Abstract
The aim of the present study was to investigate the expression and function of forkhead box protein 3 (FOXP3) in gastric cancer using a rat model. A total of 92 Wistar rats were divided into two groups: An experimental group (n=46) and a control group (n=46). In the experimental group, sarcosine ethyl ester hydrochloride and sodium nitrite carcinogens were administered for 6 months to induce gastric cancer, whereas the control group was administered saline. Reverse transcription-polymerase chain reaction, immunoblotting, immunohistochemistry and western blotting were applied to analyze FOXP3 expression in gastric cancer and normal gastric tissue in the experimental and control groups, respectively. The association between FOXP3 expression and gastric cancer pathogenesis was investigated. In the experimental group, 6/46 rats developed hyperplastic lesions (grade I), 8 rats developed precancerous lesions (grade II), 18 rats developed early stage gastric cancer (grade III) and 14 rats developed gastrointestinal invasive carcinoma (grade IV). FOXP3 transcription and expression was observed in all gastric tissues of the experimental group. FOXP3 transcription and expression levels were significantly higher in the experimental group than in the control group (P<0.05). Furthermore, in the experimental group, a higher lesion grade was associated with a higher level of FOXP3 transcription and expression (P<0.05). FOXP3 protein was predominantly distributed in the tumor nuclei of the gastric cancer tissues. In the 32 pathological slices of gastric cancer tissue obtained from the experimental group, 20 cases (62.50%) exhibited positive FOXP3 staining. In the hyperplastic (grade I) and precancerous gastric (grade II) tissues, 2 cases (33.33%) and 4 cases (50.00%) exhibited positive FOXP3 staining, respectively. However, no positive FOXP3 expression was identified in the 46 pathological gastric tissue slices obtained from the control group. In conclusion, the expression of FOXP3 exhibits a positive correlation with gastric lesion grade. Therefore, FOXP3 may exhibit an important function in the occurrence and development of gastric cancer.
Keywords: forkhead box protein 3, gastric carcinoma, correlation
Introduction
Gastric cancer is the fourth most common type of cancer worldwide and the second leading cause of cancer-associated mortalities after lung cancer. The majority of patients with early gastric cancer have no obvious symptoms, being pain and weight loss the most common clinical symptoms of advanced gastric cancer patients. The diagnostic methods for gastric cancer include laboratory tests, endoscopy and pathological observation, while the treatment methods include surgery, chemotherapy and radiation therapy. The prognosis depends on the type of gastric cancer (1,2).
Forkhead box protein 3 (FOXP3) is a member of the forkhead transcription factor family, which is considered as the molecular marker of regulatory T cells (3). Mutation of the FOXP3 gene can cause severe autoimmune diseases. Therefore, FOXP3 is considered critical for the regulation of immunological homeostasis (4,5). FOXP3, as a transcriptional regulatory factor, regulates the activity of regulatory T cells via the direct regulation of certain genes (3). Regulatory T cells are a subset of T lymphocytes with a suppressive function, which are involved in the maintenance of immunological self-tolerance (6,7). Regulatory T cells exhibit high expression of cluster of differentiation 25, and exhibit an immunosuppressive function via direct contact with cells or secretion of transforming growth factor-β and interleukin-10 (8). Regulatory T cells are important for immunological tolerance, tumor immunity and transplantation immunity (9). Regulatory T cells effectively inhibit the antitumor immune response and are involved in the process of tumor immune escape (10). The number of regulatory T cells in the peripheral blood of patients with tumors is significantly increased, and thus their presence markedly inhibits the antitumor immune response (11).A previous study has demonstrated that the number of regulatory T cells in the peripheral blood is closely associated with the survival and prognosis of gastric cancer patients (12). Depletion of regulatory T cells improves the specific immune response of tumor bearing mice to tumors and dendritic cell vaccines to low antigenicity tumors (13). Recently, the association between FOXP3 expression and gastric cancer has gained increasing attention (12,13); however, few studies have investigated the correlation between FOXP3 expression and various gastric cancer lesion grades (14).
In the present study, sarcosine ethyl ester hydrochloride and sodium nitrate were used to induce gastric cancer in a rat model to investigate the correlation between FOXP3 expression and different gastric cancer lesion types.
Materials and methods
Experimental animals
A total of 92 adult male Wistar rats (weight, 250–3000 g; age, 8–12 weeks) were purchased from the Animal Experimental Center of Fudan University (Shanghai, China) and maintained in separate cages (n=4/cage) at the vivarium of Henan University Huaihe Hospital (Henan, China) on a 12 h light/dark cycle. The rats were then weighed after 12 h of fasting and randomly divided into an experimental group (n=46) and a normal control group (n=46). The present study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA) (8th edition) (15). The animal use protocol in the present study was reviewed and approved by the Institutional Animal Care and Use Committee of Henan University Huaihe Hospital (Kaifeng, China).
Experimental method
To establish a gastric cancer model in rats, the experimental group was administered a mixture of sarcosine ethyl ester hydrochloride (2.5 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and sodium nitrite (2.5 mg/kg; Sangon Biotech Co., Ltd., Shanghai, China) by lavage, daily for 6 months. The rats of the control group were administered saline (2.5 mg/kg) by lavage, daily for 6 months. Anti-FOXP3 monoclonal antibody (catalogue number, SAB4700610; dilution, 1:100), anti-β-actin monoclonal antibody (catalogue number, A2228; dilution, 1:100) and anti-FOXP3 polyclonal antibody (catalogue number, AV32564; dilution, 1:200) were purchased from Sigma-Aldrich. PEF FOXP3 eukaryotic expression vector, RPMI 1640 medium and the pSV2neo plasmid were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Lipofectamine 2000, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, immunohistochemical SP kits and 3,3′-diaminobenzidine chromogenic reagent kit were purchased from OriGene Technologies, Inc. (Beijing, China).
The time period for establishing the gastric cancer model in rats was 6 months, and the model was evaluated via the grading of gastric lesions. Once the model was established, rats were sacrificed using anesthesia with chloral hydrate (Sangon Biotech Co., Ltd.). Serum was stored at −80°C for use, while gastrointestinal tissues were weighed and observed. A section of gastrointestinal tissue was stored at −80°C for use, while the other part of gastrointestinal tissue was fixed in 4% formaldehyde solution, and the pieces (4-mm thick tissue sections) were then embedded in paraffin wax. The expression of FOXP3 in gastrointestinal tissues was determined by immunohistochemistry with anti-FOXP3 antibodies, which were incubated at room temperature for 30 min.
Grading of gastric lesions
The grades of gastrointestinal tissue lesions in the experimental group were classified as follows: Grade I, hyperplasia (simple hyperplasia and papillary hyperplasia); grade II, precancerous lesions (papilloma, endogenous epithelioma and dysplasia); grade III, early cancer (basal cell carcinoma, carcinoma in situ and early invasive carcinoma); grade IV, invasive carcinoma (12). Gastric cancer lesions in the experimental group rats were assessed according to the Borrmann classification (16): Type I (nodular); type II (circumscribed ulcerative type), tumor with larger ulcers, infiltrating into the surrounding tissues; type III (infiltrating ulcerative type) tumor with major ulcers, with evident edge uplift, unclear boundaries and evident infiltration into the surrounding tissues; and type IV (diffuse infiltration type), tumor with diffuse invasive growth and unclear boundaries (13).
The intensity of FOXP3 expression was divided into four categories according to the number of positive cells in the gastrointestinal tract: negative (−), <5% positive cells; weak positive (+), 5–19% positive cells; positive (++), 20–60% positive cells; and strong positive (+++), >60% positive cells (17).
Gene transfection
The experiment was divided into three groups as follows: Group A, PEF FOXP3 and pSV2neo co-transfection; group B, no transfection; and group C, PEF and pSV2neo co-transfection group. Groups B and C served as the control groups. Transfection was performed according to the manufacturer's protocol. The positive clone group was formed after 3 weeks, then amplified, cultured and reserved (18).
TriPure Isolation reagent (Roche Diagnostics, Basel, Switzerland) was used to extract total RNA from the gastric cells obtained from the rats. A total of 3 µg total RNA was obtained from each group to perform reverse transcription-polymerase chain reaction (RT-PCR). A total of 8 µl RT product was then used to perform PCR. FOXP3 fragment specific primers (Da An Gene Co., Ltd., Guangzhou, China) were synthesized according to the FOXP3 gene RNA sequence, and β-actin was used as the reference gene. The primer sequences were as follows: Forward, 5′-CACAACATGCGACCCCCTTTCACC-3′ and reverse, 5′-AGGTTGTGGCGGATGGCGTTCTTC-3′ for FOXP3 (synthetic product, 167 bp); forward, 5′-GGCACCACACCTTCTACA-3′ and reverse, 5′-AGGAAGGCTGGAAGAGTG-3′ for β-actin (synthetic product, 540 bp). The PCR was conducted according to the following conditions: pre-denaturation at 94°C for 30 sec; 30 cycles of denaturation at 94°C for 30 sec, annealing at 63°C for 30 sec and extension at 72°C for 40 sec; and terminal extension at 72°C for 3 min. FOXP3 expression levels were analyzed by 1.5% agarose gel electrophoresis. The results were analyzed by gel imaging and Quantity One version 4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data was analyzed using SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). Categorical data were analyzed by t-test, while continuous data were analyzed by analysis of variance. Results of immunohistochemistry and RT-PCR were analyzed by χ2 test. The correlation between the expression of FOXP3 and the lesion classification of gastric tissue was analyzed by Cochran-Mantel-Haenszel test. P<0.05 was considered to indicate a statistically significant difference.
Results
Modeling results and classification of gastrointestinal lesions
Of the 46 rats in the experimental group, 6 rats developed hyperplastic lesions (grade I), 8 rats developed precancerous lesions (grade II), 17 rats developed early gastric cancer (grade III) and 14 rats developed infiltrative gastric carcinoma (grade IV). In all gastric tissues obtained from the experimental group, the whole gastrointestinal tract exhibited ulcerative infiltration. Part of the marginal ulcer was infiltrated and destructed, the boundary of which became not clear, and a number of ulcer-surrounding tissues were infiltrated. Microscopy revealed gastric carcinoma cancer cells of various sizes, arranged in nests, with decreased mesenchyme, hyperchromatic nuclei and increased mitotic activity, indicating that gastric cancer was successfully induced in the rat model by sarcosine ethyl ester hydrochloride and sodium nitrite.
FOXP3 transcription in gastric tissues of the experimental and control groups
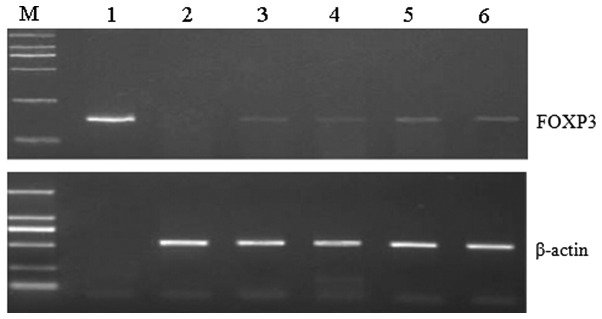
RT-PCR revealed that the transcription levels of FOXP3 were different in grade IV gastric lesions compared with the control group. The results revealed that, in the experimental group, FOXP3 transcription levels were positively correlated with gastric lesion grade. However, no FOXP3 expression was observed in the gastric mucosa of the control group (Figs. 1 and 2).
Figure 1.
Reverse transcription-polymerase chain reaction analysis of FOXP3 gene transcription revealed high levels of FOXP3 in advanced gastric cancer. Lane M, DL2000 DNA marker; lane 1, FOXP3 expression plasmid (control group); lane 2, control group; lane 3, experimental group (grade I); lane 4, experimental group (grade II); lane 5, experimental group (grade III); lane 6, experimental group (grade IV). Sizes: β-actin, 540 bp; FOXP3, 167 bp. FOXP3, forkhead box protein 3.
Figure 2.
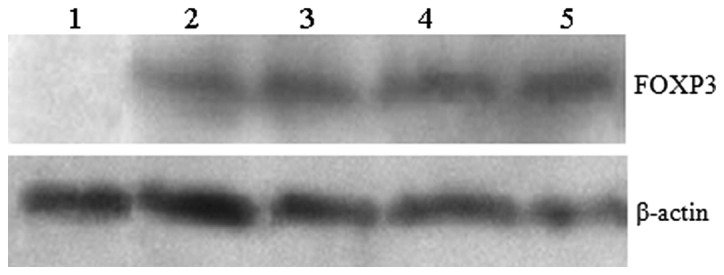
FOXP3 protein expression in the experimental and control groups was analyzed by western blot analysis. The results demonstrated high expression levels of FOXP3 in advanced gastric cancer. Lane 1, control group; lane 2, experimental group (grade I); lane 3, experimental group (grade II); lane 4, experimental group (grade III); lane 5, experimental group (grade IV). FOXP3, forkhead box protein 3.
FOXP3 expression in gastric cancer cells
Western blot analysis revealed that FOXP3 protein expression levels were different in grade IV gastric lesions compared with the control group. Furthermore, in the experimental group, FOXP3 protein levels were positively correlated with gastric lesion grade. However, no FOXP3 protein expression was identified in gastric mucosa of the control group (P<0.05) (Figs. 2 and 3).
Figure 3.
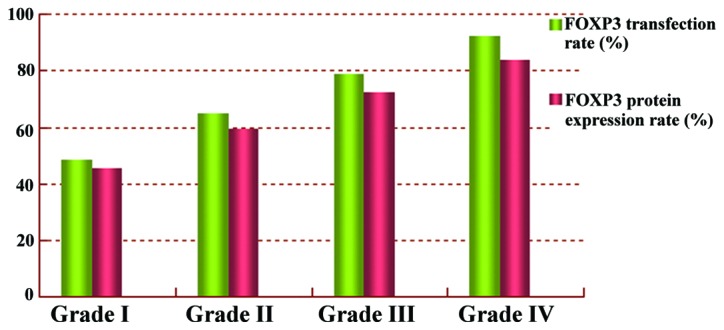
The transcription and expression rates of forkhead box protein 3 increased with the severity of disease, as indicated by the gastric lesion grade. FOXP3, forkhead box protein 3.
FOXP3 protein distribution and immunohistochemical staining in gastric cancer lesions of the experimental group
In the experimental group, FOXP3 protein expression was identified in the gastric tissues, which was mainly distributed in the nuclei of gastric cancer cells. Infiltrative cancer (grade III and IV lesions) exhibited strong FOXP3 staining, whereas early cancer and precancerous lesions (grades I and II) exhibited weak FOXP3 staining (Fig. 4).
Figure 4.
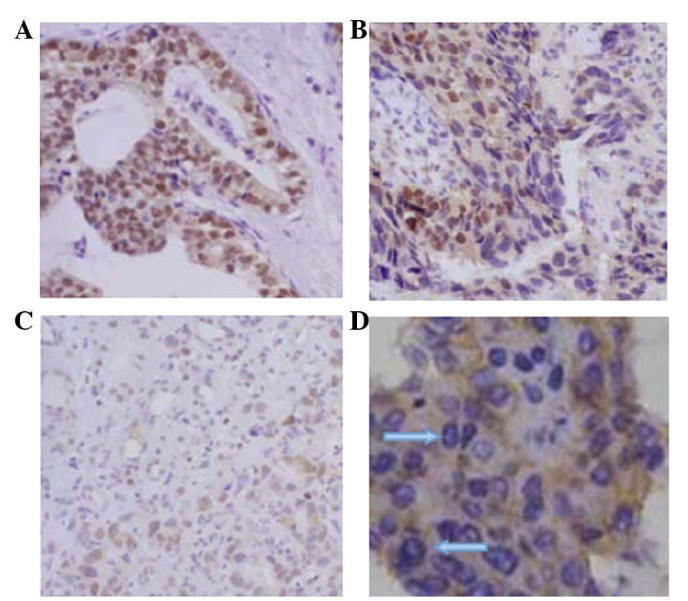
Immunohistochemistry staining revealing FOXP3 protein expression in gastric lesion tissues. (A) Nuclear FOXP3 staining in (A) invasive carcinoma, (B) early invasive carcinoma and (C) carcinoma in situ tissues (stain, HE; magnification, ×200). (D) Nuclear FOXP3 staining in early invasive carcinoma (arrow) (stain, HE; magnification, ×100). FOXP3, forkhead box protein 3; HE, hematoxylin and eosin.
Of the 32 gastric cancer pathological sections obtained (which included grade III, early cancer and grade IV, invasive carcinoma.), 20 cases (62.5%) exhibited positive FOXP3 staining, of which, 4 cases exhibited positive nuclear and cytoplasmic staining and 16 cases exhibited positive nuclear staining. Of the 8 precancerous lesion sections, 4 cases (50.00%) exhibited positive FOXP3 staining. Of the 6 proliferative lesion sections, 2 cases (33.33%) exhibited positive FOXP3 staining. No positive FOXP3 staining was observed in any of the 46 control tissue sections. In the experimental group, FOXP3 was predominantly distributed in the nuclei of gastric cancer cells, and the expression of FOXP3 positively correlated with the gastric lesion grade. These results indicated that FOXP3 may exhibit an important function in the formation and development of gastric cancer.
Discussion
The incidence rate of gastric cancer continues to be the highest among all malignancies of the digestive system, despite having decreased in recent years (19). The prognosis of gastric cancer is closely associated with cancer stage and treatment, and >90% of patients with early gastric cancer survive for >5 years and may be cured following adequate treatment (20). However, the 5-year survival rate of advanced gastric cancer patients is <5% following treatment (21). Therefore, early diagnosis is critical to improve patient prognosis and increase the survival rate. Gastric cancer patients usually present with symptoms at a late stage, and the incidence and mortality rates of the disease in China are two-fold higher than the global incidence (15.86/100,000) and mortality rates (7.20/100,000) (22). Therefore, constructing animal models of gastric cancer to simulate the pathological state and pathophysiological processes of gastric cancer patients represents a useful approach to investigate the underlying mechanism and to identify novel treatments for the disease (23,24).
It has been previously demonstrated that the level and function of regulatory T cells in the majority of gastric cancer patients may be useful to predict patient survival and prognosis (25). FOXP3 is an important protein marker of regulatory T cells, which exhibits important functions in development and maturation of regulatory T cells (3). In recent years, FOXP3 expression in gastric cancer tissue has become a research focus (26). However, few studies have investigated the association between FOXP3 expression and different types of gastric cancer lesions, and the potential underlying mechanism remains unknown (27,28).
In the present study, a gastric cancer rat model was established using sarcosine ethyl ester hydrochloride and sodium nitrite. FOXP3 expression and localization was investigated using RT-PCR, western blot analysis and immunohistochemistry. The transcription levels of FOXP3 were different in grade IV gastric cancer lesions compared with the control group. The levels of FOXP3 transcription were positively correlated with gastric cancer stage. The transcription level of FOXP3 in normal gastric tissue was not significantly different from that in gastric cancer. Notably, the level of FOXP3 expression was closely associated with the gastric cancer lesion grade. Previous studies have revealed that FOXP3 expression was localized to the nucleus and cytoplasm in pancreatic and colon cancer cells (29,30), which is consistent with the results of the present study, which demonstrated that FOXP3 expression in gastric cancer tissues was mainly localized to the nuclei and cytoplasm. However, in the present study, no FOXP3 expression was identified in normal gastric tissue by immunohistochemical analysis. In addition, the expression level of FOXP3 was positively correlated with gastric lesion grade. Of the 32 gastric cancer tissue sections, 20 cases (62.50%) exhibited positive FOXP3 protein expression, including 4 cases with positive nuclear and cytoplasmic FOXP3 staining and 16 cases with positive nuclear staining. Of the 8 precancerous gastric tissues, 4 cases (50.00%) exhibited positive FOXP3 protein expression. Of the 6 proliferative lesion tissue sections, 2 cases (33.33%) exhibited positive FOXP3 protein expression. These results indicated that FOXP3 may exhibit a modulatory function in the formation and development of gastric carcinoma.
A previous study has reported that gastric cancer cells with increased FOXP3 expression demonstrate increased drug resistance, indicating that FOXP3 may be involved in the signal transduction pathways that are associated with drug resistance, and may interact with and key molecules of these pathways (31). The combined application of various chemotherapeutic drugs was commonly used as a method to inhibit the growth of tumor cells in the clinic. However, gastric cancer, as one of the most common tumors in China, exhibits obvious characteristics of drug resistance, and the recurrence rate of gastric cancer following surgical resection is high (32). Therefore, detailed investigation regarding the generation and development of gastric carcinoma resistance may improve chemotherapy and immunotherapy of gastric cancer. The factors that influence the development and prognosis of gastric cancer are complex, including tumor growth pattern, tumor types, cancer gene expression, clinical stage and lymph node metastasis (20). Advances in molecular biology and immunohistochemistry have increased the understanding of the close association between gastric cancer, pathology and immunology (33).
In conclusion, in the present study, FOXP3 expression was identified in gastric cancer cell lines and tissues by establishing a gastric cancer rat model, and performing RT-PCR, western blot analysis and immunohistochemistry. In addition, the results revealed that the expression of FOXP3 was associated with gastric lesion grade, indicating that the expression of FOXP3 may exhibit an important function in the formation, development and prognosis of gastric cancer. In present study, the mechanism responsible for the effect of FOXP3 on gastric cancer has not been completely clarified due to certain limitations on the experimental conditions. Therefore, the functions and detailed mechanism of FOXP3 in gastric carcinoma requires further investigation.
References
- 1.Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905. doi: 10.1016/j.bpg.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Smid D, Skalicky T, Dolezal J, Kubackova D, Fichtl J. Surgical treatment of gastric cancer. Bratisl Lek Listy. 2015;116:666–670. doi: 10.4149/bll_2015_129. [DOI] [PubMed] [Google Scholar]
- 3.de Reuver PR, Mehta S, Gill P, Andrici J, D'Urso L, Clarkson A, Mittal A, Hugh TJ, Samra JS, Gill AJ. Immunoregulatory forkhead box protein p3-positive lymphocytes are associated with overall survival in patients with pancreatic neuroendocrine tumors. J Am Coll Surg. 2016;222:281–287. doi: 10.1016/j.jamcollsurg.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171:1216–1223. doi: 10.4049/jimmunol.171.3.1216. [DOI] [PubMed] [Google Scholar]
- 5.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, Ziegler SF. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol. 2006;177:3133–3142. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- 7.Buckner JH, Ziegler SF. Functional analysis of FOXP3. Ann N Y Acad Sci. 2008;1143:151–169. doi: 10.1196/annals.1443.014. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Wang X, Xu L, Wang N, Cai P, Liang T, Hu L. Foxp3 gene polymorphisms and haplotypes associate with susceptibility of Graves' disease in Chinese Han population. Int Immunopharmacol. 2015;25:425–431. doi: 10.1016/j.intimp.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Safinia N, Scotta C, Vaikunthanathan T, Lechler RI, Lombardi G. Regulatory T cells: Serious contenders in the promise for immunological tolerance in transplantation. Front Immunol. 2015;6:438. doi: 10.3389/fimmu.2015.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabusay M. The role of regulatory T-cells in antitumor immune response. Klin Onkol. 2015;28(Suppl 4):4S23–4S27. doi: 10.14735/amko20154S23. (In Czech) [DOI] [PubMed] [Google Scholar]
- 12.Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96:284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan XL, Shen DF, Lu J, Dong P, Wang J, Li MX, Shen LS. The populations, distribution of regulatory T cells, Foxp3 mRNA expression gastric cancer patients and the association with malignant stage. Chinese Journal of Laboratory Medicine. 2008;31:378–383. (In Chinese) [Google Scholar]
- 15.Mühler MR, Clément O, Salomon LJ, Balvay D, Autret G, Vayssettes C, Cuénod CA, Siauve N. Maternofetal pharmacokinetics of a gadolinium chelate contrast agent in mice. Radiology. 2011;258:455–460. doi: 10.1148/radiol.10100652. [DOI] [PubMed] [Google Scholar]
- 16.Chen BB, Liang PC, Liu KL, Hsiao JK, Huang JC, Wong JM, Lee PH, Shun CT, Ming-Tsang Y. Preoperative diagnosis of gastric tumors by three-dimensional multidetector row CT and double contrast barium meal study: Correlation with surgical and histologic results. J Formos Med Assoc. 2007;106:943–952. doi: 10.1016/S0929-6646(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 17.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: Differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, Svingen PA, Sarmento OO, Smyrk TC, Dave M, Khanna S, Lomberk GA, Urrutia RA, Faubion WA., Jr Differential coupling of KLF10 to Sin3-HDAC and PCAF regulates the inducibility of the FOXP3 gene. Am J Physiol Regul Integr Comp Physiol. 2014;307:R608–R620. doi: 10.1152/ajpregu.00085.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant SJ, Kim J, Choi AH, Sun V, Chao J, Nelson R. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer. 2016 Feb 29; doi: 10.1007/s10120-016-0603-7. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacker U, Lordick F. Current standards in the treatment of gastric cancer. Dtsch Med Wochenschr. 2015;140:1202–1205. doi: 10.1055/s-0041-102756. (In German) [DOI] [PubMed] [Google Scholar]
- 21.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 22.Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, Tada M, Okita K. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: A pilot study. Gastrointest Endosc. 1997;46:212–216. doi: 10.1016/S0016-5107(97)70088-9. [DOI] [PubMed] [Google Scholar]
- 23.Levi E, Sochacki P, Khoury N, Patel BB, Majumdar AP. Cancer stem cells in Helicobacter pylori infection and aging: Implications for gastric carcinogenesis. World J Gastrointest Pathophysiol. 2014;5:366–372. doi: 10.4291/wjgp.v5.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ke X, Wang J, Li L, Chen IH, Wang H, Yang XF. Roles of CD4+CD25 (high) FOXP3+ Tregs in lymphomas and tumors are complex. Front Biosci. 2008;13:3986–4001. doi: 10.2741/2986. [DOI] [PubMed] [Google Scholar]
- 25.Kakinuma T, Nadiminti H, Lonsdorf AS, Murakami T, Perez BA, Kobayashi H, Finkelstein SE, Pothiawala G, Belkaid Y, Hwang ST. Small numbers of residual tumor cells at the site of primary inoculation are critical for anti-tumor immunity following challenge at a secondary location. Cancer Immunol Immunother. 2007;56:1119–1131. doi: 10.1007/s00262-006-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao Q, Zhang C, Gao Y, Wang S, Li J, Li M, Xue X, Li W, Zhang W, Zhang Y. FOXP3 inhibits NF-κB activity and hence COX2 expression in gastric cancer cells. Cell Signal. 2014;26:564–569. doi: 10.1016/j.cellsig.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Song X, Li B, Greene MI. FOXP3 and its partners: Structural and biochemical insights into the regulation of FOXP3 activity. Immunol Res. 2008;42:19–28. doi: 10.1007/s12026-008-8029-x. [DOI] [PubMed] [Google Scholar]
- 28.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148–153. doi: 10.1038/sj.bjc.6604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durães C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: Prognostic, predictive or targets of therapy? Virchows Arch. 2014;464:367–378. doi: 10.1007/s00428-013-1533-y. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]