Abstract
Artemisia apiacea (also known as Artemisia annua L) is a herb commonly used in traditional Chinese medicine. In the early 1970s, artemisinin was isolated and identified as the active antimalarial ingredient, and thereafter, A. apiacea and artemisinin have been studied extensively, such as anti-inflammation and antipyresis, antibacteria, antiparasitic and immunosuppression effects of A. apiacea extract. The present study investigated the extracts anti-allergic effect obtained from the dried flowering tips of A. apiacea in rats. A systemic anaphylactic reaction model was induced in rats using compound 48/80. Artemisia extract was administered 1 h prior to the injection of compound 48/80. Artemisia was extracted from dried flowering tips of A. deserti using 80% ethanol. Subsequently, the systemic anaphylactic shock, histamine release, scratching behavior and vascular permeability induced by compound 48/80 were evaluated. The administration of Artemisia extract at 200 and 400 mg/kg doses suppressed the systemic anaphylactic shock induced by compound 48/80 in a dose-dependent manner. Overall, the Artemisia extract was able to effectively decrease systemic anaphylactic shock, histamine release, scratching behavior and vascular permeability induced by compound 48/80 in a dose-dependent manner.
Keywords: artemisia extract, systemic anaphylactic reaction, allergic models
Introduction
Hypersensitivity reaction is a type of B-type adverse reaction, which is an abnormal reaction unrelated to dose and conventional pharmacological effects and unpredictable in preclinical experiments, with low incidence and high mortality (1). Hypersensitivity reaction is referred to as anaphylaxis (also known as Type I hypersensitivity) and is a strong reaction produced by the body when the immune system is stimulated by a certain allergen repeatedly. Type I hypersensitivity is usually caused by an immunogenic macromolecule substance or hapten molecules capable of being combined with a macromolecular carrier (2). Allergens may stimulate lymphocytes to generate antibody IgE, which is combined with high-affinity IgE receptor (FcεRI) of mast cells or basophils via the circulatory system. Once the allergen enters the body, its combination with IgE leads to FcεRI coupling, thus triggering the degranulation of mast cells or basophils, and the release of allergic mediators causes local or systemic reactions (3).
The majority of drug-induced allergic reactions are Type I hypersensitivity reactions ranging from the local to the body; local reactions include allergic dermatitis, and systemic allergic reactions may lead to anaphylactic shock and patient fatality (4). Artemisinin is an antimalarial active chemical component isolated from Artemisia extract. Artemisia apiacea (also known as Artemisia annua L) contains >140 types of chemical substance, of which >30 have anti-malarial activity. A recently identified chemical substance in A. apiacea extract, isolated by the China Traditional Chinese Medicine Academy (http://www.catcm.ac.cn/), has shown a good antimalarial effect that is equal to artemisinin (5–7). A. apiacea is a herb commonly used in traditional Chinese medicine, which exhibits anti-inflammatory and antipyresis, antibacterial, antiparasitic and immunosuppressive effects (8). Therefore, the aim of the present study was to evaluate the effect of Artemisia extract on the allergic response induced by compound 48/80 in rats.
Materials and methods
Animals and grouping
A total of 60 male Wistar rats (weight, 230–280 g; age, 7 weeks) were obtained from the Animal Experimental Center of Southern Medical University (Guangzhou, China) and were maintained at a constant temperature (24±2°C) with a relative humidity of 55±15% in a 12-h light/dark cycle. All rats were administered standard laboratory rodent feed and water ad libitum. A total of 50 rats were randomly assigned into 5 groups: A spontaneous group (n =10), a control (n=10), 100 mg/kg of Artemisia extract-treated group (n=10), 200 mg/kg of Artemisia extract-treated group (n=10), 400 mg/kg of Artemisia extract-treated group (n=10). This study was in accordance with the Southern Medical University Guidelines and Regulations on the Use and Care of Lab Animals. Ethical approval was obtained for this study from the Southern Medical University.
Preparation of the extract
The dried flowering tips of A. apiacea were obtained from Kunming Institute of Medicine (Kunming, China) and were ground into fine powder using an electric blender (Thermo Fisher Scientific Inc., Waltham, MA, USA). Next, 20 g powder was ground and refluxed with 80% ethanol (600 ml) using a Soxhlet extractor (Thermo Fisher Scientific Inc.) for 8 h. The extraction process was repeated twice. The extract was separated by passing the mixture through filter paper. and the resulting Artemisia extract was freshly dissolved in a 5% gum arabic (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solution prior to use.
Compound 48/80-induced systemic anaphylactic reaction
All rats received an intraperitoneal (i.p.) injection of compound 48/80 (8 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) to induce a systemic anaphylactic reaction. The systemic anaphylactic reaction model was conducted according to the method previously described by Shin et al (9). Artemisia extract was administered 1 h prior to the injection of compound 48/80. Following the induction of anaphylactic shock, mortality rate was recorded over a 1-h period.
Compound 48/80-induced histamine release from isolated rat peritoneal mast cells
The rats were cervically dislocated under anesthesia with 40 mg/kg pentobarbital sodium). Peritoneal mast cells were harvested from male Wistar strain rats using decollation under 350 mg/kg chloral hydrate. A total of 5 ml of medium was injected into enterocoelia of every mouse and the abdomen was gently massaged for 5–10 min. The cells were purified using Percoll density centrifugation (Thermo Fisher Scientific, Inc.) at 8,000xg at 4°C for 10 min, as described previously (10). All cells (3×104 cells/tube) were incubated in physiological buffer solution (Beyotime Institute of Biotechnology, Haimen, China) for 20 min at 37°C. Artemisia extract, 0.1 ml phosphate-buffered saline (Wuhan Procell Science and Technology Co., Ltd., Wuhan, China) and 0.5 mg/ml compound 48/80 were blended altogether. The miscible liquids were incubated for 20 min on ice. The histamine content was measured by means of a fluorometric assay (ELISTA kit; E-EL-0032c, Elabscience, Wuhan, China) by an ELx800 microplate reader (Biotek Instruments, Inc., Winooski, VT, USA). Briefly, the miscible liquids were incubated for 20 min on ice and 80 µl of affinity chain enzyme-HRP at 37°C was added for 1 h. Next, every well was washed with scrubbing solution. The histamine content was measured by means of a fluorometric assay (Synergy 2 Microplate Reader, Bio-Tek, USA) at 450 nm.
Scratching behavior
Scratching behavior was surveyed using a previously described method (11). The Artemisia extract was administered orally and scratching behavior was observed for 1 h. Next, 10 µg/0.02 ml compound 48/80, 100 nmol/0.02 ml histamine and 100 nmol/0.02 ml serotonin (both from Sigma-Aldrich) were injected intradermally into the rostral part of the back of the rats. Scratching behavior of rat was observed for a further 1 h, and evaluated using a HF-200 camera (Canon Legria, Canon Inc., Tokyo, Japan).
Vascular permeability of the skin
Artemisia extract was administered orally and 1 h passed before subsequent experiments. Next, 0.5 mg/0.02 ml compound 48/80 (i.p.), 10 nmol/0.02 ml histamine (i.p.) or 10 nmol/0.02 ml serotonin (i.p.) was administered into the rostral part of the back. In addition, 2% Evans blue solution (Beyotime Institute of Biotechnology) was intravenously injected into each animal. After a pentobarbital sodium injection (1.5%; Sigma-Aldrich) the rats were sacrificed using cervical dislocation and after 30 min, then the ‘bluing’ reaction diameter was measured at the injection site.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Statistical analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Statistical significance was tested using one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of Artemisia extract on the systemic anaphylactic shock induced by compound 48/80
To determine the effect of artemisia extract on the systemic anaphylactic shock induced by compound 48/80, the mortality rate within 1 h after compound 48/80 injection was recorded. The administration of 8 mg/kg compound 48/80 (i.p.) increased the mortality rate in the allergic model rats (Table I). However, the Artemisia extract pretreatment reduced the mortality rate of allergic model rats in a concentration- and time-dependent manner (Table I).
Table I.
Effect of Artemisia extract on the systemic anaphylactic shock induced by compound 48/80.
| Mortality rate (%) | ||||
|---|---|---|---|---|
| Group | 10 min | 20 min | 60 min | |
| Control | 10 | 60 | 100 | |
| Artemisia extract (mg/kg) | ||||
| 100 | 0 | 30 | 70 | |
| 200 | 0 | 20 | 50 | |
| 400 | 0 | 10 | 40 | |
Mortality within 1 h after compound 48/80 injection is presented as the number of dead rats/total number rats (n=10).
Effect of Artemisia extract on the histamine released from rat peritoneal mast cells induced by compound 48/80
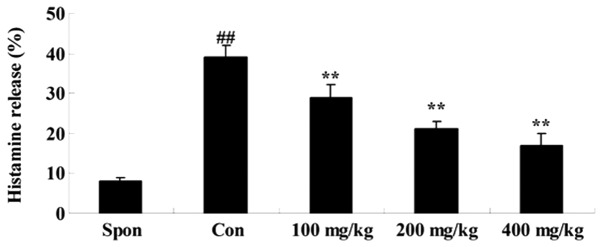
A fluorometric assay was performed to investigate the effect of Artemisia extract on histamine release in the allergic model rats. Treatment with 0.5 mg/ml compound 48/80 significantly increased the histamine release in the allergic model rats (Fig. 1). Artemisia extract pretreatment (100, 200 and 400 mg/kg) significantly reduced the histamine release in the allergic model rats, compared with the untreated control (Fig. 1).
Figure 1.
Effect of artemisia extract on the histamine release from rat peritoneal mast cells induced by compound 48/80. ##P<0.01 vs. Spon group, **P<0.01 vs. Con group. Spon, spontaneous-group; Con, control-group; 100 mg/kg, Artemisia extract (100 mg/kg)-treated group; 200 mg/kg, Artemisia extract (200 mg/kg)-treated group; 400 mg/kg, Artemisia extract (400 mg/kg)-treated group. Statistical analysis was done with the Student's t-test and one-way analysis of variance followed by Dunnett's test.
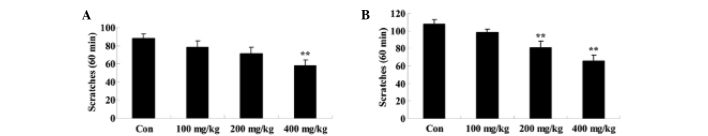
Effect of Artemisia extract on the scratching behavior induced by compound 48/80
To elucidate the effect of Artemisia extract on the scratching behavior of allergic model rats, the scratching behavior was induced by treatment with compound 48/80. Furthermore, the Artemisia extract pretreatment (200 and 400 mg/kg) significantly reduced the scratching behavior of the allergic model rats (Fig. 2).
Figure 2.
Effect of Artemisia extract on the scratching behavior. **P<0.01 vs. Con group. Con, control-group; 100 mg/kg, Artemisia extract (100 mg/kg)-treated group; 200 mg/kg, Artemisia extract (200 mg/kg)-treated group; 400 mg/kg, Artemisia extract (400 mg/kg)-treated group. Statistical analysis was done with the Student's t-test and one-way analysis of variance followed by Dunnett's test.
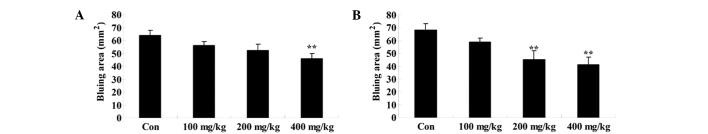
Effect of Artemisia extract on the vascular permeability induced by compound 48/80
To assess the effect of Artemisia extract on the vascular permeability of allergic model rats, the vascular permeability was evaluated following 0.5 mg/0.02 ml compound 48/80 injection. Compound 48/80 effectually augmented the vascular permeability of allergic model rats (Fig. 3). The Artemisia extract pretreatment (200 and 400 mg/kg) significantly reduced the vascular permeability of allergic model rats (Fig. 3).
Figure 3.
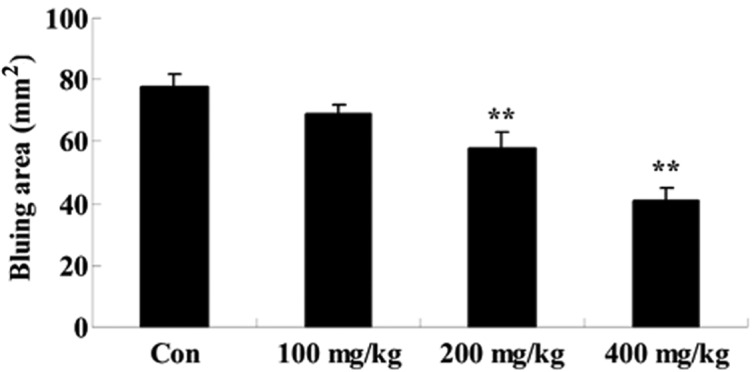
Effect of Artemisia extract on the vascular permeability. **P<0.01 vs. Con group. Con, control-group; 100 mg/kg, Artemisia extract (100 mg/kg)-treated group; 200 mg/kg, Artemisia extract (200 mg/kg)-treated group; 400 mg/kg, Artemisia extract (400 mg/kg)-treated group. Statistical analysis was done with the Student's t-test and one-way analysis of variance followed by Dunnett's test.
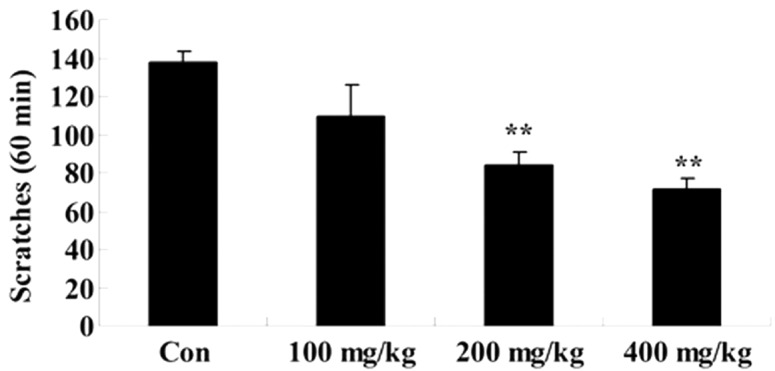
Effects of Artemisia extract on the scratching behavior induced by histamine and serotonin
To examine the effect of Artemisia extract on the scratching behavior of allergic model rats, scratching behavior was induced with 100 nmol/0.02 ml histamine or serotonin injection. Histamine or serotonin significanlty increased the scratching behavior of allergic model rats (Fig. 4A and B). The Artemisia extract pretreatment (400 mg/kg) significantly reduced the scratching behavior induced by histamine (Fig. 4A). Furthermore, the Artemisia extract pretreatment (200 and 400 mg/kg) significantly reduced the scratching behavior induced by serotonin (Fig. 4B).
Figure 4.
Effects of Artemisia extract on the scratching behavior induced by histamine and serotonin. Effects of artemisia extract on the scratching behavior induced by (A) histamine and (B) serotonin. **P<0.01 vs. Con group. Con, control-group; 100 mg/kg, Artemisia extract (100 mg/kg)-treated group; 200 mg/kg, Artemisia extract (200 mg/kg)-treated group; 400 mg/kg, Artemisia extract (400 mg/kg)-treated group. Statistical analysis was done with the Student's t-test and one-way analysis of variance followed by Dunnett's test.
Effect of artemisia extract on the vascular permeability induced by histamine and serotonin
To analyze the effect of Artemisia extract on the vascular permeability of allergic model rats, the vascular permeability was investigated after 10 nmol/0.02 ml histamine or serotonin injection. Histamine or serotonin markedly increased the vascular permeability of allergic model rats (Fig. 5A and B). The Artemisia extract pretreatment (400 mg/kg) significantly reduced the vascular permeability induced by histamine (Fig. 5A). Furthermore, the Artemisia extract pretreatment (200 and 400 mg/kg) significantly reduced the vascular permeability induced by serotonin (Fig. 5B).
Figure 5.
Effect of Artemisia extract on the vascular permeability induced by histamine and serotonin. Effects of Artemisia extract on the vascular permeability induced by (A) histamine and (B) serotonin. **P<0.01 vs. Con group. Con, control-group; 100 mg/kg, Artemisia extract (100 mg/kg)-treated group; 200 mg/kg, Artemisia extract (200 mg/kg)-treated group; 400 mg/kg, Artemisia extract (400 mg/kg)-treated group. Statistical analysis was done with the Student's t-test and one-way analysis of variance followed by Dunnett's test.
Discussion
Hypersensitivity reaction is a type of B-type adverse reaction that is an abnormal reaction unrelated to dose and conventional pharmacological effects, and is unpredictable in preclinical experiments, with low incidence and high mortality (12). Drug-induced allergic reactions may be serious, as anaphylactic shock may lead to patient fatality (13). Establishing a sensitive and reliable animal model for the study of allergic anaphylaxis is necessary; as a basis for screening and discovering drug allergens in addition to the investigation of drug-induced allergic reaction mechanisms, that are relevant to the prevention and treatment of clinical allergic reactions to drugs (14). However, during the preclinical safety evaluation of drugs, allergic reaction is among the most difficult factors to predict due to the lack of appropriate models for predicting clinical allergic reactions (15). The establishment of appropriate animal models for allergic reaction prediction, and their successful application to predicting drug allergies are required (16,17). The present results demonstrated that Artemisia extract pretreatment is capable of reducing the systemic anaphylactic shock, histamine release, scratching behavior and vascular permeability induced by compound 48/80. Previously, Kim et al suggested that Artemisia asiatica extract improved airway inflammation of allergic asthma in mice (18).
The safety of various traditional Chinese medicines, particularly with regard to clinical allergic reaction, has led to increased their preclinical re-evaluation in China to guarantee drug safety of patients (19). The traditional Chinese medicine A. apiacea is also known as Artemisia annua L (20). Its extract is anti-allergy medicine, which has been proposed as a treatment of disease due to its speculated anti-illness effects, limited side effects and low cost (21). Recent studies show that the extract is a physiologically active traditional medicine, with anti-allergic, anti-oxidative and anti-inflammatory effects (22–24). In the present study, the anti-allergic effect of Artemisia extract markedly suppressed the scratching behavior and vascular permeability induced by histamine and serotonin.
In conclusion, the present findings suggest that the extract obtained from the dried flowering tips of A. apiacea exerts an anti-allergic effect on the allergic reaction induced by compound 48/80 in rats. This study provides an experimental basis for the investigation of Artemisia extract in the treatment of allergic diseases.
Acknowledgements
This study was supported by The National Natural Science Fund (grant no. 81202704).
References
- 1.Kiss B, Szántó M, Szklenár M, Brunyánszki A, Marosvölgyi T, Sárosi E, Remenyik É, Gergely P, Virág L, Decsi T, et al. Poly (ADP) ribose polymerase-1 ablation alters eicosanoid and docosanoid signaling and metabolism in a murine model of contact hypersensitivity. Mol Med Rep. 2015;11:2861–2867. doi: 10.3892/mmr.2014.3044. [DOI] [PubMed] [Google Scholar]
- 2.Roth-Walter F, Gomez-Casado C, Pacios LF, Mothes-Luksch N, Roth GA, Singer J, Diaz-Perales A, Jensen-Jarolim E. Bet v 1 from birch pollen is a lipocalin-like protein acting as allergen only when devoid of iron by promoting Th2 lymphocytes. J Biol Chem. 2014;289:17416–17421. doi: 10.1074/jbc.A114.567875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitoshi M, Kuriyama I, Nakayama H, Miyazato H, Sugimoto K, Kobayashi Y, Jippo T, Kuramochi K, Yoshida H, Mizushina Y. Suppression of allergic and inflammatory responses by essential oils derived from herbal plants and citrus fruits. Int J Mol Med. 2014;33:1643–1651. doi: 10.3892/ijmm.2014.1720. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Yi JS, Gong CH, Jang YJ. Development of Aspergillus protease with ovalbumin-induced allergic chronic rhinosinusitis model in the mouse. Am J Rhinol Allergy. 2014;28:465–470. doi: 10.2500/ajra.2014.28.4100. [DOI] [PubMed] [Google Scholar]
- 5.Brandys J, Grimsøen A, Nilsen BM, Paulsen BS, Park HS, Hong CS. Cross-reactivity between pollen extracts from six artemisia species. Planta Med. 1993;59:221–228. doi: 10.1055/s-2006-959656. [DOI] [PubMed] [Google Scholar]
- 6.Olsen OT, Frølund L, Heinig J, Jacobsen L, Svendsen UG. A double-blind, randomized study investigating the efficacy and specificity of immunotherapy with Artemisia vulgaris or Phleum pratense/Betula verrucosa. Allergol Immunopathol (Madr) 1995;23:73–78. [PubMed] [Google Scholar]
- 7.Subiza J, Subiza JL, Alonso M, Hinojosa M, Garcia R, Jerez M, Subiza E. Allergic conjunctivitis to chamomile tea. Ann Allergy. 1990;65:127–132. [PubMed] [Google Scholar]
- 8.Ryu JC, Park SM, Hwangbo M, Byun SH, Ku SK, Kim YW, Kim SC, Jee SY, Cho IJ. Methanol extract of Artemisia apiacea hance attenuates the expression of inflammatory mediators via NF-κB inactivation. Evid Based Complement Alternat Med. 2013 Feb 22; doi: 10.1155/2013/494681. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin TY, Park JH, Kim HM. Effect of Cryptotympana atrata extract on compound 48/80-induced anaphylactic reactions. J Ethnopharmacol. 1999;66:319–325. doi: 10.1016/S0378-8741(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 10.Noori A, Amjad L, Yazdani F. The effects of Artemisia deserti ethanolic extract on pathology and function of rat kidney. Avicenna J Phytomed. 2014;4:371–376. [PMC free article] [PubMed] [Google Scholar]
- 11.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-B. [DOI] [PubMed] [Google Scholar]
- 12.Du Q, Gu X, Cai J, Huang M, Su M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Mol Med Rep. 2012;6:100–104. doi: 10.3892/mmr.2012.893. [DOI] [PubMed] [Google Scholar]
- 13.Cheng LJ, Liu B, Ning B, Ming H, Wang C, Wan LX. High-intensity focused ultrasound for the treatment of allergic rhinitis using nasal endoscopy. Exp Ther Med. 2013;5:320–322. doi: 10.3892/etm.2012.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite-allergic children with asthma. Exp Ther Med. 2014;7:630–634. doi: 10.3892/etm.2014.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollander SM, Joo SS, Wedner HJ. Factors that predict the success of cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol. 2011;107:523–528. doi: 10.1016/j.anai.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim HH, Choi PH, Yoo JS, Jeon H, Chae BS, Park JS, Kim SH, Shin TY. Ripe fruit of Rubus coreanus inhibits mast cell-mediated allergic inflammation. Int J Mol Med. 2012;29:303–310. doi: 10.3892/ijmm.2011.829. [DOI] [PubMed] [Google Scholar]
- 17.Yong J, Chen GQ, Huang B, Wu S. Correlation between the ratio of T-bet/GATA-3 and the levels of IL-4 and IFN-γ in patients with allergic asthma. Mol Med Rep. 2011;4:663–666. doi: 10.3892/mmr.2011.469. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Kim DY, Lee YS, Lee BK, Lee KH, Ro JY. DA-9601, Artemisia asiatica herbal extract, ameliorates airway inflammation of allergic asthma in mice. Mol Cells. 2006;22:104–112. [PubMed] [Google Scholar]
- 19.Guo H, Liu MP. Mechanism of traditional Chinese medicine in the treatment of allergic rhinitis. Chin Med J (Engl) 2013;126:756–760. [PubMed] [Google Scholar]
- 20.Zhang F, Fu X, Lv Z, Shen Q, Yan T, Jiang W, Wang G, Sun X, Tang K. Type 2C phosphatase 1 of Artemisia annua L. Is a negative regulator of ABA signaling. Biomed Res Int. 2014;2014:521794. doi: 10.1155/2014/521794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen T, Xu Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Rasool R, Ganai BA, Akbar S, Kamili AN. Free radical scavenging potential of in vitro raised and greenhouse acclimatized plants of Artemisia amygdalina. Chin J Nat Med. 2013;11:377–384. doi: 10.1016/S1875-5364(13)60055-2. [DOI] [PubMed] [Google Scholar]
- 23.Park JM, Hahm KB, Kwon SO, Kim EH. The Anti-inflammatory effects of acidic polysaccharide from Artemisia capillaris on Helicobacter pylori infection. J Cancer Prev. 2013;18:161–168. doi: 10.15430/JCP.2013.18.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puc M, Wolski T. Forecasting of the selected features of Poaceae (R. Br.) Barnh., Artemisia L. and Ambrosia L. pollen season in Szczecin, north-western Poland, using Gumbel's distribution. Ann Agric Environ Med. 2013;20:36–47. [PubMed] [Google Scholar]