Abstract
The aim of this study was to determine if syndrome‐specific birth weight charts were beneficial for babies with Down syndrome in England and Wales. Birth weights of 8,825 babies with Down syndrome born in England and Wales in 1989–2010 were obtained from the National Down Syndrome Cytogenetic Register. Birth weight centiles for 30–42 weeks gestation by sex were fitted using the LMS method and were compared to those for unaffected babies from the UK‐WHO growth charts. For babies born with Down syndrome the median birth weight from 37 to 42 weeks was 2,970 g (10th–90th centile: 2,115–3,680) for boys and 2930 g (2,100–3,629) for girls, and the modal age of gestation was 38 weeks, 2 weeks earlier than for unaffected babies. At 38 weeks gestation they were only slightly lighter than unaffected babies (159 g for boys and 86 g for girls). However at 40 weeks gestation the shortfall was much greater (304 g and 239 g, respectively). In neonates with Down syndrome there is little evidence of growth restriction before 38 weeks gestation, so up to this age it is appropriate to use the UK‐WHO birth weight charts. Thereafter birth weight is below that of unaffected babies and it should be plotted on the UK Down syndrome growth charts. © 2015 The Authors. American Journal of Medical Genetics Part A Published by Wiley Periodicals, Inc.
Keywords: Down syndrome, trisomy 21, birth weight, gestational age, growth charts
INTRODUCTION
Growth charts show that early in life babies with Down syndrome gain weight more slowly than unaffected babies [Piro et al., 1990; McCoy, 1992; Myrelid et al., 2002; Styles et al., 2002]. However there is a lack of data on their prenatal growth. Since the seminal paper of Smith and McKeown [1955], using cross sectional birth weight data as a proxy measure of intrauterine growth in late pregnancy, only two others [Clementi et al., 1990; Boghossian et al., 2012] have presented data on the birth weight of babies with Down syndrome according to gestational age at birth. It is nevertheless a belief widely held that among those born with Down syndrome there is an excess of preterm birth and low birth weight [Cunningham, 2006]. Our study used cross sectional birth weight data from the National Down Syndrome Cytogenetic Register (NDSCR) to test this belief and to determine whether syndrome‐specific birth weight charts are necessary for this population.
METHODS
The NDSCR was set up on January 1st 1989, and currently holds anonymous data on over 33,000 ante‐ or postnatal diagnoses of Down syndrome obtained from all clinical cytogenetic laboratories in England and Wales [Mutton et al., 1991]. It has approval from the Confidentiality Advisory Group, under Section 251 of the NHS Act 2006, to collect, process and use data without the need for individual consent. It also has ethics approval from the Trent Medical Research Ethics Committee (MREC).
Virtually every baby with clinical features suggesting Down syndrome, and any antenatal diagnostic sample from a pregnancy suspected to have Down syndrome, receives a cytogenetic examination because the definitive test for the condition is the finding of trisomy 21. The data in the register are obtained from all clinical cytogenetic laboratories in England and Wales, which are requested to send a completed form for each such diagnosis and its variants. The form contains details of the date, place of birth, and indications for referral, maternal age, and family history. Most laboratories send a copy of this form to the referring physician for confirmation and completion. The gestational age was estimated from the date of the last menstrual period (LMP), and was usually confirmed by ultrasound.
Comparisons with other congenital anomaly registers and the Office for National Statistics show that since its inception the register has captured data on an estimated 93% of all diagnosed Down syndrome births and pregnancy terminations for residents of England and Wales [Savva and Morris, 2009].
Centiles were fitted to the birth weight data with the LMS method [Cole and Green, 1992] in R (http://www.R-project.org/) using the gamlss package [Rigby and Stasinopoulos, 2005]. The Box–Cox–Cole–Green (BCCG) distribution was used with a log link for the median. In essence the LMS method estimates for each week of gestation the median birth weight (M) and its coefficient of variation (S), allowing for non‐normality in the distribution by using a Box–Cox power transformation (L). The sex difference in median birth weight did not vary with gestation and so was fitted as a constant, this (due to the log link) corresponding to a constant percentage difference. The centiles were fitted using data from 28 to 43 weeks of gestation, and are presented from 30 to 42 weeks as tables of smoothed L, M, and S values by sex. From these values, centiles C100 α were derived using the formula
where zα is the standard deviation for tail area α under a Normal distribution. This leads to approximate 2nd, 9th, 25th, 50th, 75th, 91st, and 98th centiles using the two‐thirds of a standard deviation spacing proposed by Cole [1994].
The birth weight centiles were compared with those for unaffected babies from the revised UK‐WHO growth charts [Cole et al., [Link]], which were based on 9,443 babies with gestational age estimated by date of LMP confirmed by ultrasound.
The secular trend in birth weight was examined using linear regression of birth weight SD score on year of birth. The association between gestational age and missing birth weight was estimated using logistic regression.
RESULTS
33,767 diagnoses of Down syndrome were recorded in the register from January 1st 1989 to December 31st 2011, from which 8,825 live births with free trisomy 21 and complete information on birth weight and gestational age were extracted (see flow chart in Fig. 1).
Figure 1.
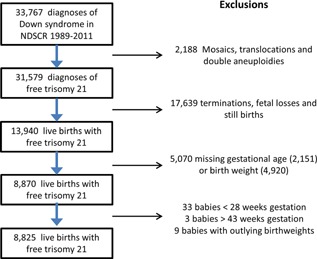
Flow chart of the selection of free trisomy 21 cases for inclusion in the analysis. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmga].
Figure 2 shows the distribution of gestational age at birth for babies with Down syndrome compared to unaffected babies born in England and Wales in 2010 [Office for National Statistics, 2012], scaled to adjust for the different sample sizes. The modal gestational ages were 38 weeks for Down syndrome babies and 40 weeks for unaffected babies.
Figure 2.
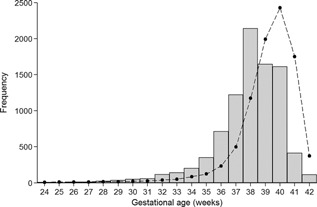
The distribution of gestational age at birth in babies with Down syndrome (gray bars) compared to unaffected babies (dashed line) [Office for National Statistics, 2012].
Table I gives the sample sizes and fitted LMS parameters for birth weight by sex and gestational age. The skewness (L) and coefficient of variation (S) parameters were the same by sex, while median birth weight (M) was 2.4% (95%CI: 1.7–3.1) less in girls than boys at all gestations. There was no evidence of a secular trend in birth weight from 1989 to 2011 (regression coefficient 0.3 g per year, 95%CI −2 g to +2.6 g).
Table I.
Sample Sizes and LMS Parameters for Birth Weight in Down Syndrome by Sex and Gestational Age (n = 8,825)
| Gestational age | Number of births | M | ||||
|---|---|---|---|---|---|---|
| (weeks) | Boys | Girls | Boys | Girls | L | S |
| 28 | 11 | 10 | 960 | 937 | −0.12 | 0.266 |
| 29 | 16 | 19 | 1132 | 1105 | −0.03 | 0.253 |
| 30 | 26 | 23 | 1319 | 1288 | 0.06 | 0.243 |
| 31 | 29 | 27 | 1518 | 1482 | 0.15 | 0.237 |
| 32 | 73 | 41 | 1719 | 1678 | 0.23 | 0.233 |
| 33 | 84 | 54 | 1915 | 1869 | 0.31 | 0.228 |
| 34 | 117 | 85 | 2113 | 2063 | 0.39 | 0.220 |
| 35 | 192 | 156 | 2333 | 2277 | 0.47 | 0.206 |
| 36 | 414 | 298 | 2567 | 2506 | 0.55 | 0.189 |
| 37 | 693 | 525 | 2800 | 2733 | 0.62 | 0.174 |
| 38 | 1219 | 921 | 3019 | 2947 | 0.69 | 0.162 |
| 39 | 822 | 824 | 3164 | 3088 | 0.76 | 0.153 |
| 40 | 797 | 809 | 3251 | 3174 | 0.83 | 0.150 |
| 41 | 204 | 207 | 3304 | 3225 | 0.89 | 0.151 |
| 42 | 55 | 56 | 3318 | 3239 | 0.96 | 0.152 |
| 43 | 8 | 10 | 3300 | 3221 | 1.02 | 0.153 |
M, Median: L, skewness; S, coefficient of variation.
Tables II and III present the fitted birth weight centiles by sex and gestational age. At 38 weeks gestation, median weight was 2,567 g for boys and 2,506 g for girls.
Table II.
Birth Weight (g) Centiles for Boys With Down Syndrome According to Gestational Age
| Gestational age (weeks) | 2nd | 9th | 25th | 50th | 75th | 91st | 98th |
|---|---|---|---|---|---|---|---|
| 28 | 574 | 679 | 806 | 960 | 1149 | 1380 | 1665 |
| 29 | 685 | 809 | 957 | 1132 | 1341 | 1590 | 1887 |
| 30 | 805 | 951 | 1121 | 1319 | 1550 | 1819 | 2131 |
| 31 | 929 | 1098 | 1294 | 1518 | 1775 | 2068 | 2402 |
| 32 | 1049 | 1245 | 1467 | 1719 | 2003 | 2322 | 2678 |
| 33 | 1170 | 1390 | 1638 | 1915 | 2222 | 2561 | 2934 |
| 34 | 1304 | 1548 | 1817 | 2113 | 2437 | 2789 | 3170 |
| 35 | 1476 | 1739 | 2024 | 2333 | 2665 | 3020 | 3399 |
| 36 | 1682 | 1958 | 2253 | 2567 | 2899 | 3250 | 3618 |
| 37 | 1893 | 2181 | 2483 | 2800 | 3131 | 3476 | 3835 |
| 38 | 2092 | 2389 | 2698 | 3019 | 3350 | 3692 | 4044 |
| 39 | 2232 | 2534 | 2845 | 3164 | 3491 | 3826 | 4168 |
| 40 | 2302 | 2612 | 2929 | 3251 | 3580 | 3913 | 4252 |
| 41 | 2325 | 2647 | 2974 | 3304 | 3638 | 3976 | 4316 |
| 42 | 2317 | 2649 | 2983 | 3318 | 3655 | 3993 | 4332 |
| 43 | 2289 | 2627 | 2964 | 3300 | 3636 | 3971 | 4305 |
Table III.
Birth Weight (g) Centiles for Girls With Down Syndrome According to Gestational Age
| Gestational age (weeks) | 2nd | 9th | 25th | 50th | 75th | 91st | 98th |
|---|---|---|---|---|---|---|---|
| 28 | 560 | 662 | 786 | 937 | 1121 | 1347 | 1625 |
| 29 | 668 | 790 | 934 | 1105 | 1309 | 1552 | 1842 |
| 30 | 786 | 928 | 1094 | 1288 | 1513 | 1776 | 2080 |
| 31 | 906 | 1072 | 1263 | 1482 | 1733 | 2019 | 2344 |
| 32 | 1024 | 1215 | 1432 | 1678 | 1955 | 2266 | 2614 |
| 33 | 1142 | 1357 | 1599 | 1869 | 2169 | 2500 | 2864 |
| 34 | 1273 | 1511 | 1774 | 2063 | 2378 | 2722 | 3094 |
| 35 | 1441 | 1697 | 1976 | 2277 | 2601 | 2948 | 3318 |
| 36 | 1641 | 1911 | 2199 | 2506 | 2830 | 3172 | 3532 |
| 37 | 1848 | 2128 | 2424 | 2733 | 3056 | 3393 | 3744 |
| 38 | 2042 | 2332 | 2634 | 2947 | 3270 | 3604 | 3948 |
| 39 | 2179 | 2473 | 2777 | 3088 | 3408 | 3734 | 4068 |
| 40 | 2247 | 2550 | 2859 | 3174 | 3494 | 3820 | 4150 |
| 41 | 2270 | 2584 | 2903 | 3225 | 3552 | 3881 | 4213 |
| 42 | 2262 | 2586 | 2912 | 3239 | 3568 | 3898 | 4229 |
| 43 | 2234 | 2564 | 2893 | 3221 | 3549 | 3876 | 4202 |
Figure 3 shows the birth weight centiles by gestational age for babies with Down syndrome compared to unaffected babies. From 30 to 38 weeks median birth weight for Down syndrome babies was slightly but consistently lower than for unaffected babies. At 38 weeks the difference was 159 g for boys and 86 g for girls. But after 38 weeks the two median curves diverged, and by 40 weeks the shortfall was much greater (304 g for boys and 239 g for girls). The Down syndrome babies showed greater variation than the unaffected babies at all gestations, but particularly before 34 weeks when the centiles are positively skew, with a much wider gap between the 91st and 98th centiles than between the 2nd and 9th. This corresponds to the L value being well below one at early gestations.
Figure 3.
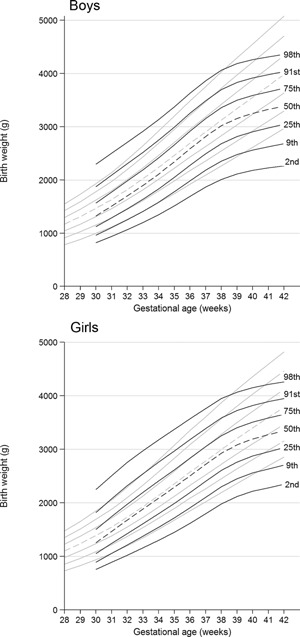
Birth weight centiles for boys and girls: Down syndrome (black lines) compared with revised UK‐WHO growth charts (gray lines) [Cole et al., [Link]].
Figure 4 shows the distribution of birth weight at 38 weeks gestation for babies with Down syndrome compared to unaffected babies, with good agreement.
Figure 4.
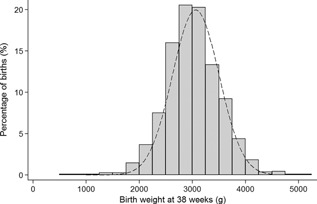
The distribution of birth weight at 38 weeks in babies with Down syndrome (grey bars) compared to unaffected babies (dashed line) [Office for National Statistics, 2012].
DISCUSSION
Our study shows that babies with Down syndrome born near to term (39–41 weeks) were lighter than unaffected babies (Fig. 3). However the modal age at delivery was 38 weeks gestation (Fig. 2). This concurs with findings by Smith and McKeown [1955] and it was also shown though not mentioned by Clementi et al. [1990] and Boghossian et al. [2012]. Hence this is the first time in 60 years that attention has been drawn to the fact that modal gestational age in Down syndrome is 38 weeks, when mean birth weight was within 150 g of that for unaffected babies (Fig. 2). Hence there was little evidence of significant growth restriction in the first 38 weeks of pregnancy. After 38 weeks babies with Down syndrome were increasingly lighter than unaffected babies on average, suggesting that they were postmature and that intrauterine growth was slowing.
There is a relatively high rate of spontaneous fetal loss in Down syndrome pregnancies [Savva et al., 2006] with 3.3% of births being stillborn. The gestational‐age‐adjusted weight of stillborn babies was less than that of live births (data not shown), indicating that birth weight of live births is a biased measure of intrauterine growth, with the smaller fetuses being excluded. However, as the proportion of stillbirths did not alter from 38 to 42 weeks gestation, ignoring them would not explain the observed divergence in birth weight. So there is probably some intrauterine growth restriction amongst fetuses with Down syndrome at all gestations, but compared to unaffected fetuses it is greater from 38 weeks gestation.
The birth weight charts for US babies with Down syndrome derived by Boghossian et al. [2012] gave similar results to ours (e.g., at 38 weeks gestation median birth weight for boys was 3,048 g vs. 2,947 g in this study and for girls 3,092 g vs. 3,019 g). Median birth weight for unaffected babies is greater in North America than England and Wales, so a larger difference might have been expected. Against that, Boghossian only included babies admitted to hospital at birth or dying before admission; this might be expected to be a “sicker” population, biasing the weight centiles downwards, which might explain why the differences between the studies were small.
Heart anomalies are also relevant, as 44% of babies with Down syndrome have a heart anomaly [Morris et al., 2014]. Babies with heart anomalies are 100–200g lighter than unaffected babies born at the same gestational age [Rosenthal et al., 1991; Rosenthal, 1996]. This will account for some but not all of the difference in birth weight, and the observation of growth restriction after 38 weeks gestation remains relevant.
Smith and McKeown [1955] questioned whether the low birth weight of those with Down syndrome was due to shorter gestation or slower prenatal growth. In a study hampered by small numbers (n = 103) their difference in birth weight at 38 weeks was greater than in our study − 0.6 Lb (272 g). On this basis they concluded that there must be some intrauterine growth restriction prior to 38 weeks. They did however record placental weights and found these to be similar to those in their control population. Hence they suggested that the apparent growth restriction was likely to be due to a “lowered growth capacity of the foetus rather than inability of the intrauterine environment to support its growth”. However studies of first trimester growth restriction and aneuploidy using crown rump measurements [Bahado‐Singh et al., 1997; Schemmer et al., 1997] have shown that those with Down syndrome grow normally in the first trimester. Our own data suggest that from 30‐38 weeks the average intrauterine growth of those with Down syndrome differs little from that of other babies. Hence intrauterine growth restriction appears to be confined to gestations beyond 38 weeks.
In unaffected babies, slowing of intrauterine growth after the modal gestational age of 40 weeks is considered a surrogate marker for incipient postmaturity and signals a need for enhanced obstetric vigilance and possible intervention. For babies with Down syndrome, slowing of intrauterine growth appears to occur from the modal gestational age of 38 weeks, hence there may be an earlier onset of incipient postmaturity in this population and enhanced vigilance may be necessary from this time.
Strengths and Weaknesses
Direct measures of intrauterine growth in late pregnancy are not available for babies with Down syndrome. Hence in our study and those of others [Clementi et al., 1990; Smith and McKeown, 1995; Boghossian et al., 2012] cross sectional birth weight data is used as a proxy measure. A strength of the study is the large sample size derived from a national register over 22 years with an estimated ascertainment rate of 93%. There was no evidence of a trend in birth weight over this time. A weakness is that 36% of the 13,940 live births recorded in the register had missing data for gestational age and/or birth weight. The register receives information from cytogenetic laboratories and then contacts the referral clinicians for further information. For some cytogenetic laboratories it is not possible to contact the referral clinicians and therefore the missing information is unlikely to be a source of bias as it is missing for administrative reasons. There was no association between gestational age and missing birth weight (P = 0.2). Mode of delivery was also unavailable and it may be that growth restriction was an indication for induced delivery before 38 weeks. However this is unlikely because mean birth weight was similar to that for unaffected babies up to 38 weeks. Detailed information on other associated anomalies (particularly heart anomalies) was not available.
Implications for Perinatal Clinical Practice
Timing of elective delivery
Marlow has recently challenged the accepted view that for the general population the optimum time for delivery is 37–41 weeks gestation (full term) [Marlow, 2012]. He provides evidence of increased morbidity and mortality among those born in early term (37–38 weeks), agreeing with Clark that for the general population perinatal risk is a continuum for adverse outcomes that is minimal at 39–41 weeks of gestation [Clark and Fleischman, 2011]. Our findings suggest that in Down syndrome the optimum time for delivery may be earlier than for other babies, though there is currently no other evidence to support this. We suggest nevertheless that clinicians should be mindful of this possibility when a foetus with Down syndrome is still in utero at 40–41 weeks. In this situation they may wish to consider induction of labour. However, they need to balance this against the early weight gain/loss in newborns with DS, for which there is very little robust information.
There is a need for information about the associations between gestational age at delivery and short and long‐term outcome measures in babies with Down syndrome. Some of this might be available by linking existing cohorts and registers.
Preterm Birth Weight Charts for Babies With Down Syndrome
The widely used UK‐WHO growth charts include a birth weight chart for 32–42 weeks gestation [Cole et al., [Link]]. Our findings show that median birth weight for those with Down syndrome is only slightly less than for UK‐WHO until 38 weeks gestation. However the centile lines are further apart, so there is a greater chance of Down syndrome babies being small or large for dates. We suggest that birth weights of preterm babies with Down syndrome should be plotted on the UK‐WHO charts up to 38 weeks gestation, and for later gestations at age 0 on the 2011 edition of the UK Down syndrome growth charts [Styles et al., 2002] (http://www.dsmig.org.uk/publications/growthchart.html). The distribution of birth weight in this study was similar to that of Styles et al (medians 3.06 and 3.00 kg respectively).
CONCLUSION
The modal age at delivery in babies with Down syndrome is 38 weeks. For gestations up to 38 weeks their median birth weight is similar to that for unaffected babies, but after 38 weeks their median birth weight rises less fast than for unaffected babies. This may have implications for perinatal clinical practice.
FUNDING
TJC was funded by UK Medical Research Council grant MR/M012069/1.
ACKNOWLEDGMENTS
We would like to thank Haiyan Wu for ensuring the accuracy of the data from the NDSCR.
Morris JK, Cole TJ, Springett AL, Dennis J. 2015. Down syndrome birth weight in England and Wales: Implications for clinical practice. Am J Med Genet Part A 167A:3070–3075.
Conflict of interest: none.
REFERENCES
- Bahado‐Singh RO, Lynch L, Deren O, Morroti R, Copel JA, Mahoney MJ, Williams J. 1997. First‐trimester growth restriction and fetal aneuploidy: The effect of type of aneuploidy and gestational age. Am J Obstet Gynecol 176:976–980. [DOI] [PubMed] [Google Scholar]
- Boghossian NS, Horbar JD, Murray JC, Carpenter JH. for the Vermont Oxford Network 2012. Anthropometric charts for infants with trisomies 21, 18, or 13 born between 22 weeks gestation and term: The VON charts. Am J Med Genet Part A 158A:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, Fleischman AR. 2011. Term pregnancy: Time for a redefinition. Clinics in Perinatology 38:557–564. [DOI] [PubMed] [Google Scholar]
- Clementi M, Calzolari E, Turolla L, Volpato S, Tenconi R. 1990. Neonatal growth patterns in a population of consecutively born Down syndrome children. Am J Med Genet 7:71–74. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Green PJ. 1992. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat Med 11:1305–1319. [DOI] [PubMed] [Google Scholar]
- Cole TJ. 1994. Do growth chart centiles need a face lift? BMJ 308:641–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Williams AF, CM Wright. RCPCH Growth Chart Expert Group. 2011. Revised birth centiles for weight, length and head circumference in the UK‐WHO growth charts. Ann Hum Biol 38:7–11. [DOI] [PubMed] [Google Scholar]
- Cunningham C. 2006. In Down Syndrome: An introduction for parents. Human Horizon series. Souvenir Press. 174. ISBN 0285636979.
- Marlow N. 2012. Full term: An artificial concept. Arch Dis Child Fetal Neonatal Ed 97:F158–F159. [DOI] [PubMed] [Google Scholar]
- McCoy EE. 1992. Growth Patterns in Down's Syndrome In: McCoy EE, editor. Down Syndrome: Advances in Medical Care. NewYork: Wiley‐Liss, Inc. ISBN 0471561843. [Google Scholar]
- Morris JK, Garne E, Wellesley D, Addor MC, Arriola L, Barisic I, Beres J, Bianchi F, Budd H, Dias CM, Gatt M, Klungsoyr K, Khoshnood B, Latos‐Bielenska A, Mullaney C, Nelen V, Neville AJ, O'Mahony M, Queisser‐Luft Am Randrianaivo H, Rankin J, Rissmann A, Rounding C, Sipek A, Stoianova S, Tucker D, de Walle H, Yevtushok L, Loane M, Dolk H. 2014. Major congenital anomalies in babies born with Down syndrome: A population‐based registry study. Am Med Genet Part 164A:2979–2986. [DOI] [PubMed] [Google Scholar]
- Mutton DE, Alberman E, Ide R, Bobrow M. 1991. Results of first year (1989) of a national register of Down's syndrome in England and Wales. BMJ 303:1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrelid A, Gustafsson J, Ollars B, Anneren G. 2002. Growth charts for Down's syndrome from birth to 18 years of age. Arch Dis Child 87:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. 2012. Statistics Bulletin: Gestation‐specific Infant Mortality, 2010.
- Piro E, Pennino C, Cammarata M, Corsello G, Grenci A, Giudice CL, Morabito M, Piccione M, Giuffre L. 1990. Growth charts of Down syndrome in Sicily: Evaluation of 382 children 0–14 years of age. Am J Med Genet 7:66–70. [DOI] [PubMed] [Google Scholar]
- Rigby RA, Stasinopoulos DM. 2005. Generalized additive models for location, scale and shape (with discussion). Applied Statistics 54:507–554. [Google Scholar]
- Rosenthal GL, Wilson PD, Permutt T, Boughman JA, Ferencz C. 1991. Birth weight and cardiovascular malformations: A population based study. The Baltimore‐Washington Infant Study. Am J Epidemiol 133:1273–1281. [DOI] [PubMed] [Google Scholar]
- Rosenthal GL. 1996. Patterns of prenatal growth among infants with cardiovascular malformations: Possible fetal hemodynamic effects. Am J Epidemiol 143:505–513. [DOI] [PubMed] [Google Scholar]
- Savva GM, Morris JK, Mutton DE, Alberman E. 2006. Maternal age‐specific fetal loss rates in Down syndrome pregnancies. Prenat Diagn 26:499–504. [DOI] [PubMed] [Google Scholar]
- Savva GM, Morris JK. 2009. Ascertainment and accuracy of Down syndrome cases reported in congenital anomaly registers in England and Wales. Arch Dis Child Fetal Neonatal Ed 94:F23–F27. [DOI] [PubMed] [Google Scholar]
- Schemmer G, Wapner RJ, Johnson A, Schemmer M, Norton HJ, Anderson WE. 1997. First‐trimester growth patterns of aneuploid fetuses. Prenat Diagn 17:155–159. [PubMed] [Google Scholar]
- Smith A, McKeown T. 1955. Prenatal growth of mongoloid defectives. Arch Dis Child 30:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles ME, Cole TJ, Dennis J, Preece MA. 2002. New cross sectional stature, weight, and head circumference references for Down's syndrome in the UK and Republic of Ireland. Arch Dis Child 87:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]


