O2 levels were detected in H441 cells exposed to 100 μg/mL of nano-TiO2 for 1 h as compared to controls (4.78 ± 0.09 μM vs. 1.47 ± 0.06 μM, p<0.01; 3-fold increase as shown in Figure 2b). Exposure of H441 cells to 10 μg/mL of TiO2 did not increase H2O2 concentrations in the extracellular medium (1.59 ± 0.08 μM vs. 1.47 ± 0.06 μM).
Nano-TiO2 have been reported to produce increased intracellular stress depending on the particle size, cell type and duration of exposure (Horie et al., 2010; Sohaebuddin et al., 2010), although there are no data on their effects on H441 cells. In our next series of experiments, we incubated H441 cells with the redox sensitive dye DCFH-DA dye for 30 min and subsequently with 0,10, and 100 μg/mL of nano-TiO2 for 1 h. Cells incubated for 1 h with 100 but not 10 μg/mL of nano-TiO2 exhibited significant fluorescence (Figure 2c) indicative of the existence of reactive intermediates. However, no significant increase of lipid peroxidation was observed in western blots probing for MDA adducts (data not shown).
3.4 Short-term exposure of H441 cells to nano-TiO2 decreases their ability to adhere to fibronectin through ERK1/2 phosphorylation
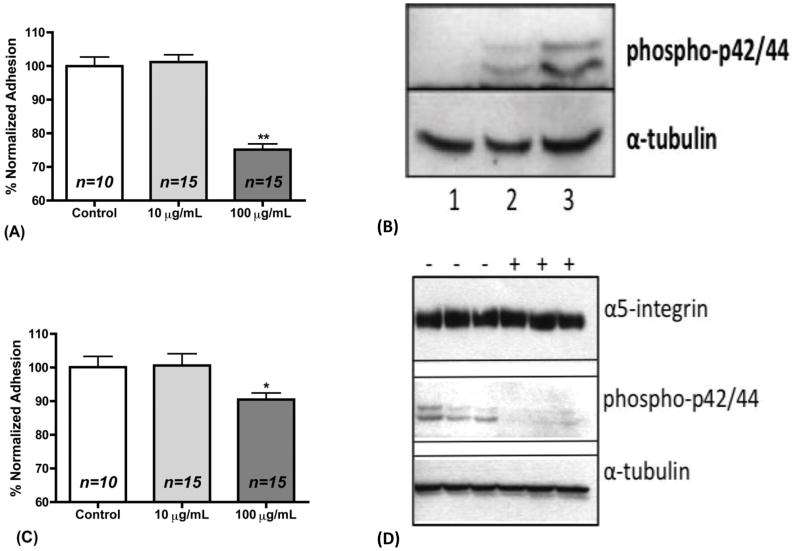
Clara cells are considered progenitor cells of the small airways and play a pivotal role in the injury repair of the bronchi and the alveolus, by migrating and adhering to the site of injury (Atkinson et al., 2008; Dombu et al., 2010). We assessed the effect of TiO2 nanoparticles on the adhesion of H441 cells to fibronectin, a key component of the extracellular matrix. Clara cells express alpha5 integrin and exhibit higher adhesion towards fibronectin than any other extracellular matrix substrate (Atkinson et al., 2008). Exposure to low (10 μg/mL) TiO2 concentration did not alter the ability of cells to adhere to the fibronectin substrate as compared to control measurement. However, as shown in Figure 3a, high (100 μg/mL) concentrations of TiO2 significantly impaired the ability of H441 cells to adhere to fibronectin by approximately 25% (p<0.01).
Figure 3. Nano-TiO2 decrease adhesion to fibronectin partially due to ERK1/2 activation.
(a) Normalized % adhesion of H441 cells that were allowed to adhere for 1 h in fibronectin substrate while being incubated with 0, 10, and 100 μg/mL TiO2 for 1 h. (b) Western Blot analysis of alpha-5 integrin, phosphor-ERK1/2 and α-tubulin in H441 cell lysates after incubation with nano-TiO2 for 1 h. Lanes 1: 0 μg/mL, 2: 10 μg/mL, and 3: 100 μg/mL. α-tubulin was examined as a loading control. (c) Normalized % adhesion of H441 cells that were pretreated with the ERK inhibitor UO126 for 3 h and then allowed to adhere for 1 h in fibronectin substrate while being incubated with 0, 10, and 100 μg/mL TiO2 for 1 h. (d) Western Blot analysis of alpha-5 integrin, phosphor-ERK1/2 and α-tubulin in H441 cell lysates after pre-treatment with ERK1/2 inhibitor UO126 (10μM) for 3 h and subsequent incubation with 100 μg/mL nano-TiO2 for 1 h. (−) No UO126, (+) UO126. α-tubulin was examined as a loading control.
Reactive oxygen species and extracellular H2O2 are known to induce the mitogen activated protein kinase (MAPK) signaling pathways (Song, 2004; MCCUBREY et al., 2006). Furthermore, it has recently been shown in neutrophils that nano-TiO2 induce the phosphorylation of ERK1/2 kinase and p38 MAPK (Gonçalves et al., 2010). On the other hand, the cellular fibronectin receptor is α5 integrin and is required for adhesion to fibronectin, and ERK1/2 activation reduces the membrane levels of α5 integrin (Chen et al., 2008). Therefore, we performed western blot analysis to find out whether phosphorylation of ERK1/2 and p38 occurs after nano-TiO2 incubation for 1h at the concentrations of 0, 10 and 100 μg/mL. α-Tubulin was used as a loading control. As seen in a typical experiment in Figure 3b phospho-ERK1/2 is increased 5-fold (100 μg/mL) and 2-fold (10 μg/mL) respectively as compared to the control. We did not detect any changes in the phospho-p38 or total p-38 (data not shown). In order to assess whether ERK1/2 inhibition would restore the adherence properties of H441 cells, we pre-incubated them with the ERK1/2 inhibitor UO126 (10 μM) for 3 h and repeated the adhesion experiment. As seen in Figure 3c the adhesion was partially restored to 90.5% of control values (p<0.05).
ERK1/2 activation is an established factor of α5 integrin activation (Hughes et al., 1997). Since we observed reduced cell adhesion to fibronectin after the exposure of H441 cells to 100 μg/mL we tested whether inhibition of ERK1/2 would have any effect on the protein expression levels of α5 integrin. We found that after inhibition of ERK1/2 the levels of the expression of α5 integrin were not altered as shown in Figure 3d. This finding along with the increased ERK1/2 activation can account for the observed decreases in adhesion. In support of our data, it has also been reported that nano-TiO2 may impair microtubule formation by decreasing tubulin polymerization which is necessary for adhesion (Gheshlaghi et al., 2008).
Our study also has some limitations such as the fact that our studies were performed using a cell line while the actual responses in vivo or even in situ may be completely different. Additionally, we have not used a negative or positive control in our experiments since throughout the experimental procedure our results were concentration specific (only 100 μg/mL exhibited biological effects, while 10 μg/mL had no effects). This observation implies that these cell responses are not generalized nanoparticle responses and that there is a specific threshold concentration of nano-TiO2 that leads to deleterious effects on H441 cells. Finally, our study focused on adhesion effects and association with ERK1/2 activation. We did not examine thoroughly the component of reactive oxygen species scavenging and associated effects on adhesion recovery, which is the objective of an ongoing project of our group.
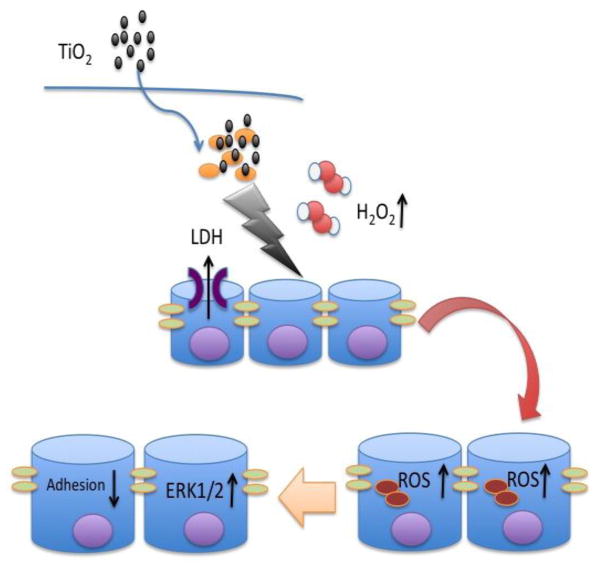
In conclusion, as shown in the schematic of Figure 4, our data show that short-term exposure of H441 cells to TiO2 anatase nanoparticles in concentration of 100 μg/mL leads to increased extracellular and intracellular oxidative stress that activates ERK1/2 and results in impairment of the adhesion towards fibronectin. These results stress the need for further investigation of the toxic effects of TiO2 nanoparticles in the respiratory system.
Figure 4. Proposed mechanism of nano-TiO2 effects on cell adhesion to fibronectin.
Schematic depicting the proposed mechanism through which incubation of H441 cells with 100 μg/mL nano-TiO2 for 1 h results in impaired adhesion towards fibronectin. More specifically, nano-TiO2 increases extracellular H2O2 and intracellular reactive oxygen species production, induces ERK1/2 activation and decreases adhesion of H441 cells to fibronectin.
Highlights.
Extra- and intra- cellular oxidative stress.
Activation of ERK1/2.
Decreased adherence to fibronectin substrate.
Acknowledgments
This work was supported by a grant from the Department of Commerce [NA09OAR417-199] and a European Respiratory Society Fellowship (LTRF58-2010) to SGZ. The authors thank Dr. Ahmed Lazrak for help with DCF imaging, Dr. Gang Liu for provision of the alpha5 integrin antibody, Mr. Larry Lawson for help with initial experiments and Ms. Gloria Son for editing the manuscript.
Footnotes
Competing Interest
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althaus M, Pichl A, Clauss WG, Seeger W, Fronius M, Morty RE. Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells. American Journal of Respiratory Cell and Molecular Biology. 2011;44:53–65. doi: 10.1165/2009-0335OC. [DOI] [PubMed] [Google Scholar]
- Ao Y, Xu J, Fu D, Shen X, Yuan C. Low temperature preparation of anatase TiO2-coated activated carbon. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;312:125–130. [Google Scholar]
- Atkinson JJ, Adair-Kirk TL, Kelley DG, deMello D, Senior RM. Clara cell adhesion and migration to extracellular matrix. Respir Res. 2008;9:1. doi: 10.1186/1465-9921-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffan M, Rose J, Bottero JY, von Lowry G, Jolivet J-P, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnology. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Davoren M, Boertz J, Schins RP, Hoffmann E, Dopp E. Titanium dioxide nanoparticles induce oxidative stress and DNAadduct formation but not DNA-breakage in human lung cells. Part Fibre Toxicol. 2009;6:17. doi: 10.1186/1743-8977-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Casanova D, Bouzigues C, Nguyên TL, Ramodiharilafy RO, Bouzhir-Sima L, Gacoin T, Boilot JP, Tharaux PL, Alexandrou A. Single europium-doped nanoparticles measure temporal pattern of reactive oxygen species production inside cells. Nature Nanotechnology. 2009;4:581–585. doi: 10.1038/nnano.2009.200. [DOI] [PubMed] [Google Scholar]
- Chen HW, Su SF, Chien CT, Lin WH, Yu SL, Chou CC, Chen JJW, Yang PC. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. The FASEB Journal. 2006;20:2393–2395. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- Chen J, Dong X, Zhao J, Tang G. In vivoacute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29:330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- Chen Q, Meng LH, Zhu CH, Lin LP, Lu H, Ding J. ADAM15 suppresses cell motility by driving integrin α5β1 cell surface expression via Erk inactivation. The International Journal of Biochemistry & Cell Biology. 2008;40:2164–2173. doi: 10.1016/j.biocel.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Dombu CY, Kroubi M, Zibouche R, Matran R, Betbeder D. Characterization of endocytosis and exocytosis of cationic nanoparticles in airway epithelium cells. Nanotechnology. 2010;21:355102. doi: 10.1088/0957-4484/21/35/355102. [DOI] [PubMed] [Google Scholar]
- Falck G, Lindberg H, Suhonen S, Vippola M, Vanhala E, Catalan J, Savolainen K, Norppa H. Genotoxic effects of nanosized and fine TiO2. Human & Experimental Toxicology. 2009;28:339–352. doi: 10.1177/0960327109105163. [DOI] [PubMed] [Google Scholar]
- Gheshlaghi ZN, Riazi GH, Ahmadian S, Ghafari M, Mahinpour R. Toxicity and interaction of titanium dioxide nanoparticles with microtubule protein. Acta Biochim Biophys Sin (Shanghai) 2008;40:777–782. [PubMed] [Google Scholar]
- Gonçalves DM, Chiasson S, Girard D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicology in Vitro. 2010;24:1002–1008. doi: 10.1016/j.tiv.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Nishio K, Fujita K, Kato H, Endoh S, Suzuki M, Nakamura A, Miyauchi A, Kinugasa S, Yamamoto K, Iwahashi H, Murayama H, Niki E, Yoshida Y. Cellular responses by stable and uniform ultrafine titanium dioxide particles in culture-medium dispersions when secondary particle size was 100nm or less. Toxicology in Vitro. 2010;24:1629–1638. doi: 10.1016/j.tiv.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Institute of Occupational Medicine, HSE. RR274 - Nanoparticles: An occupational hygiene review. 2004:1–113. [Google Scholar]
- Jin C, Tang Y, Yang FG, Li XL, Xu S, Fan XY, Huang YY, Yang YJ. Cellular Toxicity of TiO2 Nanoparticles in Anatase and Rutile Crystal Phase. Biol Trace Elem Res. 2010 doi: 10.1007/s12011-010-8707-0. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim KS, Kwak NJ, Lee KH, Kweon SA, Lim Y. Cytotoxicity of yellow sand in lung epithelial cells. J Biosci. 2003;28:77–81. doi: 10.1007/BF02970135. [DOI] [PubMed] [Google Scholar]
- Lanone S, Boczkowski J. Titanium and gold nanoparticles in asthma: the bad and the ugly. European Respiratory Journal. 2011;37:225–227. doi: 10.1183/09031936.00140110. [DOI] [PubMed] [Google Scholar]
- Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. The FASEB Journal. 2009;23:3829–3842. doi: 10.1096/fj.09-135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazrak A, Matalon S. cAMP-induced changes of apical membrane potentials of confluent H441 monolayers. Am J Physiol Lung Cell Mol Physiol. 2003;285:L443–50. doi: 10.1152/ajplung.00412.2002. [DOI] [PubMed] [Google Scholar]
- Madl AK, Pinkerton KE. Health effects of inhaled engineered and incidental nanoparticles. Critical Reviews in Toxicology. 2009;39:629–658. doi: 10.1080/10408440903133788. [DOI] [PubMed] [Google Scholar]
- MCCUBREY JA, LaHAIR MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Msiska Z, Pacurari M, Mishra A, Leonard SS, Castranova V, Vallyathan V. DNA Double-Strand Breaks by Asbestos, Silica, and Titanium Dioxide: Possible Biomarker of Carcinogenic Potential? American Journal of Respiratory Cell and Molecular Biology. 2010;43:210–219. doi: 10.1165/rcmb.2009-0062OC. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdorster E, Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010;7:22. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ. Hydrogen Peroxide-Induced Extracellular Signal-Regulated Kinase Activation in Cultured Feline Ileal Smooth Muscle Cells. Journal of Pharmacology and Experimental Therapeutics. 2004;312:391–398. doi: 10.1124/jpet.104.074401. [DOI] [PubMed] [Google Scholar]
- Suryanarayana C. X-Ray diffraction: a practical approach - Google Books. 1998. [Google Scholar]
- Tong T, Zhang J, Tian B, Chen F, He D. Preparation and characterization of anatase TiO2 microspheres with porous frameworks via controlled hydrolysis of titanium alkoxide followed by hydrothermal treatment. Materials Letters. 2008;62:2970–2972. [Google Scholar]
- Vainio EW. Monograph No 2 – Titanium dioxide. 2006. pp. 1–5. [Google Scholar]
- Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4:2. doi: 10.1186/1743-8977-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and Deferoxamine Administration Post Chlorine Exposure Decrease Mortality and Lung Injury in Mice. American Journal of Respiratory Cell and Molecular Biology. 2011;45:386–392. doi: 10.1165/rcmb.2010-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]