Abstract
Immunotherapy with tumor lysate-pulsed dendritic cells (DCs) is one of the breakthrough strategies used in the treatment of cancer. However, DC-based immunotherapies for osteosarcoma are limited. In the present study, preclinical studies of a C3H osteosarcoma mouse model (produced by subcutaneous injection of LM8 murine osteosarcoma cells) validated the concept that LM8 cell lysate-pulsed bone marrow-derived DCs may evoke a more potent immune response compared with DCs that have been matured using polyinosinic:polycytidylic acid (poly I:C). A cytotoxic T lymphocyte (CTL) response was established using two groups of C3H mice (n=9) with osteosarcoma; the treatment group consisted of LM8 cell lysate-pulsed DCs and the control group consisted of DCs matured using poly I:C. Each group was immunized with doses of 1×106 cells twice per week for 3 weeks. No difference in the expression of cluster of differentiation markers was identified in the two groups. DCs pulsed with LM8 cell lysate were associated with the increased induction of CTL activity. Serum interferon-γ levels were increased in mice that received DCs pulsed with LM8 cell lysate compared with that in the poly I:C-matured DC group (P<0.041). Serum interleukin-4 was decreased in the treatment group vs. the control group (P<0.033). A mixed lymphocyte reaction assay confirmed that LM8-DC immunotherapy may evoke a significant antigen-specific immune response in a mouse model. The present study reveals promising data on efficacy of a DC-based immunotherapy in the treatment of osteosarcoma; however, further clinical studies are warranted.
Keywords: immunotherapy, dendritic cells, cytotoxic immune response, cancer
Introduction
Osteosarcoma is the most common primary malignant tumor of the bone and represents a significant therapeutic challenge. This type of bone and soft tissue sarcoma predominantly affects children and young adults (1). Rapid advancements have occurred in the treatment of osteosarcoma, including the introduction of adjuvant chemotherapy, conformal radiotherapy and improved surgical excision (2–5), and the 5-year survival rate in patients with localized disease is 50–70% (6). However, prognosis remains poor in relapsed disease and in patients that are resistant to the standard treatment modalities (7). These poor outcomes have resulted in the search for novel treatments that are more effective and less toxic, including dendritic cell (DC)-based immunotherapy for disease management.
In contrast to recent studies that have described successful immunotherapy for certain solid tumors (8–10), the number of reports on immunotherapy for osteosarcoma is limited and this topic has received less attention (11,12). DCs are professional antigen-presenting cells (APCs) that are able to activate naive T cells and to induce the generation and expansion of certain cytotoxic T lymphocytes (CTLs) and T helper cells (8). The ability of DCs to internalize, process and present antigens, mediated by major histocompatibility complex class I and class II molecules, provides a basis for the development of cancer vaccines (8). Extensive studies with DC-based vaccination for the treatment of various types of malignant tumor have been conducted, and have produced evidence of immune and clinical responses, particularly in patients with melanoma and renal cell carcinoma (8,9,13). However, there have been fewer investigations into DC-based cancer vaccination for osteosarcoma (9,10). For several reasons, osteosarcoma provides a rational target to evaluate the potential to induce tumor-specific immunity by DC vaccination. Firstly, a randomized phase III study previously indicated that muramyl tripeptide, an immune modulatory agent, in combination with chemotherapy had a positive effect on survival (14). Secondly, the long and indolent disease courses often observed in patients with pulmonary metastases from osteosarcoma may be indicative of natural tumor immunity; however, this remains to be confirmed histologically or immunologically (15). Thirdly, tissue for use in vaccine generation is commonly available, as surgical resection is often performed to remove pulmonary metastatic lesions in patients with disease relapse.
The purpose of the present study was to examine an in vitro model for the generation and expansion of a DC-mediated CTL response for the prospective treatment of osteosarcoma. The generation of systemic antitumor immunity by LM8 murine osteosarcoma cell lysate-pulsed DCs was investigated in mouse models with osteosarcoma, and the hallmarks of a potent CTL response were also explored.
Materials and methods
Cell culture and generation of DCs
LM8 cells, derived from Dunn osteosarcoma, were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The cells were cultured in complete medium consisting of RPMI-1640 (Lonza Group Ltd., Basel, Switzerland) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml streptomycin (Lonza Group Ltd.) and 100 U/ml penicillin (Lonza Group Ltd.). Cells were cultured at 37°C in an atmosphere of 5% CO2.
Bone marrow-derived DCs were generated according to the protocol described by Inaba et al (16), with minor alterations incorporated. Briefly, erythrocyte-depleted mouse bone marrow cells were collected from flushed marrow cavities (1×106 cells/ml) and were cultured in complete medium with 20 ng/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Inc., Minneapolis, MN, USA) in 10-cm tissue culture dishes at 37°C in an atmosphere containing 50 ml CO2/l. LM8 cells (6×106) were lysed by rapidly freezing 3-day cultured LM8 cells in liquid nitrogen and thawing in a water bath at 37°C 3 times. The thawed LM8 cell lysate was added to the DC cultures on day 6 at a ratio of 1:5, and incubated at 37°C in an atmosphere containing 50 ml CO2/l. Following 24 h of incubation, non-adherent cells were harvested by gentle pipetting. The cells were washed and resuspended in phosphate-buffered saline (PBS) for subcutaneous immunizations.
Animal experiments
All experimental procedures involving animals were conducted under a protocol reviewed and approved by the Ethics Committee of Tai'an City Central Hospital (Tai'an, China). All efforts were made to minimize the number of animals used and to ameliorate suffering. For in vivo studies, C3H mice were obtained from Sankyo Labo Service Corporation, Inc. (Toyama, Japan) and maintained in pathogen-free conditions. A total of 5×106 LM8 cells were subcutaneously implanted into the gluteal region of 18 C3H mice (female; age, 6–8 weeks) for the development of tumors. Following the development of the tumors, two groups consisting of 9 mice each were immunized with 1×106 DCs pulsed in LM8 cell lysate, or with polyinosinic:polycytidylic acid (poly I:C, a Toll-like receptor 3 agonist that acts as a maturation stimulus)-matured DCs; the freshly prepared vaccine was diluted to the appropriate concentration and delivered intradermally with a needle in a total volume of 200 µl into the animal's groin, twice per week for 3 weeks.
Immunophenotyping
The phenotype of immature and mature DCs (iDCs and mDCs) was studied using single-color fluorescence analysis. Cells (3×105) were resuspended in 50 µl of buffer (PBS, 2% FBS, and 1% sodium azide) and incubated for 30 min at 4°C with 10 µl of the appropriate fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies. The following primary FITC-tagged antibodies were used to characterize the cells: CD34 (#MCA1825GA; clone MEC14.7; mouse IgG1; dilution, 1:1,000), CD80 (#IMCA2462F; clone RM80; mouse IgG1; dilution, 1:1,000) from Beckman Coulter (Brea, CA, USA), CD83 (#MCA2747F; clone 3D11; mouse IgG1; dilution, 1:2,000) and CD86 (#553691; clone RMMP-2; mouse IgG1; dilution, 1:1,000) from AbD Serotec (Raleigh, NC, USA); CD205 (#130 102 906; clone NLDC 145; rat IgG2a anti-mouse; dilution, 1:1,000) from Miltenyi Biotec, Inc. (Singapore); and CD209 (#11-2092-80; clone LWC06; rat IgG2B anti-mouse; dilution, 1:1,000) from eBioscience, Inc. (San Diego, CA, USA). FITC-conjugated F(ab')2 goat anti-rat IgG (#IC108F; dilution, 1:1,000; R&D Systems, Inc.) was used as secondary antibody. Following incubation, the cells were washed twice and then were resuspended in 500 µl of BD Pharmingen™ Stain Buffer (#554656; BD Biosciences). Fluorescence was studied using a FACSCalibur™ flow cytometer and analyzed using BD CellQuest™ software (both from BD Biosciences). For each sample, 20,000 events were recorded, and the percentage of positive cells was reported. SigmaStat™ version 4.0 software (Systat Software, Inc., San Jose, CA, USA) was used for statistical analysis.
Quantification of antigen uptake
In brief, 2×105 cells were equilibrated to 37°C for 45 min and pulsed with FITC-conjugated dextran (40,000 molecular mass; Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 1 mg/ml. Cold staining buffer (BD Biosciences) was added to stop the reaction. The cells were washed 3 times and stained with mouse phycoerythrin-conjugated anti-CD11c antibody (1:1,000; #ab210309; Abcam, Cambridge, UK), and then analyzed using BD CellQuest software version 5.1 with the FACSCalibur™ flow cytometer. To obtain a background level, the non-specific binding of dextran to DCs was determined by the incubation of DCs with FITC-conjugated dextran for 30 min at 37°C. The medium used in the cultures with dextran stimulation was supplemented with GM-CSF, which is required for DCs to capture the antigen.
Measurement of CTL activity and cytokine profile
At the end of the study, blood was collected using a heart puncture and mice were euthanized via an intraperitoneal injection of 5 ml sodium pentobarbital (Jinan Haohua Industry Co., Ltd., Shandong, China). Murine interferon (IFN)-γ and interleukin (IL)-4 release were measured by enzyme-linked immunosorbent assay (ELISA) with IFN-γ ELISA kit (#EM10012; Thermo Fisher Scientific, Inc.) and IL-4 ELISA kit (#EMIL4; Thermo Fisher Scientific, Inc.), respectively, according to the manufacturer's protocol, using an iMark Microplate Absorbance Reader (Bio-Rad, Laboratories, Inc., Hercules, CA, USA).
Pooled spleen cells from immunized mice were stimulated by three parallel sets of cultures in the presence of 20 µl/ml mouse IL-2 (R&D Systems, Inc.). Equivalent spleen cells were co-cultured with DCs pulsed with LM8 cell lysate, poly I:C-matured DC or LM8 cell lysate alone (control). The stimulated T cells were harvested at 48 h, separated by passing through nylon wool and used as effector cells in the CTL assay. The LM8 cell lysate-loaded DCs, poly I:C-matured DCs and LM8 cells were labelled with 51Cr for 60 min at 37°C and plated in round-bottom 96-well plates at 2×104 cells/well. Effector cells were added at various effector/target cell ratios in triplicate, in a total volume of 200 µl. After 5 h incubation, 25 µl of cell-free supernatants was collected from each well and assayed in a gamma counter (Wizard 1470-005; Perkin Elmer, Inc., Waltham, MaA, USA) for 51Cr release. The spontaneous release of 51Cr was assessed by incubation of the targets in the absence of effectors. Maximum or total release of 51Cr was determined by incubation of the targets in 0.1% Triton X-100 in distilled water. The percentage of specific 51Cr release was determined by the following calculation: Percentage of specific release = [(experimental - spontaneous)/(maximum - spontaneous)] × 100%.
Mixed lymphocyte reaction (MLR) assay
An allogeneic MLR assay was performed using mDCs that were irradiated with 3,000 rad as a stimulator and allogeneic T cells as responder cells in the ratios of 1:1, 1:10, 1:25 and 1:100. The positive and negative controls used were 2.5% phytohemagglutinin-stimulated T cells (GlaxoSmithKline, Brentford, UK), and the DCs or T cells alone, respectively. Cultures were made in V bottom 96-well plates at a final volume of 200 µl of complete medium supplemented with 10% human AB serum (Sigma-Aldrich) for 5 days and 3H-thymidine was added at a concentration of 1 µCurie/well at 18 h prior to the end of the culture. Proliferative responses were measured by a liquid scintillation counter (PerkinElmer, Inc.) and expressed as the mean count/min obtained for triplicate wells.
Statistical analysis
The differences between the two groups were determined using analysis of variance and the Scheffe test. All analyses were conducted using SigmaStat™ version 4.0 software. Results were expressed as the mean ± standard deviation, and P<0.05 was considered to indicate a statistically significant difference.
Results
Immunophenotyping
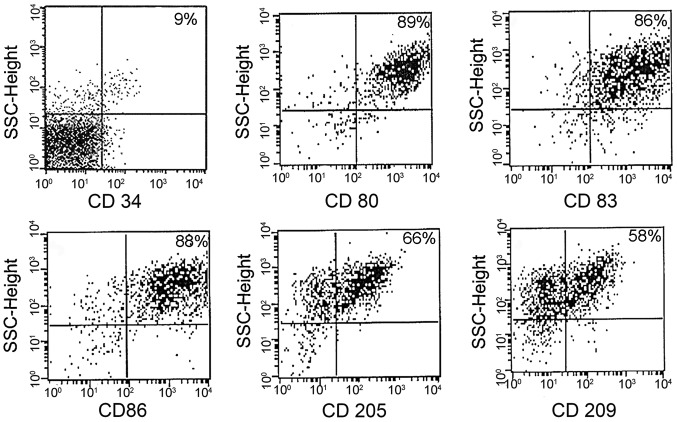
DCs were assessed for cell surface phenotypes by flow cytometry. A greater degree of maturation was observed in LM8-pulsed DCs than in poly I:C-matured DCs. The results showed that DCs expressed a high level of function of associated surface antigens in the LM8-pulsed mDCs, including the following: CD80+, 89%; CD83+, 86%; CD86+, 88%; CD205+, 66%; and CD209+, 58% (Fig. 1). The DCs matured with the poly I:C antigen (control) showed similar surface antigen expression (Fig. 2), and no significant difference was indicated in the expression profile of the two groups.
Figure 1.
Fluorescence-activated cell sorting analysis of LM8 cell lysate-loaded dendritic cells. One representative result from three experiments is shown. CD, cluster of differentiation.
Figure 2.
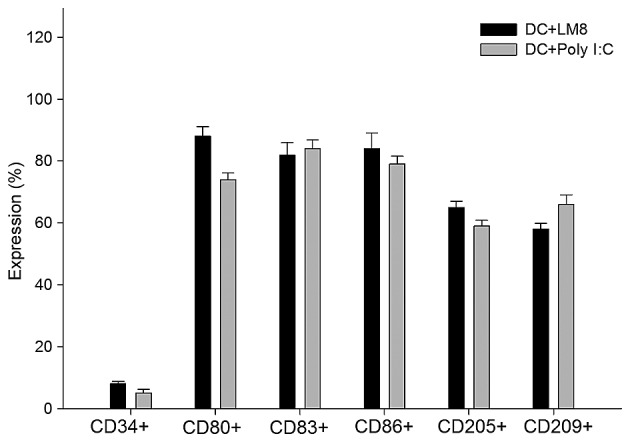
Percentage DCs expressing cell surface markers, as measured by flow cytometry. DCs were pulsed with LM8 cell lysate (DC+LM8) or matured in poly I:C without cell lysate (DC+Poly I:C). Results represent the mean of three experiments ± standard deviation. DC, dendritic cell; Poly I:C, polyinosinic:polycytidylic acid; CD, cluster of differentiation.
Quantitation of antigen uptake
Endocytosis is a functional feature of iDCs which decreases with maturation (17). The iDCs obtained on day 6 demonstrated high endocytotic capability. As expected, an increased dextran uptake occurred by iDCs (day 6 culture) compared with the day 8 mDCs, but the increase was not statistically significant (P=0.12) (Fig. 3). However, compared with the LM8 cells, a statistically significant increase in dextran uptake was observed in the day 6 and 8 cells (P<0.026).
Figure 3.
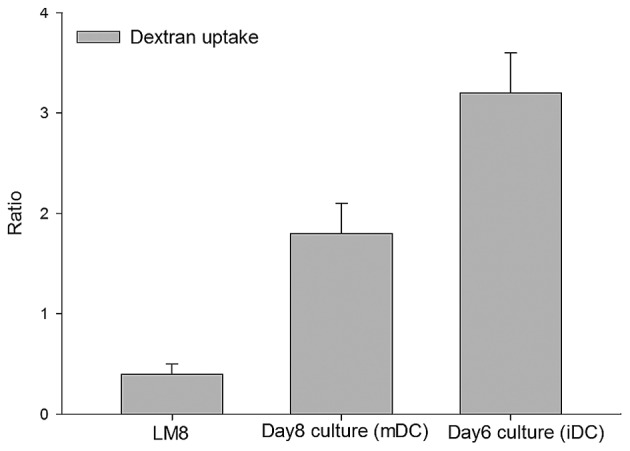
Dextran uptake of the day 6 culture iDCs, day 8 culture mDCs and LM8 cells. Dextran uptake of each sample was detected by flow cytometry following 1 h of incubation at 37°C, and mean fluorescence intensity ratio is presented. Results are shown as mean ± standard deviation of at least three samples tested. iDC, immature dendritic cell; mDC, mature dendritic cell.
Measurement of CTL activity
To determine the ability of LM8 cell lysate-pulsed DCs to generate LM8-specific CTLs against tumor cells, in vitro CTL assays with splenocytes from C3H mice immunized with the DC vaccine were performed. Mice that were previously injected with LM8 cells to develop a tumor but were not administered DCs acted as controls. As shown in Fig. 4, the highest CTL activity was noted in mice immunized with LM8 cell lysate-pulsed DCs in vivo. Mice immunized with poly I:C-matured DCs demonstrated a low level of CTL activity, whereas mice injected with only LM8 cells demonstrated further decreased LM8-specific lysis. Thus, LM8-specific CTL activity was significantly enhanced in mice immunized with LM8 cell lysate-matured DCs compared with poly I:C-matured DCs (P<0.046).
Figure 4.
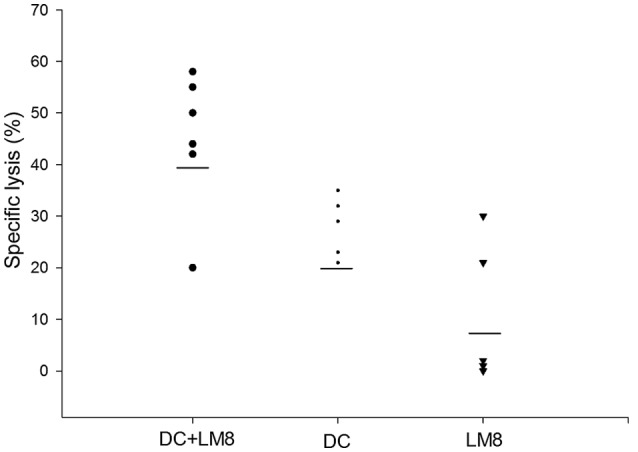
LM8-specific cytotoxic T lymphocytes. Percentage of specific lysis (exhibited by mouse spleen cells when treated with LM8-pulsed DCs or poly I:C-matured DCs. LM8 cells were used as a control. DC, dendritic cell.
Serum IFN-γ levels were increased in mice that received LM8 cell lysate-pulsed DCs (116.0±5.15 pg/ml) compared with the poly I:C-matured DC group (57.33±2.31 pg/ml; P<0.05) (Fig. 4). Serum IL-4 was decreased in the mice that received DCs pulsed with LM8 cell lysate, at 12.23±4.75 pg/ml vs. 41.06±2.51 pg/ml in poly I:C mDCs (P<0.047) (Fig. 5).
Figure 5.
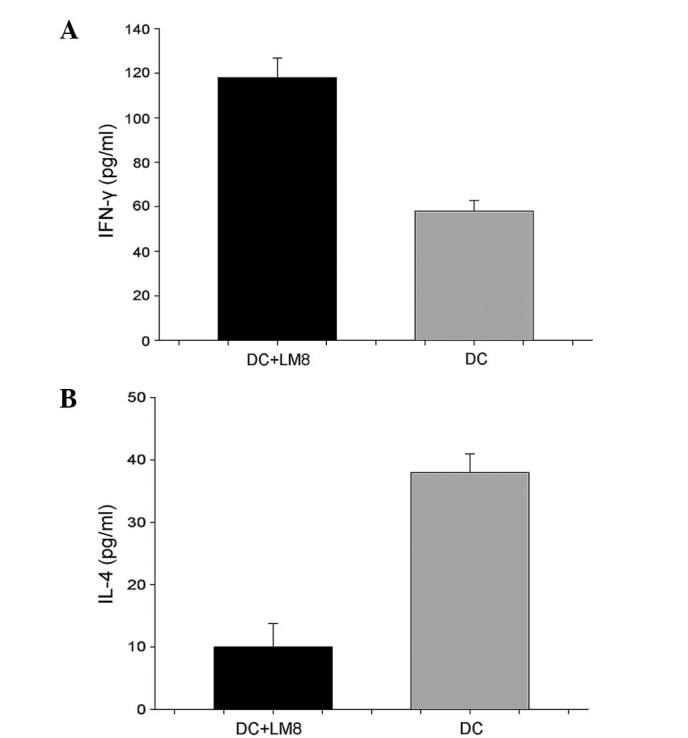
Release of (A) IFN-γ and (B) IL-4 from effector T cells against LM8-pulsed DCs or poly I:C-matured DCs. Data are expressed as mean ± standard deviation. DC, dendritic cell; IFN, interferon; IL, interleukin.
MLR assay
The effect of the two DC populations on the allogeneic responder lymphocyte was assessed using an MLR assay. The capacity of the DC subsets to stimulate allogeneic T cells was associated with the levels of co-stimulatory molecules CD86, CD83, CD205 and CD209 of the DC subpopulations (18). In order to investigate the critical function of mDCs to activate T cell proliferation through antigen presentation, the present study determined whether LM8 cell lysate-pulsed DCs could induce antigen-specific CD4+ and CD8+ T cell responses. The results revealed that priming of DCs with LM8 effectively enhanced CD4+ and CD8+ T cell proliferative responses at a DC:T cell ratio of 1:1, 1:10 and 1:25, compared with LM8 cells alone (Fig. 6).
Figure 6.
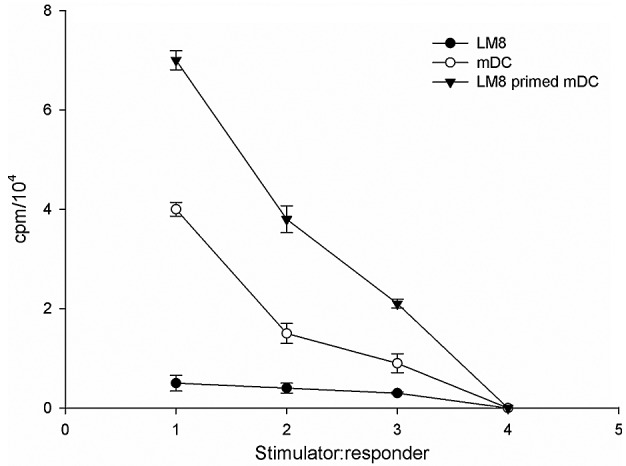
Allogenic MLR response induced by mDCs, LM8-pulsed mDCs and LM8 cells. Effect of dendritic cells' stimulation on the proliferation of T lymphocytes in MLR. The T-cell stimulatory capacity of mDCs that were irradiated with 3,000 rad as a stimulator and allogeneic T cells as responder cells in the ratios of 1:1, 1:10, 1:25 and 1:100 was assessed by MLR. The proliferative response was evaluated by 3H-thymidine incorporation and expressed as counts per minute. Data are presented as mean ± standard deviation. mDC, mature dendritic cells; MLR, mixed lymphocyte reaction; cpm, counts per minute.
Discussion
DCs exposed to tumor antigens serve crucial roles in antitumor immunity by inducing tumor-specific T cell responses. The current study used lysate from LM8 osteosarcoma cells to pulse DCs and elicit a CTL response in a mouse osteosarcoma model; this strategy has also been studied in other solid tumors (18–20). LM8 is an osteosarcoma cell line, originally from the C3H/He mouse, that has high metastatic potential and has been shown to cause metastatic osteosarcoma in C3H mice (21). Unlike specific and known tumor-associated peptide antigens, whole tumor cells or their derivatives may be used to stimulate T cells in a variety of tumors for which few or no defined tumor-specific antigens are available, including colon cancer and osteosarcoma (10,19). Furthermore, the application of whole tumor cell immunizations may result in the polyvalent stimulation of CD8+ CTLs and CD4+ T cells against a broad range of antigens. Experiments in vitro and in mouse models support the notion that tumor-specific T cells may be activated and expanded in vitro, and that they may inhibit tumor growth (18).
In the current study, LM8 lysate-pulsed DCs and poly I:C-matured DCs derived from bone marrow were found to express CD80, CD83, CD86, CD205 and CD209, which is consistent with the properties of APCs and earlier studies (22). Although the poly I:C-based protocol has been proposed as a potentially superior alternative to the conventional method proposed by Inaba et al (16), the present study has, for the first time, shown that minor modifications to the whole tumor cell lysate strategy may elicit a better CTL response.
DCs without sufficient tumor-associated antigens lack the ability to target tumor cells and present the antigen to T lymphocytes. Therefore, the present study evaluated endocytosis, using FITC-labelled dextran, as a functional feature of DC. As expected, an increased dextran uptake by iDCs (day 6 culture) was observed compared with the day 8 mDCs; however, the increase was not statistically significant. Various studies have indicated that the uptake of dextran may be a measure of immune response (23–25). The present results also support earlier studies that suggest that increased antigen uptake is associated with IL-12 and IFN-γ secretion, and CTL response (17,26,27). LM8-specific CTL activity was significantly enhanced in mice immunized with LM8 cell lysate-pulsed DCs compared with poly I:C-matured DCs (P<0.026).
The capacity of the DC subsets to stimulate allogeneic T cells was associated with the levels of the co-stimulatory molecules CD86, CD83, CD205 and CD209 in the DC subpopulations (18). Pulsing of DCs with LM8 cell lysate effectively enhanced CD4+ and CD8+ T cell proliferative responses compared with LM8 cells alone. The group treated with the combination of LM8 cell lysate-pulsed DCs showed increased expression of serum IFN-γ levels compared with the poly I:C-matured DC group. Serum IL-4 was decreased in the mice that received DCs pulsed with LM8 cell lysate vs. the poly I:C-matured DCs.
The present study was subject to certain limitations as the clinical setting used in humans was not completely replicated using the mouse model, and synergistic effects with chemotherapy were not assessed.
In summary, the present study expands the validation of the whole tumor cell lysate-based strategy in the active immunotherapy of patients with osteosarcoma. Additional validation in a clinical setting may support the use of its approach in conjunction with chemotherapy.
References
- 1.Renard AJ, Veth RP, Schreuder HW, Pruszczynski M, Bökkerink JP, van Hoesel QG, van Der Staak FJ. Osteosarcoma: Oncologic and functional results. A single institutional report covering 22 years. J Surg Oncol. 1999;72:124–129. doi: 10.1002/(SICI)1096-9098(199911)72:3<124::AID-JSO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Dominkus M, Lang S, Bodmer N, Jundt G, Klingebiel T, Jürgens H, Gadner H, Bielack S. COSS Study Group: Osteosarcoma in very young children: Experience of the cooperative osteosarcoma study group. Cancer. 2010;116:5316–5324. doi: 10.1002/cncr.25287. [DOI] [PubMed] [Google Scholar]
- 4.Lewis VO. What's new in musculoskeletal oncology. J Bone Joint Surg Am. 2007;89:1399–1407. doi: 10.2106/JBJS.G.00075. [DOI] [PubMed] [Google Scholar]
- 5.Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the automated childhood cancer information system project. Eur J Cancer. 2006;42:2124–2135. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, Donati D, Lari S, Forni C, DePaolis M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: Long-term results of the Rizzoli's 4th protocol. Eur J Cancer. 2001;37:2030–2039. doi: 10.1016/S0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: Recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–1134. doi: 10.1093/annonc/mdg286. [DOI] [PubMed] [Google Scholar]
- 8.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 9.Berntsen A, Geertsen PF, Svane IM. Therapeutic dendritic cell vaccination of patients with renal cell carcinoma. Eur Urol. 2006;50:34–43. doi: 10.1016/j.eururo.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Suminoe A, Matsuzaki A, Hattori H, Koga Y, Hara T. Immunotherapy with autologous dendritic cells and tumor antigens for children with refractory malignant solid tumors. Pediatr Transplant. 2009;13:746–753. doi: 10.1111/j.1399-3046.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 11.Campbell CJ, Cohen J, Enneking WF. Editorial: New therapies for osteogenic sarcoma. J Bone Joint Surg Am. 1975;57:143–144. [PubMed] [Google Scholar]
- 12.Kawaguchi S, Wada T, Tsukahara T, Ida K, Torigoe T, Sato N, Yamashita T. A quest for therapeutic antigens in bone and soft tissue sarcoma. J Transl Med. 2005;3:31. doi: 10.1186/1479-5876-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival-a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini E, Staals EL, Ferrari S, Rinaldi R, Alberghini M, Mercuri M, Bacci G. Nonresectable multiple lung metastases of high-grade osteosarcoma of the humerus: Stable after twelve years. A case report. J Bone Joint Surg Am. 2008;90:2240–2244. doi: 10.2106/JBJS.G.01619. [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang CL, Hagemann AR, Leskowitz R, Mick R, Garrabrant T, Czerniecki BJ, Kandalaft LE, Powell DJ, Jr, Coukos G. Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PLoS One. 2011;6:e28732. doi: 10.1371/journal.pone.0028732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R, Melcher AA. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother. 2008;31:620–632. doi: 10.1097/CJI.0b013e31818213df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alaniz L, Rizzo MM, Mazzolini G. Pulsing dendritic cells with whole tumor cell lysates. Methods Mol Biol. 2014;1139:27–31. doi: 10.1007/978-1-4939-0345-0_3. [DOI] [PubMed] [Google Scholar]
- 20.Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozawa E, Sugiura H, Wasa J, Kohyama K, Yamada K, Nishioka A, Nishida Y, Ishiguro N, Taguchi O. Suppression of tumour metastasis in a murine osteosarcoma model with anti-CD25 monoclonal antibody treatment. Anticancer Res. 2010;30:5019–5022. [PubMed] [Google Scholar]
- 22.Thomas-Kaskel AK, Zeiser R, Jochim R, Robbel C, Schultze-Seemann W, Waller CF, Veelken H. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119:2428–2434. doi: 10.1002/ijc.22097. [DOI] [PubMed] [Google Scholar]
- 23.Napoletano C, Pinto D, Bellati F, Taurino F, Rahimi H, Tomao F, Panici PB, Rughetti A, Frati L, Nuti M. A comparative analysis of serum and serum-free media for generation of clinical grade DCs. J Immunother. 2007;30:567–576. doi: 10.1097/CJI.0b013e318046f396. [DOI] [PubMed] [Google Scholar]
- 24.Coester C, Nayyar P, Samuel J. In vitro uptake of gelatin nanoparticles by murine dendritic cells and their intracellular localisation. Eur J Pharm Biopharm. 2006;62:306–314. doi: 10.1016/j.ejpb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Araki H, Katayama N, Mitani H, Suzuki H, Nishikawa H, Masuya M, Ikuta Y, Hoshino N, Miyashita H, Nishii K, et al. Efficient ex vivo generation of dendritic cells from CD14+ blood monocytes in the presence of human serum albumin for use in clinical vaccine trials. Br J Haematol. 2001;114:681–689. doi: 10.1046/j.1365-2141.2001.02973.x. [DOI] [PubMed] [Google Scholar]
- 26.Mahdian R, Kokhaei P, Najar HM, Derkow K, Choudhury A, Mellstedt H. Dendritic cells, pulsed with lysate of allogeneic tumor cells, are capable of stimulating MHC-restricted antigen-specific antitumor T cells. Med Oncol. 2006;23:273–282. doi: 10.1385/MO:23:2:273. [DOI] [PubMed] [Google Scholar]
- 27.Vegh Z, Mazumder A. Generation of tumor cell lysate-loaded dendritic cells preprogrammed for IL-12 production and augmented T cell response. Cancer Immunol Immunother. 2003;52:67–79. doi: 10.1007/s00262-002-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]