Abstract
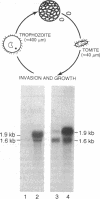
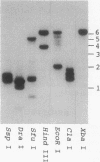
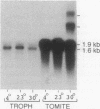
A 1.2-kilobase (kb) cDNA encoding a major surface antigen of the holotrich ciliate Ichthyophthirius multifiliis (an obligate parasite of fish) has been isolated and used as a probe to examine the expression of immobilization antigen (i-antigen) genes in this system. The cDNA encodes a predicted protein of 394 amino acids with a tandemly repeated structure characteristic of the i-antigens of the related free-living ciliates Paramecium and Tetrahymena. As shown by Northern hybridization analysis with both total and poly(A)+ RNAs, the 1.2-kb cDNA recognizes distinct transcripts of 1.6 and 1.9 kb which are differentially expressed through the parasite life cycle. During the transition from the host-associated trophozoite stage to the infective tomite stage, steady-state levels of the 1.9-kb RNA undergo a marked increase of greater than or equal to 50-fold, while the 1.6-kb transcript increases only slightly. The absolute amounts of RNA encoding the i-antigen have been quantitated and were found to reach extremely high levels equivalent to approximately 6% of the poly(A)+ RNA of I. multifiliis tomites. Southern hybridization analysis with I. multifiliis genomic DNA suggests that at least two genes encode the i-antigen transcripts. In experiments to examine the effects of temperature on the expression of I. multifiliis i-antigen genes, levels of the 1.6- and 1.9-kb transcripts were found to remain relatively constant in cells maintained at different temperature extremes. These studies indicate that genes encoding i-antigens of I. multifiliis are developmentally regulated, and they suggest the existence of alternative mechanisms for the control of surface antigen expression in ciliates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Caron F., Meyer E. Molecular basis of surface antigen variation in paramecia. Annu Rev Microbiol. 1989;43:23–42. doi: 10.1146/annurev.mi.43.100189.000323. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Clark T. G., Dickerson H. W., Findly R. C. Immune response of channel catfish to ciliary antigens of Ichthyophthirius multifiliis. Dev Comp Immunol. 1988 Summer;12(3):581–594. doi: 10.1016/0145-305x(88)90074-2. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Dickerson H. W., Clark T. G., Findly R. C. Icthyophthirius multifiliis has membrane-associated immobilization antigens. J Protozool. 1989 Mar-Apr;36(2):159–164. doi: 10.1111/j.1550-7408.1989.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kasper L. H., Bradley M. S., Pfefferkorn E. R. Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol. 1984 Jan;132(1):443–449. [PubMed] [Google Scholar]
- Kavenoff R., Zimm B. H. Chromosome-sized DNA molecules from Drosophila. Chromosoma. 1973;41(1):1–27. doi: 10.1007/BF00284071. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Costlow N. A. A molecular titration assay to measure transcript prevalence levels. Methods Enzymol. 1987;152:633–648. doi: 10.1016/0076-6879(87)52070-5. [DOI] [PubMed] [Google Scholar]
- Lin T. L., Dickerson H. W. Purification and partial characterization of immobilization antigens from Ichthyophthirius multifiliis. J Protozool. 1992 Jul-Aug;39(4):457–463. doi: 10.1111/j.1550-7408.1992.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M., Lin V. K. Comparison of two different hybridization systems in northern transfer analysis. Biotechniques. 1989 Apr;7(4):331-2, 334. [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S. Stage-specific antigens on the surface membrane of sporozoites of malaria parasites. Nature. 1978 Jul 6;274(5666):55–57. doi: 10.1038/274055a0. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Wrightsman R. A., Manning J. E. Cloning of a major surface-antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature. 1986 Aug 7;322(6079):566–568. doi: 10.1038/322566a0. [DOI] [PubMed] [Google Scholar]
- Prat A., Katinka M., Caron F., Meyer E. Nucleotide sequence of the Paramecium primaurelia G surface protein. A huge protein with a highly periodic structure. J Mol Biol. 1986 May 5;189(1):47–60. doi: 10.1016/0022-2836(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., Grange T., Pictet R. The use of NaOH as transfer solution of DNA onto nylon membrane decreases the hybridization efficiency. Nucleic Acids Res. 1987 Jan 26;15(2):857–857. doi: 10.1093/nar/15.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Haron J. A., Rothblum K. N., Dugaiczyk A. Regulation of muscle differentiation: cloning of sequences from alpha-actin messenger ribonucleic acid. Biochemistry. 1980 Dec 9;19(25):5883–5890. doi: 10.1021/bi00566a034. [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., Willis R. L., Love H. D., Jr, Bannon G. A. Molecular characterization of SerH3, a Tetrahymena thermophila gene encoding a temperature-regulated surface antigen. Mol Cell Biol. 1990 Nov;10(11):6091–6096. doi: 10.1128/mcb.10.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]