Abstract
Introduction
Cognitive tests and nonamyloid imaging biomarkers do not consistently identify preclinical AD. The objective of this study was to evaluate whether white matter hyperintensity (WMH) volume, a cerebrovascular disease marker, is more associated with preclinical AD than conventional AD biomarkers and cognitive tests.
Methods
Elderly controls enrolled in the Alzheimer's Disease Neuroimaging Initiative (ADNI, n = 158) underwent florbetapir-PET scans, psychometric testing, neuroimaging with MRI and PET, and APOE genetic testing. Elderly controls the Parkinson's progression markers initiative (PPMI, n = 58) had WMH volume, cerebrospinal fluid (CSF) Aβ1–42, and APOE status measured.
Results
In the ADNI cohort, only WMH volume and APOE ε4 status were associated with cerebral Aβ (standardized β = 0.44 and 1.25, P = .03 and .002). The association between WMH volume and APOE ε4 status with cerebral Aβ (standardized β = 1.12 and 0.26, P = .048 and .045) was confirmed in the PPMI cohort.
Discussion
WMH volume is more highly associated with preclinical AD than other AD biomarkers.
Keywords: Alzheimer's disease, Aging, MRI, PET, White matter, Leukoaraiosis, Preclinical Alzheimer's disease, Amyloid
1. Introduction
Owing to the recent failures of several clinical trials in treating symptomatic Alzheimer's disease (AD) [1], focus in therapeutic trials is shifting from reversing the effects of AD to preventing cognitive decline due to AD at the preclinical stage, before any noticeable cognitive change has occurred [2]. Preclinical AD is defined based on the presence of cerebral amyloidosis, detected by either amyloid PET or measurement of cerebrospinal Aβ1–42 [3]. We focus here on preclinical AD, which is simply defined as presence of cerebral Aβ [3]. Presence of preclinical AD does not necessarily imply that clinical AD will result but does appear to come with a higher risk of developing clinical AD [4]. Because of the importance of preclinical AD, an accurate and thorough understanding of the cognitive and brain changes at this stage is critical. Furthermore, predictors of preclinical AD are potentially valuable in the context of clinical trials to enrich populations before the use of more expensive or invasive amyloid measurement.
Within cognitively normal older adults, two predictors of amyloid status have already been relatively well established: age and apolipoprotein E (APOE) status [5], [6]. Beyond these risks, it is possible that other neurodegenerative biomarkers and cognitive changes that presumptively represent the downstream effect of the presence of cerebral Aβ, such as hippocampal atrophy, hypometabolism, and subjective cognitive impairment, may also be sensitive to preclinical AD [7], [8], [9]. Although these markers clearly predict conversion from mild cognitive impairment (MCI) to probable AD [10], [11], [12], [13], [14] and the presence of cerebral amyloid in MCI to varying degrees [15], their value in preclinical disease is less well established.
One neuroimaging measure that has received less, but growing, attention in relationship to AD is the presence of white matter hyperintensity (WMH) volume. WMH volume has been associated with clinical AD [16], [17], cognitive ability [18], cortical atrophy [19], and AD pathology in cognitively normal populations [20], but no study has examined the association of WMH volume with preclinical AD in the context of more established imaging and cognitive AD biomarkers. Here, we compare the association of a variety of biomarkers, including neurodegenerative, genetic, functional, and cognitive biomarkers, as well as WMH volume, with preclinical AD. This comparison sheds light on the pathogenesis of AD and can inform subsequent studies on longitudinal trajectories of AD biomarkers.
2. Methods
2.1. Clinical data
2.1.1. Subjects
Data used in the preparation of this article were obtained from two publicly available data repositories: the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) and the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and nonprofit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early Alzheimer's disease (AD). For up-to-date information on the PPMI study, visit www.ppmi-info.org.
Data used in this article were downloaded from the ADNI website in November 2014. We included all cognitively normal subjects from ADNI2 and ADNI-GO who had undergone florbetapir-PET scans to obtain a measure of cerebral amyloidosis, APOE genotyping, FDG-PET, structural magnetic resonance (MR) imaging, and all cognitive tests examined. Only subjects with Freesurfer cortical and hippocampal segmentations judged acceptable by the structural MR processing core were included. Inclusion criteria for the study and diagnostic criteria for establishing disease state were as previously reported [21]. For up-to-date information on specific inclusion and exclusion criteria, please see www.adni-info.org. Data were also downloaded from the PPMI website, October 2014. Inclusion criteria for these study data included a baseline diagnosis of cognitively normal, a T1-weighted and Flair MRI, CSF analysis of AD biomarkers, and APOE genotyping. For up-to-date information on the PPMI study, visit www.ppmi-info.org.
2.2. Psychometric testing
The following measures were included in the analysis: the mini-mental state examination [22], Rey Auditory Verbal Learning Test [23], immediate and delayed recall of the Logical Memory Test [24], the Trail Making Test [trails A and trails B] [25], category fluency [animals [26]], and Boston Naming Test [27]. Given the importance of memory in prodromal AD, we examined several of the AVLT measures, which depend on differential aspects of episodic and working memory [28]. For the present study, we analyzed performance on the fifth immediate memory trial (AVLT Trial 5 Recall), 5-minute and 30-minute delayed recall (AVLT 5-min Recall, AVLT 30-min Recall), and recognition memory discrimination (AVLT recognition discrimination). To account for false alarms to nonstudied items, we calculated a measure of discriminability, d-prime (d'), in a standard fashion [29].
In addition to psychometric measures, we also examined a measure of cognitive complaints via the Everyday Cognition (ECog) questionnaire [30], [31], using both informant-report and self-report data. Informants and participants are separately queried as to the degree to which particular everyday functioning has changed compared to 10 years earlier. Responses for ADNI were obtained on a five-point scale, with increasing values indicating more complaints and 5 indicating “do not know”. The global scores were averaged separately over informant-rated and self-rated scales, excluding values of 5.
2.3. Determination of amyloid status
Florbetapir-PET was administered in accordance with the ADNI PET protocols available online (http://adni.loni.usc.edu/data-samples/pet), and image processing was performed by the ADNI core laboratory as described previously [32]. Briefly, a PET scan was acquired 50–70 minutes after injection of florbetapir. Images were smoothed and aligned to an MPRAGE anatomic image to obtain a cortical segmentation. Mean florbetapir uptake in lateral and medial frontal, anterior and posterior cingulate, lateral parietal, and lateral temporal regions was normalized to uptake in the cerebellum to obtain a mean cortical standardized uptake value ratio (SUVr). Cortical florbetapir uptake of ≥1.11 was considered “positive” for cortical Aβ [32]. APOE genotyping was performed as described on the ADNI website (http://adni.loni.usc.edu/data-samples/genetic-data/).
2.4. Neuroimaging biomarkers
Processing of neuroimaging data was performed by ADNI cores and made publicly available. FDG-PET scans were acquired and analyzed in accordance with a standard protocol [33]. Mean FDG uptake was averaged >5 ROI's that are sensitive to AD-related changes in metabolism, including right and left angular gyri, right and left inferior temporal regions, and bilateral posterior cingulate. Cortical thickness and hippocampal volume measurement based on MRI scans were performed according to the standard ADNI Freesurfer [34] processing pipeline and downloaded from the ADNI website. Only images that passed ADNI quality control for the temporal, occipital, and parietal lobe were included. Cortical thickness in the caudal portion of the middle frontal gyrus, medial portion of the orbital frontal cortex, inferior parietal lobule, lateral portion of the occipital cortex, inferior temporal gyrus, entorhinal cortex, temporal pole, and the isthmus of the cingulate cortex were averaged to form a meta-ROI thought sensitive to early AD-related neurodegeneration, as previously suggested [35]. WMH volumes were computed by the ADNI core laboratory in accordance with previously published protocols [36]. Briefly, FLAIR MR images were corrected for inhomogeneity and warped to T1 images to provide a segmentation. WMHs are seeded at points that are >3.5 standard deviations from the mean signal in white matter, and final segmentation is based on a Bayesian approach, combining spatial priors and tissue class constraints. The WMH segmentation also included segmentations of white matter, gray matter, and CSF; the sum of the tissue volumes was used as a surrogate for intracranial volume. For analysis, WMH volumes were normalized to intracranial volume and transformed using the natural logarithm.
2.5. PPMI analysis
To analyze evidence for the presence of evidence for cerebral amyloidosis, we evaluated CSF amyloid-beta (Aβ1–42). PPMI has completed two CSF analyses, Project 101 and Project 103, and overall Aβ1–42 were significantly elevated in the latter. To maximize sample sizes, we adapted a linear regression transformation method for CSF Aβ1–42 to transform the elevated values from Project 103 to match values from Project 101, as previously reported [37]: Transformed Aβ1–42 = 1.82994 + (Aβ1–42 × 0.61562). In our transformed CSF series, we observed that a Aβ1–42 CSF cutoff of 198 pg/mL marked the first point of deviation from the normal distribution of Aβ1–42 CSF values, so we selected this cutoff to classify participants as being positive or negative for CSF Aβ.
We applied an automated MR image processing pipeline for quantifying WMH volume in the PPMI cohort. The T1-weighted scan of each subject was first preprocessed for correction of intensity inhomogeneities [38]. A multiatlas skull-stripping algorithm was applied using study-specific atlases for the extraction of the brain tissue [39]. Images with quality issues, such as low T1 resolution, were excluded from the analysis and brain masks with errors were manually corrected. A multi-atlas label-fusion method, which uses nonlinear registration for transferring atlas labels to subject space, was applied to form the basis of the white and gray matter segmentations [40], [41]. Regions of WMH were segmented using a multimodal segmentation method, white matter lesion segmentation (WMLS), using T1-weighted and fluid-attenuated inversion recovery (FLAIR) images [42]. WMLS is a supervised learning method that trains on lesions manually delineated by an expert radiologist. The lesion segmentation involves data preprocessing via histogram standardization and co-registration, feature extraction, training a voxelwise discriminative model, voxelwise label assignment, and false-positive elimination. Quality control was performed on final volumetric data by overlaying each subject's lesion map on the FLAIR image. None of the lesion masks had errors that would require exclusion. There were minor errors, particularly in the determination of the boundaries of large lesions. However, we did not prefer to correct them manually, as the intra-rater and inter-rater variability associated with manual delineations could potentially bias the results.
2.6. Statistical analysis
All statistical analyses were performed using the R programming language, version 3.1.0. Two-tailed two-sample t tests with unequal variances (Welch's t test) were used to assess differences in demographic characteristics between WMH positive and WMH negative subjects. Logistic regression using a logit link function was used to assess the relationship between white matter hyperintensities and presence of cerebral Aβ. Stepwise forward regression was performed to generate an ideal multivariate linear model, using the Bayesian Information Criterion to regularize the model [43]. For all analyses, patient age, gender, and education were used as covariates. For hippocampal volume, intracranial vault volume (ICV) was used as an additional covariate.
3. Results
3.1. Subject demographics
A total of 184 cognitively normal subjects with florbetapir-PET were identified from the ADNI database. Of these, 155 subjects had complete psychometric and imaging variables as described in Methods, including acceptable cortical and hippocampal segmentations. A summary of the demographics of the study population, including the psychometric and imaging information, is given in Table 1. Aβ+ subjects were slightly older (M = 75.1, standard deviation (SD) = 5.7) than Aβ− subjects (M = 72.5, SD = 6.1), t(100) = 2.4, P = .02 and trended toward having slightly less education (for Aβ+ subjects, M = 16 and SD = 2.4 years; for Aβ− subjects, M = 17 and SD = 2.5 years; t(95) = −2, P = .05).
Table 1.
Summary of demographics, psychometric scores, and imaging data for ADNI subjects.
| Characteristic | All subjects | Aβ+ | Aβ− |
|---|---|---|---|
| Number of subjects | 158 | 49 | 109 |
| Number of males | 76 | 17 | 59 |
| Age | 73.5 ± 6.1 | 75.1 ± 5.7 | 72.7 ± 6.2 |
| Education | 16.4 ± 2.5 | 15.9 ± 2.4 | 16.7 ± 2.5 |
| AVLT trial 5 recall | 11.4 ± 2.6 | 11.2 ± 2.8 | 11.5 ± 2.5 |
| AVLT 5-min recall | 8.8 ± 3.6 | 7.9 ± 3.5 | 9.3 ± 3.6 |
| AVLT 30-min recall | 7.7 ± 4.0 | 7.1 ± 3.6 | 7.9 ± 4.2 |
| AVLT recognition discrimination | 3.1 ± 1.0 | 3.1 ± 1.0 | 3.2 ± 1.0 |
| Trail making test A | 33.3 ± 10.6 | 35.7 ± 10.4 | 32.2 ± 10.5 |
| Trail making test B | 80.3 ± 38.9 | 84.7 ± 36.7 | 78.3 ± 39.9 |
| Boston naming test | 28.1 ± 2.3 | 28.1 ± 2.0 | 28.2 ± 2.4 |
| Category fluency (animals) | 21.4 ± 5.5 | 21.7 ± 4.8 | 21.2 ± 5.8 |
| Mini-mental status examination | 29.0 ± 1.3 | 29.0 ± 1.0 | 28.9 ± 1.4 |
| Logical memory | 14.2 ± 3.0 | 14.0 ± 3.3 | 14.3 ± 2.8 |
| Logical memory, delayed | 13.4 ± 3.1 | 13.1 ± 2.9 | 13.5 ± 3.2 |
| Subject-reported ECOG score | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Study partner–reported ECOG score | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.3 |
| White-matter hyperintensity volume (mm3) | 3084 (1789–5657) | 4836 (3133–7861) | 2644 (1402–4864) |
| Mean FDG-PET SUVR of AD meta-ROI | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Hippocampal volume | 3754.7 ± 451.7 | 3640.2 ± 432.4 | 3806.2 ± 452.6 |
| Mean cortical thickness of AD meta-ROI | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 |
| Number (percent) of APOE ε4 positive | 47 (30%) | 23 (47%) | 24 (22%) |
Values are reported as mean ± standard deviation except for white-matter hyperintensities, which are reported as median and interquartile ranges.
3.1.1. Associative models
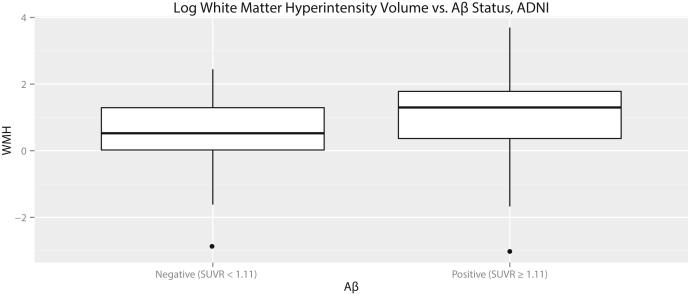
We computed a logistic regression relating each psychometric test and modality with Aβ status, while covarying for age, gender, and education (Table 2). The logistic regression results indicated that the best univariate predictor of cerebral Aβ was APOE ε4 status, followed by white matter hyperintensity (WMH) volume. All other imaging and cognitive measures, including FDG-PET, hippocampal volume, ECog, and any AVLT measure, were not significant predictors of Aβ status. WMH volume was not increased in APOE ε4-carrying subjects (t(85.8) = −0.018, P = .99). The independence between WMH volume and APOE genotype implied that both were independent predictors of Aβ status. This was confirmed by running a stepwise forward multivariate regression model, which selected only WMH volume and APOE status as independent predictors. A boxplot comparing WMH volumes in Aβ+ and Aβ− subjects is shown in Fig. 1. There was no significant association between WMH and either τ or τ/Aβ ratio (P = .56 and .09, n.s.).
Table 2.
Summary of univariate logistic regressions predicting Aβ status from each psychometric test and imaging biomarker for ADNI subjects.
| Variable | Standardized β estimate | Standard error | z value | P value |
|---|---|---|---|---|
| AVLT trial 5 recall | −0.09 | 0.17 | −0.53 | .60 |
| AVLT 5-min recall | −0.29 | 0.18 | −1.63 | .10 |
| AVLT 30-min recall | −0.17 | 0.17 | −0.95 | .34 |
| Trail making test A | 0.19 | 0.17 | 1.14 | .26 |
| Trail making test B | 0.04 | 0.17 | 0.21 | .83 |
| Boston naming test | 0.05 | 0.18 | 0.29 | .77 |
| Category fluency (animals) | 0.24 | 0.18 | 1.34 | .18 |
| Mini-mental status examination | 0.08 | 0.18 | 0.47 | .64 |
| Discrimination | −0.01 | 0.17 | −0.08 | .94 |
| Logical memory | −0.02 | 0.17 | −0.11 | .91 |
| Logical memory, delayed | −0.13 | 0.17 | −0.74 | .46 |
| Subject-reported ECOG score | −0.10 | 0.18 | −0.58 | .56 |
| Study partner–reported ECOG score | −0.17 | 0.20 | −0.85 | .40 |
| Log white-matter hyperintensity volume | 0.44 | 0.20 | 2.19 | .028∗ |
| Mean FDG-PET SUVR of AD meta-ROI | −0.18 | 0.17 | −0.77 | .44 |
| Hippocampal volume | −0.13 | 0.17 | −0.77 | .44 |
| Mean cortical thickness of AD meta-ROI | 0.01 | 0.17 | 0.07 | .94 |
| APOE ε4 status | 1.25 | 0.40 | 3.15 | .002∗∗ |
Age, gender, and education level (in years) were included as covariates. All data were scaled before regression to facilitate inspection of regression coefficients. The only variables significant at the P = .05 level were APOE status and log white-matter hyperintensity volume.
P <.05.
P <.005.
Fig. 1.
Boxplots comparing white matter hyperintensity volumes for the ADNI cohort, normalized to intracranial volume, and log transformed. Aβ+ subjects had significantly higher WMH volumes than Aβ− subjects.
3.1.2. Replication in PPMI data
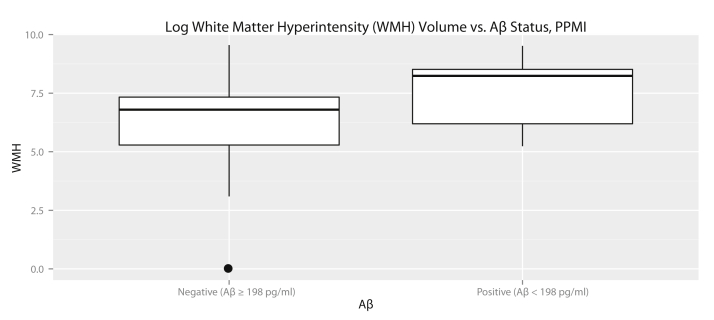
Owing to conflicting results in prior studies about the link between WMH volume and Aβ pathology, we sought to replicate correlation between WMH volume and Aβ pathology with elderly controls from the Parkinson's progression markers initiative. We identified 240 subjects with FLAIR and T1 images. A total of 207 images passed manual quality control; of these, 58 were control subjects. Log WMH volume normalized to ICV was not associated with age (ρ = 0.12, P = .37) or gender (t(38) = −1.6, P = .12). A summary of the demographics of the study population is given in Table 3. In contrast to the ADNI cohort, WMH volume was significantly increased in APOE ε4 carriers (t(24) = 2.3, P = .03). After correcting for age and gender, WMH volume was significantly predictive of Aβ status (β estimate 1.12 ± 0.57, z = 1.97, P = .048), even given the limited sample size. A boxplot showing WMH volumes in the PPMI cohort is shown in Fig. 2. However, in the PPMI cohort, a stepwise forward regression model included only APOE ε4 status as a predictor of Aβ and did not include WMH.
Table 3.
Summary of PPMI cohort demographics
| Characteristic | All subjects (mean ± standard deviation) | Aβ+ | Aβ− |
|---|---|---|---|
| Number of subjects | 58 | 12 | 46 |
| Number of males | 35 | 7 | 28 |
| Age (y) | 60 ± 13 | 62 ± 18 | 60 ± 11 |
| Number of APOE ε4 carriers | 12 | 5 | 7 |
| White-matter lesion volume (mm3) | 2654 ± 5757 | 4757 ± 7509 | 2105 ± 5168 |
Fig. 2.
Boxplots comparing log white matter hyperintensity volumes for the PPMI cohort, normalized to intracranial volume and log transformed. As in the ADNI cohort, Aβ+ subjects had significantly higher WMH volumes than Aβ− subjects.
4. Discussion
This study represents the first comprehensive comparative evaluation of a variety of biomarkers to predict presence of preclinical AD pathology in a cognitively normal population. In particular, the surprising finding that WMHs are more highly correlated with AD pathology than any of the standard AD imaging biomarkers or cognitive tests suggests that in the earliest stages of AD, vascular disease, as reflected by WMH, may play a significant role in the development of cerebral amyloidosis or be an early downstream effect of this molecular pathology. The replication of this result in an independent data set, using different image processing techniques, points to the robustness of the finding. The salient association of WMH with preclinical AD supports earlier studies [44], [45], [46] that have demonstrated a link between WMH and cognitive decline at this stage [44]. The strength of this association is in contrast to conventional thinking about this disease stage which assumes that biomarkers and, perhaps, subtle cognitive symptoms traditionally used to characterize MCI due to AD and probable AD are the same ones that would characterize preclinical AD [3], [47]. Indeed, AD biomarker cascade models do not generally include a measure of white matter integrity.
4.1. White matter hyperintensities and amyloid
The degree of association between WMH volume and amyloid deposition in nondemented control subjects is controversial. Some studies have not found a link between WMH volume or other vascular disease markers and amyloid [48], [49], [50], [51], [52], whereas other studies, including a large (n = 337) Amsterdam study [20], [53], [54], have reported such a correlation. It is possible that differences in WMH calculation, such as WM histogram normalization, thresholds for defining WMH, and the incorporation of priors for segmenting WMHs, may at least partially account for these discrepancies. Standardization and rigorous comparison of competing methods for evaluating WMH volume may help to reduce the variation in results. Differential involvement of periventricular and subcortical WMH may also contribute to differing study results [55], although the relative importance of WMH anatomic distribution as compared to total WMH volume is still a matter of debate [36].
The relationship of cerebrovascular disease to clinical AD and amyloid pathology is likely complex, and an evolving understanding is emerging [56], [57], but there are clear links between the severity of cerebrovascular disease and the risk of clinical dementia associated with AD pathology [58]. There are a number of overlapping risk factors for cerebrovascular disease and WMH with AD, including hypertension, diabetes, obesity, and tobacco use. For example, poorly controlled hypertension has been found to correlate with amyloid plaque pathology [59], and circle of Willis atherosclerosis is more highly related to AD pathology than other common proteinopathies [60], [61]. Other work has related blood pressure and systemic arterial stiffness, also associated with WMH, to amyloid burden measured by amyloid imaging [62], [63]. We did not observe statistically significant differences between mean arterial pressure, systolic, and diastolic blood pressure, body mass index, or history of cardiovascular disease, hypertension, or smoking between Aβ+ and Aβ− subjects in the ADNI cohort. Although we did not have complete medical histories of all subjects in the PPMI cohort, we did not observe differences in blood pressure between Aβ+ and Aβ− subjects in the PPMI cohort either.
Whether these are common risk factors with dissociable associations or are more directly mechanistically related is unclear. WMH volume may be a marker of cerebral amyloid due to the increased likelihood of deposition of amyloid in vessel walls leading to development of cerebral amyloid angiopathy [64]. Alternatively, there are a number of potential mechanisms by which cerebrovascular changes may directly relate to deposition of cerebral amyloid. For example, Aβ clearance may depend of the perivascular “glymphatic” system which is diminished by reduced arterial pulsatility or stiffness [65]. Furthermore, a number of factors associated with cerebrovascular alterations, including hypoperfusion, may accelerate Aβ production [66]. An enhanced understanding of these linkages may provide vascular specific therapeutic options at presymptomatic stages.
4.2. Independent association of white matter hyperintensities and APOE status with cerebral amyloid
The finding that WMH and APOE ε4 carrier status are both independently associated with amyloid, and that WMH volume is not associated with APOE implies that vascular burden increases the risk for cerebral amyloid over and above this highly significant genetic risk. This finding is in consonance with other studies that have shown that WMH volume is an independent risk factor for incident dementia [67], although not all studies have supported this result [52]. The independence of these factors is particularly interesting given the relationship of APOE with cardiovascular risk [68], [69]. Nonetheless, it is worth noting that we did not observe the same dissociation in the PPMI data set, although this could be due in part to an issue of power.
4.3. Lack of relationship with traditional AD neurodegenerative biomarkers and cognitive measures
The current findings did not support the role of neuroimaging biomarkers that have more traditionally been associated with AD in the prodromal and dementia stages of disease, including hippocampal volumes, cortical thickness, and FDG-PET. Although some prior studies have found associations between cortical thinning or volume changes and amyloid status in cognitively normal individuals [70], [71], [72], [73], [74], this has not been a consistent finding [75]. The differences in results may be due to methodologic differences, with some studies using rate of atrophy and others cross-sectional measures. The observed inconsistency may also be due to the heterogeneity among the proposed “stages” of preclinical AD, which include a spectrum of neurodegeneration and cognitive change [3]. This also may explain why in some instances cognitive measures have also been reported to be associated with preclinical AD [8], [76], [77]. It is worth pointing out that while not significant, hippocampal volume was smaller and 5-minute delayed recall on the AVLT was poorer in the group with evidence of cerebral amyloid. Finally, despite work suggesting a link between subjective complaints and amyloid status [7], we did not find either the patient or informant-based ECog of value for predicting preclinical AD; this finding resonates with results from a recent meta-analysis of amyloid status in cognitively normal adults with and without subjective symptoms [5].
Although small vessel disease is typically associated with executive dysfunction, we did not observe an association between executive function and WMH volume. This lack of association may be because we studied only cognitively normal subjects, so the variance in cognitive ability is relatively limited. It also may be the case that white matter findings associated with cerebral amyloid do not have the same impact on executive function as those unassociated with amyloid and instead accompanied by more pervasive cerebrovascular disease.
4.4. Limitations
Our study is limited by the cross-sectional nature of the data, and a longitudinal study looking at the relative timing of the development of WMH and cerebral amyloid would be informative of the direction of causality in this relationship. The characteristics of the ADNI cohort may impact the generalizability of the findings. The ADNI cohort is racially and socioeconomically homogeneous and is relatively free of cardiovascular disease and other comorbidities. It is uncertain whether the relationship between WMH and Aβ would be strengthened or weakened in a more heterogeneous cohort with more prevalent cardiovascular pathology. In addition, the lack of standardization both of FLAIR imaging methods, including resolution and WMH quantification techniques makes comparisons of different populations difficult. The clinical applicability of WMH quantification would be greatly enhanced by a standardized measurement method. Additional replication in other cohorts would bolster confidence in the observed association. Although manual quality control was performed on all hippocampal and WMH segmentations to avoid major failures, it is possible that minor errors affected the results. Finally, although cerebral Aβ has been adopted as the defining feature of preclinical AD, the lack of complete longitudinal data on ADNI 2/GO precludes any definitive conclusion on the impact of WMH on developing clinical AD in the future.
5. Conclusion
In our samples of cognitively normal controls, white matter hyperintensities (WMHs) are more highly associated with biomarker evidence of cerebral Aβ than any other recognized biomarker or cognitive test. This finding challenges the assumption that biomarkers of neurodegeneration, which are well established in later stages of disease, are reliably sensitive at the preclinical stage. As the preclinical stage of AD is increasingly recognized as a period of primary importance for preventing and ameliorating incipient neurodegeneration, the importance of accurately understanding correlates of AD pathology at this stage similarly increases. At the same time, the biological mechanism of the correlation between Aβ and WMH is not clear. A more thorough understanding of the nature of the relationship between Aβ and WMH is necessary to establish potential targets for disease-modifying interventions. Nevertheless, it is clear that WMH should be considered as a potential biomarker for preclinical AD in addition to more widely used cognitive tests and imaging biomarkers.
Research in context.
-
1.
Systematic review: We reviewed the literature using PubMed and Google Scholar searches, as well as meeting abstracts and presentations. There has been an increasing interest in the contribution of vascular disease to Alzheimer's Disease in general, and several recent studies have specifically looked at white matter hyperintensity (WMH) volume in the context of preclinical Alzheimer's disease. These studies are referenced.
-
2.
Interpretation: We found that WMH volume is more highly associated with preclinical Alzheimer's disease than any conventional biomarker. This finding supports the notion that vascular disease, as marked by WMH, is associated with cerebral amyloid deposition at early disease phases, perhaps preceding other downstream neurodegenerative changes.
-
3.
Future directions: This work raises several questions for future research. First, although WMH volume is an important marker of small vessel disease, there may be other markers of vascular health that are more sensitive to preclinical Alzheimer's. Second, and most importantly, the direction of causality between cerebrovascular disease and amyloid deposition is uncertain and deserves intensive study given implications for potential preventative interventions.
Acknowledgments
Author contributions: B.M.K. and D.A.W. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. D.A.W. and B.M.K. contributed to study concept and design. B.M.K., D.A.W., B.B.A., J.C.G., C.D., C.M., G.E., and J.D. performed the analysis and interpretation of data. B.M.K. and D.A.W. drafted the article. B.M.K., D.A.W., B.B.A., J.C.G., and C.D. performed the critical revision of the article for important intellectual content. B.M.K. and D.A.W. performed the statistical analysis of data. D.A.W., J.C.G., and C.D. obtained funding. D.A.W. and J.C.G. provided the administrative, technical, or material support. D.A.W. supervised the study.
Study funding: This research was supported by NIH grants T32-EB009384, K01-ES025432-01, R01-AG14971, R01-NS061906, AG010124, AG043503, AG040271, and the Berkman Charitable Trust. Joint funding was provided by the Michael J. Fox Foundation for Parkinson's Research, Alzheimer's Association, and Weston Brain Institute (BAND-9665). D.A.W. has performed consulting for GE Healthcare, Inc. B.M.K., B.B.A., J.C.G., C.T.M., J.M., C.D., and G.E. have no disclosures to report. The funding agencies had no say in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the article.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
PPMI, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Avid, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier, and UCB.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
References
- 1.Sperling R.A., Jack C.R., Aisen P.S. Testing the Right Target and Right Drug at the Right Stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 Study: Stopping AD before symptoms Begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris J.C., Roe C.M., Xiong C., Fagan A.M., Goate A.M., Holtzman D.M. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amariglio R.E., Becker J.A., Carmasin J., Wadsworth L.P., Lorius N., Sullivan C. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolk D.A., Mancuso L., Kliot D., Arnold S.E., Dickerson B.C. Familiarity-based memory as an early cognitive marker of preclinical and prodromal AD. Neuropsychologia. 2013;51:1094–1102. doi: 10.1016/j.neuropsychologia.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papp K.V., Amariglio R.E., Mormino E.C., Hedden T., Dekhytar M., Johnson K.A. Free and cued memory in relation to biomarker-defined abnormalities in clinically normal older adults and those at risk for Alzheimer's disease. Neuropsychologia. 2015;73:169–175. doi: 10.1016/j.neuropsychologia.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killiany R.J., Gomez-Isla T., Moss M., Kikinis R., Sandor T., Jolesz F. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 11.Davatzikos C., Bhatt P., Shaw L.M., Batmanghelich K.N., Trojanowski J.Q. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging. 2011;32:2322.e19–2322.e27. doi: 10.1016/j.neurobiolaging.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckerström C., Olsson E., Bjerke M., Malmgren H., Edman A., Wallin A. A combination of neuropsychological, neuroimaging, and cerebrospinal fluid markers predicts conversion from mild cognitive impairment to dementia. J Alzheimers Dis. 2013;36:421–431. doi: 10.3233/JAD-122440. [DOI] [PubMed] [Google Scholar]
- 13.Ewers M., Walsh C., Trojanowski J.Q., Shaw L.M., Petersen R.C., Jack C.R., Jr. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33:1203–1214.e2. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomar J.J., Bobes-Bascaran M.T., Conejero-Goldberg C., Davies P., Goldberg T.E., Alzheimer's Disease Neuroimaging Initiative UTility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the alzheimer's disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 15.Kandel B.M., Avants B.B., Gee J.C., Arnold S.E., Wolk D.A. Neuropsychological testing predicts cerebrospinal fluid amyloid-β in mild cognitive impairment. J Alzheimers Dis. 2015;46:901–912. doi: 10.3233/JAD-142943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshita M., Fletcher E., Harvey D., Ortega M., Martinez O., Mungas D.M. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provenzano F.A., Muraskin J., Tosto G., Narkhede A., Wasserman B.T., Griffith E.Y. White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son S.J., Lee K.S., Lee Y., Baek J.H., Choi S.H., Na D.L. Association between white matter hyperintensity severity and cognitive impairment according to the presence of the apolipoprotein E (APOE) ε4 allele in the elderly: retrospective analysis of data from the CREDOS study. J Clin Psychiatry. 2012;73:1555–1562. doi: 10.4088/JCP.12m07702. [DOI] [PubMed] [Google Scholar]
- 19.Barnes J., Carmichael O.T., Leung K.K., Schwarz C., Ridgway G.R., Bartlett J.W. Vascular and Alzheimer's disease markers independently predict brain atrophy rate in Alzheimer's Disease Neuroimaging Initiative controls. Neurobiol Aging. 2013;34:1996–2002. doi: 10.1016/j.neurobiolaging.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kester M.I., Goos J.D., Teunissen C.E., Benedictus M.R., Bouwman F.H., Wattjes M.P. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 2014;71:855–862. doi: 10.1001/jamaneurol.2014.754. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Rey A. Presses universitaires de France; Paris: 1964. L'examen clinique en psychologie. [Google Scholar]
- 24.Wechsler D. The Psychological Corporation, Harcourt Brace Jovanovich, Inc.; New York: 1987. Wechsler Memory Scale — Revised Manual. [Google Scholar]
- 25.Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 26.Butters N., Granholm E., Salmon D.P., Grant I., Wolfe J. Episodic and semantic memory: A comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan E., Goodglass H., Weintraub S., Goodglass H. Lea & Febiger; Philadelphia: 1983. Boston Naming Test. [Google Scholar]
- 28.Wolk D.A., Dickerson B.C. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snodgrass J.G., Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Farias S.T., Mungas D., Reed B.R., Cahn-Weiner D., Jagust W., Baynes K. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmonds E.C., Delano-Wood L., Galasko D.R., Salmon D.P., Bondi M.W. Subjective Cognitive Complaints Contribute to Misdiagnosis of Mild Cognitive Impairment. J Int Neuropsychol Soc. 2014;20:836–847. doi: 10.1017/S135561771400068X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 35.Desikan R.S., Sabuncu M.R., Schmansky N.J., Reuter M., Cabral H.J., Hess C.P. Selective Disruption of the Cerebral Neocortex in Alzheimer's Disease. PLoS One. 2010;5:e12853. doi: 10.1371/journal.pone.0012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeCarli C., Fletcher E., Ramey V., Harvey D., Jagust W.J. Anatomical Mapping of White Matter Hyperintensities (WMH) Exploring the Relationships Between Periventricular WMH, Deep WMH, and Total WMH Burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMillan C., Wolk D. Presence of Cerebral Amyloid Modulates Phenotype and Pattern of Neurodegeneration in early Parkinson's Disease (P5.023) Neurology. 2015;84(14 Supplement) doi: 10.1136/jnnp-2015-312690. P5.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A. N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doshi J., Erus G., Ou Y., Gaonkar B., Davatzikos C. Multi-Atlas Skull-Stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doshi J.J., Erus G., Ou Y., Davatzikos C. MICCAI Challenge Workshop on Segmentation: Algorithms, Theory and Applications. Nagoya, Japan. 2013. Ensemble-based medical image labeling via sampling morphological appearance manifolds. [Google Scholar]
- 41.Ou Y., Sotiras A., Paragios N., Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zacharaki E.I., Kanterakis S., Bryan R.N., Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 44.Silbert L.C., Dodge H.H., Perkins L.G., Sherbakov L., Lahna D., Erten-Lyons D. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79:741–747. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang L., Sachdev P.S., Trollor J.N., Kochan N.A., Reppermund S., Brodaty H. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology. 2012;79:748–754. doi: 10.1212/WNL.0b013e3182661f4d. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael O., Mungas D., Beckett L., Harvey D., Farias S.T., Reed B. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33:83–95.e2. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedden T., Mormino E.C., Amariglio R.E., Younger A.P., Schultz A.P., Becker J.A. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchant N.L., Reed B.R., DeCarli C.S., Madison C.M., Weiner M.W., Chui H.C. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging. 2012;33:1006.e25–1006.e36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutten-Jacobs L.C., de Leeuw F.E., Geurts-van Bon L., Gordinou de Gouberville M.C., Schepens-Franke A.N., Dederen P.J. White Matter Lesions Are Not Related to Beta-Amyloid Deposition in an Autopsy-Based Study. Curr Gerontol Geriatr Res. 2011;2011:e826862. doi: 10.1155/2011/826862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanskanen M., Kalaria R.N., Notkola I.L., Mäkelä M., Polvikoski T., Myllykangas L. Relationships between white matter hyperintensities, cerebral amyloid angiopathy and dementia in a population-based sample of the oldest old. Curr Alzheimer Res. 2013;10:1090–1097. doi: 10.2174/15672050113106660177. [DOI] [PubMed] [Google Scholar]
- 52.Lo R.Y., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thanprasertsuk S., Martinez-Ramirez S., Pontes-Neto O.M., Ni J., Ayres A., Reed A. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014;83:794–800. doi: 10.1212/WNL.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenset V., Hofoss D., Johnsen L., Berstad A.E., Negaard A., Skinningsrud A. White matter lesion load increases the risk of low CSF Aβ42 in apolipoprotein E-ɛ4 carriers attending a memory clinic. J Neuroimaging. 2011;21:e78–e82. doi: 10.1111/j.1552-6569.2009.00444.x. [DOI] [PubMed] [Google Scholar]
- 55.de Leeuw F.E., Richard F., de Groot J.C., van Duijn C.M., Hofman A., Van Gijn J. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke. 2004;35:1057–1060. doi: 10.1161/01.STR.0000125859.71051.83. [DOI] [PubMed] [Google Scholar]
- 56.Kling M.A., Trojanowski J.Q., Wolk D.A., Lee V.M., Arnold S.E. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9:76–92. doi: 10.1016/j.jalz.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes T.M., Kuller L.H., Barinas-Mitchell E.M., McDade E.M., Klunk W.E., Cohen A.D. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider J.A., Wilson R.S., Bienias J.L., Evans D.A., Bennett D.A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman L.B., Schmeidler J., Lesser G.T., Beeri M.S., Purohit D.P., Grossman H.T. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beach T.G., Wilson J.R., Sue L.I., Newell A., Poston M., Cisneros R. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 61.Yarchoan M., Xie S.X., Kling M.A., Toledo J.B., Wolk D.A., Lee E.B. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toledo J.B., Toledo E., Weiner M.W., Jack C.R., Jr., Jagust W., Lee V.M. Cardiovascular risk factors, cortisol, and amyloid-β deposition in Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2012;8:483–489. doi: 10.1016/j.jalz.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes T.M., Kuller L.H., Barinas-Mitchell E.J., Mackey R.H., McDade E.M., Klunk W.E. Pulse wave velocity is associated with -amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurol M.E., Viswanathan A., Gidicsin C., Hedden T., Martinez-Ramirez S., Dumas A. Cerebral amyloid angiopathy burden associated with leukoaraiosis: A positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013;73:529–536. doi: 10.1002/ana.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iadecola C. The Pathobiology of Vascular Dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Debette S., Beiser A., DeCarli C., Au R., Himali J.J., Kelly-Hayes M. Association of MRI Markers of Vascular Brain Injury With Incident Stroke, Mild Cognitive Impairment, Dementia, and Mortality The Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gungor Z., Anuurad E., Enkhmaa B., Zhang W., Kim K., Berglund L. Apo E4 and lipoprotein-associated phospholipase A2 synergistically increase cardiovascular risk. Atherosclerosis. 2012;223:230–234. doi: 10.1016/j.atherosclerosis.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigue K.M., Rieck J.R., Kennedy K.M., Devous M.D., Sr., Diaz-Arrastia R., Park D.C. Risk factors for β-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dickerson B.C., Stoub T.R., Shah R.C., Sperling R.A., Killiany R.J., Albert M.S. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickerson B.C., Wolk D.A. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh H., Mormino E.C., Madison C., Hayenga A., Smiljic A., Jagust W.J. β-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Amariglio R.E., Rentz D.M. Synergistic effect of β-amyloid and neurodegeneration on Cognitive Decline in Clinically Normal Individuals. JAMA Neurol. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schott J.M., Bartlett J.W., Fox N.C., Barnes J., for the Alzheimer's Disease Neuroimaging Initiative Investigators Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 75.Whitwell J.L., Tosakulwong N., Weigand S.D., Senjem M.L., Lowe V.J., Gunter J.L. Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? Neuroimage Clin. 2013;2:249–257. doi: 10.1016/j.nicl.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sperling R.A., Johnson K.A., Doraiswamy P.M., Reiman E.M., Fleisher A.S., Sabbagh M.N. Amyloid deposition detected with florbetapir F 18 (18F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013;34:822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rentz D.M., Parra Rodriguez M.A., Amariglio R., Stern Y., Sperling R., Ferris S. Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer's disease: a selective review. Alzheimers Res Ther. 2013;5:58. doi: 10.1186/alzrt222. [DOI] [PMC free article] [PubMed] [Google Scholar]