Abstract
Objective
Patients with connective tissue disease (CTD)–associated pulmonary arterial hypertension (PAH) have a poorer prognosis compared to those with idiopathic PAH, but little is known about the differences in treatment-related adverse events (AEs) and serious adverse events (SAEs) between these groups. This study was undertaken to characterize these differences.
Methods
Individual patient-level data from 10 randomized controlled trials of therapies for PAH were obtained from the US Food and Drug Administration. Patients diagnosed as having either CTD-associated PAH or idiopathic PAH were included. A treatment-by-diagnosis interaction term was used to examine whether the effect of treatment on occurrence of AEs differed between patients with CTD-associated PAH and those with idiopathic PAH. Studies were pooled using fixed-effect models.
Results
The study sample included 2,370 participants: 716 with CTD-associated PAH and 1,654 with idiopathic PAH. In the active treatment group compared to the placebo group, the risk of AEs was higher among patients with CTD-associated PAH than among those with idiopathic PAH (odds ratio [OR] 1.57, 95% confidence interval [95% CI] 1.00–2.47 versus OR 0.94, 95% CI 0.69–1.26; P for interaction = 0.061), but there was no difference in the risk of SAEs in analyses adjusted for age, race, sex, hemodynamic findings, and laboratory values. Despite the higher occurrence of AEs in patients with CTD-associated PAH assigned to active therapy compared to those receiving placebo, the risk of drug discontinuation due to an AE was similar to that in patients with idiopathic PAH assigned to active therapy (P for interaction = 0.27).
Conclusion
Patients with CTD-associated PAH experienced more treatment-related AEs compared to those with idiopathic PAH in therapeutic clinical trials. These findings suggest that the overall benefit of advanced therapies for PAH may be attenuated by the greater frequency of AEs.
Pulmonary arterial hypertension (PAH) is a severe and often fatal complication of connective tissue diseases (CTDs). Among the CTDs, systemic sclerosis (SSc) is the most common setting for PAH, with a reported prevalence of 7–12% based on the proportion of patients undergoing right-sided heart catheterization (1–3), and PAH is the leading cause of death in patients with SSc (4,5). PAH is also known to occur in systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), overlap syndromes, and, to a lesser extent, rheumatoid arthritis and Sjögren’s syndrome (2,6–9). Compared to patients with idiopathic PAH, patients with CTD-associated PAH have a higher mortality and a lower walking distance on the 6-minute walk test, higher levels of B-type natriuretic peptide, worse right ventricular function, more left-sided heart dysfunction, lower lung function, and more pericardial disease (10–20).
Clinical trials of therapies for PAH have often included both CTD-associated PAH and idiopathic PAH. Although prior studies have evaluated differences in efficacy (21,22), little attention has been paid to differences in adverse events (AEs) between CTD-associated PAH and idiopathic PAH. The reporting of AEs is an important and required component of clinical trials from both the perspective of protection of human subjects and the safety profile of an experimental drug. The US Food and Drug Administration (FDA) Code of Federal Regulations defines an AE as “any untoward medical occurrence associated with the use of a drug in humans, whether or not considered drug-related” (23). In addition, a serious AE (SAE) is an AE that, according to the investigator or sponsor, results in death, a life-threatening AE, inpatient hospitalization or prolongation of existing hospitalization, a persistent and significant inability to conduct normal life functions, or a congenital anomaly or birth defect. The nature and frequency of AEs are important factors when deciding on the regulatory approval of a new medication or when physicians and patients are making decisions regarding initiation or maintenance of treatments.
The potential for both treatment-related and non–treatment-related AEs and SAEs may be greater in patients with multiorgan systemic diseases (24,25). Understanding the AE profile in patients with CTD-associated PAH compared to patients with idiopathic PAH in clinical trials could inform the design of future clinical trials, influence the monitoring of drug toxicities in patients who are receiving therapy, provide insight into improving compliance, and better help physicians and patients consider the comparative effectiveness and risk of treatment. The purpose of this study was to compare the risk of AEs and SAEs between patients with CTD-associated PAH and those with idiopathic PAH enrolled in clinical trials.
PATIENTS AND METHODS
Study population
De-identified individual patient data were obtained from the databases of phase III placebo-controlled, randomized trials submitted to the FDA through 2013 that tested endothelin receptor antagonists (ERAs), phosphodiesterase type 5 (PDE5) inhibitors, and prostacyclin analogs for the treatment of PAH. Only patients with a diagnosis of idiopathic PAH or CTD-associated PAH, as determined by the investigators in each study, were included in the analysis.
Ten clinical trials that studied 7 agents (ambrisentan, bosentan, iloprost, macitentan, sildenafil, sitaxsentan, and treprostinil) were included (26–34). The BREATHE-2 (Bosentan: Randomized Trial of Endothelin Receptor Antagonist Therapy for Pulmonary Arterial Hypertension 2) and AIR-2 (Aerosolized Iloprost Randomized Study 2) trials were excluded, since they were not phase III trials. In addition, 5 trials that did not routinely collect baseline hemodynamic data were also excluded (35–41). All trials had at least a 12-week treatment period. Trials of treatments that were subsequently not approved by the FDA (e.g., sitaxsentan) were included, since this was a study of clinical trial populations rather than specific drugs. All included trials reported similar inclusion criteria, outcomes, followup time, and methods, except the trial investigating macitentan, which was an event-driven study design (34). Because the followup time was notably longer in the macitentan trial (mean duration of followup >1 year), we examined only the AEs that occurred in the first 18 weeks of followup, to be consistent with the other studies.
Exposure
The primary exposure was the differential treatment effect between CTD-associated PAH and idiopathic PAH. Therefore, a treatment-by-diagnosis interaction was included to assess whether diagnosis (CTD-associated PAH versus idiopathic PAH) modified the effect of treatment (active therapy versus placebo) on the outcome. A subgroup analysis was performed among patients with CTD who were diagnosed as having SSc (limited or diffuse), and these patients were compared to patients with idiopathic PAH. All trials contained CTD subtype except for the macitentan trial; for that study, CTD subtype was derived from the medical history.
Outcomes
The primary outcome of interest was the occurrence of AEs (yes versus no) during the trial period. Secondary outcomes included the rate of AEs, occurrence of an SAE (yes versus no), and the rate of SAEs. AEs and SAEs were then categorized into specific groups, such as infection, headache, and rash. The 10 most common AE and SAE types were identified and the occurrence of each of these AE and SAE types was examined. Discontinuation of the study drug due to an AE or SAE was also examined.
Statistical analysis
Patient characteristics were summarized for the entire group as well as by diagnosis (CTD-associated PAH or idiopathic PAH) and CTD subgroup (SSc-associated PAH). Results are expressed as the mean ± SD for normally distributed continuous variables, median and inter-quartile range for non-normally distributed variables, and percentages for categorical variables. Comparisons between patients with CTD-associated PAH and those with idiopathic PAH were made using Student’s t-test for continuous variables and chi-square test for categorical variables.
Evaluation for between-study heterogeneity was performed using a 2-stage inverse-variance individual participant data meta-analysis, and the I2 test for heterogeneity was determined. Due to the lack of significant heterogeneity between studies, data from the studies were pooled using a fixed-effect model. To examine the outcomes, logistic regression was used for binary outcomes, such as occurrence of AEs/SAEs and occurrence of discontinuation of the study drug due to AEs/SAEs. Negative binomial regression was used for count data (rates of AEs and SAEs) to analyze the number of AEs or SAEs during the followup period, accounting for over-dispersion. Adjustments were made a priori for age, race, sex, study, body mass index (BMI), baseline 6-minute walk distance, baseline World Health Organization (WHO) functional class, baseline hemodynamics (cardiac index, mean pulmonary arterial pressure, right atrial pressure), and baseline laboratory values (creatinine and hemoglobin levels). These potential confounders were selected on the basis of prior observations of their association with PAH outcomes (12,14,42–44). Regression models were not used to analyze the individual SAE types, given the low number of events.
The relationships between treatment allocation and outcome with effect modification by diagnosis (CTD-associated PAH and idiopathic PAH) or diagnosis subgroup (SSc-associated PAH and idiopathic PAH) were examined. If the treatment-by-diagnosis interaction term was not significant, then the association of diagnosis (CTD-associated PAH versus idiopathic PAH) with the outcome (number or rate of AEs and SAEs) was evaluated independently, with adjustment for potential confounders, including a coded value for active treatment versus placebo. Interactions were also examined after stratifying by drug type (ERA, PDE5 inhibitor, and prostacyclin analog). A significance level of 0.10 was used for interaction terms, and a significance level of 0.05 was used in all other hypothesis tests (45).
All analyses were conducted using Stata (version 12.1; StataCorp). The study was exempt from review by the institutional review board of the University of Pennsylvania because we used existing de-identified patient data, which are not considered part of the definition of human subjects research.
RESULTS
Patient characteristics
There were 2,822 patients in the 10 trials, of whom 2,370 met the inclusion criteria. There were 716 patients with CTD-associated PAH and 1,654 with idiopathic PAH (Table 1). Among the patients with CTD-associated PAH, 407 (57%) had SSc, 100 (14%) had SLE, 80 (11%) had MCTD, 69 (10%) had Sjögren’s syndrome, 21 (3%) had an overlap syndrome, and 39 (5%) were classified as having another CTD. Patients with CTD-associated PAH were significantly older, more often female, and had a significantly lower baseline 6-minute walk distance compared to patients with idiopathic PAH. The proportions of patients in each WHO functional class at baseline were similar between the 2 diagnoses, and patients with CTD-associated PAH had significantly better baseline hemodynamics, including lower right atrial pressure, lower mean pulmonary arterial pressure, higher cardiac index, and lower pulmonary vascular resistance (Table 1). There were 1,614 patients (68%) enrolled in trials of ERAs, 259 (11%) in trials of PDE5 inhibitors, and 497 (21%) in trials of prostacyclin analogs. Patients with CTD-associated PAH were more likely to be enrolled in a trial of ERAs compared to patients with idiopathic PAH (Table 1).
Table 1.
Characteristics of the study participants*
| CTD-associated PAH
|
Idiopathic PAH (n = 1,654) | P, all CTD-associated PAH vs. idiopathic PAH | |||
|---|---|---|---|---|---|
| All (n = 716) | SSc-associated PAH (n = 407) | Non–SSc-associated PAH (n = 309) | |||
| Age, years | 53 ± 14 | 59 ± 11 | 45 ± 14 | 46 ± 15 | <0.001 |
| Female sex, % | 89 | 88 | 90 | 73 | <0.001 |
| Race, % | 0.39 | ||||
| White | 71 | 88 | 49 | 73 | |
| Black | 5 | 4 | 6 | 4 | |
| Other | 25 | 8 | 45 | 23 | |
| Body mass index, kg/m2 | 26 ± 6 | 27 ± 6 | 26 ± 6 | 28 ± 7 | <0.001 |
| WHO functional class, % | 0.18 | ||||
| I–II | 47 | 37 | 59 | 44 | |
| III–IV | 53 | 63 | 41 | 56 | |
| Baseline hemodynamics | |||||
| Right atrial pressure, mm Hg | 8.0 ± 5.0 | 8.3 ± 4.9 | 7.7 ± 5.0 | 9.6 ± 5.8 | <0.001 |
| Mean pulmonary arterial pressure, mm Hg | 46 ± 12 | 45 ± 13 | 48 ± 12 | 56 ± 16 | <0.001 |
| Cardiac index, liters/minute/m2 | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.3 ± 0.8 | <0.001 |
| Pulmonary capillary wedge pressure, mm Hg | 9 ± 4 | 10 ± 3 | 9 ± 3 | 9 ± 4 | 0.70 |
| Pulmonary vascular resistance, Wood units | 10.2 ± 6.2 | 9.6 ± 6.4 | 10.9 ± 5.9 | 13.3 ± 7.8 | <0.001 |
| Baseline 6-minute walk distance, meters | 336 ± 93 | 322 ± 92 | 353 ± 91 | 347 ± 89 | <0.001 |
| Drug class, % | 0.021 | ||||
| Endothelin receptor antagonists | 71 | 72 | 69 | 67 | |
| Phosphodiesterase 5 inhibitors | 12 | 13 | 11 | 13 | |
| Prostacyclin analogs | 17 | 16 | 20 | 22 | |
Except where indicated otherwise, values are the mean ± SD.
CTD-associated PAH = connective tissue disease–associated pulmonary arterial hypertension; SSc = systemic sclerosis; WHO = World Health Organization.
Adverse events
At least 1 AE occurred in 1,944 patients (82%) overall. Among patients with idiopathic PAH, 1,346 (81%) developed an AE, and among those with CTD-associated PAH, 598 (84%) experienced an AE. The median number of AEs experienced by an individual patient was similar between CTD-associated PAH and idiopathic PAH (Table 2). The most common AEs included infection, headache, musculoskeletal issues, edema, and rash (Table 3).
Table 2.
Adverse events (AEs) and serious adverse events (SAEs) by diagnosis*
| CTD-associated PAH
|
Idiopathic PAH (n = 1,654) | P, all CTD-associated PAH vs. idiopathic PAH | |||
|---|---|---|---|---|---|
| All (n = 716) | SSc-associated PAH (n = 407) | Non–SSc-associated PAH (n = 309) | |||
| AE | |||||
| Occurrence of at least 1 AE, % | 84 | 87 | 79 | 81 | 0.20 |
| AEs per person, median (IQR) no. | 3 (1–7) | 3 (1–7) | 3 (1–6) | 3 (1–6) | 0.87 |
| SAE | |||||
| Occurrence of at least 1 SAE, % | 19 | 20 | 17 | 14 | <0.001 |
| SAEs per person, % | <0.001 | ||||
| 0 | 81 | 80 | 83 | 86 | |
| 1 | 14 | 14 | 13 | 9 | |
| >1 | 5 | 6 | 4 | 5 | |
CTD-associated PAH = connective tissue disease–associated pulmonary arterial hypertension; SSc = systemic sclerosis; IQR = interquartile range.
Table 3.
Risk of the 10 most common adverse event types in patients with CTD-associated PAH compared to patients with idiopathic PAH*
| Outcome | Odds ratio (95% CI) for CTD-associated PAH vs. idiopathic PAH | P |
|---|---|---|
| Infection | 1.65 (1.31–2.08) | <0.001 |
| Headache | 0.85 (0.65–1.13) | 0.26 |
| Musculoskeletal | 1.13 (0.87–1.48) | 0.36 |
| Edema | 1.12 (0.85–1.48) | 0.42 |
| Rash | 0.89 (0.62–1.29) | 0.54 |
| Bleeding | 1.49 (1.08–2.06) | 0.021 |
| Nausea/vomiting | 1.10 (0.78–1.54) | 0.59 |
| Dizziness | 1.09 (0.78–1.52) | 0.62 |
| Cough | 0.99 (0.66–1.49) | 0.98 |
| Dyspnea | 1.33 (0.91–1.93) | 0.14 |
Odds ratios with 95% confidence intervals (95% CIs) were determined in analyses adjusted for age, sex, race, treatment (drug versus placebo), study, body mass index, baseline 6-minute walk distance, baseline World Health Organization functional class, baseline hemodynamics (cardiac index, mean pulmonary arterial pressure, right atrial pressure), and baseline laboratory values (creatinine and hemoglobin levels). CTD-associated PAH = connective tissue disease–associated pulmonary arterial hypertension.
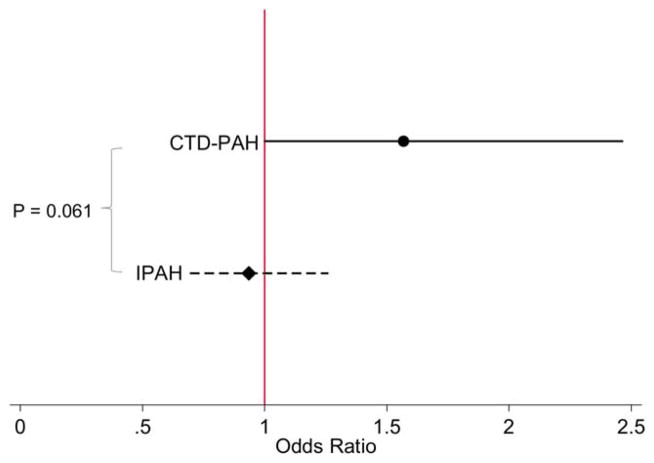
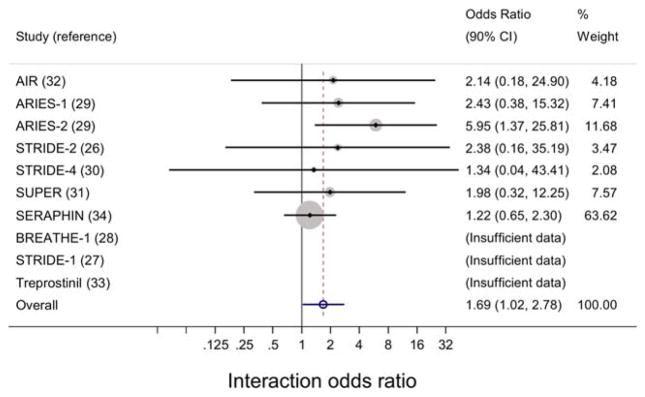
Patients with CTD-associated PAH in the active treatment group compared to the placebo group were more likely to have AEs than were patients with idiopathic PAH in the active treatment group compared to the placebo group (odds ratio [OR] 1.57, 95% confidence interval [95% CI] 1.00–2.47 versus OR 0.94, 95% CI 0.69–1.26; P for interaction = 0.061) (Figure 1). The meta-analysis evaluating the pooled estimates of interaction effect for occurrence of AEs across studies showed there was no statistically significant heterogeneity between 7 of the 10 trials (I2 = 0.0%, P = 0.82) (Figure 2). Three of the trials could not be analyzed in the test for heterogeneity because of the low number of events.
Figure 1.
Odds ratios for adverse events (AEs) in patients assigned to the active treatment group as compared to the placebo group, stratified by diagnosis (connective tissue disease–associated pulmonary arterial hypertension [CTD-PAH] and idiopathic PAH [IPAH]). Odds ratios are shown with 95% confidence intervals. Compared to patients with IPAH, patients with CTD-associated PAH were more likely to experience an AE if assigned to active treatment versus placebo. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.39220/abstract.
Figure 2.
Interaction effect estimates from individual studies and from meta-analysis of pooled studies to assess the impact of diagnosis-by-treatment interaction on adverse events in patients with connective tissue disease–associated pulmonary arterial hypertension and those with idiopathic pulmonary arterial hypertension. Forest plots show the odds ratios with 90% confidence intervals (90% CIs) for the diagnosis-by-treatment interactions and occurrence of adverse events. Three studies (the BREATHE-1 [Bosentan: Randomized Trial of Endothelin Receptor Antagonist Therapy for Pulmonary Arterial Hypertension 1], STRIDE-1 [Sitaxsentan to Relieve Impaired Exercise 1], and treprostinil studies) had an insufficient number of events and therefore could not be analyzed (I2 = 0.0%, P = 0.82). AIR = Aerosolized Iloprost Randomized Study; ARIES-1 = Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Study 1; SUPER = Sildenafil Use in Pulmonary Arterial Hypertension; SERAPHIN = Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.39220/abstract.
The treatment-by-diagnosis interaction was not significant for all other secondary AE outcomes, including edema, dyspnea, and infection. These secondary outcomes were then evaluated for an association with diagnosis (without effect modification by treatment). There was no difference in the rate of AEs in patients with CTD-associated PAH compared to those with idiopathic PAH (incidence rate ratio 1.08, 95% CI 0.96–1.21; P = 0.18). When we examined the 10 most common AEs, we found that patients with CTD-associated PAH had a significantly higher occurrence of infection (OR 1.65, 95% CI 1.31–2.08; P < 0.001) and bleeding (OR 1.49, 95% CI 1.08–2.06; P = 0.021), in analyses adjusted for age, race, sex, treatment, study, BMI, baseline 6-minute walk distance, baseline WHO functional class, baseline hemodynamics (cardiac index, mean pulmonary arterial pressure, right atrial pressure), and baseline laboratory values (creatinine and hemoglobin levels) (Table 3).
There was no interaction between treatment and diagnosis subgroup (SSc-associated PAH versus idiopathic PAH) for either the primary outcomes or any of the secondary outcomes (P for interaction>0.10 for all comparisons). No significant interactions were found in the subgroup analysis stratified by drug type—ERA, PDE5 inhibitors, and prostacyclin analogs (data not shown).
Serious adverse events
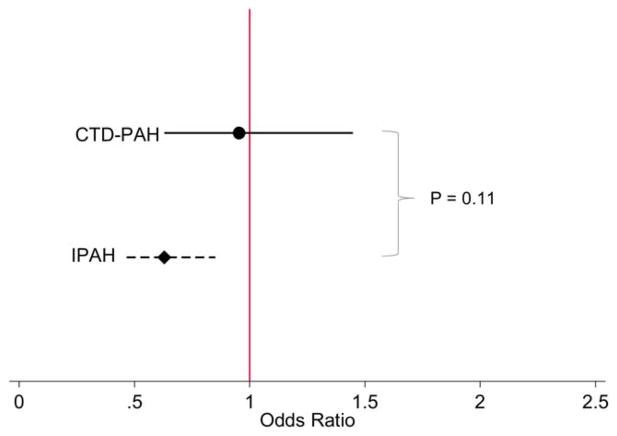
At least 1 SAE occurred in 371 patients (16%) overall, of whom 236 with idiopathic PAH (14%) and 135 with CTD-associated PAH (19%) had at least 1 SAE (Table 2). The most common SAEs included congestive heart failure, infection, worsening PAH, bleeding, and dyspnea. There was no difference in treatment-related SAEs in CTD-associated PAH compared to idiopathic PAH (P for interaction = 0.11) (Figure 3). When the relationship between diagnosis (CTD-associated PAH versus idiopathic PAH) alone and occurrence of an SAE was examined, patients with CTD-associated PAH overall were more likely to develop an SAE during the trial period compared to patients with idiopathic PAH, independent of treatment assignment, although the difference was not statistically significant (OR 1.30, 95% CI 0.99–1.72; P = 0.061). Therefore, SAEs occurred more often in patients with CTD-associated PAH than in patients with idiopathic PAH, and this difference was unrelated to therapy. There was no difference in the rate of SAEs between patients with CTD-associated PAH and those with idiopathic PAH (incidence rate ratio 1.17, 95% CI 0.82–1.66; P = 0.38).
Figure 3.
Odds ratios for serious adverse events (SAEs) in patients assigned to the active treatment group as compared to the placebo group, stratified by diagnosis (connective tissue disease–associated pulmonary arterial hypertension [CTD-PAH] and idiopathic PAH [IPAH]). Odds ratios are shown with 95% confidence intervals. There was no significant difference in the occurrence of treatment-related SAEs between the CTD-associated PAH and idiopathic PAH groups. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.39220/abstract.
We found no significant differences in the effect of treatment on occurrence of an SAE between patients with SSc-associated PAH and those with idiopathic PAH (P for interaction = 0.23). Secondary analyses of SAEs stratified by drug type found no differences in treatment-related SAEs between the 2 diagnoses within the 3 individual drug types (data not shown).
Risk of treatment discontinuation due to adverse events/serious adverse events
Among the 1,944 patients who experienced an AE, 140 (7%) had to discontinue the study drug due to the AE. There was no treatment-by-diagnosis interaction in terms of discontinuation of the drug due to an AE, indicating that the risk of treatment discontinuation among patients receiving active treatment compared to those receiving placebo was not influenced by diagnosis of CTD-associated PAH or idiopathic PAH, even after adjusting for age, sex, baseline 6-minute walk distance, and baseline pulmonary vascular resistance (P for interaction = 0.27). Furthermore, neither of the 2 diagnoses (OR 0.84, 95% CI 0.56–1.25; P = 0.39) nor treatment assignment (OR 0.86, 95% CI 0.60–1.22; P = 0.39) was independently associated with treatment discontinuation due to AEs, in analyses adjusted for all potential confounders.
Among the 371 patients who developed an SAE, 78 (21%) had to discontinue the study drug due to the SAE. The diagnosis did not modify treatment-related discontinuations of the study drug due to an SAE (P for interaction = 0.15). Furthermore, discontinuation of treatment was not associated with diagnosis (OR 0.72, 95% CI 0.40–1.29; P = 0.28) or treatment (OR 0.64, 95% CI 0.38–1.09; P = 0.10).
DISCUSSION
This study shows that the risk of treatment-related AEs is higher in participants of clinical trials diagnosed as having CTD-associated PAH than in participants with idiopathic PAH. This indicates that patients randomized to receive active therapy were more likely to develop an AE compared to those receiving placebo if they had CTD-associated PAH than if they had idiopathic PAH. Despite the higher risk of AEs in patients with CTD-associated PAH, the rate of drug discontinuation due to an AE or SAE was not different between the 2 groups.
Patient characteristics were similar to those of patients from prior studies (9,11,13,18,46,47). Patients with CTD-associated PAH were older and were more often female compared to patients with idiopathic PAH. Baseline hemodynamic measurements were less affected in CTD-associated PAH compared to idiopathic PAH, which is consistent with prior published studies (12,13,18). These differences were accounted for in the analyses, so that our findings are independent of these underlying differences. Although patients with CTD-associated PAH had less severely altered hemodynamics, the baseline 6-minute walk distance was significantly lower in those with CTD-associated PAH. An explanation for this discrepant finding may be attributed to other features of disease, such as pericardial or myocardial complications, which may cause the poorer exercise capacity in CTD-associated PAH (11,13,18). In addition, the 6-minute walk distance may be disproportionately affected by concurrent joint disease, muscle wasting, and poor overall health in CTD-associated PAH (48–51).
The higher risk of AEs in patients with CTD may be explained by the complex multiorgan involvement of their underlying systemic disease, as well as by the immunosuppressive therapies required for treatment. The presence of cytopenia, most often due to the use of immunomodulating agents as well as to the presence of intrinsic immune dysfunction, may predispose patients with CTD to infections. Vascular malformations and clotting abnormalities (e.g., thrombocytopenia) may contribute to bleeding tendencies. Immunosuppressive therapies used to treat CTDs are associated with toxicities, and potentially unknown drug interactions with therapies for PAH may have occurred. Moreover, gastrointestinal disease seen in CTDs (especially in those with SSc-associated CTD) can cause malabsorption, which may affect the bioavailability and drug concentration of these advanced vasodilator therapies; however, decreased absorption in patients with CTD-associated PAH would be expected to bias the results toward the null. Notably, there was not a higher occurrence of dyspnea in patients with CTD-associated PAH, which may have been expected given the higher prevalence of pulmonary venoocclusive disease (PVOD)—thereby conferring a higher risk of pulmonary edema in the active therapy group—in patients with CTD (52,53). Therefore, it is likely that clinically apparent PVOD was uncommon in this population of patients with CTD.
Notably, the higher occurrence of treatment-related AEs in patients with CTD-associated PAH did not translate into an increased risk of drug discontinuation due to an AE. This may indicate that treatment-related AEs in patients with CTD-associated PAH, although greater in number, were not severe enough to warrant treatment discontinuation. Alternatively, it may be possible that there were not enough events to detect a difference (inadequate power).
Understanding the heterogeneity of treatment effects within subgroups of patients has become a major research priority (54). This is the first study to evaluate issues of safety in patients with CTD-associated PAH compared to patients with idiopathic PAH within randomized controlled trials. We found that patients with CTD-associated PAH assigned to active treatment are more likely to experience AEs compared to those receiving placebo, whereas patients with idiopathic PAH had no difference in the occurrence of AEs between the treatment and placebo groups. Our study used individual patient data from 10 randomized controlled trials, which allowed us to examine patient subgroups, adjusted for potential confounders, and utilize interaction terms. Furthermore, the power to detect differences was substantially increased by pooling data from multiple studies.
Drug development programs may choose to more comprehensively distinguish between patients with CTD-associated PAH and those with idiopathic PAH, and to prospectively study differential treatment responses and toxicity profiles as well as potential drug interactions. In addition, analysis of AEs and SAEs in clinical trials may warrant stratification by diagnosis to determine whether AEs are occurring mostly in CTD-associated PAH. This study found no evidence of an increased risk of drug discontinuation due to an AE between patients with CTD-associated PAH and those with idiopathic PAH. Because patients with CTD-associated PAH have a higher risk of AEs, anticipating potential AEs and performing appropriate monitoring could minimize the occurrence and impact of AEs on patients, improving their quality of life.
Several limitations of this analysis are also important to consider. Misclassification bias may have occurred if patients with CTD were incorrectly diagnosed. A diagnosis of CTD was based on physician reporting, and standardized classification criteria were not used. However, patient characteristics, such as age, sex, race, and baseline hemodynamics, as well as differences between patients with CTD-associated PAH and those with idiopathic PAH, were similar to that reported in previous studies (11,18). In addition, we would expect that patients with CTD would be more likely misdiagnosed as having idiopathic PAH, which would bias toward the null hypothesis; therefore, the true differences between CTD-associated PAH and idiopathic PAH may be even larger than has been shown in this study.
Second, reporting bias may have occurred, since clinicians were not blinded with regard to the diagnosis and therefore may have been more prone to report AEs in those with a CTD diagnosis; however, allocation of treatment was masked, and therefore reporting bias would not explain the significant treatment-by-diagnosis interactions. The patients studied were enrolled in clinical trials and may differ from other populations, affecting generalizability. The relatively short length of followup may have limited the examination of certain AEs in these trials. Furthermore, these trials were not designed to examine differences in AEs between CTD-associated PAH and idiopathic PAH, and therefore unmeasured and residual confounders may exist; however, if proper randomization occurred, comparison of treatment and placebo groups within diagnoses should not be affected.
Another limitation is the possibility of Type I error. Selection of a higher alpha level has been recommended for studies of effect modification, given the greater power needed to assess interactions (45). However, rather than relying solely on P values, examination of estimates and confidence intervals (which are better at conveying magnitude and precision) is generally favored (55). There is clearly heterogeneity between the diagnostic groups.
No between-study heterogeneity was found with formal testing. Although 3 of the studies (the BREATHE-1, STRIDE-1 [Sitaxsentan to Relieve Impaired Exercise 1], and treprostinil studies) could not be included in the test for heterogeneity and in the formal meta-analysis (because of the low number of observations), these studies had a similar study design and similar inclusion/exclusion criteria, and therefore inclusion of the 3 trials would be unlikely to significantly change the results. In addition, a prior analysis that did include these 3 trials showed minimal statistical heterogeneity (P for heterogeneity = 0.99) (56). Furthermore, unmeasured confounders, such as concomitant medications used and existing comorbidities, also limit the interpretation of the data.
In conclusion, this study found that among patients enrolled in therapeutic clinical trials, more treatment-related AEs occurred in patients with CTD-associated PAH compared to those with idiopathic PAH, but no difference in the occurrence of SAEs or in treatment discontinuation due to an AE/SAE was observed. These findings suggest that the overall benefit of advanced therapies for PAH may be attenuated by the greater frequency of AEs. The results nevertheless support the continued enrollment of patients with CTD-associated PAH into clinical trials of therapies for PAH.
Acknowledgments
Drs. Rhee and Kawut’s work was supported by the NIH (grants T32-DK-07785 and K24-HL-103844, respectively).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Rhee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Rhee, Merkel, Kawut.
Acquisition of data. Rhee, Praestgaard, Kawut.
Analysis and interpretation of data. Rhee, Gabler, Praestgaard, Merkel, Kawut.
References
- 1.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 2.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phung S, Strange G, Chung LP, Leong J, Dalton B, Roddy J, et al. Prevalence of pulmonary arterial hypertension in an Australian scleroderma population: screening allows for earlier diagnosis. Intern Med J. 2009;39:682–91. doi: 10.1111/j.1445-5994.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 4.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516–22. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 5.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagan KA, Badesch DB. Pulmonary hypertension associated with connective tissue disease. Prog Cardiovasc Dis. 2002;45:225– 34. doi: 10.1053/pcad.2002.129975. [DOI] [PubMed] [Google Scholar]
- 7.Pan TL, Thumboo J, Boey ML. Primary and secondary pulmonary hypertension in systemic lupus erythematosus. Lupus. 2000;9:338–42. doi: 10.1191/096120300678828361. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez O, Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary hypertension secondary to connective tissue diseases. Thorax. 1999;54:273–7. doi: 10.1136/thx.54.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galie N, Manes A, Farahani KV, Pelino F, Palazzini M, Negro L, et al. Pulmonary arterial hypertension associated to connective tissue diseases. Lupus. 2005;14:713–7. doi: 10.1191/0961203305lu2206oa. [DOI] [PubMed] [Google Scholar]
- 10.Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant. 2011;30:982–9. doi: 10.1016/j.healun.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Clements PJ, Tan M, McLaughlin VV, Oudiz RJ, Tapson VF, Channick RN, et al. on behalf of the Pulmonary Arterial Hypertension Quality Enhancement Research Initiative (PAH-QuERI) Investigators. The Pulmonary Arterial Hypertension Quality Enhancement Research Initiative: comparison of patients with idiopathic PAH to patients with systemic sclerosis-associated PAH. Ann Rheum Dis. 2012;71:249–52. doi: 10.1136/annrheumdis-2011-200265. [DOI] [PubMed] [Google Scholar]
- 12.Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JS, Vrapi F, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179:151–7. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–50. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 15.Johnson SR, Granton JT. Pulmonary hypertension in systemic sclerosis and systemic lupus erythematosus. Eur Respir Rev. 2011;20:277–86. doi: 10.1183/09059180.00003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Launay D, Hachulla E, Hatron PY, Jais X, Simonneau G, Humbert M. Pulmonary arterial hypertension: a rare complication of primary Sjögren syndrome: report of 9 new cases and review of the literature. Medicine (Baltimore) 2007;86:299–315. doi: 10.1097/MD.0b013e3181579781. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi H, Kataoka M, Yanagisawa R, Kawakami T, Tamura Y, Fukuda K, et al. Platelet level as a new prognostic factor for idiopathic pulmonary arterial hypertension in the era of combination therapy. Circ J. 2012;76:1494–500. doi: 10.1253/circj.cj-11-1223. [DOI] [PubMed] [Google Scholar]
- 18.Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383–94. doi: 10.1378/chest.10-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overbeek MJ, Lankhaar JW, Westerhof N, Voskuyl AE, Boonstra A, Bronzwaer JG, et al. Right ventricular contractility in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. Eur Respir J. 2008;31:1160–6. doi: 10.1183/09031936.00135407. [DOI] [PubMed] [Google Scholar]
- 20.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–63. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avouac J, Wipff J, Kahan A, Allanore Y. Effects of oral treatments on exercise capacity in systemic sclerosis related pulmonary arterial hypertension: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:808–14. doi: 10.1136/ard.2007.077149. [DOI] [PubMed] [Google Scholar]
- 22.Kuwana M, Watanabe H, Matsuoka N, Sugiyama N. Pulmonary arterial hypertension associated with connective tissue disease: meta-analysis of clinical trials. BMJ Open. 2013;3:e003113. doi: 10.1136/bmjopen-2013-003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Code of federal regulations: 21CFR312.32. Investigational new drug application. 2014 Apr; URL: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32.
- 24.Farber HW, Graven KK, Kokolski G, Korn JH. Pulmonary edema during acute infusion of epoprostenol in a patient with pulmonary hypertension and limited scleroderma. J Rheumatol. 1999;26:1195–6. [PubMed] [Google Scholar]
- 25.Palmer SM, Robinson LJ, Wang A, Gossage JR, Bashore T, Tapson VF. Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest. 1998;113:237–40. doi: 10.1378/chest.113.1.237. [DOI] [PubMed] [Google Scholar]
- 26.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–56. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 27.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 28.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 29.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. for the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008;117:3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 30.Sandoval J, Torbicki A, Souza R, Ramirez A, Kurzyna M, Jardim C, et al. Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. 2012;25:33–9. doi: 10.1016/j.pupt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 32.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 33.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. for the Treprostinil Study Group. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 34.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 35.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 36.Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med. 2010;104:731–40. doi: 10.1016/j.rmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;42:1383–90. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 40.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–33. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 41.Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. on behalf of the FREEDOM-C2 Study Team. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–8. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 42.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 43.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–6. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefevre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, et al. Survival and prognostic factors in systemic sclerosis–associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 2013;65:2412–23. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 45.Selvin S. Statistical analysis of epidemiologic data. New York: Oxford University Press; 1996. [Google Scholar]
- 46.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–50. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 47.Schachna L, Wigley FM, Chang B, White B, Wise RA, Gelber AC. Age and risk of pulmonary arterial hypertension in scleroderma. Chest. 2003;124:2098–104. doi: 10.1378/chest.124.6.2098. [DOI] [PubMed] [Google Scholar]
- 48.Coghlan JG, Pope J, Denton CP. Assessment of endpoints in pulmonary arterial hypertension associated with connective tissue disease. Curr Opin Pulm Med. 2010;16(Suppl 1):S27–34. doi: 10.1097/01.mcp.0000370208.45756.e8. [DOI] [PubMed] [Google Scholar]
- 49.Deuschle K, Weinert K, Becker MO, Backhaus M, Huscher D, Riemekasten G. Six-minute walk distance as a marker for disability and complaints in patients with systemic sclerosis. Clin Exp Rheumatol. 2011;29:S53–9. [PubMed] [Google Scholar]
- 50.Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol. 2009;36:330–6. doi: 10.3899/jrheum.080447. [DOI] [PubMed] [Google Scholar]
- 51.Schoindre Y, Meune C, Dinh-Xuan AT, Avouac J, Kahan A, Allanore Y. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J Rheumatol. 2009;6:1481–5. doi: 10.3899/jrheum.081221. [DOI] [PubMed] [Google Scholar]
- 52.Gunther S, Jais X, Maitre S, Berezne A, Dorfmuller P, Seferian A, et al. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum. 2012;64:2995– 3005. doi: 10.1002/art.34501. [DOI] [PubMed] [Google Scholar]
- 53.Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Patient-Centered Outcomes Research Institute. Clinical effectiveness research (CER) 2015 Apr; URL: http://www.pcori.org/program/clinical-effectiveness-research-cer.
- 55.Nuzzo R. Scientific method: statistical errors. Nature. 2014;506:150–2. doi: 10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- 56.Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126:349–56. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]