Abstract
As the sister lineage of all other actinopterygians, the Middle to Late Devonian (Eifelian–Frasnian) Cheirolepis occupies a pivotal position in vertebrate phylogeny. Although the dermal skeleton of this taxon has been exhaustively described, very little of its endoskeleton is known, leaving questions of neurocranial and fin evolution in early ray‐finned fishes unresolved. The model for early actinopterygian anatomy has instead been based largely on the Late Devonian (Frasnian) Mimipiscis, preserved in stunning detail from the Gogo Formation of Australia. Here, we present re‐examinations of existing museum specimens through the use of high‐resolution laboratory‐ and synchrotron‐based computed tomography scanning, revealing new details of the neuro‐cranium, hyomandibula and pectoral fin endoskeleton for the Eifelian Cheirolepis trailli. These new data highlight traits considered uncharacteristic of early actinopterygians, including an uninvested dorsal aorta and imperforate propterygium, and corroborate the early divergence of Cheirolepis within actinopterygian phylogeny. These traits represent conspicuous differences between the endoskeletal structure of Cheirolepis and Mimipiscis. Additionally, we describe new aspects of the parasphenoid, vomer and scales, most notably that the scales display peg‐and‐socket articulation and a distinct neck. Collectively, these new data help clarify primitive conditions within ray‐finned fishes, which in turn have important implications for understanding features likely present in the last common ancestor of living osteichthyans.
Keywords: computed tomography, Devonian, neurocranium, palaeontology
Ray‐finned fishes (Actinopterygii) account for nearly half of living vertebrate diversity (Nelson 2006; Faircloth et al. 2013), but understanding of their early evolution is substantially incomplete. Despite a probable date of divergence from Sarcopterygii of around 420–430 Ma (Zhu et al. 2009; Broughton et al. 2013), no unequivocal actinopterygians are known from the Silurian. Scale taxa such as Lophosteus Pander, 1856, Andreolepis Gross, 1968 and Naxilepis Wang and Dong, 1989, once considered to be primitive actinopterygians (Gross 1968; Schultze 1977; Janvier 1978; Wang and Dong 1989; Märss 2001), are now thought to branch from the osteichthyan stem (Botella et al. 2007; Friedman and Brazeau 2010; Zhu et al. 2013). Further uncertainty surrounds the affinities of Dialipina Schultze, 1968 and Ligulalepis Schultze, 1968, both originally described as actinopterygians (Ligulalepis: Basden and Young 2001; Dialipina: Schultze and Cumbaa 2001) but now more commonly recovered as stem osteichthyans (Friedman 2007; Brazeau 2009; Davis et al. 2012; Zhu et al. 2013; Dupret et al. 2014; Giles et al. 2015a). Of the handful of articulated actinopterygians known from the Devonian, the majority are described exclusively from their dermal skeletons, with only limited reports of endoskeletal remains (Gardiner and Schaeffer 1989). Two important exceptions are the early Frasnian Mimipiscis toombsi (Gardiner and Bartram, 1977) and Moythomasia durgaringa Gardiner and Bartram, 1977, described in great detail from multiple three‐dimensional, acid‐prepared specimens from the Gogo Formation, Western Australia (Gardiner and Bartram 1977; Gardiner 1984; Choo 2011). Given its exceptional preservation, Mimipiscis Choo, 2011 has understandably become the key exemplar for the primitive actinopterygian conditions (Gardiner 1984; Gardiner and Schaeffer 1989; Coates 1999; Cloutier and Arratia 2004; Gardiner et al. 2005; Friedman and Blom 2006; Long et al. 2008; Choo 2011; Friedman 2015).
Cheirolepis Agassiz, 1835 is the earliest occurring taxon that can be unequivocally placed within Actinopterygii. It is represented by articulated specimens from the late Eifelian of Scotland, the Givetian of Nevada (Reed 1992; Arratia and Cloutier 2004) and the Frasnian of Canada, as well as by scales from the Givetian of Germany (Gross 1973) and Eifelian–Givetian of Belarus, Latvia and Estonia (Blieck and Cloutier 2000; Mark‐Kurik 2000; Lukševičs et al. 2010). As part of his original description, Agassiz (1835) erected three species on the basis of Scottish material: the type species C. trailli from Orkney, C. uragus from Gamrie and C. cummingae from Cromarty. A further three species were described by M'Coy (1848): C. velox, C. macrocephalus (both from Orkney) and C. curtus, from Lethen Bar. These species were subsequently revised by Egerton (1860) and Traquair (1888), with only C. trailli retained. Whiteaves (1881) first reported Canadian material from the Frasnian Miguasha Lagerstätte and assigned it to the new species C. canadensis. An additional species, C. schultzei, was erected by Arratia and Cloutier (2004) for specimens from the Denay Limestone of Nevada, first reported by Reed (1992) as Cheirolepis cf. C. canadensis. C. trailli and C. canadensis were comprehensively reviewed by Pearson and Westoll (1979) and Arratia and Cloutier (1996).
The affinities of Cheirolepis with bony fishes generally, and actinopterygians specifically, have not always been apparent. On the basis of its micromeric, non‐overlapping scales, Agassiz (1835) grouped Cheirolepis with Acanthodes Agassiz, 1833 and Cheiracanthus Agassiz, 1835 in his Acanthodidae, a placement upheld by M'Coy (1855) and Egerton (1860). Dissenters from this view included Miller (1841) and Müller (1846), who considered the combination of characters in Cheirolepis sufficiently unique to merit placement in its own group. Similarities between Cheirolepis and ‘palaeoniscoids’ were first articulated by Pander (1860), although he too regarded Cheirolepis as a member of its own distinct group. Traquair (1875) noted conspicuous differences between the structure of Cheirolepis and acanthodians, including several points relating to scale morphology. The most compelling similarity between these groups (scale micromery) was also rejected based on Egerton's (1864) description of micrometric squamation in the Carboniferous ‘palaeoniscoid’ Myriolepis clarkei Egerton, 1864. The structure of the fins, shoulder girdle and skull bones led Traquair (1875) to align Cheirolepis with taxa that are now assigned to Actinopterygii, a position universally accepted since. More recently, cladistic analyses have consistently resolved Cheirolepis as the sister taxon of all other ray‐finned fishes (Gardiner 1984; Coates 1999; Cloutier and Arratia 2004; Gardiner et al. 2005; Friedman and Blom 2006; Friedman 2007; Friedman et al. 2007; Long et al. 2008; Brazeau 2009; Swartz 2009; Zhu et al. 2009; Choo 2011; Davis et al. 2012; Giles et al. 2015a). As only a small part of the endoskeleton of Cheirolepis has been described (Fig. 1; Pearson and Westoll 1979), very limited comparisons can be drawn with other well‐known early actinopterygians such as Mimipiscis and Moythomasia. This makes it impossible to understand the evolution of key actinopterygian characters during the early history of the group, particularly endoskeletal structures with a major impact on osteichthyan, and gnathostome, phylogeny more generally. The paucity of endoskeletal data outside of Mimipiscis and Moythomasia has led to a situation where early actinopterygian relationships are investigated almost exclusively on the basis of dermal characters (Gardiner and Schaeffer 1989; Cloutier and Arratia 2004; Friedman and Blom 2006; Long et al. 2008; Swartz 2009; Choo 2011), in stark contrast to the more comprehensive character sets used when examining early gnathostome and sarcopterygian interrelationships (Zhu and Yu 2002; Friedman 2007; Brazeau 2009; Zhu et al. 2009; Davis et al. 2012; Zhu et al. 2013; Giles et al. 2015a). Attempts to expand the actinopterygian character set (Coates 1998, 1999; Hamel and Poplin 2008; Giles and Friedman 2014) have increased the number of informative endoskeletal characters, but these are yet to be incorporated into many analyses due to the large amounts of missing data associated with taxa known principally from dermal material (Choo 2011).
Figure 1.
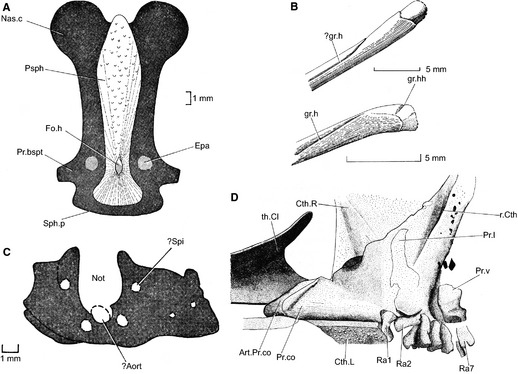
Existing interpretations of the endoskeletal structure of Cheirolepis trailli. A, sphenethmoid portion of neurocranium and parasphenoid (Pearson and Westoll 1979, fig. 1a). B, partial hyomandibula (Pearson and Westoll 1979, fig. 10c, d). C, occipital portion of neurocranium (Pearson and Westoll 1979, fig. 1c). D, shoulder girdle and fin radials (Pearson and Westoll 1979, fig. 14a; no scale given). Abbreviations (as given by Pearson and Westoll 1979): Aort, canal for dorsal aorta; Art.Pr.co, anterior articulatory surface of coracoid process; Cth.L, left cleithrum; Cth.R, right cleithrum; Epa, foramen for efferent pseudobranchial artery; Fo.h, foramen hypophyseos; gr.h, groove on median side of hyomandibula; gr.hh, possible groove on head of hyomandibula; Nas.c, nasal capsule; Not, notochordal canal; Pr.bspt, basipterygoid process; Pr.co, coracoid process of endogirdle; Pr.l, processus lateralis of endogirdle; Pr.v, processus ventralis of endogirdle; Psph, parasphenoid; Ra, radial element; r.Cth, dorsoventrally running ridge and groove on medial surface of cleithrum; Sph.p, posterior edge of sphenethmoid (position of ventral fissure); Spi, foramen for a spinal nerve; th.Cl, thickening on inner surface of clavicle. Reproduced from Pearson and Westoll (1979) with the permission of The Royal Society of Edinburgh.
Here, we use computed tomography (CT) to examine endoskeletal anatomy in Cheirolepis. This study uses material previously noted as preserving endoskeletal structures, but which could only be described on the basis of surface morphology (Pearson and Westoll 1979; Figs 1, 2, 3). Lab‐ and synchrotron‐μCT permit the description of internal features of the specimens without recourse to destructive techniques, which could not be applied to such rare material. The specimens described herein preserve a largely complete braincase, a hyomandibula and pectoral fin endoskeleton. In the light of these data, we also revisit the neurocranium and associated bones in two other Devonian actinopterygians sometimes hypothesized to diverge outside the clade comprising Mimipiscis, Moythomasia and more derived actinopterygians (Friedman and Blom 2006; Long et al. 2008; Swartz 2009; Choo 2011): the Givetian Howqualepis Long, 1988 and the Famennian Tegeolepis Miller, 1892. Collectively, these new data allow us to test the suitability of Mimipiscis as a model of primitive ray‐fin anatomy while also clarifying patterns of character evolution early in actinopterygian history.
Figure 2.

Photographs of specimens described using computed tomography (CT). A, Cheirolepis trailli NHMUK P.62908 (= Pearson and Westoll 1979, Sp.2a), specimen in which the base of the ethmosphenoid region and parasphenoid have become separated from the dorsal part of the ethmosphenoid region plus the otic region and occipital arch. B, Cheirolepis trailli NMS.1956.19, specimen preserving both pectoral fin endoskeletons and a hyomandibula. Both scale bars represent 2 cm.
Figure 3.

Specimens of Cheirolepis trailli in which the parasphenoid and/or base of the ethmosphenoid region are preserved. A, NMS.1877.30.5. B, MCZ 6039. C, NHMUK P.4051b. D, NMS.1892.8.60. Abbreviations: bhc, buccohypophyseal canal; bpt, basipterygoid process; dent, denticle field of parasphenoid; epsa, efferent pseudobranchial artery; nas.c, nasal capsule. Anterior to top. Scale bar represents 2 mm.
Material and methods
Material
Cheirolepis
The specimens of Cheirolepis trailli studied herein are housed at the NMS, NHMUK and MCZ. This material originates from the Tynet Burn and Gamrie localities of the lacustrine Achanarras Limestone, Scotland, which has been dated as late Eifelian (390.4–388.1 Ma; Gradstein et al. 2012) based on the presence of spores of Dinsosporites devonicus (Richardson and McGregor 1986). NMS.1877.30.5 is a near‐complete specimen of Cheirolepis. The head is completely disarticulated, and the anteriormost region of the specimen is preserved in part (NMS.1877.30.5) and counterpart (NHMUK P.62908; Fig. 2A; Sp.2a of Pearson and Westoll 1979). NMS.1956.19 preserves the anterior of a specimen of Cheirolepis and contains a hyomandibula and pectoral endoskeleton (Fig. 2B). This specimen shows a greater degree of three‐dimensional preservation than the individual represented by NMS.1877.30.5/NHMUK P.62908. Further examined specimens preserving the parasphenoid and/or ethmosphenoid are as follows: NHMUK P.4051a/b, from Gamrie; MCZ 6039, from Gamrie; NHMUK P.66863 (BMP.41410 of Pearson and Westoll 1979), from Tynet Burn; NMS.1892.8.60, from Gamrie (Fig. 3).
Howqualepis
The specimen of Howqualepis rostridens examined here, AMF65495, is that of a near‐complete fish, missing only the snout and the anal fin. The material is from the lacustrine Mt. Howitt locality, south‐east Australia, and has been dated by Young (2006) as Givetian (387.7–382.7 Ma; Gradstein et al. 2012) on the basis of vertebrate biostratigraphy. When first described by Long (1988), this specimen was acid‐prepared in dilute HCl, leaving a siliciclastic mould of the original bone.
Tegeolepis
The parasphenoid of Tegeolepis clarkii Newberry, 1888 is seen in peels of one specimen, CMNH 5518. The remains described as a parasphenoid by Dunkle and Schaeffer (1973, fig. 1) do not appear to represent that bone (see below). The specimen originates from the Cleveland Member of the Ohio Shale, which has been correlated by Over (2007) with the marginifera–praesulcata conodont zones (371.06–361.54 Ma; Gradstein et al. 2012).
Methods
Several specimens were selected for CT scanning. NHMUK P.62908, which preserves the braincase of Cheirolepis, was scanned using synchrotron radiation X‐ray microtomography at the I12 beamline of the Diamond Light Source, Didcot, UK, using an 80 keV monochromatic beam, CdW04 scintillator of 0.9 mm thickness, 4008 × 2672 pco.4000 camera and 3000 projections of 0.04‐second exposure collected through 180° rotation. From the projections, slice images were reconstructed with an in‐house filtered back projection reconstruction algorithm (Titarenko et al. 2010). The resulting voxel size was 12.35 μm. The part (NMS.1877.30.5), in which the parasphenoid and parts of the ethmoid region are preserved (Fig. 2A), was not scanned due to its large size and high aspect ratio. NMS.1956.19, which preserves the endoskeletal fin girdles and hyomandibula of Cheirolepis, was scanned at the Imaging and Analysis Centre, NHMUK, using a Metris X‐Tek HMX ST 225 CT System with a 2000 × 2000 pixel detector, tungsten reflection target and 3142 projections. Volumes were created with CTPro V2.1. AMF65495, which preserves a mould of the braincase and hyomandibula, as well as dermal material of Howqualepis, was scanned using the same machine at NHMUK, using a copper filter.
Following scanning, the data were reconstructed and segmented manually in Mimics version 15.01 (http://biomedical.materialise.com/mimics; Materialise, Leuven, Belgium). As the preservation in AMF65495 is mouldic, a mask of the air was generated, producing a ‘virtual’ cast. The use of this method, rather than producing a latex peel, minimizes any risk of damage to the specimen and results in a permanent record that will not deteriorate (original latex peels of the material, made in the 1980s, have degraded badly and been lost; J. Long pers. comm. 2013). Meshes were exported as .PLY surface files and were exported to and imaged in Blender (Garwood and Dunlop 2014). PLY files of the braincase of Cheirolepis, the fin and hyomandibula of Cheirolepis and the cast of the Howqualepis specimen are made available online (Giles et al. 2015b). These files can be easily opened and manipulated in free programs such as Meshlab (http://meshlab.sourceforge.net; Cignoni et al. 2008).
Meshlab was used to downsample and prepare the two portions of the Cheirolepis braincase for 3D printing. The model was upscaled by a factor of five and printed using a Zprinter 350 at the Hull York Medical School, York, UK. These models aided in the interpretation of the braincase.
The parasphenoid of Tegeolepis is preserved as a negative impression. We studied a positive cast of this mould made using two‐part flexible dental casting compound. Unlike standard latex peels, this material releases easily from matrix. For photography, this cast was coated with a sublimate of ammonium chloride.
Several specimens were studied under immersion, and photographed with a Nikon SLR camera with a polarizing filter. Line drawings were produced by hand.
Institutional abbreviations
AMF, Australian Museum, Sydney, Australia; CHMN, Cleveland Museum of Natural History, Cleveland, USA; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, USA; NHMUK, Natural History Museum, London, UK; NMS, National Museums of Scotland, Edinburgh, UK.
Systematic palaeontology
Class OSTEICHTHYES Huxley, 1880 Subclass ACTINOPTERYGII Cope, 1887 Family CHEIROLEPIDIDAE Pander, 1860 Genus CHEIROLEPIS Agassiz, 1835 Cheirolepis trailli Agassiz, 1835
Emended diagnosis
See Pearson and Westoll (1979, p. 390) with the following amendments. Cheirolepidid with lozenge‐shaped parasphenoid lacking ascending processes. Spiracle housed in groove. Open groove for dorsal aorta on basicranium. Differs from other species of Cheirolepis in the following features: extrascapulae do not contact each other at midline, head of dermohyal projects above operculum, elongate spiracular slit.
Description
Neurocranium
General features
The specimen of Cheirolepis containing the neurocranium studied here is preserved in part and counterpart (NMS.1877.30.5/NHMUK P.62908; Figs 2A, 3A). The bulk of the braincase is preserved in the part. The neurocranium has been dorsoventrally compressed, with the loss of internal anatomy, and is preserved as two parts: the base of the ethmosphenoid region, and the dorsal part of the ethmosphenoid region plus the otic region and occipital arch. The break between these two components occurred along the interorbital septum. Detachment of the parasphenoid and ethmosphenoid is common in specimens of Cheirolepis, with isolated examples found in several specimens (e.g. NMS.1877.30.5/NHMUK P.62908 (Sp.2a); NHMUK P.4051a/b; MCZ 6039; NHMUK P.66863 (BMP.41410); Fig. 3). The occipital region has rotated backwards during compaction such that the posterior face of the occiput is now oriented dorsally (Fig. 4). A similar style of preservation appears to characterize Pearson and Westoll's (1979, fig. 11a) ‘Sp. 13’ and UMZC.425 (Pearson and Westoll 1979, fig. 4a). The basioccipital plate (lying between the vestibular fontanelles, and extending from the occiput to the ventral otic fissure) has shifted slightly to the anatomical right.
Figure 4.
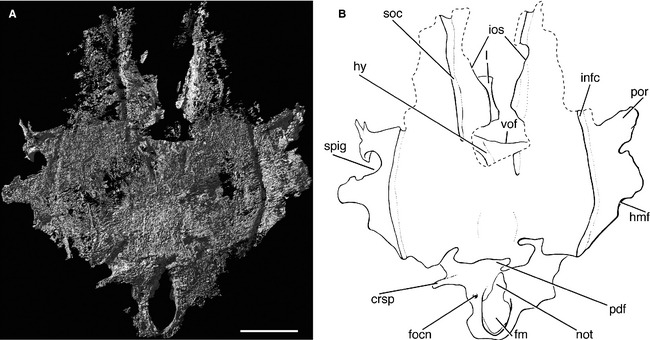
The braincase of Cheirolepis trailli NHMUK P.62908 in dorsal view. A, three‐dimensional rendering of braincase. B, interpretive drawing of braincase. Anterior to top. Abbreviations: crsp, craniospinal process; fm, foramen magnum; focn, foramen of the occipital nerve; hmf, articulation facet for hyomandibula; hy, hyoid artery; infc, infraorbital canal; ios, interorbital septum; not, notochordal canal; pdf, posterior dorsal fontanelle; por, postorbital process; soc, supraorbital canal; spig, spiracular groove; vof, ventral otic fissure; I, olfactory nerve. Scale bar represents 5 mm.
The otico‐occipital region of the braincase of Cheirolepis is formed as one ossification, as in Mimipiscis and Moythomasia (Gardiner 1984). Although the crushed nature of the specimen makes it difficult to determine the location or extent of different ossification centres, the boundaries between the occipital and otic/orbital regions of the braincase can be deduced from the presence of conspicuous fissures.
Occipital region
The occiput is preserved lying face up on the surface of the rock, with the openings for the foramen magnum and notochord clearly visible (Fig. 4). The foramen magnum (fm; Fig. 4B) is ovoid and approximately twice the size of the notochord (not; Fig. 4B). As the floor of the foramen magnum is incompletely mineralized along the midline, the two openings appear confluent. Incomplete mineralization of the floor of the foramen magnum is also typical in Mimipiscis and Moythomasia (Gardiner 1984, p. 189). Pearson and Westoll (1979, p. 345) incorrectly identified these openings as accommodating the notochord and dorsal aorta. The floor of the notochordal canal is largely complete, with the exception of a slot‐shaped cavity on the midline, which likely represents incomplete fusion of paired parachordal plates, as in the Gogo actinopterygians (Gardiner 1984; Fig. 5B). Because the braincase is flattened, the notochordal canal cannot be traced anteriorly, and the relationship of this canal with the ventral otic fissure is unknown. The posteriormost parts of the occipital arch, presumably including the articular areas for the first pharyngobranchials, are preserved in the counterpart.
Figure 5.
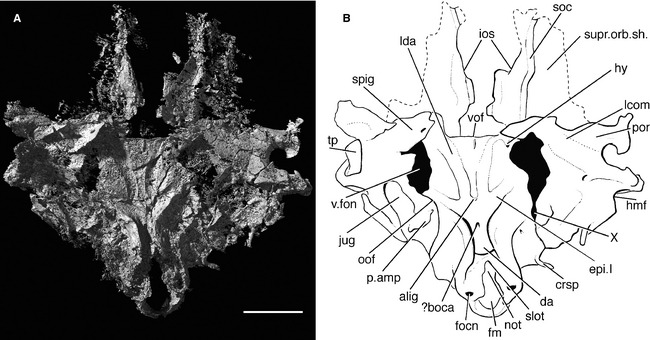
The braincase of Cheirolepis trailli NHMUK P.62908 in ventral view. A, three‐dimensional rendering of braincase. B, interpretive drawing of braincase. Anterior to top. Abbreviations: alig, attachment of aortic ligament; boca, branch of the occipital artery; crsp, craniospinal process; da, dorsal aorta; epi.I, first epibranchial artery; fm, foramen magnum; focn, foramen of the occipital nerve; hmf, articulation facet for hyomandibula; hy, hyoid artery; ios, interorbital septum; jug, jugular vein; lcom, lateral commissure; lda, lateral dorsal aorta; not, notochordal canal; oof, otico‐occipital fissure; p.amp, parampullary process; por, postorbital process; soc, supraorbital canal; slot, unmineralized floor of notochordal canal; spig, spiracular groove; supr.orb.sh, supraorbital shelf; tp, toothplate; vof, ventral otic fissure; v.fon, vestibular fontanelle; X, vagus nerve. Scale bar represents 5 mm.
Lateral to the floor of the foramen magnum, the occiput is pierced by a small canal (focn; Figs 4, 5). This travels anterolaterally to open on the lateral face of the occiput (focn; Fig. 6), and comparison with other ray fins suggests that this would have transmitted the occipital nerve. This opening was cautiously identified by Pearson and Westoll (1979, fig. 1c) as accommodating a spinal nerve. A distinct ridge on the lateral face of the occiput runs dorsally from the level of the occipital nerve (oims2; Fig. 6). Such a ridge is also present in Mimipiscis and Kansasiella Poplin, 1975 (Poplin 1974), and is tentatively identified in Cheirolepis as the ridge for the insertion of the second intermuscular septum. A modest craniospinal process is visible on the left side of the specimen, lateral to the posterior dorsal fontanelle (crsp; Figs 4, 5, 6). In life, this would have formed the dorsolateral corner of the occipital plate behind the otico‐occipital fissure.
Figure 6.
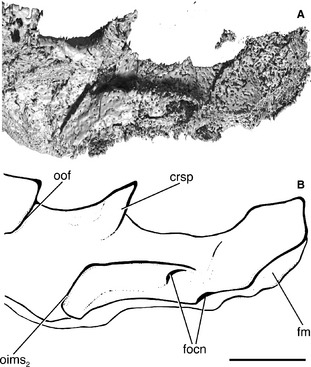
Occipital portion of the braincase of Cheirolepis trailli NHMUK P.62908 in lateral view; this part of the braincase has rotated such that the dorsalmost part of the occiput, identified by the foramen magnum, is now at the posterior edge of the specimen. A, three‐dimensional rendering of braincase. B, interpretive drawing of braincase. Anterior to left. Abbreviations: crsp, craniospinal process; fm, foramen magnum; focn, foramen of the occipital nerve; oims2, origin of second intermuscular septum; oof, otico‐occipital fissure. Scale bar represents 2 mm.
The dorsal surface of the braincase is smooth, with no evidence of a fossa bridgei (Fig. 4). Although present in later actinopterygians, a fossa bridgei is also absent in Mimipiscis and Moythomasia (Gardiner 1984), and poorly developed in Kentuckia deani (Eastman, 1908) (Rayner 1951). The dorsal roof of the braincase is poorly preserved anterior to the ventral otic fissure, particularly along the midline, and the presence or absence of an anterior dorsal fontanelle cannot be determined. Mineralization of the upper surface of the braincase is complete behind the ventral otic fissure, with the exception of the posterior dorsal fontanelle (pdf; Fig. 4B); the unfinished areas anterior and lateral to the fontanelle represent areas where the bone is either too thin or too poorly mineralized to be fully resolved by the scan.
The otico‐occipital fissure (oof; Figs 5, 6) is completely open, as in most other early osteichthyans, and can be traced anteriorly along the ventrolateral face of the braincase before intersecting the vestibular fontanelle. A slight expansion in the line of the fissure presumably marks the exit of the vagus nerve (X; Fig. 5B). It is not possible to determine whether this is divided into a dorsal and ventral portion, as in Mimipiscis and Moythomasia (Gardiner 1984). This foramen, and indeed much of the fissure, is difficult to trace on the anatomical right of the specimen, presumably due to the slight lateral displacement of the occipital plate.
The ovoid vestibular fontanelles (v.fon; Fig. 5B) are at least twice the relative length of those in Mimipiscis and Moythomasia, and are more similar in size to the vestibular fontanelles of Carboniferous actinopterygians (e.g. Kentuckia Rayner, 1951; Coccocephalichthys Whitley, 1940 (Watson 1925; Poplin and Véran 1996); Pteronisculus White, 1933 (Nielsen 1942; Patterson 1975; Coates 1998)) and sarcopterygians (e.g. Youngolepis Chang and Yu, 1981 (Chang 1982); Eusthenopteron Whiteaves, 1881 (Bjerring 1971; Jarvik 1980); Gogonasus Long, 1985 (Holland 2014)). The large fontanelles presumably formed a point of weakness about which the otic region collapsed, and the fontanelles may in fact appear slightly larger than their original size. As in Mimipiscis, Moythomasia, Kansasiella, Coccocephalichthys, Lawrenciella Poplin, 1984 (Hamel and Poplin 2008), Boreosomus Stensiö, 1921 (Nielsen 1942) and Luederia Schaeffer and Dalquest, 1978, the vestibular fontanelles are clearly separated from the ventral otic fissure by a substantial bridge of bone. The occipital portion of the ventral otic fissure (vof; Figs 4, 5, 7), which delimits the region anteriorly, is very straight.
Figure 7.
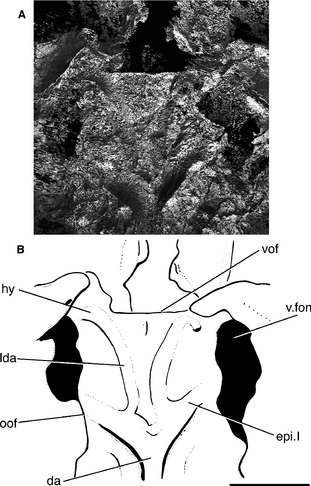
Close‐up of the ventral surface of the braincase of Cheirolepis trailli NHMUK P.62908 showing the lateral dorsal aortae and hyoid arteries. A, three‐dimensional rendering of braincase. B, interpretive drawing of braincase. Anterior to top. Abbreviations: da, dorsal aorta; epi.I, first epibranchial artery; hy, hyoid artery; oof, otico‐occipital fissure; lda, lateral dorsal aorta; vof, ventral otic fissure; v.fon, vestibular fontanelle. Scale bar represents 5 mm.
Unlike all other early actinopterygians in which the condition is known (but see below for a reinterpretation of Howqualepis), the dorsal aorta in Cheirolepis is not enclosed in a midline canal. Instead, the aorta was accommodated by a groove on the basioccipital (da; Fig. 5B), as in sarcopterygians (e.g. Youngolepis (Chang 1982) and Acanthodes (Miles 1973; Davis et al. 2012)). The aortic groove is deeper than observed in members of these outgroups. This bifurcation occurs some way posterior of the vestibular fontanelles and is positioned more posteriorly than in other early ray fins. The lateral dorsal aortae almost immediately split again; grooves for the first epibranchial artery travel anterolaterally towards the vestibular fontanelles (epi I; Figs 5, 7), and the carotids continue anteriorly towards the ventral otic fissure (lda; Figs 5, 7). Although a similar arterial branching pattern is observed in Kentuckia (Rayner 1951), Coccocephalichthys (Poplin and Véran 1996), Lawrenciella (Hamel and Poplin 2008) and Luederia (Schaeffer and Dalquest 1978), the epibranchial arteries occupy a longer portion of the basioccipital in Cheirolepis. Similarly, the proportion of the braincase carrying the lateral dorsal aortae is longer in Cheirolepis than in Mimipiscis and other early actinopterygians. As the dorsal aorta is unfloored, the position of exit of the second epibranchial arteries is unknown. In Kentuckia, Coccocephalichthys, Lawrenciella, Luederia, Kansasiella (Poplin 1974), Boreosomus and Pteronisculus (Nielsen 1942), these leave through a single or paired opening from the floor of the aortic canal. The branches of the lateral dorsal aortae and first epibranchial arteries are widely separated in Cheirolepis, and the basioccipital plate as a whole is broader than the corresponding area in Lawrenciella, Kansasiella and the Gogo actinopterygians.
Immediately posterior to the ventral otic fissure, a shallow groove branches from the lateral dorsal aorta before turning posterolaterally and entering the braincase (hy; Figs 5, 7). A similarly placed groove and foramen in other early actinopterygians (e.g. Mimipiscis: Gardiner 1984, fig. 50; Lawrenciella: Hamel and Poplin 2008, fig. 9) has been interpreted as housing the orbital artery. Positionally, this is implausible: it is unlikely that the orbital artery would turn posteriorly, away from the orbit. The posterolateral orientation of the groove and comparison with other extant and extinct gnathostomes (e.g. Chlamydoselache Allis, 1923; Janusiscus Giles et al., 2015a) suggest that this represents the path of the efferent hyoid artery.
A small peg is located on the midline immediately after the divergence of the dorsal aorta. Comparison with Mimipiscis and Moythomasia (Gardiner 1984) indicates that this likely marks the attachment point for the aortic ligament (alig; Fig. 5B). The roof of the aortic groove is pierced by an anterodorsally directed canal that opens into the notochordal canal. The canal lies on the midline, but as it is developed as a distinct groove, it is unlikely to represent a gap between the basioccipitals, and the opening is too posterior to be the aortic ligament. It may represent a branch of the occipital artery (?boca; Fig. 5B).
Otic and orbital regions
The deformation of the specimen has caused the lateral face of the neurocranium to be flattened out onto a level with the rest of the ventral surface. Therefore, the otic region may appear somewhat wider than it would in life. The lateral commissure (the transverse otic process; see revised terminology in Giles et al. 2015a) is anteroposteriorly broader and slightly longer than in other early actinopterygians (lcom; Fig. 5B), particularly Mimipiscis (Gardiner 1984, fig. 50). Although flattened, the postorbital process (por; Figs 4B, 5B) is prominent. As in Mimipiscis, the long spiracular groove (spig; Figs 4B, 5B) extends along the lateral face of the otic region and onto the basisphenoid, behind the basipterygoid process. There is no trace of the open anterior pocket between the postorbital process and spiracular groove, as seen in the braincase attributed to Ligulalepis (Basden et al. 2000; Basden and Young 2001). The lateralmost parts of the left postorbital and transverse otic processes (i.e. those preserved dorsalmost after deformation) in Cheirolepis are preserved in the counterpart. The presence or absence of an otico‐sphenoid fossa cannot be determined.
The articular area for the hyomandibula is positioned behind the spiracular groove on the posterior face of the broad postorbital process (hmf; Figs 4B, 5B). The hyomandibular facet has been distorted and now faces posteriorly. The facet on the right of the specimen is partially obscured by a displaced, possibly spiracular, toothplate (tp; Fig. 5B).
A deep gutter marking the course of the jugular vein is visible on the lateral side of the otic region, below the shelf formed by the hyomandibular facet (jug; Fig. 5B). The groove bends dorsolaterally around the prominent parampullary process (p.amp; Fig. 5B), as in Mimipiscis and Moythomasia (Gardiner 1984), and the first suprapharyngobranchial likely articulated with this region. The posterior entrance of the jugular canal into the lateral commissure presumably also transmitted the hyomandibular trunk of the facial nerve. The exit of the glossopharyngeal nerve cannot be identified. The otic region anterior to the postorbital process has collapsed, obscuring the anterior opening of the jugular canal and the trigeminofacialis chamber.
The floor and hind walls of the orbit are not preserved. Due to the manner in which the specimen is broken, the interorbital septum (ios; Figs 4B, 5B) is largely incomplete. The precise width of the interorbital septum is unclear, but appears wider than in Mimipiscis and Moythomasia (Gardiner 1984). Details of the orbital roof are not preserved, but deep grooves are visible on the dorsal surface for the overlying supraorbital canals of the dermal skull roof (soc; Fig. 5B).
Although comparison with other early actinopterygians suggests the interorbital septum would originally have been completely mineralized, separation of the sphenoid and ethmoid from the occipital and orbitotemporal regions is fairly common in specimens of Cheirolepis. The neurocranium has broken through the ventral otic fissure and basiphenoid pillar and along the interorbital septum. Consequently, the parasphenoid and ventralmost parts of the ethmosphenoid are preserved in the counterpart.
The basiphenoid portion of the ventral otic fissure is incompletely resolved, but faint notches for the orbitonasal arteries are apparent posterior to the basipterygoid processes (nona; Fig. 8B, D), as in Mimipiscis (Gardiner 1984) and Coccocephalichthys (Poplin and Véran 1996). The basisphenoid pillar in this specimen is split open through the hypophyseal fossa, and the buccohypophyseal canal (bhc; Fig. 8B, D), which travels through the basisphenoid to open on the ventral surface of the parasphenoid, is visible. Lateral to the buccohypophyseal opening are two anterolaterally directed canals, which can also be traced on to the parasphenoid. Comparison with Mimipiscis (Gardiner 1984), Moythomasia and Lawrenciella (Hamel and Poplin 2008) suggests these transmitted the efferent pseudobranchial arteries (epsa; Fig. 8B, D). The buccohypophyseal canal and openings of the efferent pseudobranchials were both identified by Pearson and Westoll (1979, fig. 1). The entrance of the palatine artery into the basisphenoid is marked by an anteroventrally directed canal immediately in front of the ventral otic fissure (pal.a; Fig. 8B).
Figure 8.
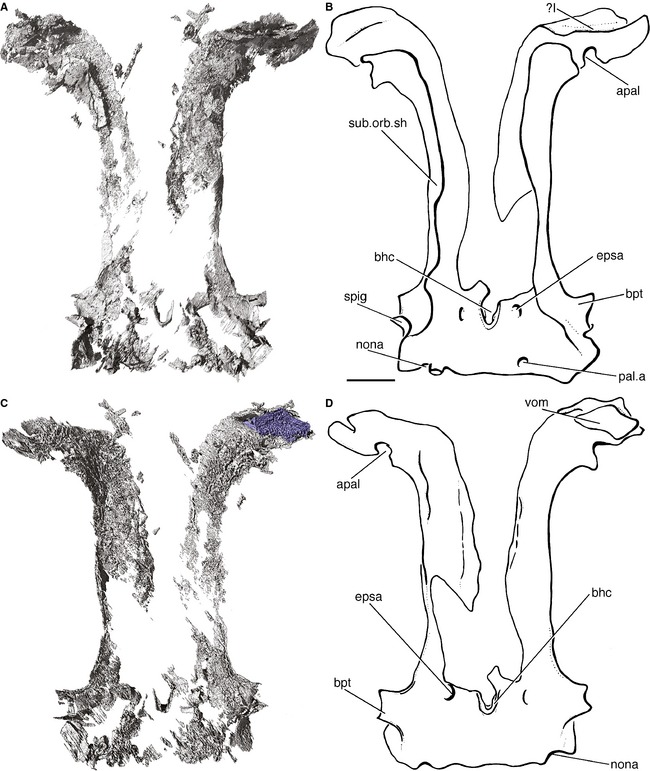
Ethmosphenoid portion of the braincase of Cheirolepis trailli NHMUK P.62908; parasphenoid preserved in counterpart. A, three‐dimensional rendering of braincase in dorsal view. B, interpretive drawing of braincase in dorsal view. C, three‐dimensional rendering of braincase in ventral view. D, interpretive drawing of braincase in ventral view. Anterior to top. Abbreviations: apal, articular facet for autopalatine; bhc, buccohypophyseal canal; bpt, basipterygoid process; epsa, efferent pseudobranchial artery; nona, notch for the orbitonasal artery; pal.a, palatine artery; spig, spiracular groove; sub.orb.sh, suborbital shelf; vom, vomer; I, olfactory nerve. Scale bar represents 2 mm. Colour online.
The sides of the basisphenoid extend laterally as small basipterygoid processes (bpt; Fig. 8B, D), as noted by Pearson and Westoll (1979). These processes are entirely endoskeletal and are not in contact with the parasphenoid; a dermal component to the basipterygoid processes is seen in Kentuckia (Rayner 1951), Kansasiella (Poplin 1974), Pteronisculus and Boreosomus (Nielsen 1942). The spiracular groove continues onto the basisphenoid (spig; Fig. 8B) behind the basipterygoid processes.
Due to the high level of dorsoventral compression, no details of the endocast can be described. As in most fish fossils preserved in carbonate matrix, the otoliths cannot be discerned, having presumably dissolved during fossilization.
Ethmoid region and parasphenoid
Very little of the ethmoid region is preserved, particularly above the level of the suborbital shelf. Additionally, much of the ventral part of the ethmoid is preserved in the counterpart. Anteriorly, the subnasal shelf flares dorsally. Two distinct notches mark the articular areas for the autopalatine (apal; Fig. 8B, D).
Parasphenoids are preserved in a number of specimens (e.g. NHMUK P.62908/NMS.1877.30.5; NHMUK P.60499; NMS.1892.8.60; NHMUK P.66863 (BMP.41410); NHMUK P.4051a/b; MCZ 6039; Fig. 3) and are separated from the bulk of the braincase in most instances. It is possible that these isolated parasphenoids represent individuals with poorly ossified or unossified endocrania. The parasphenoid is complete in NHMUK P.62908/NMS.1877.30.5 and is wholly preserved in the counterpart (Fig. 3A). As described by Pearson and Westoll (1979), this bone is simple, lacking a complex anterior margin and ascending processes, and quite unlike the ossifications seen in Mimipiscis and Moythomasia (Gardiner 1984; Choo 2011). Although the posterior part of the bone flares slightly behind the efferent pseudobranchial openings, there is no evidence of an ascending process. Ascending processes are also lacking in Mimipiscis. A shagreen of denticles covers much of the ventral surface of the parasphenoid in Cheirolepis (Fig. 3A, D).
A vomerine toothplate is preserved on the left side of the ethmoid (vom; Fig. 8D). The toothplate is lozenge shaped and is covered in fine denticles. The vomer is small, resembling those of Mimipiscis (Gardiner 1984) and Moythomasia (Gardiner and Bartram 1977), rather than the enlarged vomers of sarcopterygians (e.g. Eusthenopteron (Jarvik 1980); Powichthys (Jessen 1980; Clément and Janvier 2004)). It appears to be in life position, although it has been crushed somewhat into the ethmoid.
Hyomandibula
The left hyomandibula is preserved within NMS.1956.19 and is not visible on the surface. Although unbroken, it has been displaced to the region of the right scapulocoracoid. The hyomandibula is fairly slender and gently curved, and is firmly fused to the dermohyal (i.e. no sutures are apparent between these two ossifications in tomographs), as in Mimipiscis and Moythomasia (dhy; Fig. 9D). The dermohyal appears proportionately longer than in other early actinopterygians, although as the distal part of the hyomandibula appears to be unmineralized, its precise proportion cannot be determined. A distinct process on the medial face of the hyomandibular projects above the dermohyal (proc, Fig. 9C, D). Comparison with other actinopterygians suggests that this is too proximal to be the opercular process, and reference to Pearson and Westoll's (1979, fig. 20a) reconstruction suggests that this process may have contacted the internal face of the supratemporal. A deep gutter traverses the medial face of the hyomandibula. The shaft of the hyomandibula is imperforate, with the hyomandibular trunk of the facial nerve presumably passing behind and bifurcating distally, as in the extant Acipenser Linneaus, 1758 (Jollie 1980), some sarcopterygians (Andrews et al. 2006), acanthodians (Miles 1973), chondrichthyans (Maisey 1989; Coates and Sequeira 2001) and placoderms (Stensiö 1963; Forey and Gardiner 1986). No toothplates are evident on the hyomandibula of Cheirolepis, although these may have been present distally. The partial hyomandibulae described by Pearson and Westoll (1979, p. 362, fig. 10c, d) in NHMUK P.36061 and NMS.1877.30.5 appear to be fragments of other ossifications, although their correct attribution cannot be identified with any certainty.
Figure 9.
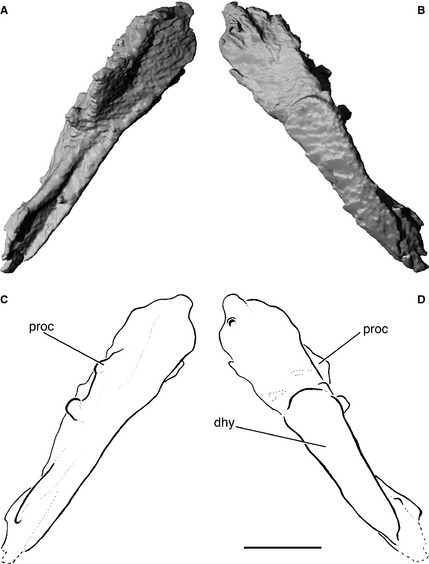
Left hyomandibula of Cheirolepis trailli NMS.1956.19. A, three‐dimensional rendering of hyomandibula in medial view. B, three‐dimensional rendering of hyomandibula in lateral view. C, interpretive drawing of hyomandibula in medial view. D, interpretive drawing of hyomandibula in lateral view. Abbreviations: dhy, dermohyal; proc, hyomandibula process. Scale bar represents 10 mm.
Pectoral fin endoskeleton
Both pectoral endoskeletal girdles are preserved in NMS.1956.19, although both are broken and thus cannot be exhaustively described. The left girdle is visible on the surface and was partially described by Pearson and Westoll (1979, fig. 14a), although their reconstruction of a complete scapulocoracoid (Pearson and Westoll 1979, fig. 12) is almost entirely hypothetical. The preserved scapulocoracoid in Cheirolepis is mineralized as a single ossification (scpc; Fig. 10C). The area of attachment to the cleithrum is fairly broad and elongate (art.cleith; Fig. 10D). There is no evidence of a ventral process nor a mesocoracoid arch. Thus, the scapulocoracoid appears to have had only a simple attachment to the cleithrum (art.cleith; Fig. 10D), unlike the tripartite attachment seen in Mimipiscis, Moythomasia (Gardiner 1984) and other actinopterygians (Nielsen 1942), although this may be due to poor mineralization or preservation in this specimen of Cheirolepis. The scapulocoracoid does not articulate with the clavicle (contra Pearson and Westoll 1979). While tripartite scapulocoracoids are also seen in sarcopterygians (e.g. Eusthenopteron, Jarvik 1980), members of the stem group, such as Psarolepis Yu, 1998 and Achoania Zhu et al., 2001, appear to have had simple attachments to the cleithrum (Zhu and Yu 2009). The anterior process (apr; Fig. 10D) in Cheirolepis appears extensive, although it is fragmented in both girdles. As a result, it is difficult to trace the course of any blood vessels. The processus lateralis identified by Pearson and Westoll (1979, fig. 14, Pr.l) appears to be an artefact on the surface of the specimen, and the processus ventralis (fig. 14, Pr.v) is in fact the misplaced metapterygium.
Figure 10.
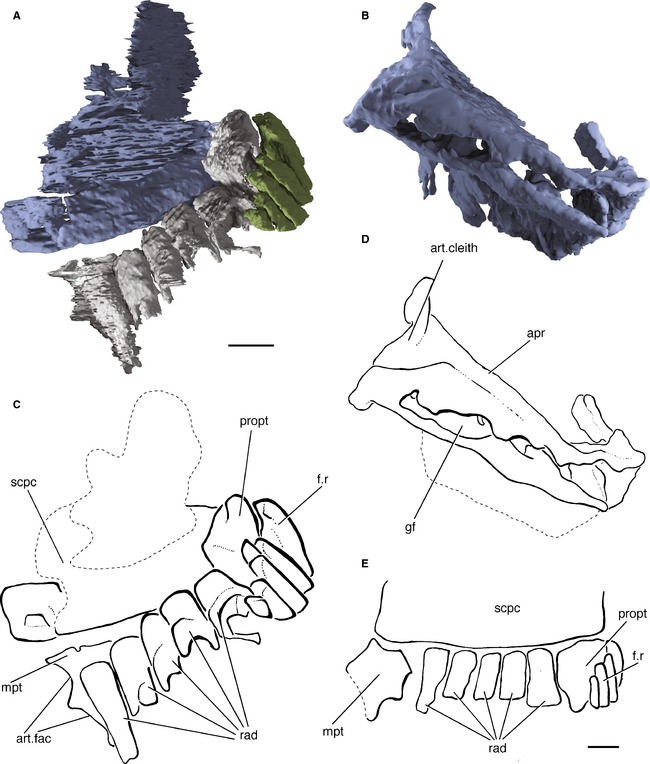
Left pectoral fin endoskeleton of Cheirolepis trailli NMS.1956.19. A, three‐dimensional rendering of fin endoskeleton in ventroposterior view; lateral edge of fin to right. B, three‐dimensional rendering of fin endoskeleton in posterior view; lateral edge of fin to left. C, interpretive drawing of fin endoskeleton in ventroposterior view; lateral edge of fin to right. D, interpretive drawing of fin endoskeleton in posterior view; lateral edge of fin to left. E, reconstruction of the left pectoral fin endoskeleton in ventral view; lateral edge of fin to right. Abbreviations: apr, anterior process of scapulocoracoid; art.cleith, articular area for cleithrum; art.fac, articular facets for secondary radials; f.r, fin rays; gf, glenoid fossa; mpt, metapterygium; propt, propterygium; rad, radial; scpc, scapulocoracoid. Both scale bars represent 2 mm.
The glenoid fossa is narrow and elongate (gf; Fig. 10D). It bears several moderately well‐developed articular facets for the radials. The radials are preserved as hollow perichondral sheaths, and those of the left fin have been shifted out of the scapulocoracoid plane. The radials of the right fin are completely disarticulated. The propterygium is stout and is clasped by four fin rays (propt, f.r; Fig. 10C). Unlike all other early actinopterygians, the propterygium is not pierced by the propterygial canal. The metapterygium is large and robust, although not noticeably elongate (mpt; Fig. 10C). It bears articular facets to support two distal radials (art.fac; Fig. 10C), although these are not preserved; these were probably cartilaginous, as in Polypterus and possibly Mimipiscis (Gardiner 1984). The shape of the metapterygium is more similar to that of actinopterygian outgroups such as Acanthodes (Coates 2003, fig. 3b) than other actinopterygians such as Mimipiscis (Gardiner 1984, fig. 137). However, the presence of an elongate metapterygium in sarcopterygians makes it difficult to assess whether Cheirolepis has retained the primitive condition or if its metapterygium is secondarily reduced. Five radials sit between the propterygium and metapterygium (rad; Fig. 10C).
Body scales
Several patches of body scales are preserved in NHMUK P.62908, including a number found in articulation with each other but inside the braincase (Fig. 11). Although the external surface of the scales is poorly resolved, the internal surface is well preserved. The scales are rhomboid, with a pronounced anterodorsal process, and bear a distinct midline keel (ant.d, k; Fig. 11B). Most importantly, the scales display peg‐and‐socket articulation, a feature previously considered absent in Cheirolepis trailli (Pearson and Westoll 1979; Gardiner 1984; Arratia and Cloutier 1996). As in Psarolepis and acanthodians, but unlike the scales of most osteichthyans (Qu et al. 2013), the rhomboid scales of Cheirolepis bear a distinct neck between the crown and base (n; Fig. 11B).
Figure 11.
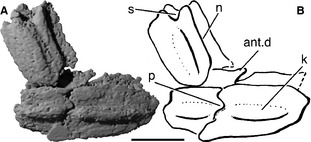
Scales of Cheirolepis trailli NHMUK P.62908 in ventral view. A, three‐dimensional rendering of scales. B, interpretive drawing of scales. Abbreviations: ant.d, anterodorsal process; k, keel; n, neck; p, peg; s, socket. Anterior to right. Scale bar represents 0.5 mm.
Comparative morphology
Howqualepis
The braincase of Howqualepis is known in most detail from AMF65495, where it is preserved in ventral view but is dorsoventrally compressed (Long 1988, fig. 16). As described by Long, the ascending processes are elongate, and end in a fairly blunt edge rather than tapering (asc.pr; Fig. 12B). Although depicted as having a pointed apex in Moythomasia (Gardiner 1984, fig. 7), unbroken ascending processes in this genus also tend to terminate rather bluntly (SG pers. obs.). The processes in Howqualepis do not appear to be denticulated. The basipterygoid processes are well developed and are entirely endoskeletal (bpt; Fig. 12B). The shape of the parasphenoid denticle field is difficult to interpret, but the denticles are largely restricted to the anterior portion of the parasphenoid. The postorbital process in Howqualepis is incompletely preserved. The median dorsal aorta is housed in an open groove, rather than an enclosed canal (da; Fig. 12B).
Figure 12.

Virtual cast of the braincase of Howqualepis rostridens AMF65495 in ventral view. A, three‐dimensional rendering of braincase. B, interpretive drawing of braincase. Anterior to top. Abbreviations: apal, articular facet for autopalatine; asc.pr, ascending process; bhc, buccohypophyseal canal; bpt, basipterygoid process; da, dorsal aorta; epsa, efferent pseudobranchial artery; lda, lateral dorsal aorta; oof, otico‐occipital fissure; por, postorbital process; psp, parasphenoid; vof, ventral otic fissure; v.fon, vestibular fontanelle. Scale bar represents 5 mm.
Tegeolepis
The parasphenoid of Tegeolepis is preserved in dorsal view in CMNH 5518. It is slender and is more similar in shape to the parasphenoid of Cheirolepis than that of any other early actinopterygian (Fig. 13). The parasphenoid is narrow anteriorly and tapers to its narrowest point just in front of the buccohypophyseal canal. It bears two small basipterygoid processes (?bpt; Fig. 13B), but their skeletal origin is unclear. Posteriorly, the parasphenoid flares into two short ascending processes (asc.pr; Fig. 13B), reminiscent of those of Howqualepis. No details of the parasphenoid denticle field can be described.
Figure 13.
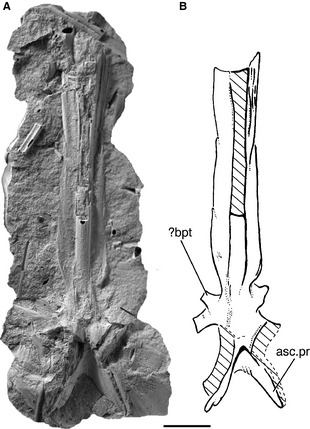
Parasphenoid of Tegeolepis clarkii CMNH 5518 in dorsal view. A, photograph of parasphenoid. B, interpretive drawing of parasphenoid. Anterior to top. Abbreviations: asc.pr, ascending process; bpt, basipterygoid process. Scale bar represents 5 mm.
The parasphenoid described here bears little relation to that reconstructed by Dunkle and Schaeffer (1973, fig. 1) for another specimen (CMNH 8244), in which the lateral edges of the bone are strongly fluted, the ascending processes are very narrow, and the posterior of the bone is developed as a long stalk that would have extended below the otic and occipital regions. Based on observations of this specimen, we question the interpretation of CMNH 8244 as a parasphenoid, and note that the structure in question is not intact.
Discussion
Cheirolepis and other early actinopterygians
Although broadly resembling other that of actinopterygians, the braincase of Cheirolepis bears clear similarities with members of actinopterygian outgroups, in particular the sarcopterygians. These features, outlined below, are presumably osteichthyan symplesiomorphies (Fig. 14). The most conspicuous of these is the uninvested dorsal aorta; an enclosed midline canal for the aorta has previously been considered an actinopterygian synapomorphy (Rayner 1951; Miles 1973; Patterson 1975; Gardiner 1984; Long 1988; Friedman and Brazeau 2010). Although present in all other early actinopterygians (with the exception of Howqualepis), the dorsal aorta is uninvested in sarcopterygians (e.g. Eusthenopteron, Jarvik 1980; Youngolepis, Chang 1982) and acanthodians (e.g. Acanthodes, Miles 1973). The condition of the dorsal aorta in the early osteichthyans Ligulalepis (Basden and Young 2001) and Psarolepis (Yu 1998) is unknown, as the only described braincases for these taxa lack the ventral occipital plate. Other features that appear plesiomorphic for actinopterygians apparent in Cheirolepis include the relative position of bifurcation of the dorsal aorta on the basioccipital, which bears a greater resemblance to sarcopterygians such as Youngolepis; and the imperforate propterygium (as noted by Pearson and Westoll 1979; Friedman and Brazeau 2010; contra Sallan 2014), a character shared with chondrichthyans, with the presence of a propterygial canal previously considered to be an actinopterygian synapomorphy. The large vestibular fontanelles of Cheirolepis resemble those of Onychodus Newberry, 1857 (Andrews et al. 2006), Youngolepis (Chang 1982), Spodichthys Jarvik, 1985 (Snitting 2008) and Eusthenopteron (Jarvik 1980), in contrast to the much smaller fontanelles of Mimipiscis (Gardiner 1984), suggesting that this feature might also be primitive for actinopterygians. However, the lack of preservation of the occipital plate in stem sarcopterygians, coupled with the absence of vestibular fontanelles in many sarcopterygian taxa (e.g. Qingmenodus Lu and Zhu, 2010; Styloichthys Zhu and Yu, 2002; coelacanths, Forey 1998; megalichthyid tetrapodomorphs, Fox et al. 1995), leaves open the question of whether the large fontanelles of Cheirolepis are plesiomorphic or secondarily derived. In addition to differences in the endoskeleton, the dermal skeleton of Cheirolepis has also long been considered atypical of other actinopterygians, given the large number of plesiomorphic osteichthyan features it retains. These features include unconsolidated rostral bones, a tectal, elongate jaws, lobed base to pectoral fins, epichordal lobe in caudal fin, and lack of acrodin tooth caps (Gardiner 1963; Pearson and Westoll 1979; Pearson 1982; Friedman and Brazeau 2010).
Figure 14.
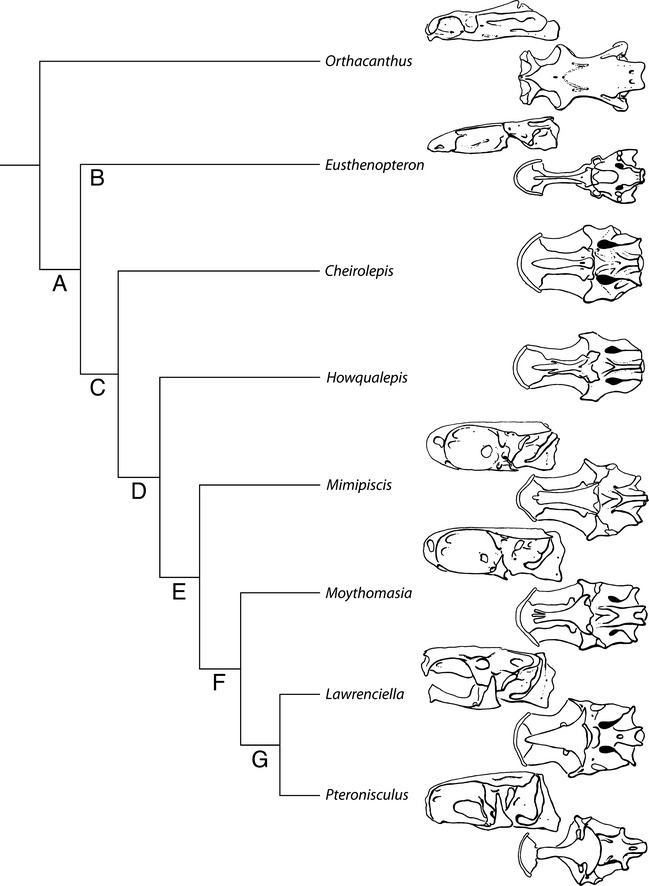
Cladogram of early vertebrate relationships, with an emphasis on actinopterygians, showing potential apomorphies in the endoskeleton. Cladogram based on Choo (2011), Zhu et al. (2013) and Xu et al. (2014). See Discussion for a list of proposed synapomorphies at lettered nodes. Braincase images redrawn from Orthacanthus, Schaeffer 1981; Eusthenopteron, Jarvik 1980; Mimipiscis, Gardiner 1984, Choo 2011; Moythomasia, Long and Trinajstic 2010; Lawrenciella, Hamel and Poplin 2008; Pteronisculus, Nielsen 1942.
Despite differing from Mimipiscis and other actinopterygians in certain key respects, Cheirolepis possesses several features that unite it with other actinopterygians: the presence of a basipterygoid fenestra in the palatoquadrate (Pearson and Westoll 1979, fig. 7c, d), a single dorsal fin (Pearson and Westoll 1979, fig. 16; Arratia and Cloutier 1996, fig. 4a) and the absence of a jugal canal (Pearson and Westoll 1979, fig. 20; Arratia and Cloutier 1996, fig. 6; Arratia and Cloutier 2004, fig. 4; Friedman and Brazeau 2010). The generalized conditions outlined above confirm the position of Cheirolepis as an early diverging member of Actinopterygii.
Parasphenoid evolution in early actinopterygians
New data on the parasphenoid in Tegeolepis, as well as new descriptions of the parasphenoids of Mimipiscis toomsbi and M. bartrami Choo, 2011, allow a detailed understanding of the evolution of the parasphenoid in early actinopterygians. Ascending processes of the parasphenoid, previously considered to be a derived actinopterygian character confined to actinopterygians crownwards of Mimipiscis (Patterson 1982; Gardiner 1984; Coates 1999; Gardiner et al. 2005), are present in all actinopterygians crownwards of Cheirolepis (Node D of Fig. 14; Choo 2011). The slight lateral extensions posterior to the buccohypophyseal canal in Cheirolepis may represent homologues of the ascending process of later actinopterygians. The absence of this process in Mimipiscis thus appears to be a secondary loss. Several groups of sarcopterygians bear ascending processes of the parasphenoid, or expanded denticle plates that occupy a similar region as actinopterygian ascending processes (Jarvik 1980; Jessen 1980; Chang 1982). However, patterns of character distribution given current hypotheses of osteichthyans interrelationships suggest that these are independently derived relative to ascending processes in actinopterygians.
Implications for the actinopterygian character suite
The recognition of features previously thought to be restricted to sarcopterygians in the braincase of an early actinopterygian, most particularly the uninvested dorsal aorta, indicates that these characters represent osteichthyan plesiomorphies. Cheirolepis lacks several key features previously identified as actinopterygian synapomorphies, allowing a new understanding of the sequence of character evolution early in the history of the group (Fig. 14). Because these data do not change the phylogenetic position of Cheirolepis, only reinforce existing interpretations, this character distribution can be summarized by mapping key neurocranial and endocast characters onto a cladogram based on recent analyses of early actinopterygian and osteichthyan relationships (Choo 2011; Zhu et al. 2013; Xu et al. 2014; Fig. 14).
The key morphological changes in the endoskeleton at successive nodes can be summarized as follows:
Node A, Osteichthyes: long olfactory tracts (Coates 1999; Friedman and Brazeau 2010; Brazeau and Friedman 2014; Giles and Friedman 2014; reversals in tetrapodomorphs and some post‐Devonian actinopterygians); horizontal semicircular canal joins labyrinth level with ampulla for posterior semicircular posterior canal (Davis et al. 2012; Brazeau and Friedman 2014; Giles and Friedman 2014); co‐mineralized ethmoid and sphenoid completely enclosing nasal capsules (Friedman and Brazeau 2010; reversals in some lungfishes, limb‐bearing tetrapods and post‐Devonian actinopterygians); endocranial cavity dorsally restricted within sphenoid (Brazeau and Friedman 2014).
Node B, Sarcopterygii: endoskeletal intracranial joint (Janvier 1996; Friedman and Brazeau 2010; reversals in some lungfishes and limb‐bearing tetrapods); basicranial fenestra (Friedman and Brazeau 2010; reversal in some lungfishes); broad or bipartite hyomandibular articulation (Miles 1977; Lauder and Liem 1983; Gardiner 1984; Friedman and Brazeau 2010); vertical component to basipterygoid process (Davis et al. 2012).
Node C, Actinopterygii: basipterygoid fenestra in palatoquadrate (Friedman 2007; Friedman and Brazeau 2010; reversal in some post‐Carboniferous actinopterygians); single dorsal fin; dermohyal fused to hyomandibula (Patterson 1982; Gardiner 1984; Coates 1999; Gardiner et al. 2005; reversals in Tegeolepis and some post‐Devonian actinopterygians).
Node D: ascending process of the parasphenoid (Patterson 1982; Gardiner 1984; Coates 1999; Gardiner et al. 2005; reversals in Mimipiscis and Gogosardina).
Node E: enclosed dorsal aorta (Miles 1973; Coates 1999; Friedman and Brazeau 2010); propterygium pierced by propterygial canal (condition unknown in Howqualepis and Tegeolepis; Patterson 1982; Gardiner 1984; Gardiner and Schaeffer 1989; Coates 1999; Gardiner et al. 2005; Friedman and Brazeau 2010); pectoral fin endoskeleton does not project outside of body (Coates 1999; reversal in some post‐Carboniferous actinopterygians).
Node F: enclosed spiracular canal (Patterson 1982; Gardiner 1984; Gardiner and Schaeffer 1989; Coates 1999; Gardiner et al. 2005).
Node G: anteriorly (as opposed to anterolaterally) directed olfactory tracts (Coates 1999; Giles and Friedman 2014); olfactory nerves carried in single midline tube (Coates 1999; Giles and Friedman 2014; reversal in some post‐Carboniferous actinopterygians); optic lobes same width as cerebellum (Giles and Friedman 2014); corpus cerebellum of anterior and posterior semicircular canals ventral to endocranial roof (Giles and Friedman 2014); fossa bridgei (Miles 1973; Gardiner 1984; Gardiner and Schaeffer 1989; Coates 1999); expanded anterior dorsal fontanelle. (NB some or all of the latter four characters may occur at Node F, but as an endocast for Moythomasia has not been described this cannot be determined.)
The endoskeleton of Cheirolepis differs from that of Mimipiscis in several important respects, including an uninvested dorsal aorta; large vestibular fontanelles; elongate basioccipital portion of the braincase; unelaborated parasphenoid lacking a multifid anterior margin and ascending processes; pectoral endoskeleton protruding from the body; and imperforate propterygium. In all of these respects, Cheirolepis more closely resembles early members of actinopterygian outgroups than Mimipiscis and other Devonian actinopterygians. The recognition of apomorphic features in the braincase of Mimipiscis, for example the loss of ascending processes of the parasphenoid, the presence of an elongate, fully enclosed dorsal aorta and the very small vestibular fontanelles (Gardiner 1984), further bring into question the suitability of Mimipiscis as representative of the plesiomorphic actinopterygian condition.
The description of the braincase and other endoskeletal anatomy of Cheirolepis adds more detail to our understanding of endoskeletal evolution in early actinopterygians. However, further data, for example from Howqualepis (Long 1988) and Gogosardina Choo et al., 2009 are needed to fully understand the early evolution of the group. Despite distinct apomorphies in its dermal skeleton (such as micromeric squamation), the endoskeleton of Cheirolepis appears to largely reflect the primitive osteichthyan condition. Cladistic analyses of early actinopterygian relationships have tended to rely almost exclusively on dermal characters (Gardiner and Schaeffer 1989; Cloutier and Arratia 2004; Friedman and Blom 2006), reflecting Gardiner and Schaeffer's (1989) view that neurocrania were too anatomically conserved to be of use in determining early actinopterygian relationships. Consequently, placement of taxa known largely or exclusively from endoskeletal remains impossible (e.g. Lawrenciella, Hamel and Poplin 2008; Kansasiella, Poplin 1974). Further problems arise from the variable nature of the dermal skeleton. Many dermal characters reflect the overall nature of the dermal skeleton as either an armour of regularly patterned plates (as in placoderms and osteichthyans) or fields of only regionally differentiated denticles or tesserae (as in acanthodians and chondrichthyans). Neurocrania are, by contrast, present in all vertebrates, and their rich complement of characters is thus comparable across almost the entirety of vertebrate diversity. The expansion of the actinopterygian character set to include more endoskeletal characters will help place problematic taxa and facilitate their routine inclusion into phylogenetic analyses of early actinopterygian relationships, thus increasing the sample set of Devonian and Carboniferous actinopterygians. Since Gardiner and Schaeffer's (1989) denouncement of neurocranial characters, the discovery of additional taxa preserving braincases (e.g. Lawrenciella, Hamel and Poplin 2008; Gogosardina, Choo et al. 2009), as well as work revealing new diagnostic characters within these structures (Coates 1998, 1999; Giles and Friedman 2014), has made clear their importance for unravelling early actinopterygian relationships. The utility of neurocranial character sets has also been established as a tool in resolving systematic issues in other taxonomic groups (e.g. chondrichthyans, Coates and Sequeira 1998, 2001, Maisey and Anderson 2001; lungfishes, Friedman 2007; tetrapodomorph fishes, Coates and Friedman 2010).
Conclusion
The endoskeleton of the Eifelian Cheirolepis trailli, from the Middle Old Red Sandstone of Scotland, is described here on the basis of lab‐ and synchrotron‐based μCT. The unique combination of primitive osteichthyan and derived actinopterygian characters revealed in the endoskeleton confirms the position of Cheirolepis as the sister taxon to all other actinopterygians, and mirrors the atypical actinopterygian morphology seen in the dermal skeleton (Pearson and Westoll 1979). The presence of ascending processes of the parasphenoid in Howqualepis and Tegeolepis confirms that these appeared fairly early in actinopterygian evolution, and were secondarily lost or reduced in Mimipiscis, as concluded by Choo (2011).
The emerging picture of the endoskeleton in early ray‐finned fishes is that it looked broadly like that of early sarcopterygians, with an uninvested dorsal aorta, large vestibular fontanelles, and an elongate basioccipital region. This is far removed from the endoskeletal anatomy of Mimipiscis, which shares more features with stratigraphically younger actinopterygians, as well as having secondarily lost or reduced key features. The endoskeleton of early sarcopterygians perhaps more accurately reflects aspects of the primitive osteichthyan, including those features outlined above. The discovery of further specimens of Cheirolepis preserving the braincase, particularly material that is three‐dimensionally preserved, may provide a way of further corroborating these observations.
Acknowledgements
We thank E. Bernard (Natural History Museum), S. Walsh (National Museums of Scotland), J. Cundiff (Museum of Comparative Zoology), Y.Y. Zhen (Australian Museum) and M. Ryan (Cleveland Museum of Natural History) for access to material. Assistance with synchrotron‐based CT scanning was kindly provided by M. Drakopoulos (Diamond Light Source) and lab‐based CT scanning by F. Ahmed, D. Sykes and R. Summerfield (Natural History Museum, London). We are grateful to P. Cox and S. Cobb (Hull York Medical School) for assistance with 3D printing. SG was supported by the National Environment Research Council (NERC cohort NE/J500045/1). RJG is an 1851 Royal Commission Research Fellow. MDB was supported by the European Research Council under the European Union's Seventh Framework Programme (FP/2007‐2013)/ERC Grant Agreement number 311092. Facilities for the processing of CT data were supported by a Fell Fund Grant and Philip Leverhulme Prize to MF.
Data archiving statement
Data for this study are available in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8bs2b
Contributor Information
Sam Giles, Email: sam.giles@earth.ox.ac.uk.
Michael I. Coates, Email: mcoates@uchicago.edu
Russell J. Garwood, Email: russell.garwood@manchester.ac.uk
Martin D. Brazeau, Email: m.brazeau@imperial.ac.uk
Robert Atwood, Email: robert.atwood@diamond.ac.uk.
Zerina Johanson, Email: z.johanson@nhm.ac.uk.
Matt Friedman, Email: mattf@earth.ox.ac.uk.
References
- Agassiz, L. 1833. –1844. Recherches sur les Poissons fossiles, 5 volumes. Imprimerie de Petitpierre et Prince, Neuchâtel, 1420 pp. [Google Scholar]
- Allis, E. P. 1923. The cranial anatomy of Chlamydoselachus anguineus . Acta Zoologica, 4, 123–221. [Google Scholar]
- Andrews, S. M. , Long, J. A. , Ahlberg, P. E. , Barwick, R. and Campbell, K. 2006. The structure of the sarcopterygian Onychodus jandemarrai n. sp. from Gogo, Western Australia: with a functional interpretation of the skeleton. Transactions of the Royal Society of Edinburgh, 96, 197–307. [Google Scholar]
- Arratia, G. and Cloutier, R. 1996. Reassessment of the morphology of Cheirolepis canadensis (Actinopterygii) 165–197. In Schultze H.‐P. and Cloutier R. (eds). Devonian fishes and plants of Miguasha, Quebec, Canada. Verlag Dr Frederich Pfeil, Munich, 374 pp. [Google Scholar]
- Arratia, G. and Cloutier, R. 2004. A new cheirolepidid fish from the Middle–Upper Devonian of Red Hill, Nevada, USA 583–598. In Arratia G., Wilson M. V. H. and Cloutier R. (eds). Recent advances in the origin and early radiation of vertebrates. Verlag Dr Friedrich Pfeil, Munich, 703 pp. [Google Scholar]
- Basden, A. M. and Young, G. C. 2001. A primitive actinopterygian neurocranium from the Early Devonian of southeastern Australia. Journal of Vertebrate Paleontology, 21, 754–766. [Google Scholar]
- Basden, A. M. , Young, G. C. , Coates, M. I. and Ritchie, A. 2000. The most primitive osteichthyan braincase? Nature, 403, 185–188. [DOI] [PubMed] [Google Scholar]
- Bjerring, H. C. 1971. The nerve supply to the second metamere basicranial muscle in Osteolepiform vertebrates, with some remarks on the basic composition of the endocranium. Acta Zoologica, 52, 189–225. [Google Scholar]
- Blieck, A. and Cloutier, R. 2000. Biostratigraphical correlations of Early Devonian vertebrate assemblages of the Old Red Sandstone Continent. Courier Forschungsinstitut Senckenberg, 223, 223–269. [Google Scholar]
- Botella, H. , Blom, H. , Dorka, M. , Ahlberg, P. E. and Janvier, P. 2007. Jaws and teeth of the earliest bony fishes. Nature, 448, 583–586. [DOI] [PubMed] [Google Scholar]
- Brazeau, M. D. 2009. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature, 457, 305–308. [DOI] [PubMed] [Google Scholar]
- Brazeau, M. D. and Friedman, M. F. 2014. The characters of Palaeozoic jawed vertebrates. Zoological Journal of the Linnean Society, 170, 779–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton, R. E. , Betancur, R. , Li, C. , Arratia, G. and Ortí, G. 2013. Multi‐locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLoS Currents, Tree of Life, 5. doi:10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M.‐M. 1982. The braincase of Youngolepis, a Lower Devonian crossopterygian from Yunnan, south‐western China. Unpublished PhD thesis, University of Stockholm. [Google Scholar]
- Chang, M.‐M. and Yu, X. B. 1981. A new crossopterygian, Youngolepis praecursor, gen. et sp. nov., from Lower Devonian of E. Yunnan, China. Scientia Sinica, 24, 89–97. [Google Scholar]
- Choo, B. 2011. Revision of the actinopterygian genus Mimipiscis (= Mimia) from the Upper Devonian Gogo Formation of Western Australia and the interrelationships of the early Actinopterygii. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 102, 77–104. [Google Scholar]
- Choo, B. , Long, J. A. and Trinajstic, K. 2009. A new genus and species of basal actinopterygian fish from the Upper Devonian Gogo Formation of Western Australia. Acta Zoologica, Supplement, 90, 194–210. [Google Scholar]
- Cignoni, P. , Corsini, M. and Ranzuglia, G. 2008. Meshlab: an open‐source 3D mesh processing system. ERCIM News, 63, 45–46. [Google Scholar]
- Clément, G. and Janvier, P. 2004. Powichthys spitsbergensis sp. nov., a new member of the Dipnomorpha (Sarcopterygii, lobe‐finned fishes) from the Lower Devonian of Spitsbergen, with remarks on basal dipnomorph anatomy. Fossils and Strata, 50, 92–112. [Google Scholar]
- Cloutier, R. and Arratia, G. 2004. Early diversification of actinopterygians 217–270. In Arratia G., Wilson M. V. H. and Cloutier R. (eds). Recent advances in the origin and early radiation of vertebrates. Verlag Dr Friedrich Pfeil, Munich, 703 pp. [Google Scholar]
- Coates, M. I. 1998. Actinopterygians from the Namurian of Bearsden, Scotland, with comments on early actinopterygian neurocrania. Zoological Journal of the Linnean Society, 122, 27–59. [Google Scholar]
- Coates, M. I. 1999. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, UK, and the interrelationships of primitive actinopterygians. Philosophical Transactions of the Royal Society of London, Series B, 354, 435–462. [Google Scholar]
- Coates, M. I. 2003. The evolution of paired fins. Theory in Biosciences, 122, 266–287. [Google Scholar]
- Coates, M. I. and Friedman, M. 2010. Litoptychus bryanti and characteristics of stem tetrapod neurocrania 389–416. In Elliot G. K., Maisey J. G., Yu K. and Miao D. (eds). Morphology, phylogeny and paleobiogeo‐graphy of fossil fishes. Verlag, Dr Friedrich Pfeil, Munich, 472 pp. [Google Scholar]
- Coates, M. I. and Sequeira, S. E. K. 1998. The braincase of a primitive shark. Transactions of the Royal Society of Edinburgh: Earth Sciences, 89, 63–85. [Google Scholar]
- Coates, M. I. and Sequeira, S. E. K. 2001. A new stethacanthid chondrichthyan from the Lower Carboniferous of Bearsden, Scotland. Journal of Vertebrate Paleontology, 21, 438–459. [Google Scholar]
- Cope, E. D. 1887. Geology and palaeontology. American Naturalist, 1887, 1014–1019. [Google Scholar]
- Davis, S. P. , Finarelli, J. A. and Coates, M. I. 2012. Acanthodes and shark‐like conditions in the last common ancestor of modern gnathostomes. Nature, 486, 247–250. [DOI] [PubMed] [Google Scholar]
- Dunkle, D. H. and Schaeffer, B. 1973. Tegeolepis clarki (Newberry), a palaeonisciform from the Upper Devonian Ohio Shale. Palaeontographica Abteilung A, 143, 151–158. [Google Scholar]
- Dupret, V. , Sanchez, S. , Goujet, D. , Tafforeau, P. and Ahlberg, P. E. 2014. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature, 507, 500–503. [DOI] [PubMed] [Google Scholar]
- Eastman, C. R. 1908. Devonian fishes of Iowa. Iowa Geological Survey Annual Report, 18, 29–386. [Google Scholar]
- Egerton, P. M. G. 1860. Palichthyological Notes, No. 12: Remarks on the nomenclature of the Devonian Fishes. Quarterly Journal of the Geological Society of London, 16, 119–136. [Google Scholar]
- Egerton, P. M. G. 1864. On some ichthyolites from New South Wales. Quarterly Journal of the Geological Society of London, 20, 1–5. [Google Scholar]
- Faircloth, B. C. , Sorenson, L. , Santini, F. and Alfaro, M. E. 2013. A phylogenomic perspective on the radiation of ray‐finned fishes based upon targeted sequencing of ultraconserved elements (UCEs). PLoS One, 8, e65923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forey, P. L. 1998. History of the coelacanth fishes. Chapman & Hall, London, 419 pp. [Google Scholar]
- Forey, P. L. and Gardiner, B. G. 1986. Observations on Ctenurella (Ptyctodontida) and the classification of placoderm fishes. Zoological Journal of the Linnean Society, 86, 43–74. [Google Scholar]
- Fox, R. C. , Campbell, K. S. W. , Barwick, R. E. and Long, J. A. 1995. A new osteolepiform fish from the Lower Carboniferous Raymond Formation, Drummond Basin, Queensland. Memoirs of the Queensland Museum, 38, 99–221. [Google Scholar]
- Friedman, M. 2007. Styloichthys as the oldest coelacanth: implications for early osteichthyan interrelationships. Journal of Systematic Palaeontology, 5, 289–343. [Google Scholar]
- Friedman, M. 2015. The early evolution of ray‐finned fishes. Palaeontology, 58, 213–228. [Google Scholar]
- Friedman, M. and Blom, H. 2006. A new actinopterygian from the Famennian of East Greenland and the interrelationships of Devonian ray‐finned fishes. Journal of Paleontology, 80, 1186–1204. [Google Scholar]
- Friedman, M. and Brazeau, M. D. 2010. A reappraisal of the origin and basal radiation of the Osteichthyes. Journal of Vertebrate Paleontology, 30, 36–56. [Google Scholar]
- Friedman, M. , Coates, M. I. and Anderson, P. 2007. First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evolution & Development, 9, 329–337. [DOI] [PubMed] [Google Scholar]
- Gardiner, B. G. 1963. Certain palaeoniscoid fishes and the evolution of the snout in actinopterygians. Bulletin of the British Museum (Natural History): Geology, 8, 254–325. [Google Scholar]
- Gardiner, B. G. 1984. The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bulletin of the British Museum (Natural History): Geology, 37, 173–428. [Google Scholar]
- Gardiner, B. G. and Bartram, A. W. H. 1977. The homologies of ventral cranial fissures in osteichthyans 227–245. In Andrews S. M., Miles R. S. and Walker A. D. (eds). Problems in vertebrate evolution. Academic Press, London, 411 pp. [Google Scholar]
- Gardiner, B. G. and Schaeffer, B. 1989. Interrelationships of lower actinopterygian fishes. Zoological Journal of the Linnean Society, 97, 135–187. [Google Scholar]
- Gardiner, B. G. , Schaeffer, B. and Masserie, J. A. 2005. A review of the lower actinopterygian phylogeny. Zoological Journal of the Linnean Society, 144, 511–525. [Google Scholar]
- Garwood, R. and Dunlop, J. 2014. The walking dead: Blender as a tool for paleontologists with a case study on extinct arachnids. Journal of Paleontology, 88, 735–746. [Google Scholar]
- Giles, S. and Friedman, M. 2014. Virtual reconstruction of endocast anatomy in early ray‐finned fishes (Osteichthyes, Actinopterygii). Journal of Paleontology, 88, 636–651. [Google Scholar]
- Giles, S. , Friedman, M. and Brazeau, M. D. 2015a. Osteichthyan‐like cranial conditions in an Early Devonian stem gnathostome. Nature, 520, 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles, S. , Coates, M. I. , Garwood, R. J. , Brazeau, M. D. , Atwood, R. , Johanson, Z. and Friedman, M. 2015b. Data from: Endoskeletal structure in Cheirolepis (Osteichthyes, Actinopterygii), the earliest ray‐finned fish. Dryad Digital Repository. doi:10.5061/dryad.8bs2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradstein, F. M. , Ogg, J. G. , Schmitz, M. and Ogg, G. 2012. The geologic time scale 2012. Elsevier Science & Technology, Oxford, 1176 pp. [Google Scholar]
- Gross, W. 1968. Fragliche Actinopterygier‐Schuppen aus den Silur Gotlands. Lethaia, 1, 184–218. [Google Scholar]
- Gross, W. 1973. Kleinschuppen, Flossenstacheln un Zähne von Fischen aus europäischen und nordamerikanischen Bonebeds des Devons. Palaeontographica Abteilung A, 142, 51–155. [Google Scholar]
- Hamel, M.‐H. and Poplin, C. 2008. The braincase anatomy of Lawrenciella schaefferi, actinopterygian from the Upper Carboniferous of Kansas (USA). Journal of Vertebrate Paleontology, 28, 989–1006. [Google Scholar]
- Holland, T. 2014. The endocranial anatomy of Gogonasus andrewsae Long, 1985 revealed through micro CT‐scanning. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 105, 9–34. [Google Scholar]
- Huxley, T. H. 1880. On the applications of the laws of evolution to the arrangement of the Vertebrata and more particularly of the Mammalia. Proceedings of the Zoological Society of London, 43, 649–662. [Google Scholar]
- Janvier, P. 1978. On the oldest known teleostome fish Andeolepis hedei Gross (Ludlow of Gotland) and the systematic position of the lophosteids. Eesti NSV Teaduste Akadeemia Toimetised, Geoloogia, 27, 86–95. [Google Scholar]
- Janvier, P. 1996. Early vertebrates. Clarendon Press, Oxford, 393 pp. [Google Scholar]
- Jarvik, E. 1980. Basic structure and evolution of vertebrates, Vol. 1. Academic Press, London, 575 pp. [Google Scholar]
- Jarvik, E. 1985. Devonian osteolepiform fishes from Greenland. Meddelelser om Grönland, 13, 1–52. [Google Scholar]
- Jessen, H. 1980. Lower Devonian porolepiformes from the Canadian Arctic with special reference to Powichthys thorsteinssoni . Palaeontographica Abteilung A, 167, 180–214. [Google Scholar]
- Jollie, M. 1980. Development of head and pectoral girdle skeleton and scales in Acipenser . Copeia, 2, 226–249. [Google Scholar]
- Lauder, G. V. and Liem, K. F. 1983. The evolution and interrelationships of the actinopterygian fishes. Bulletin of the Museum of Comparative Zoology, 150, 95–197. [Google Scholar]
- Long, J. A. 1985. A new osteolepidid fish from the Upper Devonian Gogo Formation, Western Australia. Records of the Western Australian Museum, 12, 361–377. [Google Scholar]
- Long, J. A. 1988. New palaeoniscoid fishes from the Late Devonian and early Carboniferous of Victoria. Memoir of the Australasian Association of Palaeontologists, 7, 1–64. [Google Scholar]
- Long, J. A. and Trinajstic, K. 2010. The Late Devonian Gogo Formation Lägerstatte of Western Australia: exceptional early vertebrate preservation and diversity. Annual Review of Earth and Planetary Sciences, 38, 255–279. [Google Scholar]
- Long, J. A. , Choo, B. and Young, G. C. 2008. A new basal actinopterygian from the Middle Devonian Aztec Siltstone of Antarctica. Antarctic Science, 20, 393–412. [Google Scholar]
- Lu, J. and Zhu, M. 2010. An onychodont fish (Osteichthyes, Sarcopterygii) from the Early Devonian of China, and the evolution of the Onychodontiformes. Proceedings of the Royal Society B, 277, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukševičs, E. , Lebedev, O. A. and Zakharenko, G. V. 2010. Palaeozoogeographical connections of the Devonian vertebrate communities of the Baltica Province. Part I. Eifelian–Givetian. Palaeoworld, 19, 94–107. [Google Scholar]
- Maisey, J. G. 1989. Hamiltonichthys mapesi g. & sp. nov. (Chondrichthyes; Elasmobranchii), from the Upper Pennsylvanian of Kansas. Novitates, 2931, 1–42. [Google Scholar]
- Maisey, J. G. and Anderson, M. E. 2001. A primitive chondrichthyan braincase from the Early Devonian of South Africa. Journal of Vertebrate Paleontology, 21, 702–713. [Google Scholar]
- Mark‐Kurik, E. 2000. The Middle Devonian fishes of the Baltic States (Estonia, Latvia) and Belarus 309–324. In Blieck A. and Turner S. (eds). Palaeozoic vertebrate biochronology and global marine/non‐marine correlation, Vol. 223. Courier Forschungs Institut, Senckenberg, 575 pp. [Google Scholar]
- Märss, T. 2001. Andreolepis (Actinopterygii) in the Upper Silurian of northern Eurasia. Proceedings of the Estonian Academy of Sciences: Geology, 50, 174–189. [Google Scholar]
- M'Coy, F. 1848. On some new Ichthyolites from the Scotch Old Red Sandstone. Journal of Natural History, 2, 297–312. [Google Scholar]
- M'Coy, F. 1855. A synopsis of the classification of the British Palaeozoic rocks, with a systematic description of the British Palaeozoic Fossils. University Press, Cambridge, 661 pp. [Google Scholar]
- Miles, R. S. 1973. Relationships of acanthodians 63–103. In Greenwood P. H., Miles R. S. and Patterson C. (eds). Interrelationships of fishes. Academic Press, London, 536 pp. [Google Scholar]
- Miles, R. S. 1977. Dipnoan (lungfish) skulls and the relationships of the group: a study based on new species from the Devonian of Australia. Zoological Journal of the Linnean Society, 61, 1–328. [Google Scholar]
- Miller, H. 1841. The Old Red Sandstone. J. Johnson, Edinburgh, 324 pp. [Google Scholar]
- Miller, S. A. 1892. North American geology for the use of amateurs, students, and scientists. Western Methodist Book Concern, Cincinnati, OH, 718 pp. [Google Scholar]
- Müller, J. 1846. Ueber den Bau und die Grenzen der Ganoiden. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, 1844, 119–216. [Google Scholar]
- Nelson, J. S. 2006. Fishes of the world, Fourth edition John Wiley & Sons, Hoboken, New Jersey, 601 pp. [Google Scholar]
- Newberry, J. S. 1857. New fossil fishes from the Devonian rocks of Ohio. American Journal of Science, Series 2, 24, 147–149. [Google Scholar]
- Newberry, J. S. 1888. On the fossil fishes of the Erie Shale of Ohio. Transactions of the New York Academy of Science, 7, 178–180. [Google Scholar]
- Nielsen, E. 1942. Studies on Trassic fishes from East Greenland I. Glaucolepis and Boreosomus . Meddelelser om Grønland, 146, 1–309. [Google Scholar]
- Over, D. J. 2007. Conodont biostratigraphy of the Chattanooga Shale, middle and upper Devonian, southern Appalachian Basin, Eastern United States. Journal of Paleontology, 81, 1194–1217. [Google Scholar]
- Pander, C. H. 1856. Monographie der fossilen Fische des silurischen Systems der Russisch‐Baltischen Gouvernements. Akademie der Wissenschaften, St Petersburg, 91 pp. [Google Scholar]
- Pander, C. H. 1860. Über die Saurodipterinen, Dendrodonten, Glyptolepiden und Cheirolepiden des Devonischen Systems. Kaiserlichen Akademis des Wissenschaften, St Petersburg, 100 pp. [Google Scholar]
- Patterson, C. 1975. The braincase of the pholidophorid and leptolepid fishes, with a review of the actinopterygian braincase. Philosophical Transactions of the Royal Society of London, Series B, 269, 275–579. [DOI] [PubMed] [Google Scholar]
- Patterson, C. 1982. Morphology and interrelationships of primitive actinopterygian fishes. American Zoologist, 22, 241–259. [Google Scholar]
- Pearson, D. M. 1982. Primitive bony fishes, with especial reference to Cheirolepis and palaeonisciform actinopterygians. Zoological Journal of the Linnean Society, 74, 35–67. [Google Scholar]
- Pearson, D. M. and Westoll, T. S. 1979. The Devonian actinopterygian Cheirolepis Agassiz. Transactions of the Royal Society of Edinburgh, 70, 337–399. [Google Scholar]
- Poplin, C. 1974. Étude de quelques Paléoniscidés pennsylvaniens du Kansas. Cahiers de Paléontologie (Section Vertébrés), Paris, 151 pp. [Google Scholar]
- Poplin, C. 1975. Kansasiella nomen novum remplaçant Kansasia Poplin 1974 (Poissons: Palaeonisciformes). Bulletin de la Société Géologique de France, 17, 26. [Google Scholar]
- Poplin, C. 1984. Lawrenciella schaefferi n.g., n.sp. (Pisces: Actinopterygii) and the use of endocranial characters in the classification of the Palaeonisciformes. Journal of Vertebrate Paleontology, 4, 413–421. [Google Scholar]
- Poplin, C. and Véran, M. 1996. A revision of the actinopterygian fish Coccocephalus wildi from the Upper Carboniferous of Lancashire. Special Papers in Palaeontology, 52, 7–29. [Google Scholar]
- Qu, Q. , Zhu, M. and Wang, W. 2013. Scales and dermal skeletal histology of an early bony fish Psarolepis romeri and their bearing on the evolution of rhombic scales and hard tissues. PLoS One, 8, e61485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, D. H. 1951. On the cranial structure of an early palaeoniscid, Kentuckia gen. nov. Transactions of the Royal Society of Edinburgh, 62, 58–83. [Google Scholar]
- Reed, J. W. 1992. The actinopterygian Cheirolepis from the Devonian of Red Hill, Nevada, and its implications for acanthodian–actinopterygian relationships 243–249. In Mark‐Kurik E. (ed.). Fossil fishes as living animals. Academy of Sciences of Estonia, Talinn, 299 pp. [Google Scholar]
- Richardson, J. B. and McGregor, D. C. 1986. Silurian and Devonian spore zones of the Old Red Sandstone Continent and adjacent regions. Geological Survey of Canada, Bulletin, 364, 1–79. [Google Scholar]
- Sallan, L. C. 2014. Major issues in the origins of ray‐finned fish (Actinopterygii) biodiversity. Biological Reviews, 89, 950–971. [DOI] [PubMed] [Google Scholar]
- Schaeffer, B. 1981. The xenacanth neurocranium, with comments on elasmobranch monophyly. Bulletin of the American Museum of Natural History, 169, 1–66. [Google Scholar]
- Schaeffer, B. and Dalquest, W. W. 1978. A palaeonisciform braincase from the Permian of Texas, with comments on cranial fissures and the posterior myodome. Novitates, 2658, 1–15. [Google Scholar]
- Schultze, H. P. 1968. Palaeoniscoidea‐Schuppen aus dem Unterdevon Australiens und Kanadas und aus dem Mittel‐devon Spitzbergens. Bulletin of the British Museum (Natural History): Geology, 16, 343–376. [Google Scholar]
- Schultze, H. P. 1977. Ausgangsform und Entwicklung der rhombischen Schuppen der Osteichthyes (Pisces). Paläontologische Zeitschrift, 51, 152–168. [Google Scholar]
- Schultze, H. P. and Cumbaa, S. L. 2001. Dialipina and the characters of basal actinopterygians 316–332. In Ahlberg P. E. (ed.). Major events in early vertebrate evolution: phylogeny, genetics and development. Taylor & Francis, London, 418 pp. [Google Scholar]
- Snitting, D. 2008. A redescription of the anatomy of the Late Devonian Spodichthys buetleri Jarvik, 1985 (Sarcopterygii, Tetrapodomorpha) from East Greenland. Journal of Vertebrate Paleontology, 28, 637–655. [Google Scholar]
- Stensiö, E. A. 1921. Triassic fishes from Spitzbergen, Vol. 1A. Holhausen, Vienna, 307 pp. [Google Scholar]
- Stensiö, E. A. 1963. Anatomical studies on the arthrodiran head. Pt 1. Preface, geological and geographical distribution, and organisation of the arthrodires, the anatomy of the head in the Dolichothoraci, Coccosteomorphi and Pachyosteomorphi. Kungliga Svenska Vetenskapsakademiens Handlingar, 9, 1–419. [Google Scholar]
- Swartz, B. A. 2009. Devonian actinopterygian phylogeny and evolution based on a redescription of Stegotrachelus finlayi . Zoological Journal of the Linnean Society, 56, 750–784. [Google Scholar]
- Titarenko, V. , Bradley, R. , Martin, C. , Withers, P. J. and Titarenko, S. 2010. Regularization methods for inverse problems in x‐ray tomography In Stock S. R. (ed.). Developments in x‐ray tomography VII. Proceedings SPIE 7804, 780440Z. doi:10.1117/12.860260 [Google Scholar]
- Traquair, R. H. 1875. On the structure and systematic position of the genus Cheirolepis . Annals and Magazine of Natural History, 15, 237–249. [Google Scholar]
- Traquair, R. H. 1888. Notes on the nomenclature of the fishes of the Old Red Sandstone of Great Britain. Geological Magazine, 5, 507–517. [Google Scholar]
- Wang, N. Z. and Dong, Z. Z. 1989. Discovery of Late Silurian microfossils of Agnatha and fishes from Yunnan, China. Acta Palaeontologica Sinica, 28, 192–206. [Google Scholar]
- Watson, D. M. S. 1925. The structure of certain palæoniscids and the relationships of that group with other bony fish. Proceedings of the Zoological Society of London, 54, 815–870. [Google Scholar]
- White, E. I. 1933. New Triassic Palæoniscoids from Madagascar. Annals and Magazine of Natural History, 11, 118–128. [Google Scholar]
- Whiteaves, J. F. 1881. On some remarkable fossil fishes from the Devonian rocks of Scaumenac Bay, P.Q., with description of a new genus and three new species. Canadian Naturalist, Montreal, 10, 27–35. [Google Scholar]
- Whitley, G. P. 1940. The Nomenclator Zoologicus and some new fish names. Australian Naturalist, 10, 241–243. [Google Scholar]
- Xu, G.‐H. , Gao, K.‐Q. and Finarelli, J. A. 2014. A revision of the Middle Triassic scanilepiform fish Fukangichthys longidorsalis from Xinjiang, China, with comments on the phylogeny of the Actinopteri. Journal of Vertebrate Paleonto‐logy, 34, 747–759. [Google Scholar]
- Young, G. C. 2006. Biostratigraphic and biogeographic context for tetrapod origins during the Devonian Period: Australian evidence. Alcheringa Special Issue, 1, 409–428. [Google Scholar]
- Yu, X. 1998. A new porolepiform‐like fish, Psarolepis romeri, gen. et sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. Journal of Vertebrate Paleontology, 18, 261–274. [Google Scholar]
- Zhu, M. and Yu, X. 2002. A primitive fish close to the common ancestor of tetrapods and lungfish. Nature, 418, 767–770. [DOI] [PubMed] [Google Scholar]
- Zhu, M. and Yu, X. 2009. Stem sarcopterygians have primitive polybasal fin articulation. Biology Letters, 5, 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. , Yu, X. and Ahlberg, P. E. 2001. A primitive sarcopterygian fish with an eyestalk. Nature, 410, 81–84. [DOI] [PubMed] [Google Scholar]
- Zhu, M. , Zhao, W. , Jia, L. , Lu, J. , Qiao, T. and Qu, Q. 2009. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature, 458, 469–474. [DOI] [PubMed] [Google Scholar]
- Zhu, M. , Yu, X. , Ahlberg, P. E. , Choo, B. , Lu, J. , Qiao, T. , Qu, Q. , Zhao, W. , Jia, L. , Blom, H. and Zhu, Y. 2013. A Silurian placoderm with osteichthyan‐like marginal jaw bones. Nature, 502, 188–193. [DOI] [PubMed] [Google Scholar]


