Summary
Dispersal is a key step in land plant life cycles, usually via formation of spores or seeds. Regulation of spore‐ or seed‐germination allows control over the timing of transition from one generation to the next, enabling plant dispersal. A combination of environmental and genetic factors determines when seed germination occurs. Endogenous hormones mediate this decision in response to the environment. Less is known about how spore germination is controlled in earlier‐evolving nonseed plants.
Here, we present an in‐depth analysis of the environmental and hormonal regulation of spore germination in the model bryophyte Physcomitrella patens (Aphanoregma patens).
Our data suggest that the environmental signals regulating germination are conserved, but also that downstream hormone integration pathways mediating these responses in seeds were acquired after the evolution of the bryophyte lineage. Moreover, the role of abscisic acid and diterpenes (gibberellins) in germination assumed much greater importance as land plant evolution progressed.
We conclude that the endogenous hormone signalling networks mediating germination in response to the environment may have evolved independently in spores and seeds. This paves the way for future research about how the mechanisms of plant dispersal on land evolved.
Keywords: abscisic acid (ABA), ent‐kaurenes, ethylene, high temperature, light, Physcomitrella, spore germination, strigolactones
Introduction
Transition from one generation to the next in land plants is mediated by the formation of desiccation‐resistant dispersal units (Finch‐Savage & Leubner‐Metzger, 2006). Within the spermatophyte lineage, these dispersal units are multicellular seeds, whereas in bryophytes and nonseed vascular plants (lycophytes and ferns) the functionally equivalent dispersal units are unicellular spores (Linkies et al., 2010). How and when germination is initiated in a seed or spore is critical for plant and species reproduction, movement and survival.
Hormonal and environmental factors are both well‐established as key players in the regulation of seed germination (Holdsworth et al., 2008a). The regulation of seed germination is highly complex, and involves integration of environmental signals by hormones within the seed and within different seed compartments and cell types (Yamaguchi et al., 2001; Holdsworth et al., 2008b; Linkies et al., 2010; Dekkers et al., 2013). Much is still unknown about exactly how seed germination is controlled at a cellular level (Nonogaki et al., 2010; Bassel et al., 2014).
Spores, unlike seeds, are haploid and are derived from the sporophyte stage of the plant life cycle via meiosis (reviewed in Rubinstein et al., 2010). Despite the different developmental origins of spores and seeds, previous work suggests that at least some aspects of germination regulation may be conserved between the two types of dispersal unit, as outlined later. Because spores are unicellular structures, understanding spore germination provides us with a simplified system for the study of the cellular and hormonal basis of germination regulation, and how this has evolved.
Environmental regulation of seed germination in both monocots and dicots is controlled in part by a phytochrome‐mediated reversible system, with red (R) light promoting germination, and even brief exposure to far‐red (FR) light inhibiting R light‐induced germination (Borthwick et al., 1952; Shinomura et al., 1994; Hennig et al., 2002), although this trait has been bred out of some commercial cereal crops (Barrero et al., 2012). A similar R–FR reversible system regulates spore germination in several ferns (Mohr et al., 1964; Raghavan, 1973; Wayne & Hepler, 1984; Scheuerlein et al., 1989; Tsuboi et al., 2012). In the earliest‐evolving land plant lineage, bryophytes, complete inhibition of spore germination by FR light, and reversal of this inhibition by R light via phytochromes, has been demonstrated (Possart & Hiltbrunner, 2013). Phytochrome regulation of spore germination is likely to be extremely ancient, as it appears to exist outside the land plant lineage, also, including in spores of the Charophycean algae Spirogyra and Chara, and in fungi (Calpouzos & Chang, 1971; Takatori & Imahori, 1971; Lucas et al., 1975; Mathews, 2006; Agrawal, 2009). The mechanisms downstream of phytochromes that regulate the control of germination throughout the plant lineage are poorly understood.
The onset of germination in seeds is closely regulated by the balance between plant hormone signalling pathways of gibberellin (GA) and abscisic acid (ABA), which interact at multiple levels (Karssen & Lacka, 1986; Holdsworth et al., 2008a). We have recently shown that ABA inhibits spore germination in Physcomitrella and that conserved proteins modulate ABA‐mediated germination responses in both spores and seeds (Moody et al., 2016). This suggests that downstream signalling components regulating germination may be conserved between spores and angiosperm seeds. In seeds, gibberellins are required for germination: seeds of the Arabidopsis ga1 mutant, which lacks the first enzyme in the GA biosynthesis pathway, are unable to germinate without exogenously supplied gibberellin (Koornneef & van der Veen, 1980), whereas GA receptor (GID) mutants cannot germinate fully (Voegele et al., 2011). GA overcomes the inhibitory effects of ABA to allow seed germination (Holdsworth et al., 2008a).
Conflicting results relating to the role of GA and ABA in the control of plant spore germination have been reported. Mosses biosynthesize the diterpenes at the start of the GA biosynthesis pathway, ent‐kaurene and ent‐kaurenoic acid, but they lack the enzyme that further converts ent‐kaurenoic acid into bioactive gibberellins (as occurs in seed plants). Thus, the identity of bioactive diterpenes in spore‐bearing plants is not yet fully characterized (Von Schwartzenberg et al., 2004; Hayashi et al., 2010; Zhan et al., 2015). Microarray analysis of spore germination in the fern Ceratopteris implicated involvement of GA signalling and downregulation of ABA signalling in this process, similarly to seeds (Yao et al., 2008). However, different fern species' spores have different sensitivities to GA and ABA application (Weinberg & Voeller, 1969; Chia & Raghavan, 1982; Singh et al., 1990; Kagawa & Michizo, 1991; Haas et al., 1992). The GA biosynthesis inhibitor AMO‐1618, which blocks the first step(s) in the GA biosynthesis pathway (Rademacher, 2000), can inhibit some (but not all) light‐induced fern spore germination (Weinberg & Voeller, 1969; Nester & Coolbaugh, 1986; Kagawa & Michizo, 1991). In the best‐studied model bryophyte, the moss Physcomitrella patens (Aphanoregma patens), a copalyl‐diphosphate synthase/kaurene synthase (cps/ks) mutant, which lacks the CYP88A enzyme that catalyses the key step of ent‐kaurenoic acid oxidation in gibberellin biosynthesis and hence makes no diterpenes, had no reported spore germination phenotype (Hayashi et al., 2010). However, Physcomitrella spore germination can be inhibited by AMO‐1618, although AMO‐1618 may have targets in addition to the CPS enzyme (Anterola et al., 2009). Reports in other bryophytes detail the conflicting effects of exogenously applied gibberellins on spores of different species at different concentrations (Chopra & Kumra, 1988).
ABA is central to dormancy establishment and maintenance in Arabidopsis (Finkelstein et al., 2008). Freshly harvested Arabidopsis seeds contain high levels of ABA and show primary dormancy. ABA is also implicated in the imposition of secondary dormancy by, for example, high temperatures, via de novo synthesis of this hormone (Finch‐Savage & Leubner‐Metzger, 2006; Toh et al., 2008, 2012). The majority of bryophyte species’ spores have not been reported to show primary dormancy (McLetchie, 1999; Glime, 2015). Whether spores can have secondary dormancy imposed on them is currently not well‐characterized (Glime, 2015).
ABA also protects plants against abiotic stresses such as desiccation and freezing (Lee & Luan, 2012; Dekkers et al., 2015). Formation of both mature seeds and spores involves desiccation, and aspects of ABA signalling during abiotic stress responses in bryophytes are conserved with angiosperms (Knight et al., 1995; Cuming et al., 2007; Khandelwal et al., 2010). ABA‐mediated stress tolerance in bryophytes occurs at least in part via accumulation of soluble sugars including sucrose (Burch & Wilkinson, 2002; Nagao et al., 2006; Oldenhof et al., 2006; Bhyan et al., 2012; Erxleben et al., 2012). Notably, soluble sugars (sucrose, glucose) can inhibit germination in Arabidopsis seeds (Dekkers et al., 2004; Li et al., 2012).
Several additional hormones regulate seed germination. Strigolactones (SLs) produced by host plants are potent promoters of parasitic plant seed germination (Bouwmeester et al., 2003; Yoneyama et al., 2010). In Arabidopsis, strigolactone signalling pathway mutants show reduced seed germination (Stanga et al., 2013) and strigolactone can overcome the secondary dormancy imposed by exposing Arabidopsis seeds to high temperatures (Toh et al., 2012). Ethylene also promotes seed germination via multiple routes (Stewart & Freebairn, 1969; Logan & Stewart, 1991; Linkies & Leubner‐Metzger, 2012), whereas an inhibitory role for cytokinin in germination is implied in Arabidopsis (Riefler et al., 2006). A role for auxin in seed germination under normal conditions has not been demonstrated (Holdsworth et al., 2008a; Park et al., 2011); neither has the effect of these hormones on moss spore germination been investigated systematically (Chopra & Kumra, 1988; Glime, 2015).
Here, we sought for the first time to define comprehensively how hormones and environmental processes regulate spore germination in the bryophyte lineage by using Physcomitrella as a model system.
Materials and Methods
Physcomitrella culture and spore generation
Physcomitrella patens (Gransden wild‐type (WT) strain, and Ppcps/ks and ccd8 mutants) was cultured and sporulation induced as in Moody et al. (2012). Sporophytes were harvested after maturation (dark brown sporophytes with a slightly ‘sparkly’ appearance) using sterile forceps under a SMZ645 dissecting microscope (Nikon, Tokyo, Japan) and air‐dried in sterile tubes for c. 1 wk before storage at room temperature.
Spore germination assays
Spores from a minimum of three sporophytes (all of the same age) of a particular genotype were used within each assay. For larger assays, three sporophytes’ worth of spores were used for every 10 Petri dishes (9 cm diameter). For assays comparing WT with a mutant, spores of each genotype were chosen to be of the same age, harvested at the same time.
Sporophytes were bleached in groups of two to three in 1 ml 25% Parozone™ (Jeyes Group, Thetford, UK) for 10 min and then washed three times in 1 ml sterile distilled water (10 min each) in a sterile flow cabinet. The sporophytes were then crushed in 100–200 μl of sterile water to release the spores. Spores were diluted down in sufficient sterile distilled water to allow plating of 500 μl of spore solution per Petri dish. Spores were plated on cellophane‐overlaid BCD supplemented with 5 mM CaCl2 and 5 mM ammonium tartrate, or on water agarose for the dormancy experiments in Fig. 1. Cellophane discs (A.A. Packaging Ltd, Preston, UK) were autoclaved for 15 min at 121°C, before use.
Figure 1.
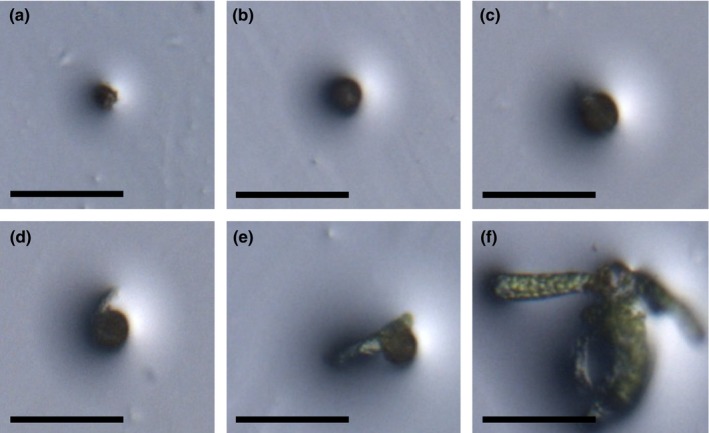
Physcomitrella patens spore germination stages. (a) Dry spore. (b) Imbibed spore. (c) Spore coat cracking. (d) Protrusion of one protonemal (chloronemal) filament. (e) Protrusion of two protonemal filaments. (f) Established protonemal colony. Bars, 100 μm.
For hormone/chemical treatments, the treatment in the relevant solvent (or a solvent‐only control) was added to 1 ml of sterile water, which was then added to BCD medium just before plate‐pouring to achieve the desired final concentration in the plates. Within each assay, all solvent‐containing plates were matched so that all contained exactly the same volume of solvent, even if the hormone concentration varied. The solvents used were methanol for diterpenoids and norflurazon, acetone for GR24 and water for ethylene.
Plated spores were air‐dried in a laminar flow hood, sealed with micropore tape, and placed at 22°C in long‐day conditions. Spores were counted daily under the ×4 objective of a Leica compound microscope with a ×10 eyepiece; total magnification ×40. A minimum of 200 spores per plate was counted to define the number of germinated and ungerminated spores. Data were expressed as percentage germination, that is: (germinated spores/total spores counted) × 100. A spore was defined as having germinated as soon as the very first deformation of the spore coat by the emerging protonemal filament was observed.
Two to three technical replicates were contained within each assay, and each assay was repeated a minimum of three times on different dates, using different batches of spores and medium, to provide biological replicates. Data from one representative assay is shown in each figure.
RNA preparation
RNA was prepared from dry spores (c. 250 harvested sporophytes), imbibed spores (c. 250 sporophytes bleached then soaked in liquid BCD for 18 h), germinating spores (spores from c. 250 bleached sporophytes plated at high density and left for 7 d until c. 50% germination was seen) and 100 mg vegetative (protonemal or leafy) tissue using the Bioline Isolate II Plant RNA preparation kit according to the manufacturer's instructions (London, UK). Average RNA yield was c. 300 ng μl−1, with OD260/280 > 2 and average OD 260/230 c. 1.8.
RT‐PCR
Reverse transcription polymerase chain reaction (RT‐PCR) was carried out on 20 ng RNA from each sample using the Bioline MyTaq™ one step RT‐PCR kit according to the manufacturer's instructions. Primer sequences are detailed in Supporting Information Table S1.
Generation of Physcomitrella patens lines with disrupted PpCPS/KS functionality
The moss line pCL755#29 is described in Zhan et al. (2015), generated using the methods described in (Bach et al., 2014). Briefly, a cassette containing p35S‐nptII‐CaMVter, expressing Neomycin Phosphotransferase II (conferring resistance to G418), was excised from pMBL6 (http://www.biology.wustl.edu/moss/pmbl6.jpg) using XhoI and inserted into the XhoI site in pDONR201:CPS/KS, generating the knock‐out construct pDONR201:CPS/KS‐nptII. Following transformation into P. patens of this construct, one line – pCL755#29 – with disrupted PpCPS/KS functionality was used for further studies.
A second line, pBK3, was generated by disrupting PpCPS/KS via targeted gene replacement using the pBK3 vector first described in Pan et al. (2015) and utilizing the method described in King et al. (2016), which contains a p35S‐aph4‐CaMVter cassette flanked by 5′‐ and 3′‐genomic sequence of PpCPS/KS on its 5′ and 3′ end, respectively. Therefore, genomic sequence of PpCPS/KS was replaced with p35S‐aph4‐CaMVter, which gives hygromycin resistance. The two lines were genotyped by PCR with the combination of primers that specifically bind to genomic DNA or selection marker cassette to distinguish knock‐out mosses from WT (Fig. S1).
GC‐MS analysis of diterpenoids
All GC‐MS analyses were performed on a Shimadzu GCMS‐QP2010 plus (GC‐2010) with a CTC auto sampler AOC‐5000, with cooled trays, agitation oven, and needle bake‐out.
GC‐MS analysis utilizing solid‐phase microextraction fibers was previously published (Drew et al., 2012; Andersen et al., 2015). Briefly, the injection port temperature was set to 230°C, with a sampling time of 1 min. The flow control mode was pressure control with a total flow of 2.3 ml min−1, with H2 as carrier gas, and a purge flow of 1.0 ml min−1. The column was a 30 m HP‐5MS column. The oven temperature program was 35°C for 3 min, rising by 10°C per min to 230°C and a hold for 3 min. The MS settings were: Ion source temperature 260°C, interface temperature 280°C and the scan range from m/z 50 to m/z 350 with 70 eV electrical ionization.
All data were analysed using the Shimadzu software Lab Solutions, GCMS solutions v.2.70, using the libraries provided by NIST (NIST 08) and wiley (wiley 8.0). Obtained spectra were compared with the spectra in the mass spectral libraries. Compounds were identified comparing the data with library information of MS and retention indices (I). All reference Is were taken from Adams (2007).
Results
Physcomitrella spores are nondormant and do not require cold‐stratification or after‐ripening treatment for germination
We define a Physcomitrella imbibed spore as having ‘germinated’ as soon as the first visible deformation of the spore coat occurs and the spore no longer appears spherical. This marks the first protrusion of protonemal filament(s), which grow with the subsequent formation of further filaments (Fig. 1a–f). Spores typically begin to germinate 2–7 d after imbibition/plating. We found that there was no correlation between spore age (time of dry storage post‐harvest) and speed of germination (Fig. 2a), suggesting that after‐ripening does not occur as it does in seeds. Moreover, a period of chilling, which breaks dormancy in seeds, did not affect spore germination (Fig. 2b,c). This suggests an absence of primary dormancy or after‐ripening in Physcomitrella spores.
Figure 2.
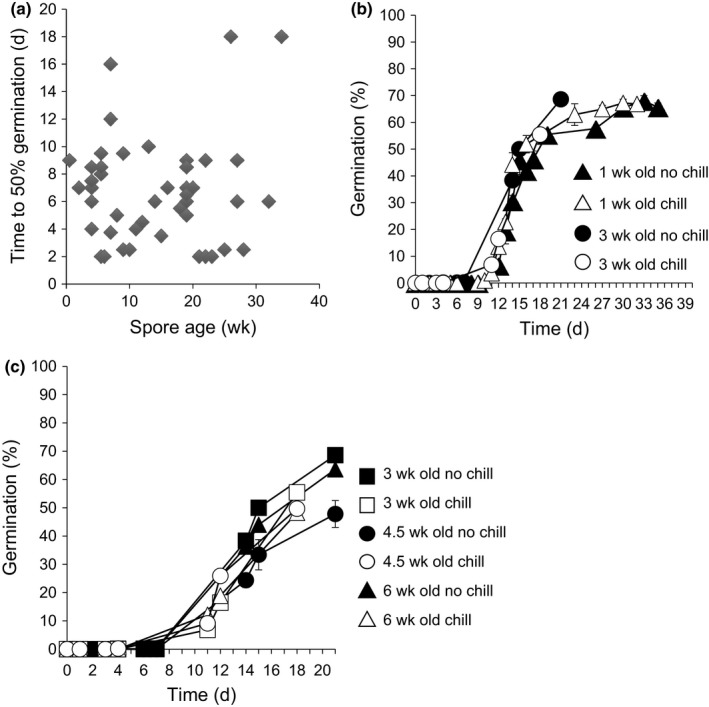
Physcomitrella spores do not appear to show primary dormancy and after‐ripening. (a) There is no correlation between dry spore age and time to 50% germination. Pearson–Spearman correlation test, R 2 = 0.004. (b) There is no effect of ageing or chilling on a single spore population. Spores from more than one sporophyte harvested from WT plants on the same day were germinated on water agarose with or without 3 d chilling at 4°C, 1 wk after collection and again at 3 wk after collection. Chilling does not accelerate germination. Error bars, ± SEM. (c) There is no effect of chilling on three independent batches of spores. Spores (from more than one sporophyte) from three independent harvests of different ages were germinated on water agarose with or without 3 d chilling at 4°C. Chilling does not accelerate germination. Error bars, ± SEM.
Far‐red inhibition of Physcomitrella spore germination is not rescued by application of diterpenes or inhibition of ABA synthesis
Although Physcomitrella spores appear not to have primary dormancy, we investigated whether their germination could be inhibited by environmental signals, as occurs in seeds (imposition of secondary dormancy in otherwise germination‐competent seeds). In both seeds and Physcomitrella spores, germination can be fully inhibited by a pulse of FR light (Seo et al., 2009; Possart & Hiltbrunner, 2013). In eudicot seeds, FR‐inhibition of germination can be fully rescued by treatment with bioactive GAs or by inhibition of ABA biosynthesis (Ikuma & Thimann, 1960; Schopfer et al., 2001; Oh et al., 2006; Seo et al., 2006; Lee et al., 2012).
In order to investigate whether the interface between light‐ and diterpene/ABA‐signalling in Physcomitrella spores is conserved with the regulation seen in Arabidopsis seeds, we FR‐treated spores in the presence of a diterpenoid known to be bioactive in moss (Hayashi et al., 2010), the fern antheridiogen GA9‐methyl ester, and the carotenoid biosynthesis inhibitor norflurazon, which blocks ABA biosynthesis in flowering plants (Chamovitz et al., 1991). We found that neither GA9‐methyl ester nor norflurazon were able to rescue the germination‐inhibitory effects of a pulse of FR light on Physcomitrella spores, even when control spores had germinated to 100% (Fig. 3a). This suggests that diterpenes and ABA do not facilitate spores’ responses to the environmental signal of light at certain wavelengths, and thus the hormonal control of Physcomitrella spores and dicot seeds is not conserved.
Figure 3.
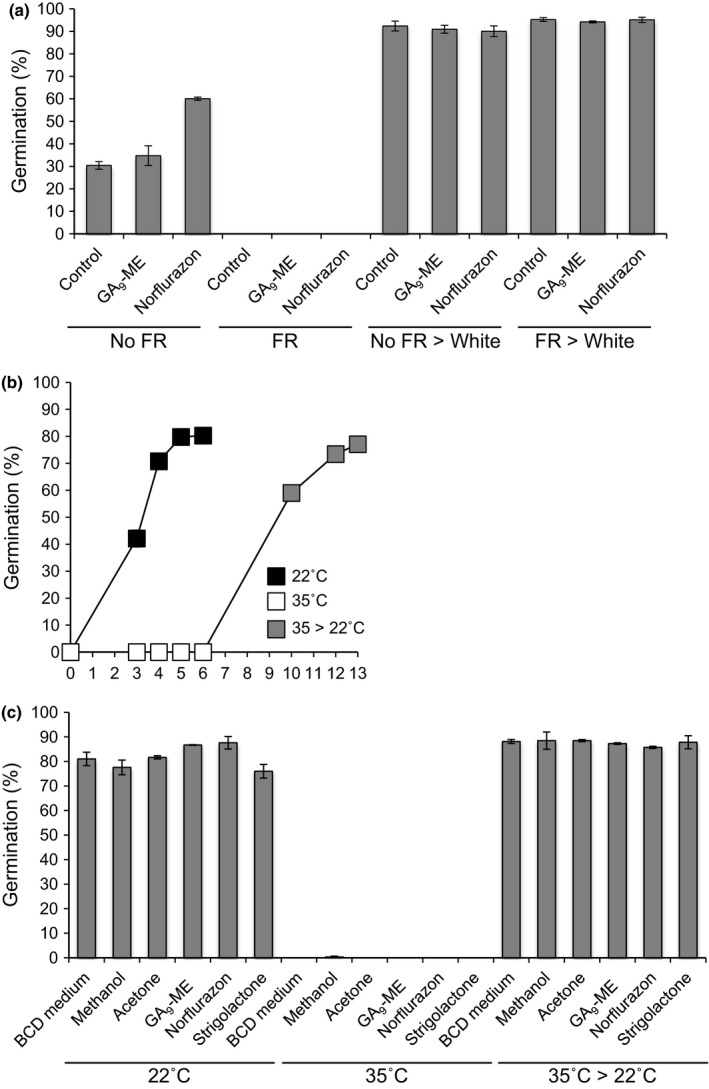
Effects of environmental signals on Physcomitrella spore germination. (a) A pulse of far‐red (FR) light inhibits spore germination and this cannot be rescued by norflurazon or GA9‐methyl ester (GA, gibberellin). Spores plated on control medium or GA9‐methyl ester (GA9‐ME) or norflurazon were treated ± FR light (‘No FR’ and ‘FR’, respectively) and immediately placed in the dark for 7 d. The spores’ germination percentage was measured 7 d after treatment. All spores were then moved to white light for a further 7 d (‘No FR > White’ and ‘FR > White’, under which conditions all spores germinated to completion, showing that the effect of FR light is completely reversible. A Kruskal–Wallis test indicates differences between FR and white light‐treated samples, P < 0.05. Error bars, ± SEM. (b) A temperature of 35°C inhibits spore germination and this is completely reversible when spores are returned to 22°C. (c) High‐temperature inhibition of spore germination cannot be rescued by norflurazon, GA9‐methyl ester or the synthetic strigolactone analogue GR24 (‘strigolactone’ on the graph). Spores were germinated for 7 d at either 22°C or 35°C. The 35°C‐treated spores were then transferred to 22°C for another 7 d (‘35°C > 22°C’). A Kruskal–Wallis test indicated differences between 35°C and 22°C spores, P < 0.05. Error bars, ± SEM.
Physcomitrella spore germination can be inhibited by high temperatures, but this cannot be rescued by ABA‐inhibition, or application of diterpenes or strigolactones
In order to further explore the role of diterpenes and ABA in the control of environmentally regulated germination, we inhibited spore germination using another environmental trigger: high temperature (thermoinhibition). Substantial and reversible thermoinhibition of seed germination (imposition of secondary dormancy) is seen at 32°C in Arabidopsis (Tamura et al., 2006; Toh et al., 2008), and this can be rescued by GA3, norflurazon or strigolactone application (Toh et al., 2012).
Incubation of spores at 35°C (but not 32 or 34°C; data not shown) caused complete inhibition of germination that was fully reversible upon return to normal growth conditions (22°C) (Fig. 3b). Thermoinhibition of Physcomitrella spores at 35°C could not be alleviated at all by GA9‐methyl ester, norflurazon or the synthetic strigolactone GR24 (Fig. 3c). Although high temperatures can inhibit germination in Physcomitrella spores, as in Arabidopsis seeds, the hormones mediating this response in Arabidopsis are not the same as in Physcomitrella, as was also seen with the FR light response.
Diterpenoids can promote Physcomitrella spore germination
Our previous work suggested conservation of some hormone function in spore and seed germination, via an ABA‐ARABIDILLO/PHYSCODILLO signalling module (Moody et al., 2016). Thus, we further explored the effects of diterpene hormones in Physcomitrella, to compare their effects with those of GAs in seeds. We examined Physcomitrella mutants in the gene encoding the first enzyme in the putative moss gibberellin biosynthesis pathway, ent‐COPALYL DIPHOSPHATE SYNTHASE/ent‐KAURENE SYNTHASE (CPS/KS), which makes no ent‐kaurene and hence no bioactive diterpenoids/gibberellins (Ppcps/ks; Hayashi et al., 2010; Fig. S1), similar to the mutants used by Hayashi et al. (2010). Two different Ppcps/ks mutant alleles showed a reduced germination speed compared with WT: they attained a lower percentage of germination at any given time on the upwards slope of the graph, although they eventually attained 100% germination (Figs 4a, S1a), and this phenotype could be rescued by application of two diterpenoids known to be bioactive in moss (Hayashi et al., 2010): the fern antheridiogen GA9‐methyl ester or ent‐kaurene (Fig. 4b,c). This result indicates that bioactive diterpenoid hormones in Physcomitrella have a positive effect on spore germination.
Figure 4.
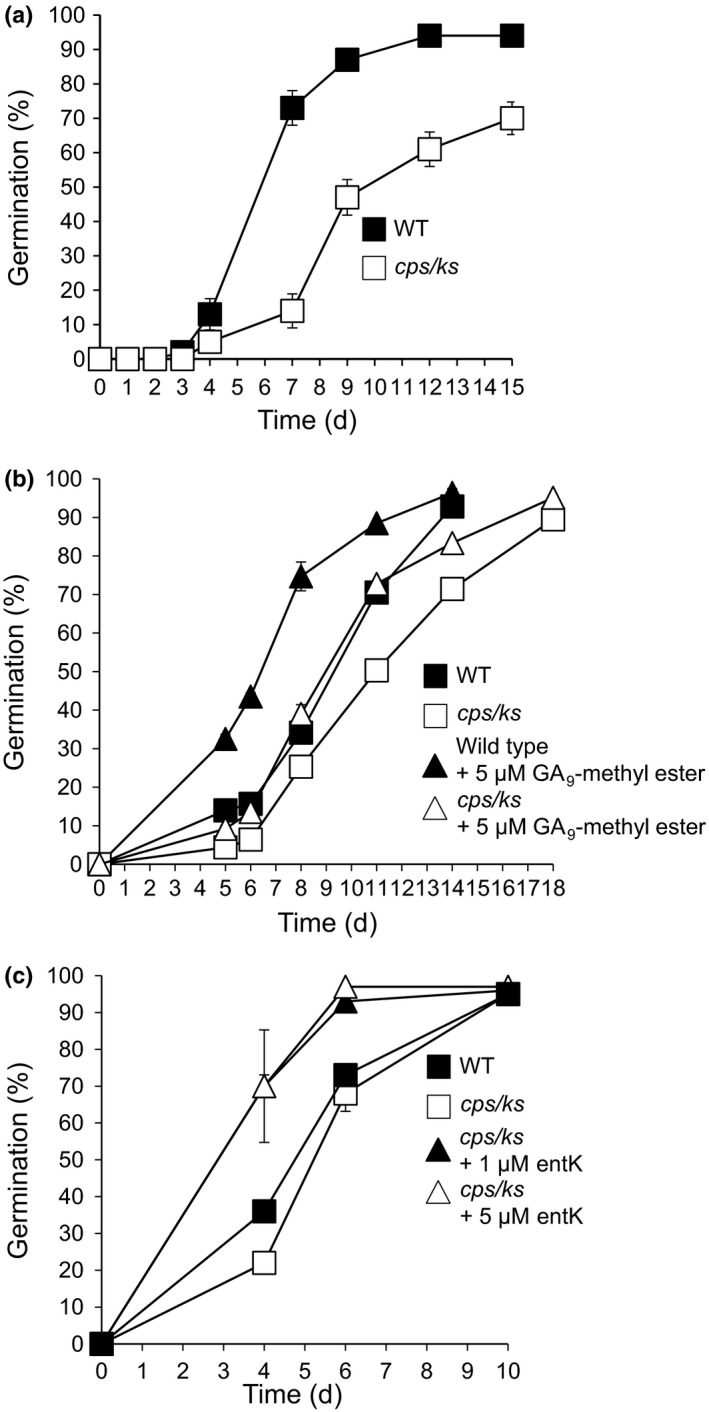
Diterpenes promote Physcomitrella spore germination. (a) Comparison of wild‐type (WT) and copalyl‐diphosphate synthase/kaurene synthase (cps/ks) mutant (Zhan et al., 2015) spore germination. (b) Effect of exogenous GA9‐methyl ester on WT and cps/ks mutant spores (GA, gibberellin). (c) Effect of exogenous ent‐kaurene (entK) on cps/ks mutant spores. Error bars, ± SEM.
In order to further investigate this possibility, we examined the effect of diterpenes on WT Physcomitrella spore germination. GA9‐methyl ester and ent‐kaurene both enhanced spore germination (Figs 4b,c, S1b), whereas GA3 did not (Fig. S1c). Conversely, the diterpenoid hormones that promote moss spore germination cannot fully rescue the germination defect of the Arabidopsis ga1‐3 mutant (Fig. S2). Together, these data: (i) show that diterpenoid hormones are not absolutely required for spore germination, unlike in seeds, corroborating Hayashi et al. (2010); (ii) indicate that diterpenoid hormones increase Physcomitrella spore germination speed, thus have a positive effect on germination; and (iii) lend support to the notion that bryophyte bioactive diterpenoid hormones differ from those in seed plants.
ABA reduces Physcomitrella spore germination acting synergistically with sucrose
We have shown previously that ABA inhibits Physcomitrella spore germination in a dose‐dependent manner (Moody et al., 2016). Physcomitrella spores require approximately five‐fold higher concentrations of ABA for strong inhibition of germination than Arabidopsis (Finkelstein, 1994).
In order to examine the effect of inhibiting ABA biosynthesis on spore germination, we treated WT spores with norflurazon. We saw a small but reproducible promotion of germination (Fig. 5a; see also Fig. 3a). To investigate a potential link between diterpenoid hormones and ABA‐regulation of spore germination, we first tested whether the cps/ks mutant phenotype can be rescued via inhibition of ABA biosynthesis in moss spores. Norflurazon enhanced the germination of cps/ks mutant spores (Fig. 5b). Moreover, exogenously applied diterpenoid hormones could reverse the inhibitory effect of ABA on spore germination (Fig. 5c). This suggests that the balance of ABA and diterpenoid hormone levels may be important for regulating spore germination, but not with the same prominent role that these hormones have assumed in seed germination.
Figure 5.
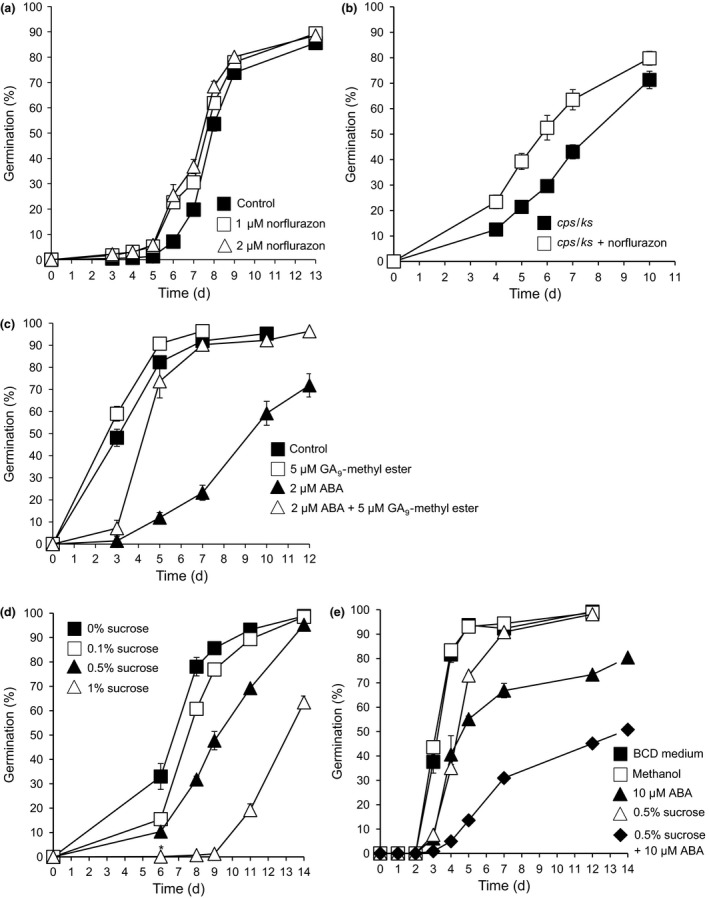
Abscisic acid (ABA) and sucrose synergistically inhibit Physcomitrella spore germination. (a) Effect of norflurazon on wild‐type (WT) spore germination. Five micromolar norflurazon has no further effect (data not shown). (b) Effect of 2 μM norflurazon on cps spores. (c) GA9‐methyl ester can rescue the inhibition of germination by ABA (GA, gibberellin). (d) Dose‐dependent inhibition of germination by sucrose. Sucrose was dissolved in BCD medium. (e) Synergistic inhibitory effect of ABA and sucrose on spore germination: an intermediate concentration of both ABA and sucrose was used; the 0.5% sucrose control also contains matched solvent. Error bars, ± SEM.
As ABA‐mediated stress responses in Physcomitrella vegetative tissue involve accumulation of soluble sugars (Burch & Wilkinson, 2002; Nagao et al., 2006; Oldenhof et al., 2006; Bhyan et al., 2012; Erxleben et al., 2012), we tested the effect of low concentrations of sucrose (0.1–1%) on spore germination. Sucrose inhibited spore germination in a dose‐dependent manner (Fig. 5d) and acted synergistically with ABA (Fig. 5e). Together these data show that ABA at relatively high concentrations reduces moss spore germination, as it does (although not as strongly as in) in seed germination, and suggest that Physcomitrella ABA‐mediated inhibition of spore germination could share downstream mechanisms with ABA‐mediated desiccation and freezing tolerance responses in the Physcomitrella gametophyte.
Ent‐kaurene and ABA biosynthesis and signalling genes are expressed in spores
Our data suggest that diterpenoid hormones and ABA have subtle effects on spore germination compared with the absolute requirement for these hormones in regulating seed germination. To extend these findings, we asked whether the putative homologues of genes encoding the proteins responsible for biosynthesis and signal transduction of diterpenes and ABA are expressed in spores or during spore germination. We extracted RNA from dry spores, imbibed spores, germinating spores, protonemal filaments and leafy gametophytes. We performed semi‐quantitative RT‐PCR to detect expression of the Physcomitrella homologues of the ent‐kaurene biosynthesis genes CPS/KS and CYP701A3 (ent‐KO) (Hayashi et al., 2006; Miyazaki et al., 2011), the first and second enzymes (respectively) in the putative moss diterpenoid hormone biosynthesis pathway (Hayashi et al., 2006). The CPS/KS transcript was detectable during spore germination and was absent from dry spores, whereas the ent‐KO transcript was detectable largely in dry spores (Fig. 6a), suggesting that spatial and temporal regulation of different stages of diterpene biosynthesis occurs during the Physcomitrella life cycle.
Figure 6.
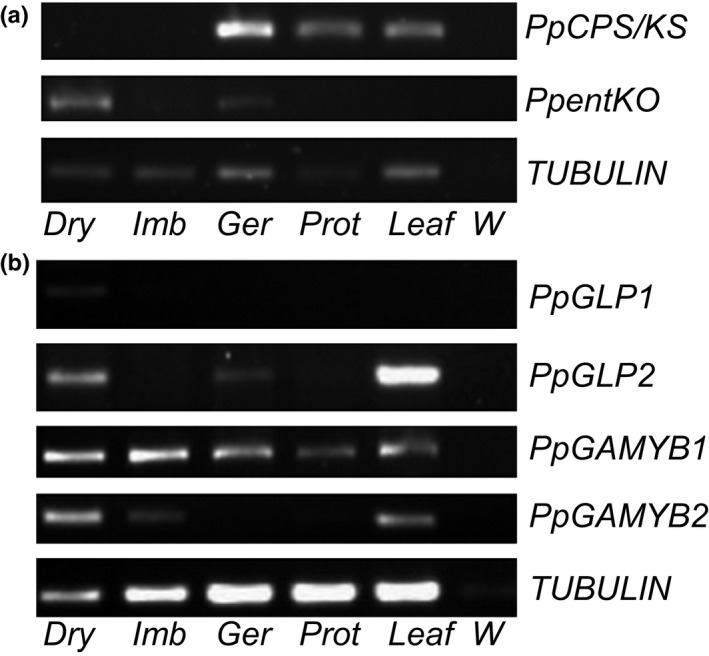
Expression of diterpene biosynthesis and putative response genes in Physcomitrella tissues. (a) Reverse transcription (RT)‐PCR of the Physcomitrella diterpene biosynthesis gene PpCPS/KS and putative diterpene biosynthesis gene PpentKO in Physcomitrella tissues compared to a PpTUBULIN control. (b) RT‐PCR of the putative Physcomitrella diterpene response genes PpGLP1 and PpGLP2 and PpGAMYB1 and PpGAMYB2 in Physcomitrella tissues compared to a PpTUBULIN control. Dry, Dry spores; Imb, imbibed spores; Ger, germinating spores; Prot, protonema; Leaf, Leafy gametophores; W, water control.
We also examined the expression of the putative gibberellin receptors GLP1 and GLP2 (Yasumura et al., 2007), and the two PpGAMYB transcription factors PpGAMYB1 and PpGAMYB2 (Aya et al., 2011). The putative gibberellin receptor PpGLP1, like CYP701A3 (ent‐KO), showed its highest expression in dry spores, decreasing upon imbibition and undetectable once germination occurs (Fig. 6b). The second putative gibberellin receptor, PpGLP2, was expressed more strongly than PpGLP1, but again showed strong expression in dry spores, decreasing markedly during imbibition (Fig. 6b). PpGLP2 expression also was detected in germinating spores and, later, in leafy tissue (Fig. 5b). PpGAMYB1 was expressed in all tissues tested, whereas PpGAMYB2, similarly to the PpGLPs and ent‐KO, was detected in dry spores but decreased upon imbibition (Fig. 6b). PpGAMYB2 was absent from germinating spores but present in protonemal and gametophyte tissues (Fig. 6b), corroborating Aya et al. (2011).
We also assessed the expression of the putative genes encoding the final two (cytosolic, ABA‐specific) steps in the ABA biosynthesis pathway, namely two putative Physcomitrella ABA DEFICIENT2 (ABA2) homologues and two putative Physcomitrella ABSCISIC ALDEHYDE OXIDASE3 (AAO3) homologues (Hanada et al., 2011). All genes showed expression in dry spores, germinating spores and leafy tissue, with one PpABA2 and one PpAAO3 also present in imbibed spores and one PpAAO3 also present in protonema (Fig. 7a). We also tested the expression of putative ABA signalling genes. The four putative PYRABACTIN‐RESISTANCE 1/PYRABACTIN RESISTANCE 1‐RELATED/REGULATORY COMPONENT OF ABA RECEPTOR (PYR/PYL/RCAR) ABA receptors (Takezawa et al., 2011) were expressed in all tissues tested (Fig. 7b), as were the two putative Class II SnRK phosphatases that were detectable in this assay (Fig. 7c) (out of the total six SnRKs in Physcomitrella; Takezawa et al., 2011). Out of the two Physcomitrella ABI‐INSENSITIVE1 (ABI1) protein phosphatases (Komatsu et al., 2013), PpABI1a was expressed ubiquitously (Fig. 7d), whereas PpABI1b was highly expressed in dry spores and leafy tissue (Fig. 7d). Out of the three ABA‐regulated transcription factors Physcomitrella ABA INSENSITIVE3A, ‐3B and ‐3C (PpABI3A, ‐3B and ‐3C)(Khandelwal et al., 2010), PpABI3A and PpABI3C were expressed in all tissues tested, whereas PpABI3b was largely absent from imbibed spores but present in other tissues (Fig. 7e).
Figure 7.
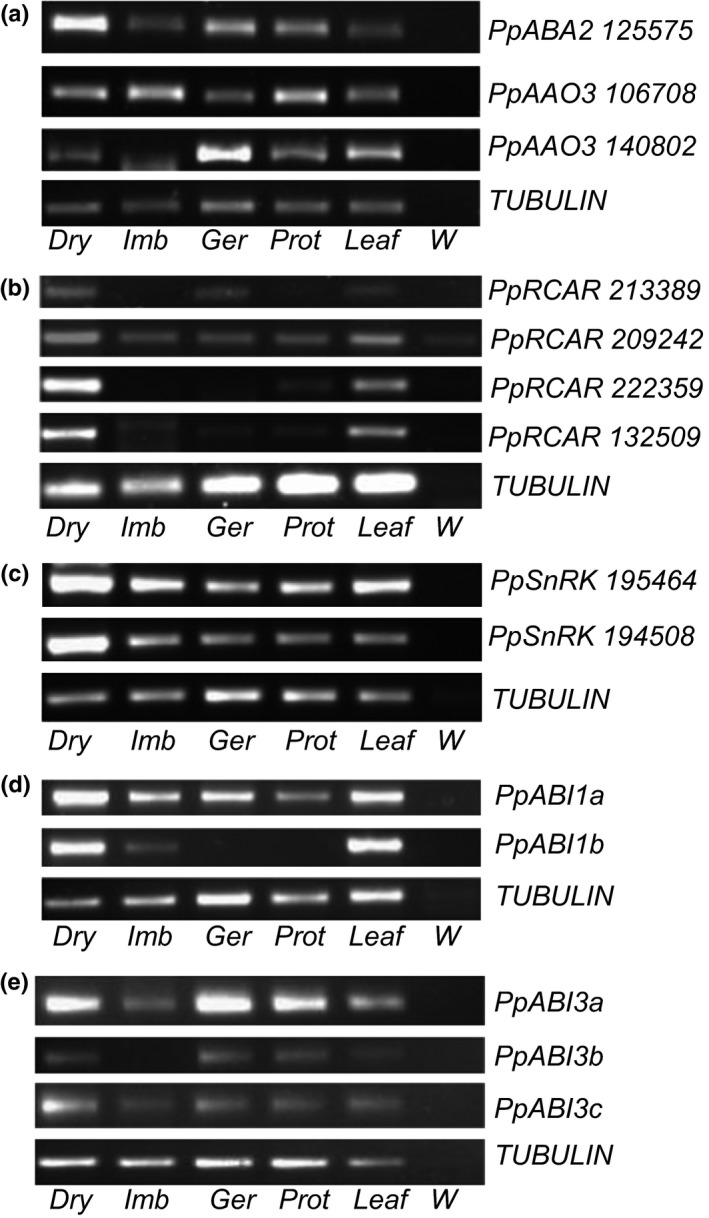
Expression of abscisic acid (ABA) biosynthesis and response genes in Physcomitrella tissues. (a) Reverse transcription (RT)‐PCR of the putative Physcomitrella ABA biosynthesis genes PpABA2 and PpAAO3 (two homologues; Hanada et al., 2011) in Physcomitrella tissues. (b) RT‐PCR of the four putative Physcomitrella ABA receptors (RCARs; Hanada et al., 2011) in Physcomitrella tissues. (c) RT‐PCR of the two putative Physcomitrella ABA signalling kinases (SnRKs; Hanada et al., 2011) in Physcomitrella tissues. (d) RT‐PCR of the Physcomitrella ABI1 ABA‐response genes (Sakata et al., 2009) in Physcomitrella tissues. (e) RT‐PCR of the Physcomitrella ABI3 ABA‐response genes (Khandelwal et al., 2010) in Physcomitrella tissues. All gene expression was compared to a PpTUBULIN control. Dry, dry spores; Imb, imbibed spores; Ger, germinating spores; Prot, protonema; Leaf, Leafy gametophores; W, water control.
In summary, all putative ABA biosynthesis genes were expressed in dry spores, with lower levels during imbibition, as is the putative second gene in the diterpene biosynthesis pathway. The initial diterpene biosynthesis gene transcript, PpCPS/KS, was detected only after imbibition during germination and growth. Putative ABA signalling genes were largely expressed ubiquitously, although many were expressed more highly in dry spores than imbibed spores. Putative GA signalling genes (receptors and GAMYBs) were all expressed in dry spores but largely decreasee in expression level during imbibition.
Strigolactones inhibit Physcomitrella spore germination
In order to extend our findings around the hormonal control of spore germination, we examined the effect of other hormones known to affect seed germination. Strigolactones promote seed germination in a variety of plants (Akiyama & Hayashi, 2006) and have been suggested to affect Physcomitrella spore germination (Proust et al., 2011). We tested whether strigolactones affected spore germination by comparing the germination of WT spores with those of the Physcomitrella ccd8 mutant, which cannot synthesize SLs (Proust et al., 2011). The ccd8 mutant showed increased germination (Fig. 8a), corroborating an unpublished observation by Proust et al. (2011). The ccd8 mutant's increased germination could be reduced to levels closer to that of WT spores by exogenous application of GR24 (Fig. 8b). Moreover, exogenous GR24 reduced the germination speed of WT Physcomitrella spores at concentrations of 0.1 μM (Fig. 8b). This indicates that in Physcomitrella, unlike in Arabidopsis and parasitic plants, strigolactones have an inhibitory role in the germination process.
Figure 8.
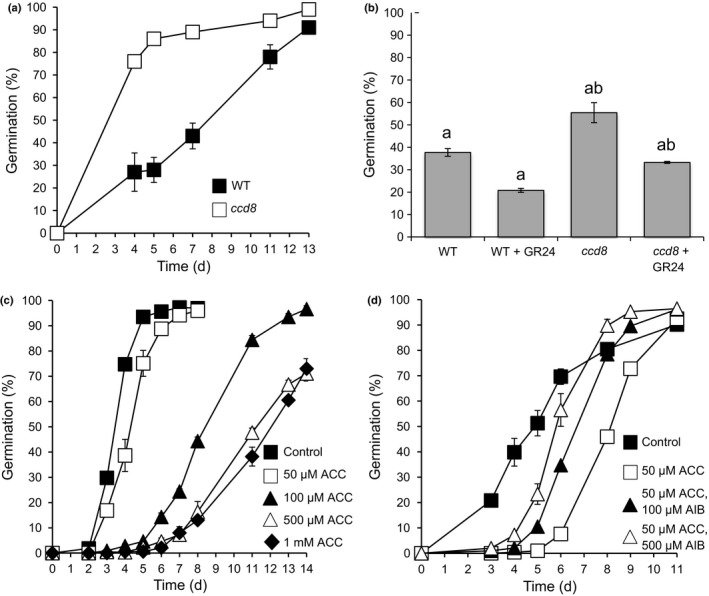
Strigolactones and an ethylene precursor reduce Physcomitrella spore germination rate. (a) Ppccd8 strigolactone biosynthesis mutants (Proust et al., 2011) germinate faster than their corresponding wild‐type (WT). (b) Ppccd8 mutant germination can be inhibited by exogenously applied synthetic strigolactone GR24, which also inhibits WT spore germination. Day 5 data shown; a Kruskal–Wallis test indicates significant differences between samples, P < 0.05 as indicated by the letters on the graph. (c) The ethylene precursor 1‐aminocyclopropane‐1‐carboxylic acid (ACC) inhibits WT spore germination rate. (d) The ethylene inhibitor aminoisobutyric acid (AIB) can rescue the effects of ACC on spore germination. Error bars ± SEM.
The ethylene precursor ACC inhibits Physcomitrella spore germination
Like strigolactone, ethylene has been shown to promote seed germination in Arabidopsis and other dicots, acting antagonistically to ABA. We showed that application of 50 μM–1 mM 1‐aminocyclopropane‐1‐carboxylic acid (ACC), the ethylene precursor that is cleaved by ACC oxidase to release ethylene, inhibited Physcomitrella spore germination in a dose‐dependent manner (Fig. 8c), which is the converse of its effects in seeds (where experiments commonly use a dose of 1 mM ACC), but similar to strigolactone's effects on spores. Moreover, the ethylene biosynthesis inhibitor aminoisobutyric acid, which competitively inhibits ACC oxidase, can overcome the effects of ACC (Fig. 8d).
Discussion
Environmental regulation of germination by light and high temperature is not integrated by the same hormones in Physcomitrella spores compared with seeds
We have shown that unlike most seeds, Physcomitrella spores grown in laboratory conditions do not show primary dormancy and after‐ripening. A lack of primary dormancy has been reported in several moss and liverwort species (McLetchie, 1999; Glime, 2015).
We have also shown that, in a similar way to seeds, germination‐competent Physcomitrella spores can be prevented from germinating using environmental cues. This is in line with evidence that conditional (secondary) dormancy exists in Sphagnum allowing formation of a spore bank (Sundberg & Rydin, 2000) and in Physcomitrium sphaericum (Furness & Hall, 1981). The interface of environmental signals with known hormonal networks is not conserved between Physcomitrella spores and flowering plant seeds.
Seed germination and spore germination can both be reversibly inhibited by a pulse of far‐red (FR) light (e.g. Seo et al., 2006; Possart & Hiltbrunner, 2013) or elevated temperatures (32°C in Arabidopsis, Toh et al., 2008; 35°C in Physcomitrella, this work). This shows that both spores and nondormant seeds respond to environmental cues that block germination, and suggests that this is an evolutionarily early adaptation for plants to live, and move around, on land. Our experiments suggest a higher degree of temperature tolerance in Physcomitrella spores compared with seeds: Physcomitrella vegetative tissue is also more tolerant to abiotic stress than that of seed plants (Frank et al., 2005). Temperatures of 35°C are known to inhibit germination in the moss Physcomitrium (Furness & Hall, 1981) and in two species of liverwort (Chopra & Kumra, 1988), although this was not shown to be reversible (Chopra & Kumra, 1988).
Unlike in seeds (Ikuma & Thimann, 1960; Schopfer et al., 2001; Oh et al., 2006; Seo et al., 2006; Nelson et al., 2009), FR‐inhibition of Physcomitrella spore germination cannot be rescued by addition of diterpenes or inhibition of abscisic acid (ABA) synthesis. Furthermore, unlike in seeds (Toh et al., 2012), addition of diterpenes, norflurazon or strigolactones (SLs) cannot rescue the germination of spores inhibited by high temperature. Thus, a rescue mechanism for FR‐ and thermo‐inhibition in Physcomitrella spores is divergent compared with seeds, and remains to be elucidated.
In seeds, environmentally regulated inhibition of germination impinges on ABA and gibberellin (GA) synthesis and metabolism (Seo et al., 2006; Toh et al., 2012). Crosstalk between light and GA/ABA signalling in seeds occurs at multiple levels, including via effects on hormone metabolism (and subsequent hormone levels), via transcriptional changes in signalling genes (reviewed in Piskurewicz et al., 2009), and via interactions between transcription factors from different pathways (e.g. Richter et al., 2010; Casal, 2013; Tang et al., 2013). For example, FR light blocks germination in dicots by repressing the expression of GA 3 ‐oxidase (Toyomasu et al., 1998; Yamauchi et al., 2004), which catalyses a step in gibberellin biosynthesis that does not exist in Physcomitrella (Stewart & Freebairn, 1969; Hayashi et al., 2010; Zhan et al., 2015). Moreover, Physcomitrella seems not to have clear orthologues of FHY3/FAR1 or ABI5 (Rensing et al., 2008) (which integrate ABA and light signalling in Arabidopsis; Tang et al., 2013) and has divergent DELLA proteins that have not been shown to transduce gibberellin signalling (Yasumura et al., 2007). Thus, the ‘wiring’ of the interface between environmental and hormonal regulation of seed germination evolved after the divergence of the bryophyte lineage. Our work suggests that a novel trigger for light‐induced germination exists in moss. Furthermore, divergent molecular networks mediate conserved developmental responses to environmental stimuli in spores and seeds to enable plant movement on land.
Diterpenes and ABA affect germination in Physcomitrella spores but appear to have a modulatory role, in contrast to the critical role of GA and ABA in seeds
We showed, on the one hand, that certain diterpenoid hormones have a positive effect on germination in Physcomitrella spores and, on the other, that ABA has a negative effect on spore germination. Furthermore, Physcomitrella ent‐KO, ABA synthesis genes and putative diterpene‐ and ABA‐signal transduction pathway genes were expressed in spores. Corroborating previous work (Hayashi et al., 2010; Zhan et al., 2015), we saw that the bioactive diterpenes in Physcomitrella are those at an early step in the biosynthesis pathway (ent‐kaurene), or those that show activity in ferns and also spore‐bearing plants (GA9‐methyl ester), rather than those active in seed plants. We found that diterpenoids are not required for Physcomitrella spore germination (corroborating Hayashi et al., 2010), but that they do influence germination in a positive way. Although Hayashi et al. (2010) did not report a germination phenotype for their cps mutant, their mutant spores were compared with wild‐type (WT) only at a single time point, so no measure of germination rate was made and thus relatively subtle differences were probably overlooked. Physcomitrella GAMYB proteins, homologues of which are regulated by gibberellin signalling in flowering plants, are required for correct spore coat formation (Aya et al., 2011).
Mutant analysis in Arabidopsis has demonstrated that bioactive gibberellins and gibberellin signalling are absolutely required for seed germination to occur (Koornneef & van der Veen, 1980), whereas Arabidopsis seed germination is completely inhibited by concentrations of ABA as low as 5 μM (Finkelstein, 1994). The effects of gibberellins and ABA in Physcomitrella spores were not as extreme: the cps mutant has a slower germination rate than WT, but cps mutant spores can eventually germinate to the same level as WT controls, demonstrating no loss of germination potential. The concentration of ABA required for strong inhibition of Physcomitrella spore germination was also five‐ to 10‐fold higher than for Arabidopsis seeds, although different spore batches vary in their ABA sensitivity, as is the case for Arabidopsis seeds (Finkelstein, 1994), as might be expected in an ephemeral species (Glime, 2013).
The effect of norflurazon on Physcomitrella spores was subtle, which may be due to the lack of primary dormancy in spores, which are essentially ‘ready to germinate’. The extent to which norflurazon reduces ABA levels in Physcomitrella is not known.
This suggests that diterpenes and ABA perform a modulatory role during Physcomitrella spore germination, and gained a more prominent and complex role in multicellular seeds after co‐option into the sporophyte during the evolution of seed plants (e.g. Piskurewicz et al., 2009). Our data comparing the behaviour of physcodillo mutant spores and arabidillo mutant seeds on ABA suggest that these Armadillo‐related protein homologues may represent a conserved node in an ancient regulatory network (Moody et al., 2016).
The function of ABA in Physcomitrella previously has been studied in gametophytic vegetative tissues, where a role in stress responses (such as drought) has been demonstrated, as in the flowering plant sporophyte (Knight et al., 1995; Cuming et al., 2007; Khandelwal et al., 2010; Takezawa et al., 2011). Several studies have demonstrated a role for sugars (including sucrose) acting synergistically with ABA during moss abiotic stress resistance (Burch & Wilkinson, 2002; Nagao et al., 2006; Oldenhof et al., 2006; Bhyan et al., 2012; Erxleben et al., 2012). We demonstrated that a similar synergism may exist during spore germination. The level of sucrose that can decrease spore germination rate (0.1–1% in this study) is 10‐ to 100‐fold lower than that used to cause osmotic stress in moss (10%: Garrocho‐Villegas & Arredondo‐Peter, 2008). Evidence exists for a regulatory role of sugars during seed germination: an inhibitory effect of low levels of sucrose (0.5–3%) on Arabidopsis seed germination up to 5 d has been observed (Chen et al., 2006; Li et al., 2012). This effect requires functional ABA biosynthesis, because aba2 mutant seeds are sucrose‐insensitive (Li et al., 2012). However, Finkelstein & Lynch (2000) showed that 1–2% sucrose could counteract the effects of 3 μM ABA on Arabidopsis seed germination after 7 d, suggesting that different interactions between sucrose and ABA may occur at different times.
A novel role for SLs and ethylene in inhibiting spore germination in Physcomitrella
We showed that SLs have a negative effect on Physcomitrella spore germination. The role of SLs in seed plant germination is a positive one: they act as signals to promote seed germination either between plants (via root exudates) or within one plant (e.g. during thermoinhibition) (Bouwmeester et al., 2003; Yoneyama et al., 2010; Toh et al., 2012; Stanga et al., 2013). This occurs via regulation of gibberellin biosynthesis (Nelson et al., 2009), lending weight to the hypothesis that SLs have a different mechanism of action in Physcomitrella germination. SLs are well known as regulators of shoot branching in seed plants (Waldie et al., 2014). This branching function is conserved in the Physcomitrella gametophyte (Proust et al., 2011). In addition, SLs in moss promote ‘self‐awareness’ and delimit colony spread, acting as quorum‐sensing molecules (Proust et al., 2011), suggesting that the ‘between‐plant’ communication function of SLs arose early in land plant evolution. Perhaps in bryophytes the inhibitory role of SLs in spore germination arose as a quorum‐sensing function; thus, when spores are released from the plant, the formation of one colony from a spore could prevent the germination of a second colony‐forming spore close by, and hence aid colony establishment without competition for resources.
Interestingly, ethylene also has a positive role in seed germination and the ethylene precursor ACC has a negative role in spore germination: as ethylene is also a small, gaseous and easily diffusible hormone, its presence in a developing moss gametophyte could also signal to neighbouring spores and prevent their germination. Ethylene affects seed germination via crosstalk with ABA signalling/synthesis, via synergism with GA signalling and via direct effects on cell separation of the endosperm in a number of plant species (Linkies & Leubner‐Metzger, 2012). This suggests that ethylene may have been co‐opted separately into spores and seeds to perform different roles.
Comparing the regulation of germination in Physcomitrella spores and nondormant seeds
The regulation of equivalent developmental processes between gametophyte and sporophyte seems to show an amazing degree of similarity in comparative studies on key model organisms. For example, Physcomitrella rhizoids are developmentally equivalent to Arabidopsis root hairs (Menand et al., 2007), and GAMYB functions in spore and reproductive organ development are equivalent (Aya et al., 2011), whereas the liverwort Marchantia polymorpha shows circadian regulation of the vegetative‐to‐reproductive transition, as in the seed plant sporophyte (Kubota et al., 2014). We have shown that spores and seeds respond to the same environmental cues to generate the same developmental output, but via different mechanisms.
Our results suggest evolution of novel hormonal regulation of germination between Physcomitrella spores and nondormant/after‐ripened seeds, with ABA and GA assuming much greater importance in seed plants, multilevel crosstalk between environmental and hormone pathways evolving in seeds, and some hormones being co‐opted into different roles in spores and seeds. Similar network rewiring has been identified between root hairs and rhizoids, between RHD SIX‐LIKE transcription factors and auxin signalling (Jang et al., 2011; Pires et al., 2013).
One possibility is that the multicellular nature of seeds may have led to a requirement for complex, coordinated hormonal regulation of the different tissues during germination. Determining the molecular nature of the signalling pathways that regulate spore germination is now a key target for future research. Alternatively, as spores appear not to show dormancy, there may be no requirement for a complex hormonal regime regulating dispersal. It is possible that a ‘bet‐hedging’ strategy controlling germination rates due to life history may exist in Physcomitrella spores as in Arabidopsis (Springthorpe & Penfield, 2015). The absence of observed dormancy in moss spores also raises the question of how these dispersal units function to colonize new environments.
Author contributions
E.F.V., Y.S., L.A.M., G.W.B., H.T.S. and J.C.C. planned and designed the research and conceived the experiments. E.F.V., Y.S., L.A.M., D.H., A.W., S.N., A.C., B.B., D.M., S.J.B., H.B., B.C.K. and J.C.C. performed the research. E.F.V., Y.S., L.A.M., D.H., A.W., S.N., H.B., B.C.K., G.W.B., H.T.S. and J.C.C. analysed data. E.F.V., L.A.M., H.B., G.W.B., H.T.S. and J.C.C. wrote the paper.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Moss bioactive gibberellins promote Physcomitrella spore germination.
Fig. S2 Gibberellins that are bioactive in Physcomitrella cannot rescue the Arabidopsis ga1‐3 mutant seed germination phenotype and substitute for GA3.
Table S1 Primers used for RT‐PCR analysis
Acknowledgements
We thank Peter Hedden for providing extensive supplies of Gibberellin A9‐methyl ester and ent‐kaurene, and Catherine Rameau for providing ccd8 spores. We thank Daniel Gibbs and Petra Stamm for providing critical comments on the manuscript, Ottoline Leyser and Binne Zwanenburg for providing GR24, Steve Penfield for use of the Far‐Red LED array and Andy Cuming for initial discussions around bryophyte spore germination. We thank Heinjo Düring, Ralf Reski and Janice Glime for discussions about spore dormancy. E.F.V. was supported by a NERC Doctoral Training Scholarship. Y.S. and S.J.B. were supported by Leverhulme Trust Grant F/00094/BA. L.A.M. was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) Doctoral Training Scholarship. D.H., A.W., S.N., A.C., B.B. and D.M. performed research using University of Birmingham Funds. H.B. was supported by a grant from the Danish Council of Independent Research – Technology and Production. B.C.K. and H.T.S. were supported by SpotLight, a grant from the Danish Council for Strategic Research. G.W.B. was supported by BBSRC grants BB/L0102321/1 and BB/J017604/1 and J.C.C. was supported by a Royal Society‐Leverhulme Trust Fellowship (2013‐14).
References
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation. [Google Scholar]
- Agrawal SC. 2009. Factors affecting spore germination in algae – review. Folia Microbiologica 54: 273–302. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Hayashi H. 2006. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Annals of Botany 97: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TB, Cozzi F, Simonsen HT. 2015. Optimization of biochemical screening methods for volatile and unstable sesquiterpenoids using HS‐SPME‐GC‐MS. Chromatography 2: 277–292. [Google Scholar]
- Anterola A, Shanle E, Mansouri K, Schuette S, Renzaglia K. 2009. Gibberellin precursor is involved in spore germination in the moss Physcomitrella patens . Planta 229: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Aya K, Hiwatashi Y, Kojima M, Sakakibara H, Ueguchi‐Tanaka M, Hasebe M, Matsuoka M. 2011. The Gibberellin perception system evolved to regulate a pre‐existing GAMYB‐mediated system during land plant evolution. Nature Communications 2: 544. [DOI] [PubMed] [Google Scholar]
- Bach SS, King BC, Zhan X, Simonsen HT, Hamberger B. 2014. Heterologous stable expression of terpenoid biosynthetic genes using the moss Physcomitrella patens . Methods in Molecular Biology 1153: 257–271. [DOI] [PubMed] [Google Scholar]
- Barrero JM, Jacobsen JV, Talbot MJ, White RG, Swain SM, Garvin DF, Gubler F. 2012. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon . New Phytologist 193: 376–386. [DOI] [PubMed] [Google Scholar]
- Bassel GW, Stamm P, Mosca G, Barbier de Reuille P, Gibbs DJ, Winter R, Janka A, Holdsworth MJ, Smith RS. 2014. Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. Proceedings of the National Academy of Sciences, USA 111: 8685–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhyan SB, Minami A, Kaneko Y, Suzuki S, Arakawa K, Sakata Y, Takezawa D. 2012. Cold acclimation in the moss Physcomitrella patens involves abscisic acid‐dependent signaling. Journal of Plant Physiology 169: 137–145. [DOI] [PubMed] [Google Scholar]
- Borthwick HA, Hendricks SB, Parker MW, Toole EH, Toole VK. 1952. A reversible photoreaction controlling seed germination. Proceedings of the National Academy of Sciences, USA 38: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. 2003. Secondary metabolite signalling in host–parasitic plant interactions. Current Opinion in Plant Biology 6: 358–364. [DOI] [PubMed] [Google Scholar]
- Burch J, Wilkinson T. 2002. Cryopreservation of protonemata of Ditrichum cornubicum (paton) comparing the effectiveness of four cryoprotectant pretreatments. Cryo Letters 23: 197–208. [PubMed] [Google Scholar]
- Calpouzos L, Chang HS. 1971. Fungus spore germination inhibited by blue and far red radiation. Plant Physiology 47: 729–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. 2013. Photoreceptor signaling networks in plant responses to shade. Annual Review of Plant Biology 64: 403–427. [DOI] [PubMed] [Google Scholar]
- Chamovitz D, Pecker I, Hirschberg J. 1991. The molecular basis of resistance to the herbicide norflurazon. Plant Molecular Biology 16: 967–974. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ji F, Xie H, Liang J, Zhang J. 2006. The regulator of G‐protein signaling proteins involved in sugar and abscisic acid signaling in Arabidopsis seed germination. Plant Physiology 140: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SH, Raghavan V. 1982. Abscisic acid effects on spore germination and protonemal growth in the fern, Mohria caffrorum . New Phytologist 92: 31–37. [Google Scholar]
- Chopra RN, Kumra PK. 1988. Biology of bryophytes. Chapter 1. New Delhi, India: New Age International Publishers, 1–38. [Google Scholar]
- Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS. 2007. Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens . New Phytologist 176: 275–287. [DOI] [PubMed] [Google Scholar]
- Dekkers BJ, Costa MC, Maia J, Bentsink L, Ligterink W, Hilhorst HW. 2015. Acquisition and loss of desiccation tolerance in seeds: from experimental model to biological relevance. Planta 241: 563–577. [DOI] [PubMed] [Google Scholar]
- Dekkers BJ, Pearce S, van Bolderen‐Veldkamp RP, Marshall A, Widera P, Gilbert J, Drost HG, Bassel GW, Muller K, King JR et al 2013. Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiology 163: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. 2004. Glucose delays seed germination in Arabidopsis thaliana . Planta 218: 579–588. [DOI] [PubMed] [Google Scholar]
- Drew DP, Rasmussen SK, Avato P, Simonsen HT. 2012. A comparison of headspace solid‐phase microextraction and classic hydrodistillation for the identification of volatile constituents from Thapsia spp. provides insights into guaianolide biosynthesis in Apiaceae. Phytochemical Analysis 23: 44–51. [DOI] [PubMed] [Google Scholar]
- Erxleben A, Gessler A, Vervliet‐Scheebaum M, Reski R. 2012. Metabolite profiling of the moss Physcomitrella patens reveals evolutionary conservation of osmoprotective substances. Plant Cell Reports 31: 427–436. [DOI] [PubMed] [Google Scholar]
- Finch‐Savage WE, Leubner‐Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR. 1994. Maternal effects govern variable dominance of two abscisic acid response mutations in Arabidopsis thaliana . Plant Physiology 105: 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. 2000. Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiology 122: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy. Annual Review of Plant Biology 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Frank W, Ratnadewi D, Reski R. 2005. Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 220: 384–394. [DOI] [PubMed] [Google Scholar]
- Furness SB, Hall RH. 1981. An explanation of the intermittent occurrence of Physcomitrium sphaericum (Hedw.) Brid. Journal of bryology 11: 733–742. [Google Scholar]
- Garrocho‐Villegas V, Arredondo‐Peter R. 2008. Molecular cloning and characterization of a moss (Ceratodon purpureus) nonsymbiotic hemoglobin provides insight into the early evolution of plant nonsymbiotic hemoglobins. Molecular Biology and Evolution 25: 1482–1487. [DOI] [PubMed] [Google Scholar]
- Glime JM. 2013. Adaptive strategies: life cycles In: Bryophyte ecology vol 1. Physiological ecology: adaptive strategies. Houghton, MI, USA: Ebook sponsored by Michigan Technological University and the International Association of Bryologists, 1–17. [Google Scholar]
- Glime JM. 2015. Spore germination In: Bryophyte ecology vol 1. Physiological ecology: ecophysiology of development. Houghton, MI, USA: Ebook sponsored by Michigan Technological University and the International Association of Bryologists, 2–24. [Google Scholar]
- Haas CJ, Kopp H, Scheruerlein R. 1992. Nitrate effect on Pfr‐mediated and GA3‐induced germination in spores of Anemia phyllitidis (L.) Sw. Photochemistry and Photobiology 56: 633–640. [Google Scholar]
- Hanada K, Hase T, Toyoda T, Shinozaki K, Okamoto M. 2011. Origin and evolution of genes related to ABA metabolism and its signaling pathways. Journal of Plant Research 124: 455–465. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Horie K, Hiwatashi Y, Kawaide H, Yamaguchi S, Hanada A, Nakashima T, Nakajima M, Mander LN, Yamane H et al 2010. Endogenous diterpenes derived from ent‐kaurene, a common gibberellin precursor, regulate protonema differentiation of the moss Physcomitrella patens . Plant Physiology 153: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Kawaide H, Notomi M, Sakigi Y, Matsuo A, Nozaki H. 2006. Identification and functional analysis of bifunctional ent‐kaurene synthase from the moss Physcomitrella patens . FEBS Letters 580: 6175–6181. [DOI] [PubMed] [Google Scholar]
- Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schafer E. 2002. Phytochrome E controls light‐induced germination of Arabidopsis . Plant Physiology 128: 194–200. [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJ. 2008a. Molecular networks regulating Arabidopsis seed maturation, after‐ripening, dormancy and germination. New Phytologist 179: 33–54. [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Finch‐Savage WE, Grappin P, Job D. 2008b. Post‐genomics dissection of seed dormancy and germination. Trends in Plant Science 13: 7–13. [DOI] [PubMed] [Google Scholar]
- Ikuma H, Thimann KV. 1960. Action of gibberellic acid on lettuce seed germination. Plant Physiology 35: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G, Yi K, Pires ND, Menand B, Dolan L. 2011. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 138: 2273–2281. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Michizo S. 1991. Involvement of gibberellic acid in phytochrome‐mediated spore germination of the fern Lygodium japonicum . Journal of Plant Physiology 138: 299–303. [Google Scholar]
- Karssen CM, Lacka E. 1986. A revision of the hormone balance theory of seed dormancy: studies on gibberellin and/or abscisic acid‐deficient mutants of Arabidopsis thaliana In: Bopp M, ed. Plant growth substances 1985. Berlin, Germany: Springer, 315–323. [Google Scholar]
- Khandelwal A, Cho SH, Marella H, Sakata Y, Perroud PF, Pan A, Quatrano RS. 2010. Role of ABA and ABI3 in desiccation tolerance. Science 327: 546. [DOI] [PubMed] [Google Scholar]
- King BC, Vavitsas K, Ikram NKBK, Schrøder J, Scharff LB, Hamberger B, Jensen PE, Simonsen HT. 2016. In vivo assembly of DNA‐fragments in the moss plant Physcomitrella patens . Scientific Reports 6: 25030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CD, Sehgal A, Atwal K, Wallace JC, Cove DJ, Coates D, Quatrano RS, Bahadur S, Stockley PG, Cuming AC. 1995. Molecular responses to abscisic acid and stress are conserved between moss and cereals. Plant Cell 7: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K, Suzuki N, Kuwamura M, Nishikawa Y, Nakatani M, Ohtawa H, Takezawa D, Seki M, Tanaka M, Taji T et al 2013. Group A PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nature Communications 4: 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH. 1980. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. TAG. Theoretical and Applied Genetics 58: 257–263. [DOI] [PubMed] [Google Scholar]
- Kubota A, Kita S, Ishizaki K, Nishihama R, Yamato KT, Kohchi T. 2014. Co‐option of a photoperiodic growth‐phase transition system during land plant evolution. Nature Communications 5: 3668. [DOI] [PubMed] [Google Scholar]
- Lee SC, Luan S. 2012. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell and Environment 35: 53–60. [DOI] [PubMed] [Google Scholar]
- Lee KP, Piskurewicz U, Turečková V, Carat S, Chappuis R, Strnad M, Fankhauser C, Lopez‐Molina L. 2012. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes & Development 26: 1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li LL, Fan RC, Peng CC, Sun HL, Zhu SY, Wang XF, Zhang LY, Zhang DP. 2012. Arabidopsis sucrose transporter SUT4 interacts with cytochrome b5‐2 to regulate seed germination in response to sucrose and glucose. Molecular Plant 5: 1029–1041. [DOI] [PubMed] [Google Scholar]
- Linkies A, Graeber K, Knight C, Leubner‐Metzger G. 2010. The evolution of seeds. New Phytologist 186: 817–831. [DOI] [PubMed] [Google Scholar]
- Linkies A, Leubner‐Metzger G. 2012. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports 31: 253–270. [DOI] [PubMed] [Google Scholar]
- Logan DC, Stewart GR. 1991. Role of ethylene in the germination of the hemiparasite Striga hermonthica . Plant Physiology 97: 1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Kendrick RE, Givan CV. 1975. Photocontrol of fungal spore germination. Plant Physiology 56: 847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S. 2006. Phytochrome‐mediated development in land plants: red light sensing evolves to meet the challenges of changing light environments. Molecular Ecology 15: 3483–3503. [DOI] [PubMed] [Google Scholar]
- McLetchie DN. 1999. Dormancy/nondormancy cycles in spores of the liverwort Sphaerocarpos texanus . Bryologist 102: 15–21. [Google Scholar]
- Menand B, Yi K, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. 2007. An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316: 1477–1480. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Katsumata T, Natsume M, Kawaide H. 2011. The CYP701B1 of Physcomitrella patens is an ent‐kaurene oxidase that resists inhibition by uniconazole‐P. FEBS Letters 585: 1879–1883. [DOI] [PubMed] [Google Scholar]
- Mohr H, Meyer U, Hartmann K. 1964. Die Beeinflussung der Farnsporen‐Keimung (Osmunda cinnamomea (L.) undO. claytoniana (L.)) über das Phytochromsystem und die Photosynthese. Planta 60: 483–496. [Google Scholar]
- Moody LA, Saidi Y, Gibbs DJ, Choudhary A, Holloway D, Vesty EF, Bansal KK, Bradshaw SJ, Coates JC. 2016. An ancient and conserved function for Armadillo‐related proteins in the control of spore and seed germination by abscisic acid. New Phytologist. doi: 10.1111/nph.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody LA, Saidi Y, Smiles EJ, Bradshaw SJ, Meddings M, Winn PJ, Coates JC. 2012. ARABIDILLO gene homologues in basal land plants: species‐specific gene duplication and likely functional redundancy. Planta 236: 1927–1941. [DOI] [PubMed] [Google Scholar]
- Nagao M, Oku K, Minami A, Mizuno K, Sakurai M, Arakawa K, Fujikawa S, Takezawa D. 2006. Accumulation of theanderose in association with development of freezing tolerance in the moss Physcomitrella patens . Phytochemistry 67: 702–709. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Riseborough JA, Flematti GR, Stevens J, Ghisalberti EL, Dixon KW, Smith SM. 2009. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiology 149: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester JE, Coolbaugh RC. 1986. Factors influencing spore germination and early gametophyte development in Anemia mexicana and Anemia phyllitidis . Plant Physiology 82: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H, Bassel GW, Bewley JD. 2010. Germination – still a mystery. Plant Science 179: 574–581. [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G. 2006. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis . Plant Journal 47: 124–139. [DOI] [PubMed] [Google Scholar]
- Oldenhof H, Wolkers WF, Bowman JL, Tablin F, Crowe JH. 2006. Freezing and desiccation tolerance in the moss Physcomitrella patens: an in situ Fourier transform infrared spectroscopic study. Biochimica et Biophysica Acta 1760: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Pan X‐W, Han L, Zhang Y‐H, Chen D‐F, Simonsen HT. 2015. Sclareol production in the moss Physcomitrella patens and observations on growth and terpenoid biosynthesis. Plant Biotechnology Reports 9: 149–159. [Google Scholar]
- Park J, Kim YS, Kim SG, Jung JH, Woo JC, Park CM. 2011. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis . Plant Physiology 156: 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires ND, Yi K, Breuninger H, Catarino B, Menand B, Dolan L. 2013. Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proceedings of the National Academy of Sciences, USA 110: 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U, Tureckova V, Lacombe E, Lopez‐Molina L. 2009. Far‐red light inhibits germination through DELLA‐dependent stimulation of ABA synthesis and ABI3 activity. EMBO Journal 28: 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possart A, Hiltbrunner A. 2013. An evolutionarily conserved signaling mechanism mediates far‐red light responses in land plants. Plant Cell 25: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogue F, Rameau C. 2011. Strigolactones regulate protonema branching and act as a quorum sensing‐like signal in the moss Physcomitrella patens . Development 138: 1531–1539. [DOI] [PubMed] [Google Scholar]
- Rademacher W. 2000. GROWTH RETARDANTS: effects on gibberellin biosynthesis and other metabolic pathways. Annual Review of Plant Physiology and Plant Molecular Biology 51: 501–531. [DOI] [PubMed] [Google Scholar]
- Raghavan V. 1973. Blue light interference in the phytochrome‐controlled germination of the spores of Cheilanthes farinosa . Plant Physiology 51: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y et al 2008. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. [DOI] [PubMed] [Google Scholar]
- Richter R, Behringer C, Muller IK, Schwechheimer C. 2010. The GATA‐type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME‐INTERACTING FACTORS. Genes & Development 24: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmulling T. 2006. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein CV, Gerrienne P, de la Puente GS, Astini RA, Steemans P. 2010. Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana). New Phytologist 188: 365–369. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Komatsu K, Taji T, Tanaka S. 2009. Role of PP2C‐mediated ABA signaling in the moss Physcomitrella patens . Plant Signaling & Behavior 4: 887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerlein R, Wayne R, Roux SJ. 1989. Calcium requirement of phytochrome‐mediated fern‐spore germination: no direct phytochrome‐calcium interaction in the phytochrome‐initiated transduction chain. Planta 178: 25–30. [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G. 2001. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiology 125: 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion‐Poll A, Sun TP, Koshiba T et al 2006. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant Journal 48: 354–366. [DOI] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S. 2009. Interaction of light and hormone signals in germinating seeds. Plant Molecular Biology 69: 463–472. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. 1994. The induction of seed germination in Arabidopsis thaliana is regulated principally by Phytochrome B and secondarily by Phytochrome A. Plant Physiology 104: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Bisoyi RN, Singh RP. 1990. Collection and germination of sporocarps of Azolla caroliniana . Annals of Botany 66: 51–56. [Google Scholar]
- Springthorpe V, Penfield S. 2015. Flowering time and seed dormancy control use external coincidence to generate life history strategy. Elife 4: e05557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga JP, Smith SM, Briggs WR, Nelson DC. 2013. SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiology 163: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ER, Freebairn HT. 1969. Ethylene, seed germination, and epinasty. Plant Physiology 44: 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S, Rydin H. 2000. Experimental evidence for a persistent spore bank in Sphagnum . New Phytologist 148: 105–116. [DOI] [PubMed] [Google Scholar]
- Takatori S, Imahori K. 1971. Light reactions in the control of oospore germination of Chara delicatula 1. Phycologia 10: 221–228. [Google Scholar]
- Takezawa D, Komatsu K, Sakata Y. 2011. ABA in bryophytes: how a universal growth regulator in life became a plant hormone? Journal of Plant Research 124: 437–453. [DOI] [PubMed] [Google Scholar]
- Tamura N, Yoshida T, Tanaka A, Sasaki R, Bando A, Toh S, Lepiniec L, Kawakami N. 2006. Isolation and characterization of high temperature‐resistant germination mutants of Arabidopsis thaliana . Plant and Cell Physiology 47: 1081–1094. [DOI] [PubMed] [Google Scholar]
- Tang W, Ji Q, Huang Y, Jiang Z, Bao M, Wang H, Lin R. 2013. FAR‐RED ELONGATED HYPOCOTYL3 and FAR‐RED IMPAIRED RESPONSE1 transcription factors integrate light and abscisic acid signaling in Arabidopsis . Plant Physiology 163: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N et al 2008. High temperature‐induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology 146: 1368–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tsuchiya Y. 2012. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant and Cell Physiology 53: 107–117. [DOI] [PubMed] [Google Scholar]
- Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y. 1998. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiology 118: 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi H, Nakamura S, Schafer E, Wada M. 2012. Red light‐induced phytochrome relocation into the nucleus in Adiantum capillus‐veneris . Molecular Plant 5: 611–618. [DOI] [PubMed] [Google Scholar]
- Voegele A, Linkies A, Muller K, Leubner‐Metzger G. 2011. Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. Journal of Experimental Botany 62: 5131–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Schwartzenberg K, Schultze W, Kassner H. 2004. The moss Physcomitrella patens releases a tetracyclic diterpene. Plant Cell Reports 22: 780–786. [DOI] [PubMed] [Google Scholar]
- Waldie T, McCulloch H, Leyser O. 2014. Strigolactones and the control of plant development: lessons from shoot branching. Plant Journal 79: 607–622. [DOI] [PubMed] [Google Scholar]
- Wayne R, Hepler PK. 1984. The role of calcium ions in phytochrome‐mediated germination of spores of Onoclea sensibilis L. Planta 160: 12–20. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Voeller BR. 1969. Induction of fern spore germination. Proceedings of the National Academy of Sciences, USA 64: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun T. 2001. Distinct cell‐specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant Journal 28: 443–453. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. 2004. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16: 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Chang C, Salmi ML, Hung YS, Loraine A, Roux SJ. 2008. Genome‐scale cluster analysis of replicated microarrays using shrinkage correlation coefficient. BMC Bioinformatics 9: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura Y, Crumpton‐Taylor M, Fuentes S, Harberd NP. 2007. Step‐by‐step acquisition of the gibberellin‐DELLA growth‐regulatory mechanism during land‐plant evolution. Current Biology 17: 1225–1230. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y. 2010. Strigolactones as germination stimulants for root parasitic plants. Plant and Cell Physiology 51: 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Bach SS, Hansen NL, Lunde C, Simonsen HT. 2015. Additional diterpenes from Physcomitrella patens synthesized by copalyl diphosphate/kaurene synthase (PpCPS/KS). Plant Physiology and Biochemistry 96: 110–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Moss bioactive gibberellins promote Physcomitrella spore germination.
Fig. S2 Gibberellins that are bioactive in Physcomitrella cannot rescue the Arabidopsis ga1‐3 mutant seed germination phenotype and substitute for GA3.
Table S1 Primers used for RT‐PCR analysis


