Abstract
Electrophiles are electron deficient species that form covalent bonds with electron rich nucleophiles. In biological systems, reversible electrophile-nucleophile interactions mediate basal cytophysiological functions (e.g., enzyme regulation through S-nitrosylation), whereas irreversible electrophilic adduction of cellular macromolecules is involved in pathogenic processes that underlie many disease and injury states. The nucleophiles most often targeted by electrophiles are side chains on protein amino acids (e.g., Cys, His and Lys) and aromatic nitrogen sites on DNA bases (e.g., guanine N7). The sulfhydryl thiol (RSH) side-chain of cysteine residues is a weak nucleophile that can be ionized in specific conditions to a more reactive nucleophilic thiolate (RS−). This review will focus on electrophile interactions with cysteine thiolates and the pathophysiological consequences that result from irreversible electrophile modification of this anionic sulfur. According to the Hard and Soft, Acids and Bases (HSAB) theory of Pearson, electrophiles and nucleophiles can be classified as either soft or hard depending on their relative polarizability. HSAB theory suggests that electrophiles will preferentially and more rapidly form covalent adducts with nucleophiles of comparable softness or hardness. Application of HSAB principles, in conjunction with in vitro and proteomic studies, have indicated that soft electrophiles of broad chemical classes selectively form covalent Michael-type adducts with soft, highly reactive cysteine thiolate nucleophiles. Therefore, these electrophiles exhibit a common mechanism of cytotoxicity. As we will discuss, this level of detailed mechanistic understanding is a necessary prerequisite for the rational development of effective prevention and treatment strategies for electrophile-based pathogenic states.
Keywords: unsaturated aldehyde toxicity, acrolein, 4-hydroxy-2-nonenal, mechanisms of cell injury, oxidative stress
INTRODUCTION
Electrophiles are defined as electron deficient species and are represented by a diverse group of chemicals that includes heavy metals, environmental pollutants, toxic drug metabolites, flavor enhancers, cell signaling mediators and unsaturated aldehyde products of membrane lipid peroxidation (Table 1). As electron deficient species, electrophiles will form covalent bonds with electron rich nucleophiles through a variety of chemical pathways (e.g., SN2, Schiff base formation, Michael addition). In biological systems, reversible electrophile-nucleophile interactions are critical components of basal cytophysiological processes; e.g., nitric oxide signaling and cell defense mechanisms [66], [67], [71]. Irreversible electrophile-nucleophile interactions are prominently involved in oxidative stress and other underlying pathophysiological processes that mediate organ injury, drug/chemical toxicity and disease states [23], [29], [47], [48], [52], [53], [58]. The biological nucleophiles (Table 2) most often targeted by electrophiles are side chains on protein amino acids (e.g., Cys, His, Arg and Lys) and aromatic nitrogen sites on DNA bases (e.g., guanine N7) [22], [23], [51], [73]. This review will focus on the irreversible reactions of electrophiles with nucleophilic sulfhydryl side-chain groups on cysteine residues of cellular proteins. These thiol groups (RSH) are sensitive to redox transformation and can exist in a number of oxidation states; e.g., sulfenic acid (RSOH), sulfonic acid (RSO3H) [65]. The thiol state, which is a very weak nucleophile, can also be ionized to a highly reactive nucleophilic thiolate (RS−; Table 2). This anionic sulfhydryl state is critically involved in many biochemical processes of the cell and therefore irreversible covalent modification by exogenous (e.g., environmental toxicants) or endogenous (e.g., disease-related) electrophiles can have substantial pathogenic consequences. However, a critical principle to be presented in this review is that these electrophile-nucleophile interactions do not occur indiscriminately. Rather these reactions exhibit a significant degree of selectivity in accordance with the Hard and Soft, Acids and Bases (HSAB) theory of Pearson, which states that electrophiles preferentially and more rapidly form covalent bonds with nucleophiles of comparable softness or hardness; see ahead. Such bond formation is thought to involve the respective frontier molecular orbitals (FMOs), the energies of which can be calculated using various quantum mechanical models [51]. Based on these FMO energies, relative softness (σ) and hardness (η) can be quantified for an electrophile and corresponding nucleophilic target (Table 3). Furthermore, the values for σ and η can be incorporated into algorithms to calculate electrophilic (ω) and nucleophilic (ω−) indices. The latter index indicates the propensity, in quantitative terms, of a given nucleophile to react with an electrophile (Table 3). As we will describe, our HSAB analyses, in conjunction with supporting in vitro and proteomic determinations, indicate that the sulfhydryl thiolate is a highly reactive soft nucleophile that will selectively react with soft electrophiles of broad chemical classes; e.g., methyl mercury, N-acetyl-p-benzoquinone imine, 4-hydroxy-2-nonenal. The theme to be developed in this review is that this predisposition for soft nucleophilic targets suggests that soft electrophiles initiate toxicity via a common molecular mechanism (covalent adduct formation) and site of action (cysteine thiolates). As will be evidenced, this detailed level of mechanistic understanding is a prerequisite for rational development of effective prevention and treatment strategies for electrophile-based pathogenic states. We will begin our review with a brief synopsis of electrophile-nucleophile chemistry and the application of HSAB principles.
Table 1.
Electrophilic Indices and Softness Values for Selected Electrophilesa
| Compound | Structure | Electrophilic index (ω) | Softness (σ, ev−1) | Comments |
|---|---|---|---|---|
| chloroethylene oxide |
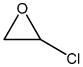
|
1.64 | 0.235 | Induces tumors via DNA adducts |
| vinyl chloride |
|
1.71 | 0.282 | Carcinogenic, neurotoxic and hepatotoxic industrial chemical |
| 2,5-hexanedione (HD) |
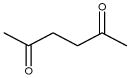
|
2.04 | 0.319 | Metabolite of n-hexane- neurotoxicant |
| Acrylamide (ACR) |
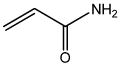
|
2.62 | 0.315 | Selective neurotoxicant, suspected carcinogenic chemical |
| methyl crotonate |
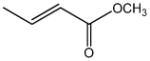
|
2.95 | 0.328 | Industrial chemical |
| 4-hydroxy-2- nonenal (HNE) |
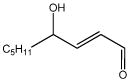
|
3.78 | 0.393 | Endogenous mediator of cellular oxidative stress |
| N-ethylmalimide (NEM) |
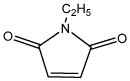
|
4.73 | 0.410 | Industrial toxicant |
| N-acetyl-p- benzoquinone imine (NAPQI) |
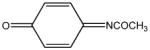
|
6.83 | 0.505 | Toxic metabolite of acetaminophen |
Ground state equilibrium geometries were calculated for each structure with DF B3LYP-6-31G* in water from 6-31G* initial geometries. Orbital energy values obtained were used to calculate HSAB parameters (see text).
Table 2.
Nucleophilic Indices and Softness Values for Selected Nucleophilesa
| Compound | Structure | Nucleophilic Index (ω−, x10−3 ev)b | Softness (σ, ev−1) | Comments |
|---|---|---|---|---|
| Cysteine Sulfhydryl (0) | HSCH2CH(NH2)CO2H | 98 | 0.282 | Thiol state (unreactive) |
| Cysteine Sulfhydryl Anion (−1) | -SCH2CH(NH2)CO2H | 261 | 0.382 | Thiolate state (reactive) |
| lysine (0) | H2N(CH2)4CH(NH2)CO2H | 126 | 0.285 | Amino acid residue |
| lysine (+1) | -(CH2)4NH3+ | 90 | 0.035 | Protonated state |
| histidine (0) |
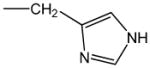
|
114 | 0.313 | Amino acid residue |
| Acetylcyclopentanone Anion (2−ACP) |
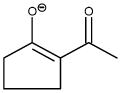
|
204 | 0.418 | Putative cytoprotectant |
Ground state equilibrium geometries were calculated for each structure with DF B3LYP-6-31G* in water from 6-31G* initial geometries. Orbital energy values obtained were used to calculate HSAB parameters (see text).
For these calculations of nucleophilicity, the reacting electrophile is acrolein.
Table 3.
HSAB parameters and respective algorithms
| Parameter | Formula | Comments |
|---|---|---|
| Hardness (η) | [ELUMO –EHOMO]/2 | Reflects ease of electron redistribution during covalent bonding |
| Softness (σ) | 1/η | Reflects ease of electron redistribution during covalent bonding |
| Chemical Potential (μ) | [ELUMO + EHOMO]/2 | Propensity to undergo chemical change |
| Electrophilic Index (ω) | μ2/2η | Measure of electrophilic reactivity |
| Nucleophilic Index (ω−) | ηA(μA−μB)2/2(ηA +ηB)2 | Reflects propensity of nucleophile (A) to form adduct with electrophile (B) |
These quantum mechanical computations can be computed using several different software packages: Gaussian (www.gaussian.com), Q-Chem (www.q-chem.com) and Spartan (www.wavefun.com). Whereas various models exist, the authors most frequently use the Density Functional Theory (DFT) models where ground state equilibrium geometries are calculated for each structure with B3LYP-6-31G* in water from 6-31G* initial geometries.
HSAB PRINCIPLES THAT DESCRIBE ELECTROPHILE-NUCLEOPHILE REACTIONS
As indicated above (Introduction), the reaction of electrophiles with nucleophiles is relatively selective as predicted by the HSAB theory. In this regard, the degree of reactant selectivity in a covalent interaction is determined by the relative polarizability (electron mobility) of the respective molecules. Specifically, the valence electrons of a hard electrophile (e.g., formaldehyde) are relatively immobile since the low electron density site (partial positive charge) is localized on the carbonyl carbon atom. In contrast, softer more polarizable electrophiles have relatively mobile electrons and the resulting delocalization leads to multiple sites of low electron density; e.g., both the carbonyl carbon atom and the β carbon atom of acrolein; see Fig. 1A. Soft nucleophiles such as the anionic side-chain sulfur atoms of cysteine residues have large atomic radii and as a consequence corresponding valence electrons are highly polarizable; see Fig. 1A. Other biological nucleophiles such as the nitrogen groups of lysine (ε-amino group side chain) and histidine residues (imidazole side chain) are harder atoms due to the more localized charge that results from their smaller atomic radii and greater electronegativity (Table 2); for detailed discussions of HSAB theory see [48], [51], [52], [53].
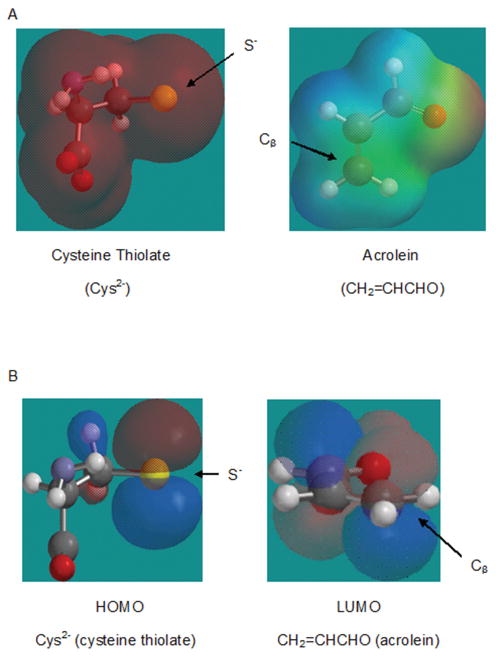
Figure 1.
(A). The concept of polarizability relevant to chemical softness/hardness is illustrated in this figure, which shows the color-coded electrostatic potential maps for cysteine thiolate and acrolein (calculated using Spartan ’14, Wavefunction Inc., Irvine, CA). The color gradient for each map illustrates how charge is distributed across the molecule and, therefore, indicates the relative degree to which the corresponding atoms attract oppositely charged atoms. Accordingly, red signifies the most negative electrostatic potential and is used for regions that attract positively charged molecules most strongly. Blue denotes areas with the most positive electrostatic potential and is used for regions that attract negatively charged molecules most strongly. The orange-yellow-green spectrum indicates intermediate (from negative to positive, respectively) electrostatic potential. In the case illustrated here negative electrostatic potential is distributed over the entire cysteine dianion (soft) and positive electrostatic potential is distributed over a broad area in acrolein (soft). Note that the area of positive electrostatic potential for acrolein encompasses Cβ, the site at which Michael addition takes place. (B). Graphical models of important molecular orbitals for Cys2− and acrolein are shown. It can be seen that the HOMO of Cys2− and the LUMO of acrolein at Cβ are in phase and able to overlap favorably to form a covalent bond. In addition, it should be noted that the most prominent lobe of the nucleophile (cysteine dianion) is, as would be expected, centered at the sulfur atom.
As indicated above, covalent bond formation is thought to occur between the respective FMOs of the reacting molecules. The reacting FMO for nucleophiles is the highest energy orbital that holds electrons and is designated as the Highest Occupied Molecular Orbital (HOMO). Correspondingly, the FMO for electrophiles is the lowest energy orbital that is vacant or the Lowest Unoccupied Molecular Orbital (LUMO). Thus, the covalent bond formation that occurs between an electrophile and a cysteine sulfhydryl thiolate can be viewed as the donation of high-energy HOMO electron density from the nucleophile into the empty LUMO of the electrophile; see Fig. 1B. The respective frontier orbital energies of the electrophile (ELUMO) and nucleophile (EHOMO) can be derived from quantum mechanical calculations and subsequently used in algorithms (Table 3) to determine softness (σ) and hardness (η). Softness, defined as the inverse of hardness (Table 3), reflects the relative ease of electron redistribution during covalent bonding. Softness or hardness, in conjunction with chemical potential (μ), an indicator of reactivity, can be used to calculate additional HSAB parameters such as the electrophilic index (ω; Table 3), which provides a measure of electrophile reactivity. A nucleophilic index (ω−) can also be calculated, which indicates the relative propensity of a nucleophile (A) to react with a designated electrophile (B; Table 3). Soft chemicals can vary in terms of reactivity; e.g., 2,5-hexanedione (HD) is a softer (higher σ value) but weaker electrophile (lower ω value) than acrylamide (ACR), whereas 4-hydroxy-2-nonenal (HNE) is softer and stronger than either of these compounds (Table 1). As indicated in the next section, such indices have proven useful in providing quantitative correlations in biologically and toxicologically relevant systems; e.g. see [51], [52].
NUCLEOPHILIC TARGETS OF SOFT ELECTROPHILES
Although notable exceptions exist, most endogenous and environmental toxicants are electrophiles that produce toxicity by interacting with biological nucleophiles [51], [68], [69]. Accordingly, HSAB concepts should have broad applicability to the fields of Molecular Toxicology and Pathophysiology. Indeed, we have applied HSAB principles and calculations of corresponding parameters to our studies of soft electrophile toxicity. Table 1 shows that quinones such as N-acetyl-p-benzoquinone imine (NAPQI), the electrophilic metabolite of acetaminophen (Tylenol™), are in general exceptionally soft, highly electrophilic chemicals (larger σ and ω values), whereas conjugated α, β-unsaturated carbonyl derivatives such as NEM, acrolein, HNE and acrylamide (ACR) exhibit significantly lower gradations of softness and electrophilicity. Calculations of corresponding electrophilicity values indicated the following rank order (Table 1): NAPQI > NEM > acrolein ~ HNE ≫ ACR. Our corroborative toxicity studies involving the unsaturated carbonyl congeners, showed that the rank order of electrophilicity (ω) was closely correlated (r2 > 0.90) to the respective second order rate constants (k) for cysteine adduct formation and to the corresponding potencies (IC50) for inhibition of neurotransmitter uptake in isolated rat synaptosomes [45], [46], [49], [50]. These data indicate that the reactivity (ω) of the electrophile directly corresponded to the respective rate constants (k) for cysteine adduction and therefore these HSAB parameters are determinants of toxic potency. However, a lack of correspondence between the experimentally derived toxic potency of a given electrophile and that predicated by the calculated electrophilicity (ω) is possible. This is because the HSAB algorithms do not consider the possible physicochemical traits of the electrophile that can affect the rate of adduct formation and toxic outcome; e.g., structural steric hindrance, solubility and acid-base equilibrium. Nonetheless, these extenuating characteristics are recognizable from the chemical structure of the electrophile, and therefore, the experimental findings can be interpreted appropriately; reviewed in [51].
As a covalent reaction, the rate of the electrophile-nucleophile interaction is also determined by the softness and reactivity of the nucleophile. Table 2 shows that, although the thiol state of cysteine is relatively unreactive, the corresponding anionic thiolate is a highly reactive soft nucleophile that will, in accordance with HSAB principles, preferentially undergo covalent reactions with soft electrophiles such as NAPQI, acrolein and 4-hydroxy-2-nonenal (HNE); see [49], [56], [82]. However, the availability of the thiolate site for adduct formation is a function of the pKa, which determines the concentration of the anionic sulfhydryl group at cellular pH (see ahead). In addition, the location of the cysteine residue within the tertiary structure of the protein determines solvent, and therefore, electrophile accessibility. In contrast to the kinetically favored soft-soft interactions, soft-hard reactions such as those that might occur between a soft electrophile and a hard nucleophile are not favored. Specifically, Table 2 shows that the nitrogen groups of the imidazole side chain of histidine and the ε-amino moiety of lysine are harder nucleophiles that are significantly less reactive with soft electrophiles. This amino acid preference has been demonstrated experimentally by comparisons of the respective second order rate constants (mean k ± SD M−1s−1). Thus, cysteine (k = 1.33±0.083) is approximately 1000-fold more reactive toward HNE, than either His (k = 0.00214±0.000312) or Lys (k = 0.00133±0.00005) [14], [15], [45], [46], [49]. Although His and Lys adducts of HNE, acrolein and other soft electrophiles have been identified in numerous cell culture and in vitro experiments, the relatively high toxicant-to-protein molar ratios and long incubation times (> 24 h) are a reflection of the correspondingly slow rate of adduct formation [17], [41], [59], [75], [76]. Thus, His, Lys and other hard amino acid residues are unlikely to be primary targets of adduct formation for NAPQI, HNE and other soft electrophiles.
MOLECULAR MECHANISMS OF SOFT ELECTROPHILE TOXICITY
Proteomic studies have provided evidence that soft electrophiles of different chemical classes (e.g., unsaturated aldehyde derivatives, quinones, methyl mercury) can impair cellular protein function by targeting specific cysteine residues; e.g. HNE inhibition of mitochondrial sirtuin 3 (SIRT3) activity by targeting Cys280 [22]; NAPQI adduction of Cys59 in the redox center of thioredoxin reductase [35]; dopamine ortho quinone inhibition of the dopamine transporter via Cys342 adduction [77] and acrolein-decreased glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity through adduction of Cys152 in the enzyme active site [56]. As discussed in the preceding section, this targeting should reflect the reaction of soft electrophiles with the highly nucleophilic sulfhydryl thiolate sites on specific cysteine residues. However, the pKa of the cysteine sulfhydryl side-chain is 8.4 and therefore, at intracellular pH ranges (7.0–7.4), these groups exist mostly in the non-nucleophilic thiol state. Nonetheless, anionic thiolate groups are present in pKa-lowering microenvironments such as cysteine-centered catalytic triads that are located within the active sites of cellular enzymes and proteins; e.g. N-ethylmaleimide sensitive factor (NSF), GAPDH, vesicular monoamine transporter [42], [43]. These sulfhydryl thiolate sites regulate protein function by playing a direct role in enzymatic catalytic processes (e.g., Cys152 of human GAPDH) and/or by acting as acceptors for nitrosating species derived from nitric oxide (NO); e.g., Cys91 and Cys264 of NSF [37], [43], [78].
Additional evidence suggests that electrophiles also cause cytotoxicity by depleting the cellular GSH pool, which can initiate redox imbalance or accelerate ongoing oxidative stress. Whereas this might be the case for highly reactive soft electrophiles (e.g., NAPQI), weaker electrophiles (e.g., acrylamide, methyl acrylate) are unlikely to react spontaneously (non-enzymatically) with GSH [32], [44]. Thus, although GSH (pKa 8.6) is a relatively strong, soft nucleophile (σ = 0.427 ev; ω− = 548 x 10−3 ev), only 6% of the corresponding sulfhydryl is in the reactive thiolate state at physiological conditions. The rate of a covalent adduct reaction is dependent not only on the reactant concentrations, but also on the respective electrophilicity and nucleophilicity which have a direct effect on the second order rate constant. As a result, the reaction of a weaker electrophile with GSH is not kinetically favored and therefore substantially slower than the GSH reaction with a stronger electrophile; see [82] for additional discussion. Whereas a spontaneous reaction with GSH is unlikely, it is possible that weak electrophiles might activate the antioxidant-responsive element (ARE), which would upregulate expression of glutathione-S-transferase (GST) and other phase II detoxification proteins. GST catalyzes the conjugation of an electrophile to GSH and could thereby reduce the GSH pool. However, our animal studies [81] indicated that weak electrophilic toxicants (e.g., acrylamide) did not affect GST protein expression in brain or liver samples. This negative finding is likely related to an inability of weak electrophiles to activate cytoprotective pathways; e.g., the toxicants might have failed to form adducts with sentinel cysteine residues (Cys151, Cys273, Cys288, Cys297) on kelch-like erythroid cell-derived protein with CNS-homology-associated protein 1 (Keap1), which promotes dissociation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and subsequent nuclear ARE activation.
The preceding data suggest that soft electrophiles cause toxicity via a common molecular mechanism involving irreversible adduct formation at regulatory cysteine thiolate residues of functionally critical proteins; see also [11], [23], [19], [45], [46], [49]. In addition, the toxicity of more reactive soft electrophiles likely involves cellular GSH depletion and secondary oxidative stress [44], [81]. It should be realized, however, that soft electrophile toxicity is unlikely to be mediated by targeted inhibition of select proteins or a specific organelle (e.g., mitochondrion). Rather, a confluence of data now indicate that soft electrophiles inhibit an electrophile-responsive proteome [4], [31], [57] comprised of specific proteins that are regulated by low pKa cysteine residues; e.g., synaptic vesicle proteome [10], [43]. Thus, an organelle might be selectively vulnerable to electrophile-induced disruption if a significant proportion of the inhibited proteins are importantly involved in organelle function. Ultimately, the toxicological manifestations of proteome inhibition are influenced by physicochemical characteristics of the electrophile that determine toxicokinetic outcomes (e.g., electrophilicity, metabolism, tissue distribution) and accessibility to individual protein targets; e.g., steric hindrance [52], [53]. In addition, cell-level responses to electrophile intoxication such as the activation of cytoprotective signaling pathways (e.g., Nrf2/Keap1 pathway) and gene expression can also shape the development of toxicity.
TOXICOLOGICAL CONSEQUENCES OF ENVIRONMENTAL EXPOSURE TO SOFT ELECTROPHILES
In this review we have discussed evidence suggesting that soft electrophiles produce cytotoxicity through a common molecular mechanism involving protein adduction of soft nucleophilic thiolate sites on cysteine residues of proteins. As a result of natural and anthropogenic production, a variety of soft electrophiles are prevalent constituents of the ambient environment; e.g., acrolein, acrylonitrile, methyl mercury and crotonaldehyde [6], [18], [20], [60] [74]. Therefore, human populations are exposed to complex mixtures of toxic electrophiles, many of which have a common mechanism of action. The chemical composition of the mixture and corresponding concentrations of individual constituents depends upon variables such as occupation, geographical location and personal habits (e.g., cigarette smoking). However, rather than recognizing the toxicological relevance of these complex environmental mixtures, regulatory agencies have tended to focus on the toxicity of individual compounds. As a result, this approach can underestimate the actual risk to human health. For example, α, β-unsaturated carbonyl derivatives such as methyl vinyl ketone, acrolein and acrylamide are members of a large chemical class known as the type-2 alkenes. Most of these compounds are soft electrophilic toxicants and are recognized as prevalent environmental pollutants [18], [20], [60]. However, in many cases, the risks to human health have been considered minimal since individual concentrations are below corresponding No Observable Adverse Effect Level (NOAEL) concentrations. Nonetheless, exposure to a mixture of environmental toxicants with a common molecular mechanism can have toxicological implications due to additive or synergistic interactions among members; e.g., see [1], [3]. At the molecular level, the toxicity induced by exposure to a mixture of soft electrophiles is theoretically due to a summation of individual rates of thiolate adduct formation that would differ among constituents as a function of respective electrophilic reactivity (ω) and steric hindrance. Regardless of the reacting soft electrophile, the formation of irreversible thiolate adducts has equivalent consequences with respect to protein inactivation and ensuing cell toxicity; see [45], [46], [49]. Based on these considerations, we have suggested that exposure to environmental soft electrophiles could represent a significant human health risk [47], [52], [53].
The ability of soft electrophiles to interact on an additive or synergistic basis also has significant implications for the onset and development of different pathogenic states. Thus, the pathophysiological consequences of cellular oxidative stress associated with a number of diseases (e.g., Alzheimer’s disease, atherosclerosis) and tissue injuries (e.g., stroke, spinal cord injury) appear to be mediated by endogenous soft electrophiles such as acrolein, 4-hydroxy-2-nonenal (HNE) and 4-oxo-2-nonenal (ONE) that are generated during membrane lipid peroxidation [16], [21], [25], [70], [79]. As noted earlier, these unsaturated aldehydes cause cytotoxicity via the soft electrophile mechanism. Given their aforementioned prevalence, it is possible that environmentally-derived soft electrophiles can interact with their endogenously produced counterparts to accelerate disease processes or impair the recovery from tissue damage. Indeed, this supposition is supported by results from several human epidemiological studies, which indicated that environmental exposure to acrolein increased the risk of cardiovascular morbidity and mortality, especially in individuals with pre-existing disease [8], [12], [26], [64]. Corroborative animal research showed that both dietary and environmental exposure to acrolein exacerbated myocardial ischemic injury and atherosclerosis in mouse models, presumably by interacting with endogenous unsaturated aldehydes generated during ongoing oxidative stress [55], [58], [63], [71]. Accordingly, it has been proposed that chronic environmental exposure to soft electrophiles such as the unsaturated aldehydes is a significant risk factor for cardiovascular diseases [58], [61].
ENOLATE CYTOPROTECTION: SOFT-SOFT INTERACTIONS
As noted above, oxidative stress is a common molecular etiology in many pathogenic states, the cytotoxic consequences of which appear to be mediated by endogenous unsaturated aldehydes. These soft electrophiles are generated by free radical-induced peroxidation of membrane lipids. Because reactive oxygen species (ROS) played a key role, initial pharmacotherapeutic strategies involved conventional (e.g., Vitamin E) and non-conventional (e.g., phytopolyphenols) antioxidant compounds that trapped free radicals. However, although preclinical animal studies indicated possible therapeutic utility, antioxidant approaches were ultimately found to be of uncertain human benefit [24], [38]. Alternatively, the likely participation of unsaturated aldehydes in oxidative stress suggested that nucleophilic chemicals that could irreversibly bind these soft electrophiles, might arrest oxidative stress-induced cell injury. In this regard, hydralazine, carnosine and other nitrogen-based nucleophiles have been shown to provide cytoprotection in numerous animal and cell culture models of oxidative stress [13], [27], [62]. However, as nitrogen-based compounds, the reaction of these relatively hard nucleophiles with soft electrophilic unsaturated aldehydes is not favorable [45], [46], [49]. The slower rate (kinetically unfavorable) of these soft-hard interactions would reduce electrophile scavenging and thereby decrease clinical efficacy and utility. Therefore, matching a soft nucleophile to the toxic soft electrophiles that mediate oxidative stress could be a more effective pharmacotherapeutic strategy. In this regard, thiol-based nucleophiles such as N-acetyl cysteine (NAC), sodium thiosulfate and diethyldithiocarbamate (DEDTC) should be cytoprotective since ionization of the thiol yields a soft thiolate nucleophile [7], [36]. However, although NAC is a significant soft nucleophile (ω− = 316 x10−3 ev), the corresponding pKa is 9.6 and consequently the sulfhydryl group does not exist in the reactive thiolate state at physiological pH; see discussion in [39]. In contrast, DEDTC is a very strong acid (pKa = 3.37), which would lead to a greater concentration of the active nucleophilic species at physiological pH (faster reaction rate), but would also yield less stable, more reversible adducts (thermodynamically unfavorable); see [50].
We have shown that acetylacetone, 2-acetylcyclopentanone (2-ACP) and other 1,3-dicarbonyl compounds provided protection in cell culture models of oxidative stress (H2O2) and electrophile (acrolein)-induced injury [50]. These compounds ionize in aqueous solutions to form soft nucleophilic enolate anions (Fig. 2; pKa = 7.8). Whereas the idea that enolate-forming 1,3-dicarbonyl compounds, such as 2-ACP, might be cytoprotective is unprecedented, the concept stems from the recognition that curcumin, a phytopolyphenol with well-documented cytoprotective capability [5], also possesses an enolizable 1,3-dicarbonyl function [50]. Furthermore, the chemistry of 1,3-dicarbonyl compounds is well known [9], [54] and in vitro mechanistic studies have shown that the nucleophilic carbon-based enolate of 2-ACP mediates cytoprotection by forming irreversible Michael adducts with acrolein, HNE and other toxic soft electrophiles involved in oxidative stress. 2-ACP and other structurally flexible 1,3-dicarbonyl compounds can also chelate metal ions (Fe2+, Cu2+) that catalyze the free-radical generating Fenton reaction; for more detailed discussions see [50], [51]. Thus, the 1,3-dicarbonyl compounds are multifunctional and could be cytoprotective by disrupting several steps in the oxidative stress cascade.
Figure 2.
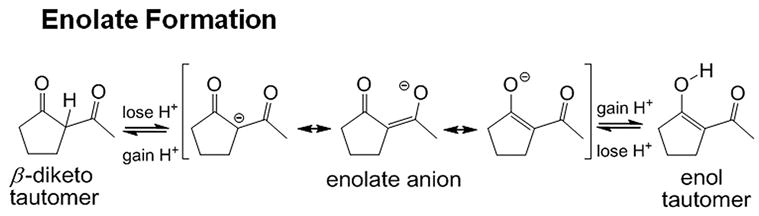
Schematic diagram showing that, loss of a proton from either the central (“α”) carbon in the diketo tautomer of 2-ACP or the hydroxyl group of the enol isomer yields the same resonance-stabilized enolate anion.
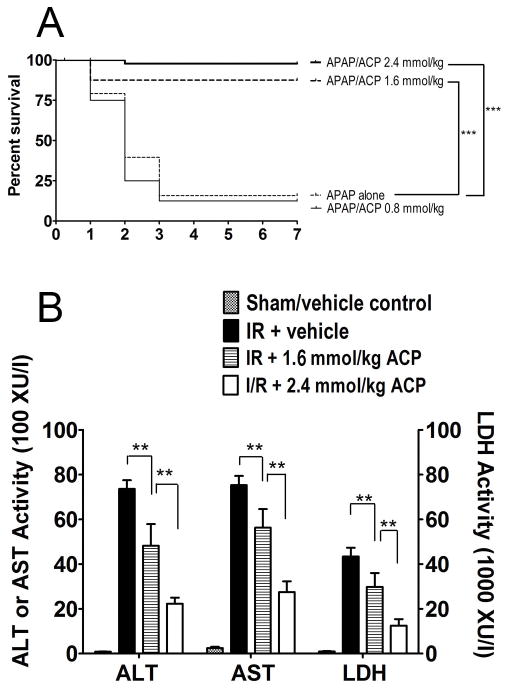
To evaluate protective efficacy in an animal model of oxidative stress, we determined the ability of 2-ACP to prevent hepatotoxicity in a mouse model of acetaminophen (Tylenol™; APAP) poisoning [82]. Experimental APAP intoxication in laboratory animals is a clinically relevant model that has been previously used to assess putative cytoprotectants [34]. Moreover, the molecular mechanism of APAP hepatoxicity is relatively well understood. Thus, in overdose, excess acetaminophen (APAP) is metabolized by the liver cytochrome P450 system to a highly reactive soft electrophile metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which causes hepatocyte damage via glutathione (GSH) depletion, covalent modification of mitochondrial proteins and secondary oxidative stress [32], [33]. Results (Fig. 3A) showed that oral APAP overdose (500 mg/kg) administered to mice was nearly 90% lethal within 72 hrs. However, we found that 2-ACP (0.80–2.40mmol/kg) given i.p. 20 minutes before or after intoxication provided dose-dependent protection against lethality over a 7 day experimental period (Fig. 3A). Measurements of several biochemical indices of hepatocyte death (e.g., plasma ALT, AST activity) and oxidative stress (HNE, malondialdehyde) suggested that 2-ACP prevented APAP-induced liver cell death. Histopathological analyses confirmed hepatocyte preservation and showed that i.p. pretreatment with 2-ACP prevented the liver centrilobular necrosis that characterizes APAP hepatotoxicity. Our in vitro studies and HSAB analyses indicated that, as a soft nucleophile, 2-ACP was able to effectively scavenge NAPQI, the reactive soft electrophilic APAP metabolite. In addition, the secondary oxidative stress initiated by NAPQI was likely curtailed through the abilities of 2-ACP to chelate metal ions and scavenge unsaturated aldehydes [82].
Figure 3.
(A). Dose-dependency of i.p. 2-ACP (0.80 – 2.40 mmol/kg) on oral APAP (500 mg/kg)-induced lethality. Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP alone, APAP/NAC and APAP/ACP groups (n= 15 mice/group). Joining line indicates statistically significant differences in treatment groups at *** p<0.001 levels of significance. (B). Dose-dependent effects of 2-ACP administered i.p.10 min prior to unclamping the portal circulation (reperfusion phase) on IRI-induced plasma appearance of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) in rat (n=10–15 /group). 2-ACP at the 0.80 mmol/kg dose was not effective relative to IRI + vehicle (data not shown). Data are expressed as mean activity ± SEM and joining lines indicate statistically significant differences in treatment groups at ** p<0.01 level.
We also determined the ability of 2-ACP to prevent the oxidative stress-induced damage associated with ischemia-reperfusion injury in rat liver [39]. IRI was induced by clamping the portal vasculature for 45 min (ischemia phase) followed by re-circulation for 180 min (reperfusion phase). This sequence was associated with substantial derangement of plasma liver enzyme activities, histopathological indices and markers of oxidative stress. 2-ACP (0.80–2.40mmol/kg), administered by intraperitoneal (i.p.) injection 10 min prior to reperfusion, provided dose-dependent cytoprotection as indicated by normalization of the IRI-altered liver histological and biochemical parameters (Fig. 3B). 2-ACP (2.40mmol/kg) was also hepatoprotective when injected before clamping the circulation (ischemia phase). In contrast, an equimolar dose of N-acetyl cysteine (NAC; 2.40mmol/kg) was not hepatoprotective when administered prior to reperfusion. Our studies to date suggest that during reperfusion the enolate nucleophile of 2-ACP limits the consequences of mitochondrial-based oxidative stress through scavenging unsaturated aldehyde electrophiles (e.g., acrolein) and chelation of metal ions that catalyze the free radical generating Fenton reaction.
These data suggest that 2-ACP and other enolate-forming 1,3-dicarbonyl analogues can block several steps of the oxidative stress cascade and thereby reduce cytotoxicity. Application of HSAB principles (soft-soft interactions) combined with knowledge of physicochemical parameters (e.g., pKa, steric hindrance) has led to the concept of enolate-based cytoprotection as well as a detailed understanding of molecular mechanism. Unlike curcumin and other phytopolyphenols (e.g., phloretin, resveratrol [28], [40], the 1,3-dicarbonyls are chemically stable, relatively water-soluble compounds that are rapidly absorbed and have large volumes of distribution [2], [50]. Furthermore, the acute animal toxicity of these chemicals is low (LD50 >800 mg/kg) and longitudinal dosing studies indicate a low incidence of systemic toxicity (e.g., 400–600 mg/kg/d x 60d [2]. The demonstrated cytoprotective properties, in conjunction with favorable pharmacokinetics and low toxicity, suggest that 2-ACP or an analogue could be developed as pharmacotherapeutic approaches drug-induced toxicities, disease processes and tissue injuries; e.g., Tylenol poisoning, stroke, donor organ failure and spinal cord injury.
SUMMARY
In accordance with HSAB concepts and supporting in vitro and proteomic studies, it is now recognized that soft electrophiles react preferentially with soft nucleophiles, which in biological systems are anionic thiolate sites. Although soft electrophiles play significant roles in a variety of normal cellular processes, in this review we have emphasized their pathophysiological significance and have presented substantial evidence that these chemicals operate via a common mechanism of toxicity. Thus, soft electrophiles from different chemical classes (e.g., α,β-unsaturated aldehydes, quinones) will form adducts with sulfhydryl thiolate groups in the active zones of enzymes. Adduction of these functionally critical nucleophiles can lead to subsequent protein inactivation, disruption of the dependent cellular process and cytotoxicity. We have also discussed how electronic (e.g., σ, η, ω, and ω−) and physicochemical (e.g., pKa, solubility and target accessibility) characteristics of the reactants can influence the reaction rate of second order soft-soft interactions. Although not discussed in this review, hard-hard interactions can also be quantitatively described by HSAB parameters, which can be used to clarify pathogenic mechanisms. Thus, we have used HSAB parameters derived from quantum chemical calculations to describe the hard-hard interactions of 2,5-hexanedione, the neurotoxic hard electrophile metabolite of n-hexane, with hard nucleophilic targets on axon cytoskeletal proteins; e.g., ε-amino nitrogens on lysine residues [80]. Perhaps most importantly, this review has delineated the possible pathophysiological consequences associated with the postulated common mechanism of soft electrophile toxicity. That is, the unrecognized potential of soft electrophiles to interact could lead to an underestimation of the human health risks associated with environmental exposure to complex mixtures of these chemicals. Furthermore, researchers have only recently realized the potential ability of environmental soft electrophiles to interact with endogenous counterparts, which could accelerate disease progression. Finally, we described the importance of understanding electrophile-nucleophile reactions at the molecular level in our development of 1,3-dicarbonyl compounds as cytoprotectants in oxidative stress-induced cell injury. Such an approach could represent a rational formula for the development of effective pharmacotherapeutic interventions.
Acknowledgments
The author’s research discussed in this review was supported by NIH grants from the National Institutes of Environmental Health Sciences (NIEHS) to R.M.L. (R01 ES03830-27; R01 ES007912-11).
Footnotes
DECLARATION OF INTEREST STATEMENT
There are no financial, consulting or personal relationships that could be construed as potential conflicts of interest.
References
- 1.Altenburger R, Nendza M, Schuurmann G. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ Toxicol Chem. 2003;22:1900–1915. doi: 10.1897/01-386. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne B, Cawley TJ. Toxicology update: 2,4-Pentanedione. J Appl Toxicol. 2001;21:165–171. doi: 10.1002/jat.739. [DOI] [PubMed] [Google Scholar]
- 3.Backhaus T, Faust M. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ Sci Tech. 2012;46:2564–2573. doi: 10.1021/es2034125. [DOI] [PubMed] [Google Scholar]
- 4.Barber DS, Stevens S, LoPachin RM. Proteomic Analyses of Rat Striatal Synaptosomes During Acrylamide Intoxication at a Low Dose-Rate. Toxicol Sci. 2007;100:156–167. doi: 10.1093/toxsci/kfm210. [DOI] [PubMed] [Google Scholar]
- 5.Begum AN, Jones MR, Lim GP. Curcumin structure-function, bioavailability and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisesi MS. Esters. 3. Esters of alkenylcarboxylic acids and monoalcohols. In: Clayton GD, Clayton FE, editors. Patty’s Industrial Hygiene and Toxicology. 4. Vol. 11. New York: John Wiley and Sons; 1994. pp. 2999–3007. [Google Scholar]
- 7.Borch RF, Pleasants ME. Inhibition of cis-platinum nephrotoxicity by diethyldithiocarbamate in a rat model. Proc Natl Acad Sci. 1979;76:6611–6614. doi: 10.1073/pnas.76.12.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope DA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 9.Bug T, Mayr H. Nucleophilic reactivities of carbonations in water: the unique behavior of the malodinitrile anion. J Am Soc Chem. 2003;125:12980–12986. doi: 10.1021/ja036838e. [DOI] [PubMed] [Google Scholar]
- 10.Burre J, Volknandt W. The synaptic vesicle proteome. J Neurochem. 2007;101:1448–1462. doi: 10.1111/j.1471-4159.2007.04453.x. [DOI] [PubMed] [Google Scholar]
- 11.Cavins JF, Friedman M. Specific modification of protein sulfhydryl groups with α, β-unsaturated compounds. J Biol Chem. 1968;243:3357–3360. [PubMed] [Google Scholar]
- 12.Dejarnett N, Conklin DJ, Riggs DW, Myers JA, O’Toole TE, Hamzeh I, Wagner S, et al. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014 doi: 10.1161/JAHA,114.000934. [DOI] [PMC free article] [PubMed]
- 13.Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30:1283–1288. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- 14.Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxynonenal and 4-oxo-2-nonenal. Chem Res Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- 15.Doorn JA, Petersen DR. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem-Biol Interact. 2003;143–144:93–100. doi: 10.1016/s0009-2797(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 16.Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007;115:13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Esterbauer H, Schaur RJ, Zollner H. Chemistry and Biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Faroon O, Roney N, Taylor J. Acrolein environmental levels and potential for human exposure. Toxicol Indust Health. 2008;24:543–564. doi: 10.1177/0748233708098124. [DOI] [PubMed] [Google Scholar]
- 19.Friedman M, Cavins JF, Wall JS. Relative nucleophilic reactivities of amino groups and mercaptide ions in addition reactions with α, β-unsaturated compounds. J Am Chem Soc. 1965;87:3672–3682. [Google Scholar]
- 20.Friedman M. Chemistry, biochemistry and safety of acrylamide. A review. J Agric Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 21.Fritz KS, Galligan JJ, Smathers RL, Roede JR, Shearn CT, Reigan P, Petersen DR. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24:651–662. doi: 10.1021/tx100355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz KS, Petersen DR. Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem Res Toxicol. 2011;24:1411–1419. doi: 10.1021/tx200169n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz KS, Petersen DR. An overview of the chemistry and biology of reactive aldehydes. Free Rad Biol Med. 2013;59:85–91. doi: 10.1016/j.freeradbiomed.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilgun-Sherki Y, Melamed E, Offen D. Antioxidant treatment in Alzheimer’s disease. J Molec Neurosci. 2003;21:1–11. doi: 10.1385/JMN:21:1:1. [DOI] [PubMed] [Google Scholar]
- 25.Grimmsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Sci. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guberan E, Raymond L. Mortality and cancer incidence in the perfumery and flavor industry of Geneva. Br J Ind Med. 1985;42:240245. doi: 10.1136/oem.42.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiotto A, Calderan A, Ruzza P, Osler A, Rubini C, Jo DG, Mattson MP, Borin G. Synthesis and evaluation of neuroprotective α,β-unsaturated aldehyde scavenger histidyl-containing analogues of carnosine. J Med Chem. 2005;48:6156–6161. doi: 10.1021/jm050507q. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurosci. 2008;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higdon AN, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signaling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012a;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higdon AN, Landar A, Barnes S, Darley-Usmar VM. The electrophile responsive proteome: Integrating proteomics and lipidomics with cellular function. Antiox Redox Signal. 2012b;17:1580–1589. doi: 10.1089/ars.2012.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Tox Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jan YH, Heck DE, Dragomir AC, Gardner CR, Laskin DL, Laskin JD. Acetaminohen reactive intermediates target hepatic thioredoxin reductase. Chem Res Toxicol. 2014;27:882–894. doi: 10.1021/tx5000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jegatheeswaran S, Siriwardena AK. Experimental and clinical evidence for modification of hepatic ischaemia-reperfusion injury by N-acetylcysteine during major liver surgery. Hepato Pancreato Biliary. 2010;13:71–78. doi: 10.1111/j.1477-2574.2010.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signaling. J Int Med. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosharskyy B, Vydyanathan A, Zhang L, Shararin N, Geohagan BC, Bivin W, Liu Q, Gavin T, LoPachin RM. 2-Acetylcyclopentanone, an Enolate-Forming 1,3-Dicarbonyl Compound, is Cytoprotective in Warm Ischemia-Reperfusion injury of Rat Liver. J Pharmacol Exp Ther. 2015;353:150–158. doi: 10.1124/jpet.114.221622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol. 2007;20:583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal. Chem Res Toxicol. 2003;16:901–911. doi: 10.1021/tx0300030. [DOI] [PubMed] [Google Scholar]
- 42.LoPachin RM, DeCaprio AP. Protein adduct formation as a molecular mechanism in neurotoxicity. Tox Appl Pharmacol. 2005;86:214–225. doi: 10.1093/toxsci/kfi197. [DOI] [PubMed] [Google Scholar]
- 43.LoPachin RM, Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Tox Sci. 2006;94:240–255. doi: 10.1093/toxsci/kfl066. [DOI] [PubMed] [Google Scholar]
- 44.LoPachin RM, He D, Soma D. Acrylamide Inhibits Dopamine Uptake in Rat Striatal Synaptic Vesicles. Toxicol Sci. 2006;89:224–234. doi: 10.1093/toxsci/kfj005. [DOI] [PubMed] [Google Scholar]
- 45.LoPachin RM, Barber DS, Geohagen BC, Gavin T, He D, Das S. Structure-toxicity analysis of Type-2 alkenes: in vitro neurotoxicity. Tox Sci. 2007a;95:136–146. doi: 10.1093/toxsci/kfl127. [DOI] [PubMed] [Google Scholar]
- 46.LoPachin RM, Gavin T, Geohagen BC, Das S. Neurotoxic mechanisms of electrophilic type-2 alkenes: soft-soft interactions described by quantum mechanical parameters. Tox Sci. 2007b;98:561–570. doi: 10.1093/toxsci/kfm127. [DOI] [PubMed] [Google Scholar]
- 47.LoPachin RM, Gavin T, Barber DS. Molecular mechanisms of the conjugated α,β-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Tox Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic and adduct formation. Chem Res Toxicol. 2009a;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LoPachin RM, Gavin T, Geohagen BC. Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal. Tox Sci. 2009b;107:171–181. doi: 10.1093/toxsci/kfn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LoPachin RM, Gavin T, Geohagen BC, Zhang L, Casper D, Lekhrag R, Barber DS. β-Dicarbonyl enolates: a new class of neuroprotectants. J Neurochem. 2011;116:132–143. doi: 10.1111/j.1471-4159.2010.07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LoPachin RM, Gavin T, DeCaprio AP, Barber DS. Application of the hard and soft acids and bases (HSAB) theory to toxicant-target interactions. Chem Res Toxicol. 2012;25:239–251. doi: 10.1021/tx2003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LoPachin RM, Gavin T. Molecular mechanisms of acrylamide neurotoxicity: lessons learned from organic chemistry. Enviorn Health Sci. 2012;120:1650–1657. doi: 10.1289/ehp.1205432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LoPachin RM, Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol. 2014;27:1081–1091. doi: 10.1021/tx5001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loudon GM. Organic Chemistry. 4. Chapt. 22. Oxford University Press; NY: 2002. Chemistry of enolate ions, enols and α,β-unsaturated carbonyl compounds; pp. 997–1068. [Google Scholar]
- 55.Luo J, Hill BG, Gu Y, Cai J, Srivastava S, Bhatnagar A, Prabhu SD. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am J Physiol Heart Circ Physiol. 2007;293:J3673–H3684. doi: 10.1152/ajpheart.00284.2007. [DOI] [PubMed] [Google Scholar]
- 56.Martyniuk CJ, Fang B, Koomen JM, Gavin T, LoPachin RM, Barber DS. Molecular mechanisms of α,β-unsaturated carbonyl toxicity: cysteine-adduct formation correlates with loss of enzyme function. Chem Res Toxicol. 2011;24:2302–2311. doi: 10.1021/tx200437y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martyniuk CJ, Feswick A, Fang B, Koomen JM, Barber DS, Gavin T, LoPachin RM. Protein Targets of Acrylamide Adduct Formation in Cultured Rat Dopaminergic Cells. Toxicol Letters. 2013;219:279–285. doi: 10.1016/j.toxlet.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain G, Joshi-Barve S. Toxicol Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nadkarni D, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem Res Toxicol. 1995;278:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien PJ, Diraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 61.O’Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Res Environ Health. 2008;23:167–202. doi: 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- 62.Park J, Zheng L, Marquis A, Walls M, Duerstock B, Pond A, Vega-Alvarez S, et al. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez CM, Ledbetter AD, Hazari MS, Haykal-Coates N, Carll AP, Winsett DW, Costa DL, Farraj AK. Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Tox Sci. 2013;132:467–477. doi: 10.1093/toxsci/kft008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittlemen M, Baliff J, Oh JA, Allen G, Monahan K, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 67.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz TW, Carlson RE, Cronin MTD, Hermens LM, Johnson R, O’Brien PJ, Roberts DW, Siraki A, Wallace KB, Veith GD. A conceptual framework for predicting the toxicity of reactive chemicals: modeling soft electrophilicity. SAR QSAR Eviron Res. 2006;17:413–428. doi: 10.1080/10629360600884371. [DOI] [PubMed] [Google Scholar]
- 69.Schwobel JAH, Koleva YK, Enoch SJ, Bajot F, Hewitt M, Madden JC, Roberts DW, Schultz TW, Cronin MTD. Measurement and estimation of electrophilic reactivity for predictive toxicology. Chem Rev. 2011;111:2562–2596. doi: 10.1021/cr100098n. [DOI] [PubMed] [Google Scholar]
- 70.Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and disease. Mol Nutr Food Res. 2011;55:1320–1331. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochim Biophys Acta. 2011;1810:741–751. doi: 10.1016/j.bbagen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava S, Sithu SD, Vladykovskaya E, Halberzettl P, Hoetkerr DJ, Siddiqui MA, Conklin DJ, et al. Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis. 2011;215:301–308. doi: 10.1016/j.atherosclerosis.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thaens D, Heinzelmann D, Bohme A, Paschke A, Schuurmann G. Chemoassay screening of DNA-reactive mutagenicity with 4-(4-nitrobenzyl)pyridine – Application to epoxides, oxethanes and sulfur heterocycles. Chem Res Toxicol. 2012;25:2092–2102. doi: 10.1021/tx3001412. [DOI] [PubMed] [Google Scholar]
- 74.Tucek M, Tenglerova J, Kollarova B. Effect of acrylate chemistry on human health. Int Arch Occup Environ Health. 2002;75:S67–S72. doi: 10.1007/s00420-002-0381-x. [DOI] [PubMed] [Google Scholar]
- 75.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uchida K, Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- 77.Whitehead RE, Ferrer JV, Javitch JA, Justice JB. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem. 76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- 78.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radical Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Wood PL, Khan MA, Moskal JR, Todd KG, Tanay VAMI, Baker G. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006;1122:184–190. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Gavin T, DeCaprio AP, LoPachin RM. γ-Diketone Neuropathy: Analysis of Cytoskeletal Motors and Highways in CNS Axons. Toxicol Sci. 2010;117:180–189. doi: 10.1093/toxsci/kfq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Gavin T, Barber DS, LoPachin RM. Role of Nrf2-ARE expression in Acrylamide Neurotoxicity. Toxicology Letters. 2011;205:1–7. doi: 10.1016/j.toxlet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang L, Gavin T, Geohagen BC, Liu Q, Downey KJ, LoPachin RM. Protective properties of 2-acetylcyclopentanone in a mouse model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2013;346:259–269. doi: 10.1124/jpet.113.205435. [DOI] [PMC free article] [PubMed] [Google Scholar]