Abstract
Background:
Pinus eldarica is a widely growing pine in Iran consisting of biologically active constituents with antioxidant properties. This study investigates the effect of hydroalcoholic extract of P. eldarica bark against oxidative damage induced by hydrogen peroxide (H2O2) in human umbilical vein endothelial cells (HUVECs).
Methods:
The total phenolic content of P. eldarica extract was determined using Folin-Ciocalteu method. The cytotoxicity of P. eldarica extract (25-1000 µg/ml) on HUVECs was assessed using 3-(4,5- Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method. Cytoprotective effect of P. eldarica extract (25-500 µg/ml) on H2O2-induced oxidative stress was also evaluated by MTT assay. The intra- and extra-cellular hydroperoxides concentration and ferric reducing antioxidant power (FRAP) were measured in pretreated cells.
Results:
The total phenolic content of P. eldarica extract was estimated as 37.04±1.8% gallic acid equivalent. P. eldarica extract (25-1000 µg/ml) had no cytotoxic effect on HUVECs viability. The pretreatment of HUVECs with P. eldarica extract at the concentrations of 50-500 µg/ml significantly reduced the cytotoxicity of H2O2. P. eldarica extract decreased hydroperoxides concentration and increased FRAP value in intra-cellular fluid at the concentration range of 100-500 µg/ml and in extra-cellular fluid at the concentration range of 25-500 µg/ml.
Conclusions:
This study revealed the antioxidant and cytoprotective effects of P. eldarica extract against H2O2-induced oxidative stress in HUVECs. Concerning the high content of phenolic compounds in P. eldarica, more research is needed to evaluate its clinical value in endothelial dysfunction and in other oxidative conditions.
Key Words: Pinus eldarica, Human umbilical vein Endothelial cells, Oxidative stress, Antioxidants
INTRODUCTION
Oxidative stress, as a result of imbalance between the production of reactive oxygen species (ROS) and antioxidant defense, is implicated in the pathogenesis of various disorders such as cancer as well as neurodegenerative and cardiovascular diseases (CVDs)[1,2]. In vasculature, oxidative damage, which is induced by several stimuli, for instance, inflammation, ischemia and reperfusion, may cause endothelial dysfunction[3]. ROS can directly deactivate nitric oxide and reduce antioxidant capacity, thus contributing to vascular stress[4]. Oxidative stress has a causal role in developing CVDs (e.g. atherosclerosis, hypertension, and heart failure) via the oxidation of low-density lipoprotein, disruption of vascular function and induction of endothelial and myocardial cell apoptosis[5,6].
Compounds or antioxidants with ability to inhibit the production of ROS or to scavenge ROS may offer therapeutic potential in the prevention or reduction of CVD. Recent studies have revealed some positive effects of antioxidant supplementation in improving endothelial function and modulating oxidative status[7-9]. Some investigations have also indicated that a high consumption of herbal antioxidants such as polyphenolics is associated with a reduced risk of CVD[10,11]. Besides, these antioxidants have shown to have anti-apoptotic effects on vascular endothelial cells following exposure to oxidizing agents like hydrogen peroxide (H2O2)[12].
Pinus eldarica Medw. is a stout evergreen tree belonged to the family Pinaceae. This tree is widely growing in Iran with a common name of “Iranian pine” or “Tehran pine”[13]. In traditional medicine, P. eldarica is used to treat skin diseases, such as wounds, allergic rashes and dermatitis as well as bronchial asthma[14,15]. In a pharmacological study, P. eldarica bark extract has been revealed to have anti-hyperglycemic activity[16]. Phytochemical analysis of the fruits and bark oil of P. eldarica have demonstrated the presence of the biologically active constituents with antioxidant activities, including α-pinene, β-caryophyllene, β-pinene, longifolene, α-humulene, δ-3-carene, and junipene[17,18]. The high concentrations of polyphenolic compounds, e.g. catechin and taxifolin, have also been observed in P. eldarica bark extract[19].
The present study aimed to investigate the antioxidant and cytoprotective effects of hydroalcoholic extract of the P. eldarica bark against oxidative stress induced by H2O2 in human umbilical vein endothelial cells (HUVECs). We also evaluated the toxicity of P. eldarica extract in HUVECs to confirm the safety of this plant extract.
MATERIALS AND METHODS
Plant material and extract preparation
The barks of P. eldarica were collected from Isfahan city, located in the Isfahan Province in the center of Iran during August 2014. After authentication of the plant by a botanist, a voucher specimen No. 3318 was deposited at the Herbarium of the School of Pharmacy and Pharmaceutical Sciences, (Isfahan, Iran) for future reference. To prepare hydroalcoholic extract, the powdered sample of air-dried barks of P. eldarica was extracted three times with ethanol (70%) using the maceration process at room temperature for 72 h. After the filtration of the extract, the solvent was removed using a rotary evaporator (Bibby RE200, UK) to produce a viscous brown residue, which was freeze-dried and stored at -20ºC. The yield of the plant extract was 21% (w/w).
Determination of total phenolic content
The total phenolic content of P. eldarica extract was determined using Folin-Ciocalteu method. Briefly, sodium bicarbonate (20%) was added to the plant samples. The mixture was then treated with diluted Folin-Ciocalteu reagent. After 2 h, the absorbance was measured at 765 nm. The total phenol content was estimated using a standard curve obtained from various concentrations of gallic acid (50, 100, 150, 250, and 500 mg/l) and was expressed in the percentage of gallic acid equivalents[20].
Cell culture
HUVECs (National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran) were cultured in Dulbecco's modified Eagle's medium (GIBCO BRL, Life Technologies, Grand Island, USA) supplemented with 10% FBS (Bioidea Company, Tehran, Iran) and 1% penicillin-streptomycin (100 U/ml penicillin and 100 µg/ml streptomycin). HUVECs were incubated at a normal culture condition (95% humidified atmosphere of 5% CO2 at 37°C) in 25-cm2 and/or 75-cm2 flasks.
Cell viability assay
The probable cytotoxicity of P. eldarica extract on HUVECs was assessed by MTT method[21] using a commercial kit (Bioidea Company, Tehran, Iran). In Brief, the cells were seeded at a concentration of 1.5×105 cells/ml in 96-well plates. Twenty four hours after plating, the cells were treated with different concentrations of freshly prepared P. eldarica extract (25 to 1000 µg/ml) or vitamin C (100 µg/ml) and incubated at 37°C for additional 24 h. Then the medium of each well was removed. After washing the cells with PBS at pH 7.4, a fresh medium, and an MTT reagent were added to each well and incubated at 37°C for 3 h. MTT reaction with living cells produced insoluble foramazan crystals with purple color. After the addition of dimethyl sulfoxide for dissolution of formazan crystals, the absorbance was measured at 570 nm by a microplate reader (BioTek Instruments, PowerWave XS, Wincoski, USA).
The cytoprotective effect of P. eldarica extract on H2O2-treated HUVECs cells was evaluated by MTT assay. Following the exposure to the plant extract (25 to 500 µg/ml) or vitamin C (100 µg/ml), the cells were washed and treated with H2O2 (0.5 mM; Merck Co., Mumbai, India) for 2 h.
The unexposed cells to the P. eldarica extract or H2O2 were considered as negative controls with the cell viability percentage of 100. Vitamin C-treated cells were used as the positive control. The viability of the treated samples was determined by a comparison between absorbance of various concentrations of the samples and negative control according to the following formula, and each experiment was performed in triplicate[21]. Cell viability (%)=(OD test-OD blank/OD negative control-OD blank)×100
Measurement of intra- and extra-cellular hydro-peroxides concentration
The effects of P. eldarica extract on intra- and extra-cellular hydroperoxides levels were determined based on the ferrous ion oxidation by xylenol orange reagent (FOX1)[22]. The FOX-1 reagent containing ammonium ferric sulfate in aqueous medium with sorbitol, was prepared according to the manufacturer’s protocol (Hakiman Shargh Research Co., Isfahan, Iran). After pretreatment with different concentrations of P. eldarica extract, HUVECs were exposed to H2O2. Then 10 μl supernatant of the cells or the cell lysates from each well was added to the 190-μl reagent and incubated at 40°C for 30 min. Absorbance was determined at 540 nm against a blank using a micro-plate reader/spectrophotometer (BioTek Instruments, PowerWave XS, Wincoski, USA). The hydroperoxides content of the samples were estimated using a standard curve of H2O2 concentrations (1-10 μM).
Measurement of cell-free and intra- and extra-cellular ferric reducing antioxidant power (FRAP)
The total antioxidant capacity of different concentrations of P. eldarica extract was determined by the evaluation of FRAP[23]. FRAP value was measured based on the reduction of ferric-tripyridyltriazine complex to ferrous form by spectro-photometric assay. The FRAP reagent containing tripyridyltriazine/ferric chloride/acetate buffer was prepared according to the manufacturer’s protocol (Hakiman Shargh Research Co., Isfahan, Iran). For each well, 10 μl sample was added to the 200 μl FRAP reagent. The samples were supernatants of the cells or the cell lysates from each well[24,25]. FRAP assay was also carried out on samples without the cells. These samples were different concentrations of P. eldarica extract in water. The mixture of sample and reagent was incubated at 40ºC for 40 min. Then the absorbance was measured at 570 nm against the blank using a microplate reader (BioTek Instruments, PowerWave XS, Wincoski, USA). The FRAP values of the samples were calculated using the standard curve acquired from various concentrations of FeSO4×7H2O (0.1-10 mM) and were expressed as μM of FeII equivalents.
Statistical analysis
Data were presented as mean±standard error of mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey's post-hoc test (SPSS software
version 16.0) was used for statistical analysis. P value <0.05 was considered statistically significant.
RESULTS
Total phenolic content
The total phenolic content was estimated as 37.04±1.8% gallic acid equivalents in dried barks of P. eldarica extract.
Effect of P. eldarica extract on human umbilical vein endothelial cells viability
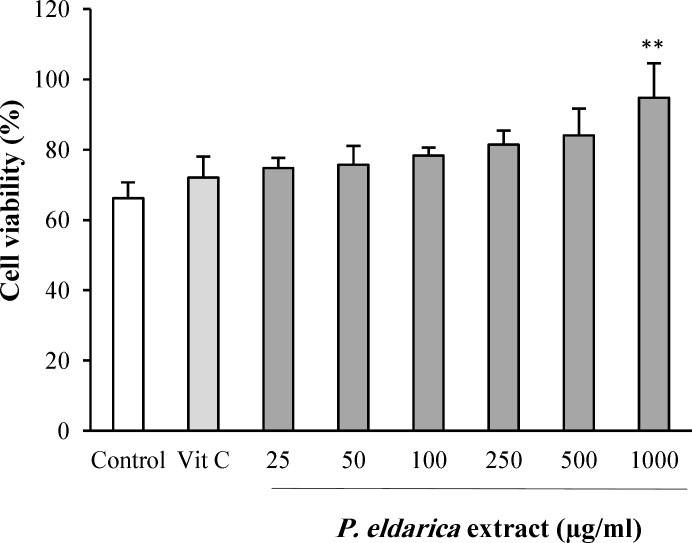
The probable cytotoxicity of P. eldarica extract on HUVECs was evaluated by MTT assay. There was no inhibitory effect on HUVECs viability after exposure to P. eldarica extract (25-1000 µg/ml) for 24 h (Fig. 1). Interestingly, P. eldarica extract at the concentration of 1000 µg/ml increased the viability of HUVECs.
Fig. 1.
The effect of P. eldarica extract on proliferation of HUVECs. Cells were incubated with different concentrations of P. eldarica extract (25-1000 µg/ml) or vitamin C (100 µg/ml) for 24 h. The cell viability was determined and compared with the control (untreated cells) by the MTT assay. Values are mean±SEM from three independent experiments in triplicate. **P<0.01 versus control (untreated cells
Cytoprotective effect of P. eldarica extract against H2O2-induced oxidative stress
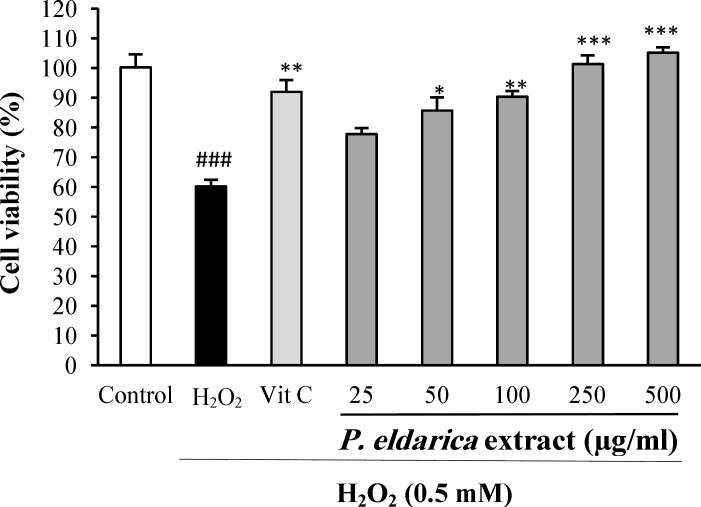
When the HUVECs were exposed to oxidative damage induced by H2O2 at 0.5 mM for 2 h, a significant drop was observed in cell viability (P<0.001). Pretreatment of HUVECs with P. eldarica extract at the concentrations of 50-500 µg/ml significantly reduced the cytotoxicity effects of H2O2 (Fig. 2).
Fig. 2.
The effect of P. eldarica extract on H2O2-induced oxidative stress in HUVECs. Cells were incubated with H2O2 (0.5 mM, 2 h) after pretreatment with different concentrations of P. eldarica extract (25-500 µg/ml) or vitamin C (100 µg/ml). The cell viability was determined by the MTT assay. Values are mean±SEM from three independent experiments in triplicate. ###P<0.001 versus control (untreated cells), *P<0.05, **P<0.01 and ***P<0.001 versus H2O2-stimulated cells
Effects of P. eldarica extract on intra- and extra-cellular hydroperoxides concentration
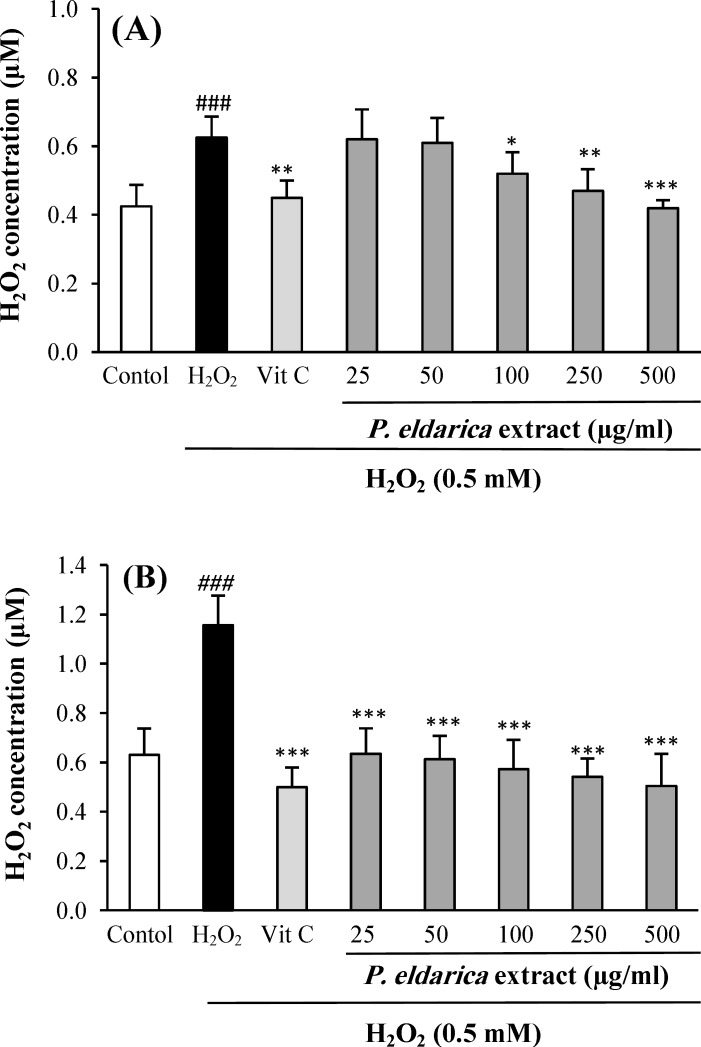
Figure 3 show the effects of P. eldarica extract on intra- and extra-cellular hydroperoxides concentration in HUVECs after exposure to the oxidative stress induced by H2O2. The incubation of HUVECs with P. eldarica extract significantly decreased the intra-cellular hydroperoxides level at the concentrations of 100-500 µg/ml as compared with the control group. Pretreatment with P. eldarica extract also reduced the extra-cellular hydroperoxides level at the concentrations of 25-500 µg/ml (P<0.001).
Fig. 3.
The effect of P. eldarica extract on intra-cellular (A) and extra-cellular (B) hydroperoxides concentration in HUVECs. Cells were incubated with H2O2 (0.5 mM, 2 h) after pretreatment with different concentrations of P. eldarica extract (25-500 µg/ml) or vitamin C (100 µg/ml). The hydroperoxides concentration was determined by FOX1 method. Values are mean±SEM from three independent experiments in triplicate. ###P<0.001 versus control (untreated cells), *P<0.05, **P<0.01 and ***P<0.001 versus H2O2-stimulated cells
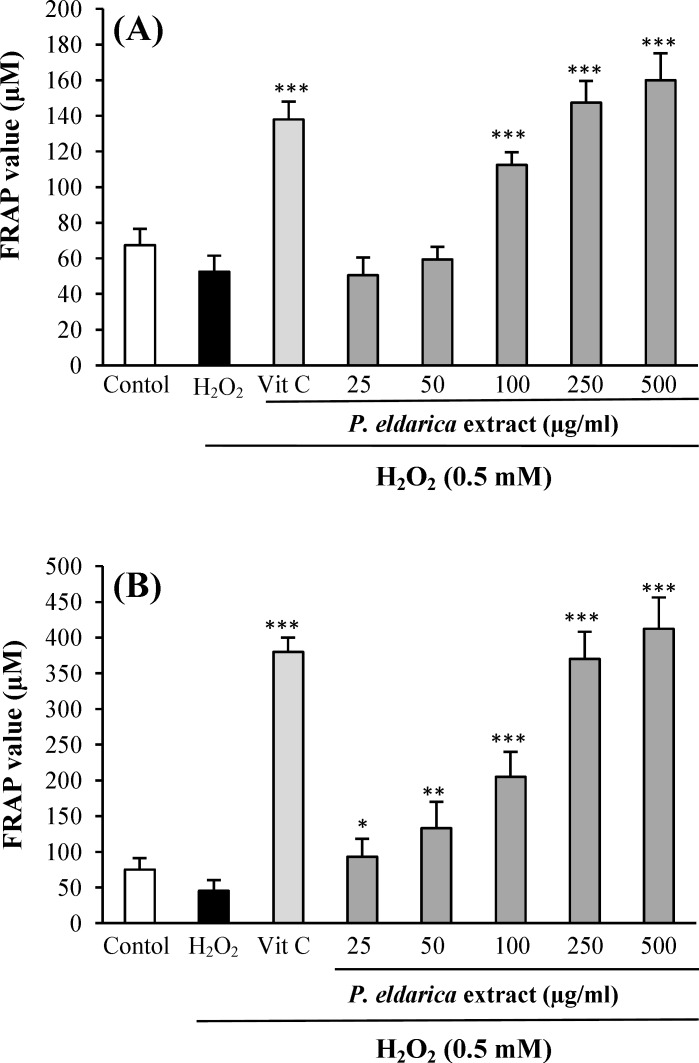
Effects of P. eldarica extract on cell-free and intra- and extra-cellular FRAP value
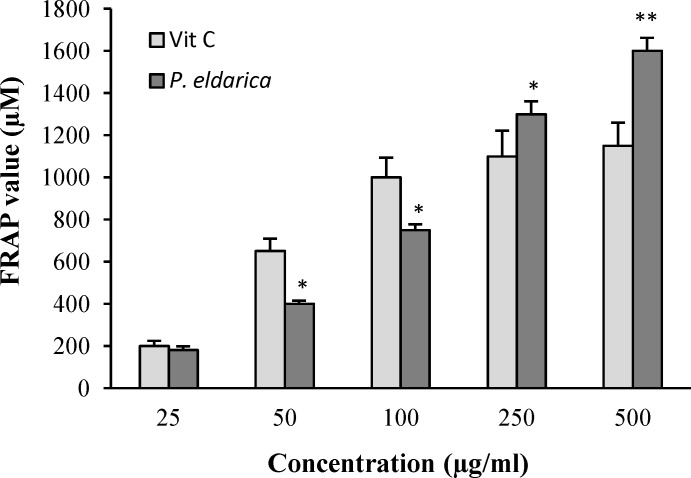
The FRAP value of P. eldarica extract without cell and also in intra- and extra-cellular fluids was evaluated. An increasing trend in FRAP value was observed with increasing P. eldarica extract concentrations in a cell-free assay (Fig. 4). The incubation of HUVECs with P. eldarica extract significantly increased the FRAP levels in the intra-cellular fluid at the concentrations of 100-500 µg/ml (Fig. 5A) and in extra-cellular fluid at the concentrations of 25-500 µg/ml (Fig. 5B).
Fig. 4.
Ferric reducing antioxidant power (FRAP) values of different concentrations of P. eldarica extract and vitamin C (25-500 µg/ml). Values are means±SEM from three independent experiments in triplicate. *P<0.05 and **P<0.01 versus vitamin C group at the same concentration
Fig. 5.
Effect of P. eldarica extract on intra-cellular (A) and extra-cellular (B) Ferric reducing antioxidant power (FRAP) value in HUVECs. Cells were incubated with H2O2 (0.5 mM, 2 h) after pretreatment with different concentrations of P. eldarica extract (25-500 µg/ml) or vitamin C (100 µg/ml). Values are means±SEM from three independent experiments in triplicate. ***P<0.001 versus H2O2-stimulated cells
DISCUSSION
The present study demonstrated the protective effect of P. eldarica extract against H2O2-induced toxicity in HUVECs at the concentration range of 25-500 µg/ml.
Also, this herbal extract did not show any cytotoxic effects at the concentration range of 25-1000 µg/ml. P. eldarica extract decreased hydroperoxides concentration and increased FRAP value in intra- and extra-cellular fluid at the concentration ranges of 100-500 µg/ml and 25-500 µg/ml, respectively.
Endothelial cells have a crucial role in the regulation of vascular physiological functions[26]. Numerous studies have indicated the role of oxidative stress in development of endothelium dysfunction[27]. H2O2, a non-free radical with oxidative ability, is widely used as a template substance to induce oxidative stress and apoptosis in various cell types such as endothelial cells[28]. As a small molecule lacking electrochemical charge, H2O2 can easily pass through the cell membrane and act as an intracellular second messenger in some vascular processes, including remodeling, inflammation, growth and apoptosis[29].
During the vascular pathological conditions, nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase and uncoupled endothelial NO synthase are the main producers of H2O2[30,31]. After the exposure of endothelial cells to H2O2, inflammatory responses including increased expression of intercellular adhesion molecule-1, platelet activating factor, and P-selectin, upregulation of monocyte chemoattractant protein-1, activation of NF-κB and also neutrophil adhesion to endothelium occur[32].
In the present study, HUVECs exposure to H2O2 (0.5 mM) induced cell growth suppression and significantly raised the intra- and extra-cellular hydroperoxides levels assessed by FOX-1 method, which is a sensitive method for estimation of hydroperoxides[33]. Oxidative stress induced by H2O2 also reduced total antioxidant capacity. Several studies have reported decreased antioxidant capacity, such as superoxide dismutase, catalase and glutathione peroxidase, and vitamins C and E in CVDs[34,35].
Pretreatment of HUVECs with P. eldarica extract significantly reduced the hydroperoxides level and increased FRAP; however, the extract provided less intra-cellular protection at low concentrations. Pinaceae is one of the largest families of conifers. The genus Pinus consists of various evergreen and aromatic trees as true pines, which is widely spread in many countries including Iran[36]. P. eldarica, as one of the most common pines in Iran composed of different parts, including needles, buds, nuts, and resin, has been commonly used in traditional medicine[16]. The high amounts of phenolic compounds, including catechin, ferulic acid, caffeic acid, and taxifolin have been identified in P. eldarica bark extract[19]. There are also high contents of monoterpenes and sesquiterpenes in composition of the bark oil of the P. eldarica[19]. Phenolic compounds are natural chemicals consisting of hydroxyl groups and possess strong antioxidant properties because of their reactivity with radical species and chelating metal ions[37]. The favorable cardiovascular effects of phenolic compounds have been shown in various studies[1,11]. The prevention of vascular oxidative stress and consequently the prevention of endothelial dysfunction has been reported in prediabetic rats following catechin intake[38]. Caffeic acid and ferulic acid belonging to cinnamic acid derivatives can reduce the risk of cardiovascular disorders by inhibiting the production of ROS[39]. Terpenoids are also plant antioxidants with helpful effects on cardiovascular system, including vasorelaxation as well as decreasing blood pressure and heart rate[40].
In summary, this study showed the cytoprotective and antioxidant effects of hydroalcoholic extract obtained from the stem bark of P. eldarica in oxidative stress conditions in HUVECs. Regarding the high content of phenolic compounds, P. eldarica extract could be a good antioxidant candidate for improving endothelial function in exposure to oxidative stress conditions.
ACKNOWLEDGMENTS
This work was financially supported by Isfahan University of Medical Sciences (grant no. 393379) (Isfahan, Iran).
CONFLICT OF INTEREST. None declared.
References
- 1.Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American oil chemists' society. 1998;72(2):199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarbishegi M, Mehraein F, Soleimani M. Antioxidant role of oleuropein on midbrain and dopaminergic neurons of substantia nigra in aged rats. Iraian Biomedical Journal. 2014;18(1):16–22. doi: 10.6091/ibj.1274.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogiatzi G, Tousoulis D, Stefanadis C. The role of oxidative stress in atherosclerosis. The hellenic journal of cardiology. 2009;50(5):402–409. [PubMed] [Google Scholar]
- 4.Schulz E, Anter E, Keaney J, John F. Oxidative stress, antioxidants, and endothelial function. Current medicinal chemistry. 2004;11(9):1093–1104. doi: 10.2174/0929867043365369. [DOI] [PubMed] [Google Scholar]
- 5.Samsam-Shariat SZ, Mostafavi SA, Khakpour F. Antioxidant effects of vitamins C and E on the low-density lipoprotein oxidation mediated by myelo-peroxidase. Iranian biomedical journal. 2013;17:22–28. doi: 10.6091/ibj.1092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertension research. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 7.Houston MC. The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Alternative therapies in health and medicine. 2013;19(1):32–49. [PubMed] [Google Scholar]
- 8.Safaeian L, Haghjoo-Javanmard SH, Ghanadian M, Seifabadi S. Cytoprotective and antioxidant effects of Echium amoenum anthocyanin-rich extract in human endothelial cells (HUVECs) Avicenna journal of phytomedicine. 2015;5(2):157–166. [PMC free article] [PubMed] [Google Scholar]
- 9.Safaeian L, Javanmard SH, Mollanoori Y, Dana N. Cytoprotective and antioxidant effects of human lactoferrin against H2O2-induced oxidative stress in human umbilical vein endothelial cells. Advanced biomedical research. 2015;4:188. doi: 10.4103/2277-9175.164010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safaeian L, Zabolian H. Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. International scholarly research notices: pharmacology. 2014 doi: 10.1155/2014/943523. Article ID 943523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Current opinion in lipidology. 2012;13(1):41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Aoki M, Nata T, Morishita R, Matsushita H, Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T, Kaneda Y. Endothelial apoptosis induced by oxidative stress through activation of NF-κB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38(1):48–55. doi: 10.1161/01.hyp.38.1.48. [DOI] [PubMed] [Google Scholar]
- 13.Zargary A. Medicinal plants. 5th Edition. Tehran: Tehran University Press; 1996. [Google Scholar]
- 14.Mamedov N, Gardner Z, Craker LE. Medicinal plants used in Russia and Central Asia for the treatment of selected skin conditions. Journal of herbs, species and medicinal plants. 2005;11(1-2):191–222. [Google Scholar]
- 15.Mamedov N, Craker LE. Medicinal plants used for the treatment of bronchial asthma in Russia and Central Asia. Journal of herbs, species and medicinal plants. 2001;8(2-3):91–117. [Google Scholar]
- 16.Fallahhuseini H, Mehrzadi S, Ghaznavi H, Tajallizadehkhoub Y, Fakhrzadeh H. Effects of Pinus eldarica Medw. Nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. Journal of medicinal plants. 2013;12(45):68–74. [Google Scholar]
- 17.Afsharypour S, Sanaty F. Essential oil constituents of leaves and fruits of Pinus eldarica Medw. Journal of essential oil research. 2005;17(3):327–328. [Google Scholar]
- 18.Zolfaghari B, Iravani S. Essential oil constituents of the bark of Pinus pinaster from Iran. Journal of essential oil bearing plants. 2012;15(3):348–351. [Google Scholar]
- 19.Iravani S, Zolfaghari B. Phytochemical analysis of Pinus eldarica bark. Research in pharmacological science. 2014;9(4):243–250. [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food chemistry. 2008;106(3):929–936. [Google Scholar]
- 21.Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Research pharmaceutical science. 2010;5(2):127–133. [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf SP. [18] Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methodsin rnzymology. 1994;233:182–189. [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Seyfizadeh N, Mahjoub S, Zabihi E, Moghadamnia AA, Pouramir M, Mir H, Khosravifarsani M, Elahimanesh F. Cytoprotective effects of arbutin against tert-butyl hydroperoxid induced toxicity in Hep-G2 cell line. World applied science journal. 2012;19(2):163–167. [Google Scholar]
- 25.Wu D, Sun MZ, Zhang C, Xin Y. Antioxidant properties of Lactobacillus and its protecting effects to oxidative stress Caco-2 cells. The journal of animal and plant sciences. 2014;24(6):1766–1771. [Google Scholar]
- 26.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: a model system for studying vascular inflammation in diabetes and atherosclerosis. Current diabetes reports. 2011;11(3):193–202. doi: 10.1007/s11892-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation research. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 28.Wei Z, Bai O, Richardson JS, Mousseau DD, Li XM. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. Journal of neuroscience research. 2003;73(3):364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 29.Pinazo-Durán MD, Gallego-Pinazo R, García-Medina JJ, Zanon-Moreno V, Nucci C, Dolz-Marco R, Martínez-Castillo S, Galbis-Estrada C, Marco-Ramirez C, Lopez-Gálvez MI, Galarreta DJ, Díaz-Llópis M. Oxidative stress and its downstream signaling in aging eyes. Journal of clinical unterventions in aging. 2014;11(9):637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. American journal of physiology heart and circulatory physiology. 2003;285(6):H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 31.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. Journal clinical investigatin. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovascular research. 2005;68(1):26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenyl phosphine. Clinica chimica acta. 2003;337(1-2):147–152. doi: 10.1016/j.cccn.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circulation journal. 2009;73(3):411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 35.Leinonen JS, Ahonen JP, Lönnrot K, Jehkonen M, Dastidar P, Molnár G, Alho H. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31(9):33–39. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- 36.Farjon A. Pines: drawings and description of the genus Pinus, 2nd revised edition. Leiden: Brill Academic Publishers; 2005. [Google Scholar]
- 37.Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed research international. 2013 doi: 10.1155/2013/639725. Article ID 639725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ihm SH, Lee JO, Kim SJ, Seung KB, Schini-Kerth VB, Chang K, Oak MH. Catechin prevents endothelial dysfunction in the prediabetic stage of OLETF rats by reducing vascular NADPH oxidase activity and expression. Atherosclerosis. 2009;206(1):47–53. doi: 10.1016/j.atherosclerosis.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Li PG, Xu JW, Ikeda K, Kobayakawa A, Kayano Y, Mitani T, Ikami T, Yamori Y. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens Research. 2005;28(4):369–377. doi: 10.1291/hypres.28.369. [DOI] [PubMed] [Google Scholar]
- 40.Santos MRV, Moreira FV, Fraga BP, De Sousa DP, Bonjardim LR, Quintans-Junior LJ. Cardiovascular effects of monoterpenes: a review. Revista Brasileira de Farmacognosia. 2011;21(4):764–771. [Google Scholar]