Abstract
Objective
To evaluate the reasons that complete remission is not achieved or maintained with original treatment in some patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) treated with rituximab (RTX) or with cyclophosphamide/azathioprine (CYC/AZA).
Methods
The Rituximab in AAV trial was a randomized, double-blind, placebo-controlled trial comparing the rate of remission induction among patients treated with RTX (n = 99) and patients treated with CYC followed by AZA (n = 98). Glucocorticoids were tapered over a period of 5 months. The primary outcome measure was lack of disease activity without glucocorticoid treatment at 6 months. To determine the most important reason for failure to achieve the primary outcome, 7 hierarchical categories of reasons were defined retrospectively (uncontrolled disease, adverse event leading to therapy discontinuation, severe flare, limited flare, Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis >0, prednisone treatment at any dosage, and other).
Results
Although remission (lack of disease activity) was achieved in 170 of the 197 patients (86%) in the first 6 months, the primary outcome measure was not achieved in 42%. There were 3 deaths. Twenty-four percent of the patients failed to achieve the primary end point due to active disease: 10 (5%) experienced uncontrolled disease in the first month and 37 (19%) experienced flares after initial improvement. In the majority of such patients, treatment with blinded crossover or according to best medical judgment led to disease control. Ninety-one percent of patients who had uncontrolled disease or experienced a severe flare had proteinase 3 (PR3)–ANCA. When patients with uncontrolled disease were excluded from analysis, those who were PR3-ANCA positive were found to experience fewer flares when treated with RTX compared to CYC/AZA (8 of 59 [14%] versus 20 of 62 [32%]; P = 0.02). Neither ANCA titers nor B cell counts predicted disease flare.
Conclusion
Current treatment regimens are largely successful in controlling AAV, but in approximately one-fourth of patients, active disease persists or recurs in the first 6 months despite treatment. PR3-ANCA positivity is a risk factor for recurrence or persistence of severe disease. ANCA titers and B cell detectability are poor predictors of both disease relapse and disease quiescence in the first 6 months.
Granulomatosis with polyangiitis (Wegener’s) (GPA) and microscopic polyangiitis (MPA) are antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAVs) that primarily affect small- and medium-sized vessels. Treatment of severe AAV with cyclophosphamide (CYC) and glucocorticoids has substantially reduced the high mortality rate previously associated with these diseases (1,2). Rituximab (RTX), an anti-CD20 monoclonal antibody, was shown to be noninferior to CYC for remission induction in the RTX in AAV (RAVE) trial, a randomized, double-blind, placebo-controlled investigation (3). The primary outcome measure of the RAVE trial, complete remission of disease (Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis [BVAS/WG] [4] 0) and successful completion of glucocorticoid taper at 6 months, set a high standard for clinical success. The glucocorticoid taper in the RAVE trial was the most rapid studied in an AAV trial to date. Although disease remission (BVAS/WG 0) was achieved in 86% of patients during the first 6 months, 42% failed to meet the primary outcome measure.
Data included in primary reports of clinical trials often fail to provide clinicians with a complete understanding of what to anticipate when the medications tested are administered in the context of usual practice. A full understanding of what to expect when prescribing either RTX- or CYC-based regimens for remission induction in AAV requires details about what happened to patients who had uncontrolled disease or disease flares, predictors of inability to achieve sustained disease control, and the contributions of adverse events to treatment discontinuation. Therefore, in the present analysis we examined the reasons the primary outcome measure was not achieved in some patients (primary treatment failure) in the RAVE trial, with the goal of better understanding and anticipating what the clinical outcome might be in individual AAV patients who are treated with RTX or CYC for remission induction.
PATIENTS AND METHODS
Study design and patients
The RAVE trial design has been reported previously (3,5). The trial enrolled ANCA-positive patients with GPA or MPA who had severe disease (BVAS/WG ≥3). Patients were assigned to receive initial treatment either with RTX or with CYC followed by azathioprine (AZA).
Treatments
Patients assigned to the RTX group received 4 weekly infusions (375 mg/m2 each), plus daily placebo CYC followed by placebo AZA upon remission. Patients assigned to the CYC/AZA group received placebo RTX infusions and oral CYC (2 mg/kg, adjusted for renal insufficiency) for 3–6 months followed by AZA (2 mg/kg) for a total of 18 months of therapy. Both groups received glucocorticoids according to the same protocol, which allowed up to 3 gm of intravenous methylprednisolone (1 gm/day for 3 days) followed by prednisone 1 mg/kg/day. Prednisone was tapered and discontinued over a period of 5–5.5 months.
Assessments
Study visits occurred weekly during the first 4 weeks, followed by visits at months 2, 4, and 6. Disease activity was assessed with the BVAS/WG. Damage was assessed with the Vasculitis Damage Index (6).
ANCA measurements
ANCA type and titer were determined by enzyme-linked immunosorbent assay (ELISA) (7). All ANCA measurements were performed simultaneously on the same ELISA plate at a single laboratory. ANCA levels were defined as having risen if there was a ≥2-fold increase from one measurement to another or an increase to ≥20 IU if the assay results had previously been negative.
B cell kinetics
B cells were measured by 5-color flow cytometry at Immune Tolerance Network facilities. B cell depletion was defined as the presence of <10 CD19+ B cells/μl, and full reconstitution as ≥69 CD19+ B cells/μl or return to baseline. B cell counts of 10–68 CD19+ B cells/μl were categorized as redetectable.
Outcome measures and disease flares
The primary outcome measure in the RAVE trial, i.e., complete remission, was defined as a BVAS/WG of 0 and no prednisone treatment at 6 months. Remission was defined as a BVAS/WG of 0 regardless of prednisone treatment or dosage. Remission could be achieved at any point before or at the 6-month time point. Secondary outcomes measured included the percentage of patients with a BVAS/WG of 0 during treatment who were taking prednisone at a dosage of <10 mg/day at 6 months, cumulative glucocorticoid doses, and adverse event rates. Patients were categorized as having uncontrolled disease if they had a new or worsening feature on the BVAS/WG, or a worsening or unchanged overall BVAS/WG 1 month after entry into the study. Disease flares were defined as an increase in the BVAS/WG of ≥1 point after the first month of therapy, regardless of whether remission had been achieved. Severe flares were defined as an increase in the BVAS/WG of >3 points or 1 new major BVAS/WG item. Patients who experienced severe flares before the 6-month time point were eligible for blinded treatment crossover. Patients who experienced limited flares were treated with prednisone. Patients who experienced an adverse event leading to treatment discontinuation or were withdrawn from the trial due to uncontrolled disease or a disease flare were treated according to best medical judgment. For such patients, in addition to the data collected according to the protocol during the study visits, some details of treatment outcomes and adverse events were collected from MedWatch forms filed with the Food and Drug Administration and from chart reviews performed by site investigators.
Adverse events
Adverse events were graded according to National Cancer Institute Common Terminology Criteria (8).
Hierarchical approach to analyzing primary outcome failures
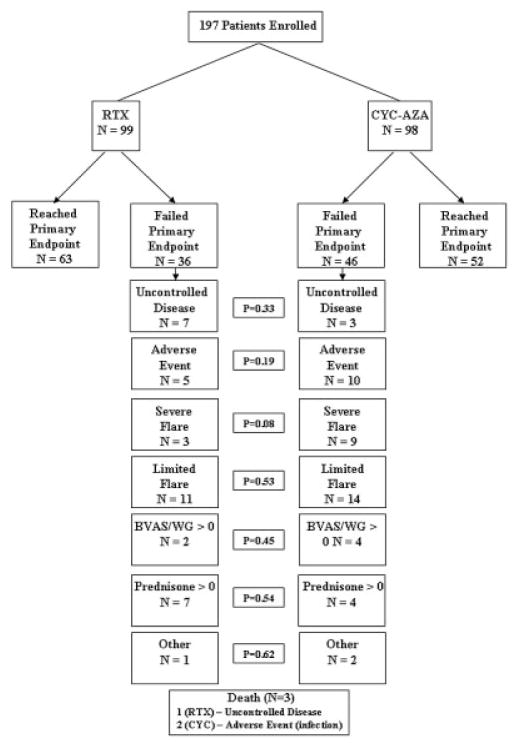
Because in some patients there was >1 potential cause for not achieving the primary outcome, we retrospectively defined 7 categories of primary treatment failure in order to identify the principal reason (Figure 1). The categories corresponded generally to the reason for failure that occurred first. The first category of treatment failure, uncontrolled disease, occurred (by definition) within 1 month of baseline. Adverse events leading to discontinuation of assigned treatment generally preceded disease flares. Severe flares were counted before limited flares in the hierarchy, to avoid underestimating the number of severe flares that required treatment crossover or treatment according to best medical judgment. The categories of primary treatment failure were, in hierarchical order: 1) uncontrolled disease at 1 month, 2) adverse event leading to cessation of assigned treatment, 3) severe flare, 4) limited flare, 5) BVAS/WG >0 at 6 months in the absence of disease flare, 6) prednisone treatment at any dosage at 6 months, and 7) other.
Figure 1.
Reasons for failure to achieve the primary outcome in the Rituximab in Antineutrophil Cytoplasmic Antibody–Associated Vasculitis trial, by treatment group. RTX = rituximab; CYC/AZA = cyclophosphamide followed by azathioprine; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis.
Statistical analysis
Binary outcomes were compared between treatment arms by chi-square or Fisher’s exact test. Continuous outcomes were compared by Wilcoxon’s rank sum test. Wald confidence limits of 95% were calculated for differences in outcomes between treatment arms at the different time points. For analyses of time to first flare, the date of the flare was assumed to be the first date of a prednisone dosage increase over the dosage in the 21 days prior to the recorded flare date. If there was no increase in prednisone dosage before the date of the recorded flare, the flare date was the date of the recorded flare. The data sets from these analyses are accessible to readers through TrialShare, a publicly accessible web site developed by the Immune Tolerance Network (www.itntrialshare.org).
RESULTS
Primary treatment was classified as having failed in 82 (42%) of the 197 patients enrolled (36 [36%] in the RTX group and 46 [47%] in the CYC/AZA group; P = 0.13). The reasons for primary treatment failure and their frequencies are listed in Figure 1 and described in detail below. Of the 82 patients with primary treatment failure, 55 (67%) reached clinical remission (BVAS/WG 0) before experiencing an event that led to categorization as primary treatment failure. Thus, only 27 patients (14 in the RTX group, 13 in the CYC/AZA group) (14% of the overall cohort) did not achieve remission in the first 6 months of therapy before being classified as having primary treatment failure.
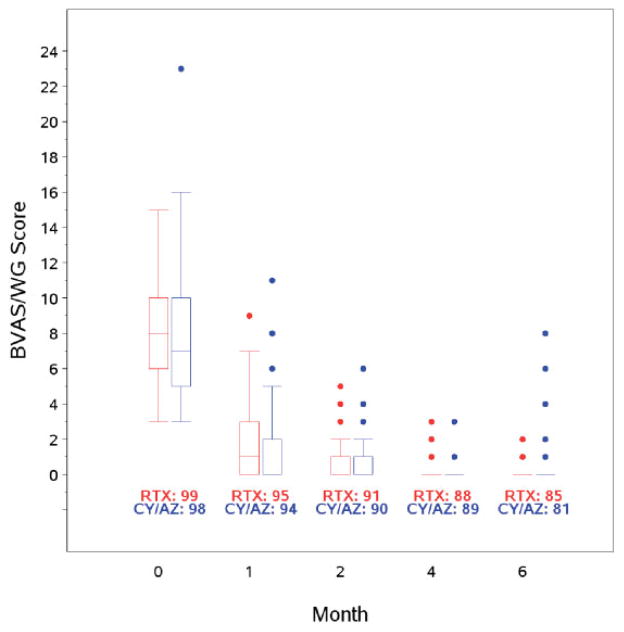
Univariate analysis demonstrated that proteinase 3 (PR3) ANCA–positive patients were at increased risk of failing to reach remission in the first 6 months, compared to those with myeloperoxidase (MPO) ANCA (18% versus 6%; P = 0.03) (Table 1). Eighty percent of the patients who achieved remission remained in remission at 6 months, and 75% remained in remission while taking <10 mg/day of prednisone. Disease activity as assessed by the BVAS/WG decreased at the same rate in both treatment groups (Figure 2).
Table 1.
Baseline characteristics of the 197 patients with AAV*
| Remission achieved in the first 6 months (n = 170) | Remission not achieved in the first 6 months (n = 27) | |
|---|---|---|
| BVAS/WG, mean | 8.0 | 8.3 |
| Serum creatinine, mean mg/dl | 1.4 | 1.6 |
| ANCA type | ||
| PR3 | 108 (82.4) | 23 (17.6)† |
| MPO | 62 (93.9) | 4 (6.1) |
| Diagnosis‡ | ||
| GPA | 125 (85.0) | 22 (15.0) |
| MPA | 44 (91.7) | 4 (8.3) |
| New diagnosis at baseline | ||
| Yes | 84 (87.5) | 12 (12.5) |
| No | 86 (85.1) | 15 (14.9) |
Except where indicated otherwise, values are the number (%) (% calculated using, as the denominator, the total number of patients [i.e., those achieving and those not achieving remission] with the given variable). AAV = antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis; PR3 = proteinase 3; GPA = granulomatosis with polyangiitis (Wegener’s); MPA = microscopic polyangiitis.
P = 0.03 versus patients with myeloperoxidase (MPO)–ANCA.
Disease type was undetermined in 2 patients.
Figure 2.
Disease activity by treatment group. Data are shown as box plots. Each box represents the upper and lower interquartile range (IQR). Lines inside the boxes represent the median. Whiskers represent 1.5 times the upper and lower IQRs. Circles indicate outliers. At the 4-month and 6-month time points, remission had been achieved in most patients. BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis; RTX = rituximab; CY/AZ = cyclophosphamide followed by azathioprine.
Uncontrolled disease
Seven patients in the RTX group (7.1%) and 3 in the CYC/AZA group (3.1%) either had worsening disease activity represented by at least 1 BVAS/WG item or failed to achieve a lower BVAS/WG during the first month of therapy (P = 0.33). All 10 patients with uncontrolled disease were PR3-ANCA positive, and all had diagnoses of GPA. Most cases of uncontrolled disease were due to glomerulonephritis (6 of 10 patients) or pulmonary hemorrhage (2 of 10); 1 patient had both. Of the remaining 3 patients, 1 had uncontrolled scleritis, 1 had progressive pulmonary nodules, and 1 had new-onset hematuria. Six of the 10 patients with uncontrolled disease had relapsing, as opposed to newly diagnosed, disease at study entry; this ratio was comparable to that in the trial overall.
Nine of the 10 patients with uncontrolled disease were treated with CYC: 3 via blinded crossover and 5 through best medical judgment (consistent with the standard of care when the trial was designed); 1 patient with uncontrolled disease continued to receive CYC (his originally assigned treatment) because, in the assessment of the investigator, the patient was on track to achieve disease control. Six of the patients treated according to best medical judgment were re-treated with intravenous methylprednisolone for 3 days and 3 underwent plasma exchange. One patient in the RTX group (the only patient with uncontrolled disease who required dialysis and had both glomerulonephritis and alveolar hemorrhage) died of septic shock at 11 weeks. After receiving 3 of 3 RTX infusions according to the study protocol, that patient also received pulse glucocorticoids, plasma exchange, and CYC according to best medical judgment. All other patients improved, with resolution of pulmonary disease and improvement in renal function.
Adverse events leading to treatment discontinuation
Fifteen patients (5 in the RTX group and 10 in the CYC/AZA group) (7.6%) discontinued treatment due to adverse events (Table 2). Four patients discontinued treatment due to leukopenia and 4 because of infection. Infections that led to treatment discontinuation included pneumonia (n = 3) and osteomyelitis (n = 1). Two patients randomized to the CYC/AZA group who had discontinued treatment due to pneumonia died during the first 6 months of the trial. One patient, who was noncompliant with trimethoprim/sulfamethoxazole prophylaxis, developed Pneumocystis jirovecii pneumonia and subsequently died of septic shock on day 55. A second patient developed Pseudomonas pneumonia, bacteremia, and sepsis in the setting of a chronic obstructive pulmonary disease flare requiring high-dose prednisone. She died on day 123.
Table 2.
Adverse events leading to drug discontinuation*
| RTX | CYC/AZA | |
|---|---|---|
| Infection | 1 | 3 |
| Leukopenia | 1 | 3 |
| Hypersensitivity reaction | 1 | 1 |
| Other† | 2 | 3 |
| Total | 5 | 10 |
Values are the number of patients.
Other adverse events in the rituximab (RTX) group included prostate cancer and microscopic colitis. Other adverse events in the cyclophosphamide followed by azathioprine (CYC/AZA) group included hemorrhagic cystitis, adverse reaction to AZA not otherwise specified, and abnormal liver function test results.
Disease flares
In 37 patients (19% of the overall cohort), the primary outcome was not achieved because of disease flares between months 1 and 6. Of these, 27 had achieved remission prior to flare and 10 experienced flares before achieving remission (but after BVAS/WG improvement in the first month of treatment). Twelve patients experienced severe flares (3 in the RTX group, 9 in the CYC/AZA group) and 25 experienced limited flares (11 and 14 in the RTX and CYC/AZA groups, respectively) (Table 3).
Table 3.
Characteristics at the time of flare in the 37 patients who experienced severe or limited flares*
| RTX | CYC/AZA | Total | |
|---|---|---|---|
| Severe flares | |||
| No. of patients | 3/98 | 9/97 | 12 |
| PR3-ANCA positive, no. of patients | 2 | 9 | 11 |
| Time to flare, mean days | 75.3 | 122.9 | 111.0† |
| Receiving CYC at time of flare, no. of patients | – | 6/9 | 6/9† |
| Prednisone dosage at time of flare, mean (range) mg/day | 15.0 (5–20) | 10.8 (0–50) | 11.8 (0–50) |
| BVAS/WG at time of flare, mean (range) | 3.3 (3–4) | 4.6 (3–8) | 4.3 (3–8) |
| Limited flares | |||
| No. of patients | 11 | 14 | 25 |
| PR3-ANCA positive, no. of patients | 6 | 11 | 17 |
| Time to flare, mean days | 121.5 | 166.4‡ | 146.7 |
| Receiving CYC at time of flare, no. of patients | – | 2/14 | 2/14 |
| Prednisone dosage at time of flare, mean (range) mg/day | 5.7 (0–20) | 6.2 (0–20) | 6.0 (0–20) |
| BVAS/WG at time of flare, mean (range) | 1.6 (1–3) | 1.4 (0–3) | 1.5 (0–3) |
CYC/AZA = cyclophosphamide followed by azathioprine; PR3 = proteinase 3; ANCA = antineutrophil cytoplasmic antibody; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis.
P = 0.02 versus limited flares.
P = 0.007 versus rituximab (RTX) group.
Ongoing treatment with CYC did not prevent disease flares. Six of the 9 patients with severe flares in the CYC/AZA group were still receiving CYC at the time of flare. In both treatment groups, the patients who experienced severe flares were receiving prednisone at an average of >10 mg/day at the time of flare. Furthermore, in 5 of the 12 patients with severe flares (4 in the CYC/AZA group, 1 in the RTX) group, remission had not been achieved prior to flare. Limited flares occurred later in the treatment course, with patients in both groups receiving prednisone at an average of 6.0 mg/day and with 12 of 14 patients in the CYC/AZA group (86%) having switched over to AZA.
Baseline ANCA status and risk of flare
Eleven of the 12 patients (92%) who experienced severe flares were PR3-ANCA positive, and 10 (83%) had GPA. Among patients with limited flares, 17 of 25 (68%) were PR3-ANCA positive and 22 of 25 (88%) had GPA. When patients with uncontrolled disease were excluded from analysis, it was found that PR3-ANCA–positive patients who were treated with RTX experienced fewer flares than did those treated with CYC/AZA (8 of 59 [14%] versus 20 of 62 [32%]; P = 0.02). In contrast, among MPO-ANCA–positive patients there was no difference in the frequency of flares between the RTX and CYC/AZA groups (6 of 33 [18%] and 3 of 33 [9%], respectively; P = 0.48). Within the CYC/AZA group, flares occurred more frequently among PR3-ANCA–positive patients than among MPO-ANCA–positive patients (20 of 62 [32%] versus 3 of 33 [9%]; P = 0.01). This difference was not observed in the RTX group, in which 8 of 59 PR3-ANCA–positive patients (14%) experienced flares, compared to 6 of 33 MPO-ANCA–positive patients (18%) (P = 0.56).
Relapsing disease at baseline and risk of flare
Among the 95 patients with relapsing disease at baseline who exhibited evidence of disease control within 1 month of randomization, severe flares occurred in 9 of the 49 patients (18%) in the CYC/AZA group, compared to none of the 46 in the RTX group (P < 0.01). This is consistent with our earlier finding that among patients with relapsing disease at entry, the primary outcome was more likely to be achieved in those randomized to the RTX group (3). In the CYC/AZA group, 17 of 49 patients with relapsing disease (35%) experienced flares between months 1 and 6, compared to only 6 of 46 (13%) who were newly diagnosed (P = 0.02). In contrast, in the RTX group the likelihood of disease flare was not different between those who had relapsing disease at baseline and those with newly diagnosed AAV (5 of 46 [11%] versus 9 of 46 [20%]; P = 0.39).
Correlation of ANCA status with disease flares
Neither ANCA positivity nor increases in ANCA titer predicted the occurrence of flares between months 1 and 6. Although ANCA results were positive at the time of flare in 10 of 14 patients in the RTX group and 18 of 23 patients in the CYC/AZA group, 44 patients with positive ANCA in the RTX group and 52 with positive ANCA in the CYC/AZA group did not experience flares in the first 6 months. Similarly, elevations in ANCA titer did not predict disease flares, particularly in the RTX group. None of the 14 flares in the RTX group was preceded by a rise in the ANCA titer, and only 10 of 23 patients (43%) in the CYC/AZA group had a rise in ANCA titer associated with a disease flare.
Correlation of B cell counts with disease flares
B cell counts did not predict flares between months 1 and 6. None of the 14 patients randomized to the RTX group who had flares between months 1 and 6 had detectable B cells. Detectable B cells were present at the time of flare in some patients randomized to the CYC/AZA group, but only in 13 of 23 (57%). B cell detectability was not associated with flare in most cases. Forty-seven patients (11 in the RTX group and 36 in the CYC/AZA group) had detectable B cells in the first 6 months without experiencing a disease flare. When B cells and ANCA status were analyzed together, we found that 7 of 37 patients (19%) (4 in the RTX group and 3 in the CYC/AZA group) had a flare in the absence of B cells and with negative ANCA.
Blinded crossover treatment of flares
Fourteen patients underwent blinded crossover treatment: 11 (3 who were randomized to receive RTX and 8 who were randomized to receive CYC/AZA) because of severe disease flares and 3 (all randomized to the RTX group) because of uncontrolled disease. Of these 14 patients, 12 remained on protocol 6 months after crossover and achieved remission within 6 months of crossover. In 8, disease remained in complete remission (BVAS/WG of 0 and no prednisone treatment) 6 months after crossover. One patient who experienced a severe flare withdrew from the study, and 1 was treated according to best medical judgment.
Treatment failures due to not meeting the BVAS/WG criterion or not meeting the glucocorticoid criterion
In 6 patients (2 in the RTX group and 4 in the CYC/AZA group) the primary outcome was not achieved due to a BVAS/WG of >0 (mean 2.0) at 6 months. None of these patients was taking prednisone at the 6-month assessment. In 11 patients (7 in the RTX group and 4 in the CYC/AZA group) the primary outcome was not achieved because they were receiving prednisone at ≥1 mg/day (mean 9.3 mg/day) at 6 months, despite having a BVAS/WG of 0.
DISCUSSION
In the past 50 years there has been extraordinary progress in the treatment of AAV. GPA and MPA are no longer routinely fatal within weeks to months, as was often the case in earlier eras. Long-term therapy with CYC has been replaced by shorter and therefore less toxic courses of CYC, followed by “step-down” therapy to AZA or other active agents. Unfortunately, however, such therapy is still not without significant risks of drug toxicity and disease relapse.
The RAVE trial established RTX plus glucocorticoids as a treatment alternative to CYC/AZA plus glucocorticoids for remission induction. Indeed, in 86% of all patients in the trial, remission (BVAS/WG 0) was recorded on at least 1 visit during the first 6 months of therapy. This indicates that current treatment regimens control severe AAV, at least initially, in the large majority of patients. Furthermore, although 11% of patients enrolled in the trial were categorized as having primary treatment failure because of either uncontrolled disease or severe flares, treatment by crossover or according to best medical judgment led to disease control in most of those patients as well. Thus, in the great majority of patients with AAV, disease activity is eventually brought under control with currently available regimens, regardless of whether RTX or CYC is used first. When the cases of uncontrolled disease and the cases of disease flare within the first 6 months are considered together, there is no evidence of any difference in the onset of action between the RTX-based regimen and the CYC-based regimen.
Despite these encouraging results, the trial’s strict primary outcome criterion (BVAS/WG of 0 without glucocorticoid treatment at 6 months) was not achieved in 42% of the patients, and nearly 25% were categorized as having primary treatment failure because of either uncontrolled disease or disease flare within the first 6 months. A small minority of patients (~5%) exhibited uncontrolled disease at 1 month despite receiving aggressive remission induction regimens. This group, characterized by PR3-ANCA positivity, comprises a subset of patients with particularly difficult-to-treat disease that failed to respond to the remission induction regimen to which they were randomized (RTX in 7, CYC/AZA in 3). Strong inferences cannot be drawn based on outcomes in only 10 patients, particularly when one considers that the duration of active disease and treatment before randomization varied among these patients. Additional studies of such patients are needed in order to understand the optimal approach to remission induction.
Severe flares also occurred preferentially in PR3-ANCA–positive patients, despite active treatment with CYC/AZA or B cell depletion with RTX. Among patients in both treatment arms, neither ANCA titers nor levels of B cells during the first 6 months of therapy were helpful in predicting disease flares. These results contrast with data from a later period in this trial—the time between 6 and 18 months—when flares were uncommon in patients who were negative for ANCA and had undetectable B cells (9).
Disease flares following the first month of treatment were the most common reason for patients to be classified as having primary treatment failure. Patients who were PR3-ANCA positive had more aggressive disease than did those with MPO-ANCA, but had fewer flares during the remission induction period if randomized to the RTX group. Both PR3-ANCA positivity and relapsing disease at baseline (with patients in the latter group having a higher rate of PR3-ANCA positivity) were associated with a higher number of flares in the CYC/AZA group. Increased rates of flare in patients with PR-ANCA positivity or GPA have been reported previously (10–14). Recent evidence from genetic studies suggests that PR3-ANCA–associated vasculitis is a different autoimmune syndrome from the disorder affecting patients whose ANCA target specificity is MPO (15).
We cannot exclude the possibility that the enrollment of patients with relapsing disease, 60% of whom had received CYC in the past, influenced our findings. However, in all of the patients treated previously with CYC, disease control had previously been achieved with that medication, and none had received CYC within 4 months of study entry. There is no indication, therefore, that the study population was enriched with patients whose disease was refractory to CYC. Patients who respond to CYC once typically respond again but most patients, regardless of whether remission is induced with regimens involving CYC or RTX, eventually are shown to experience disease relapse if followed up for a sufficient length of time.
Neither ANCA titers nor B cell measurements were useful in predicting disease flares. This was particularly true among patients in the RTX group, in whom no flares were preceded by a rise in ANCA titer or detectable B cells. These data from the remission induction period offer an important nuance in the longitudinal assessment of the likelihood of disease flares: before 6 months, when the effects of prednisone, RTX, and CYC/AZA on B cells are most intense, neither ANCA titers nor B cell concentrations are helpful in predicting disease flares. In contrast, after 6 months, the time when B cells often have begun to return, ANCA negativity and persistent peripheral B cell depletion indicate that the risk of a disease flare is low. Previous cohort studies of patients treated with RTX have yielded similar findings (16). Although RTX rapidly depletes circulating B cells, tissue B cells, which are a significantly larger population, are known to persist within the reticuloendothelial system or at sites of disease, and remain one of the possible mediators of disease activity during peripheral B cell depletion (17,18). It is also possible that other inflammatory mechanisms are more crucial to disease activity during the period when measurable B cells are not present in the peripheral blood.
Glucocorticoids were tapered over a shorter period of time in the RAVE trial compared to other randomized controlled trials in AAV (11–13,19–21). By 5 months, patients in the RAVE trial who had achieved clinical remission could discontinue prednisone completely. In theory, this comparatively rapid glucocorticoid cessation might have contributed to a high rate of treatment failure. In addition, 50% of patients in the RAVE trial had relapsing disease at baseline, and patients who have experienced relapses constitute a population that is known to be at greater risk for disease flare than patients who have not had any flares. Patients with relapsing disease have been excluded from many previous AAV trials (11–13,19–21). Despite the rapid glucocorticoid taper and the large percentage of patients with relapsing disease, the overall results in the RAVE trial during the remission induction period were comparable to those of other trials: clinical remission was achieved in 86% of the patients before the 6-month time point, and among those in whom remission was achieved, 80% remained in remission at 6 months. Given the well-known morbidity associated with prolonged glucocorticoid therapy, the optimal dosage and duration of glucocorticoid treatment in AAV requires further study.
Our study has some weaknesses, particularly with regard to certain subgroup analyses in which the numbers for some comparisons were relatively small. We have attempted to be conservative in reporting such comparisons, focusing on those for which there is some biologic rationale or consistency with other reports in the literature. The study also has several important strengths, particularly the randomized, double-blind, placebo-controlled design of the trial and the comparison of regimens that reflect the current standard of care for AAV.
In conclusion, the currently available treatment regimens are ultimately successful in controlling disease activity in severe AAV, even though one-quarter of all patients who receive these regimens do not achieve and sustain glucocorticoid-free complete remission within the first 6 months. In a small subgroup of patients with AAV, intensive remission induction regimens are unsuccessful regardless of whether the initial therapy is RTX plus glucocorticoids or CYC/AZA plus glucocorticoids. This subgroup is characterized by PR3-ANCA positivity and a tendency to progressive severe renal disease or alveolar hemorrhage. Patients with severe disease flares tend to also be PR3-ANCA positive and may experience flares even during periods of treatment with moderate-to-high doses of prednisone, B cell depletion induced by RTX, or doses of CYC/AZA that are viewed as adequate. ANCA titers and B cell subsets are poor predictors of both flares and continued disease quiescence in the first 6 months of therapy, particularly among patients treated with RTX. Both clinical trials of new treatment regimens and basic investigation must continue in the effort to improve time to remission induction, limit the risk of relapse, and diminish the morbidity and mortality that still result both from the AAVs and from their therapies.
Acknowledgments
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was performed with the support of the Immune Tolerance Network (NIH contract N01-AI-15416, protocol number ITN021AI), an international clinical research consortium headquartered at the University of California San Francisco and supported by the NIH (National Institute of Allergy and Infectious Diseases contract N01-AI-15416, protocol number ITN021AI) and the Juvenile Diabetes Research Foundation. Genentech and Biogen Idec provided the study medications and partial funding for the RAVE trial. At the Mayo Clinic, Johns Hopkins University, and Boston University School of Medicine, the RAVE trial was supported by the NIH (Mayo Clinic: National Center for Research Resources Clinical and Translational Science award RR-024150-01; Johns Hopkins University: National Center for Research Resources Clinical and Translational Science grant RR-025005 and career development awards K23-AR-052820 to Dr. Seo and K24-AR-049185 to Dr. Stone; Boston University: National Center for Research Resources Clinical and Translational Science award RR-025771, grant M01-RR-00533, and career development award K24-AR-02224 to Dr. Merkel). Dr. Miloslavsky’s work was supported by Genentech (Clinical Immunology Fellowship). Dr. Monach’s work was supported by the Arthritis Foundation (Arthritis Investigator Award).
Footnotes
ClinicalTrials.gov identifier: NCT00104299.
Dr. Hoffman has received consulting fees, speaking fees, and/or honoraria from Sanofi-Aventis, Roche, and Genentech (less than $10,000 each). Dr. Kallenberg has received speaking fees from Roche (less than $10,000). Dr. Brunetta owns stock or stock options in Genentech. Dr. Allen has received consulting fees and honoraria from Genentech for Speakers Bureau service (more than $10,000). Dr. Geetha has received consulting fees and honoraria from Genentech (more than $10,000). Dr. Stone has received consulting fees, speaking fees, and/or honoraria from Genentech and Roche (less than $10,000 each).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Stone had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Specks, Merkel, Spiera, Langford, Hoffman, Kallenberg, St.Clair, Viviano, Ding, Mieras, Iklé, Mueller, Brunetta, Allen, Fervenza, Stegeman, Stone.
Acquisition of data. Specks, Merkel, Seo, Spiera, Langford, Hoffman, Kallenberg, St.Clair, Tchao, Viviano, Ding, Sejismundo, Mieras, Iklé, Mueller, Allen, Fervenza, Geetha, Keogh, Kissin, Monach, Peikert, Stegeman, Ytterberg, Stone.
Analysis and interpretation of data. Miloslavsky, Specks, Merkel, Spiera, Langford, Hoffman, Kallenberg, St.Clair, Tchao, Viviano, Ding, Mieras, Iklé, Jepson, Mueller, Brunetta, Fervenza, Geetha, Peikert, Stone.
ROLE OF THE STUDY SPONSOR
Genentech and Biogen Idec provided study medications for the RAVE Trial and reviewed the manuscript prior to submission. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Genentech or Biogen Idec.
References
- 1.Fauci AS, Katz P, Haynes BF, Wolff SM. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979;301:235–8. doi: 10.1056/NEJM197908023010503. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. for the RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. for the International Network for the Study of the Systemic Vasculitides (INSSYS) A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Specks U, Merkel PA, Hoffman GS, Langford CA, Spiera R, Seo P, et al. for the RAVE-ITN Research Group. Design of the Rituximab in ANCA-associated Vasculitis (RAVE) Trial. Open Arthritis J. 2011;4:1–18. [Google Scholar]
- 6.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 7.Damoiseaux J, Dahnrich C, Rosemann A, Probst C, Komorowski L, Stegeman CA, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2009;68:228–33. doi: 10.1136/ard.2007.086579. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v.3. 2006 URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 9.Stone JH, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. for the RAVE-ITN Research Group. Extended follow-up of treatment with rituximab versus cyclophosphamide for remission-induction of ANCA-associated vasculitis: which subsets are at greatest risk for flare? [abstract] Arthritis Rheum. 2011;63(Suppl):S946–7. [Google Scholar]
- 10.Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J Intern Med. 1998;244:209–16. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71:955–60. doi: 10.1136/annrheumdis-2011-200477. [DOI] [PubMed] [Google Scholar]
- 12.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. for the European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 13.Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, et al. for the French Vasculitis Study Group. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359:2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M, Flossmann O, Berden A, Westman K, Hoglund P, Stegeman C, et al. on behalf of the European Vasculitis Study Group. Risk factors for relapse of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum. 2012;64:542–8. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 15.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RB, Ferraro AJ, Chaudhry AN, Brogan P, Salama AD, Smith KG, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum. 2009;60:2156–68. doi: 10.1002/art.24637. [DOI] [PubMed] [Google Scholar]
- 17.Vos K, Thurlings RM, Wijbrandts CA, van Schaardenburg D, Gerlag DM, Tak PP. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:772–8. doi: 10.1002/art.22400. [DOI] [PubMed] [Google Scholar]
- 18.Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6:2418–28. doi: 10.1111/j.1600-6143.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 19.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. for the European Vasculitis Study Group. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 20.De Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. EUVAS (European Vasculitis Study Group) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 21.Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. for the European Vasculitis Study Group (EUVAS) Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]