Abstract
Radix Paeoniae Rubra (RPR) is the dried root of Paeonia lactiflora Pallas and Paeonia veitchii Lynch, and is a herbal medicine that is widely used in traditional Chinese medicine for the treatment of blood-heat and blood-stasis syndrome, similarly to Cortex Moutan. The present study identified the same three components in RPR and Cortex Moutan extracts. In addition, it has been reported that RPR has an anti-cancer effect. Bladder cancer is the seventh most common type of cancer worldwide. Due to the high recurrence rate, identifying novel drugs for bladder cancer therapy is essential. In the present study, RPR extract was evaluated as a bladder cancer therapy in vitro and in vivo. The present results revealed that RPR extract reduced the cell viability of bladder cancer cells with a half maximal inhibitory concentration of 1–3 mg/ml, and had an extremely low cytotoxic effect on normal urothelial cells. Additionally, RPR decreased certain cell cycle populations, predominantly cells in the G1 phase, and caused a clear sub-G increase. In a mouse orthotopic bladder tumor model, intravesical application of RPR extract decreased the bladder tumor size without altering the blood biochemical parameters of the mice. In summary, the present results demonstrate the anti-proliferative properties of RPR extract on bladder cancer cells, and its anti-bladder tumor effect in vivo. Compared to Cortex Moutan extract, RPR extract may provide a more effective alternative therapeutic strategy for the intravesical therapy of superficial bladder cancer.
Keywords: bladder cancer, gallic acid, intravesical therapy, methyl gallate, mouse orthotopic bladder tumor model, paeoniflorin, Radix Paeoniae Rubra
Introduction
Radix Paeoniae Rubra (RPR), or Chishao in Chinese, is the dried root of the plant Paeonia lactiflora Pallas and Paeonia veitchii Lynch, and is a widely used Chinese medical herb, which tastes bitter. According to the traditional Chinese medicine (TCM) meridian science theory, RPR exerts a major physiological effect on the liver (1). RPR belongs to the light cold-type TCM group and has heat-clearing (through cooling and activating blood), pain-relieving and stasis-dissipating effects (2). Similarly to Cortex Moutan, RPR is normally used for the treatment of blood-heat and blood-stasis syndromes in the clinic (3). Previous studies have demonstrated that RPR has various biological and pharmacological activities, including anti-inflammatory, immunomodulatory (4), anti-angiogenic (5), anti-atherosclerosis (6), anti-platelet (7), anti-diabetic (8), anti-tumor (9), peripheral neuroprotective (10) and hepatoprotective effects (2). There are ~40 compounds that have been screened and identified from RPR extracts. The major bioactive constituents of RPR consist of monoterpene glycosides, galloyl glucoses and other phenolic compounds (11,12).
Paeoniae Radix (Shaoyao in Chinese, the dried root of Paeonia lactiflora Pallas. and/or P. veitchii Lynch.) and Cortex Moutan (Mu Dan Pi in Chinese, the dried root bark of P. suffruticosa Andr.) are obtained from plants belonging to the Paeoniaceae family and Paeonia genus. It has been reported that the dried roots of P. lactiflora and the dry root bark of P. suffruticosa contain similar compounds, including monoterpene glycosides, paeonol, paeoniflorin and gallic acid (13). Total paeony glycosides, one of the components of RPR, inhibits angiogenesis (5), human chronic myelogenous leukemia K562 cell proliferation and xenografted tumor growth (14), activates natural killer cells and elevates serum levels of interleukin (IL)-2 and IL-4, which significantly reduces tumor growth in mice in vivo (9). In addition, crude extracts of RPR reduces IL-10 and increases IL-8 levels in Bacillus Calmette-Guérin (BCG)-activated primary human blood macrophages (15). Furthermore, Bai Shao extract, which is the dried product of boiled P. lactiflora Pall. root with the bark removed, inhibits the development of rat bladder cancer, induced by N-butyl-N-(4-hydroxybutyl) nitrosamine, via apoptotic mechanisms (16).
Bladder cancer is the seventh most common type of cancer in men worldwide (17). The highest incidence (age standardized rate and proportions per 100,000) is reported in southern Europe, including 27.1 in men and 4.1 in women (18). In total, >90% of bladder cancer cases are diagnosed histologically as urothelial carcinoma (UC) (18), which is classified into two groups due to the pathogenesis: Superficial and invasive UC. Immunotherapeutic and chemotherapeutic agents are two types of intravesical therapeutic agents for bladder cancer therapy. BCG is an immunotherapeutic agent and mitomycin C is a chemotherapeutic agent (19). BCG provokes considerable and occasionally serious side-effects (20), and the recurrence rate of cancer following BCG therapy remains high in spite of intravesical chemotherapy (21). Therefore, improvement in bladder cancer therapy is required. Research concerning the identification of novel agents or methods for bladder cancer treatment remains ongoing; intravesical anti-tumor herbal medicine has demonstrated an excellent therapeutic result in a mouse orthotopic bladder tumor model (3), which provides an ideal animal bladder tumor model for studies (22).
A previous study using this animal model demonstrated that combretastatin A-4, a natural tubulin polymerization inhibitor, reduced bladder tumor growth via intravesical therapy (23). However, another natural compound, curcumin, did not demonstrate an effect in this mouse model (24). It was observed that baicalein, a natural compound derived from the root of Scutellaria baicalensis Georgi, only had a small anti-bladder tumor effect through intraperitoneal application (25). Notably, when using an extract from Cortex Moutan, which is the root bark of P. suffruticosa Andr., the anti-bladder tumor effect was increased compared with the effect observed with the aforementioned natural compounds (3). Therefore, these findings suggests that Chinese traditional medicines may provide an alternative in the treatment of UC via intravesical application. The anti-tumor effect of RPR extract, which contains similar compounds to Cortex Moutan extract, on bladder cancer remains unknown. The present study investigated the anti-cancer effect of RPR extract in bladder cancer cell lines, and in vivo in a mouse orthotopic bladder tumor model.
Materials and methods
Cell culture and RPR extract preparation
Human bladder papillary transitional cell carcinoma BFTC 905 cells were obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). Mice bladder carcinoma MB49 cells were kindly provided by Dr Timothy L. Ratliff (Purdue Cancer Center, West Lafayette, IN, USA). BFTC 905 and MB49 cells were maintained in RPMI-1640 medium supplied with 10% fetal bovine serum (FBS), 1% penicillin and 1% streptomycin. Human simian virus (SV) 40-transformed uroepithelial SV-HUC-1 cells (Bioresource Collection and Research Center) were maintained in Ham's F12 nutrient mixture supplemented with 7% FBS. All cell culture reagents were purchased from Gibco® (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were incubated in a CO2 incubator at 37°C, with 5% CO2 and 95% filtered air. RPR and Cortex Moutan were purchased from Sun Ten Pharmaceutical Co., Ltd. (New Taipei, Taiwan). RPR and Cortex Moutan were prepared by mixing each powder with RPMI-1640 medium (50 mg/ml; stock concentration), and subsequently sonicated on ice for 60 min, filtered using qualitative Millex® filter paper (0.45 µm and 0.2 µm; Merck Millipore, Darmstadt, Germany) and stored at −80°C until required. Control group cells were treated with the same volume of RPMI-1640 but without RPR.
Chromatographic separation
High-performance liquid chromatography (HPLC; Hitachi, Ltd., Tokyo, Japan) analysis was performed on a Purospher®STAR RP-18 endcapped HPLC column (internal diameter × length, 4.6×250 mm; pore size, 5 µm; Merck Millipore). Methanol, ethanol or RPMI-1640 medium were used as the solvents to dissolve gallic acid, methyl gallate and paeoniflorin, while prepared RRP and Cortex Moutan were injected directly for HPLC analysis. A binary gradient elution system composed of acetonitrile (Sigma-Aldrich) as solvent A and 0.1% phosphoric acid as solvent B was used. The gradient elution was as follows: 0–5 min, 10–15% solvent A (90–85% solvent B); 5–25 min, 15–22% solvent A (85–78% solvent B); 25–45 min, 22–70% solvent A (78–30% solvent B); 45–46 min, 70–80% solvent A (30–20% solvent B); 46–50 min, 80% solvent A (20% solvent B). The mobile phase flow rate was 0.8 ml/min and the column temperature was maintained at 25°C. The ultraviolet light detector was set at 275 nm.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) assay, according to the manufacturers's protocol, and direct cell counting. MB49 (24 h assay, 4×104 cells/well; 48 h assay, 3×104 cells/well) or BFTC 905 (24 h assay, 8×104 cells/well; 48 h assay, 4×104 cells/well) cells were cultured in 24-well plates (Nunc™; Thermo Fisher Scientific, Inc.), while SV-HUC-1 cells (24 h assay, 6×104 cells/well; 48 h assay, 4×104 cells/well) were cultured in a 96-well plate (Nunc; Thermo Fisher Scientific, Inc.). After 24 h, cells were incubated with various concentrations of RPR extract concentrations (0, 0.5, 1, 2, 3, 3.5 mg/ml)for an additional 24 and 48 h. The MTT assay is based on the conversion of the tetrazolium salt by mitochondrial dehydrogenase to a formazan product. Following cell incubation with 0.5 mg/ml MTT, the culture medium was discarded, and formazan products were dissolved in dimethyl sulfoxide (Sigma-Aldrich). Each well was measured by light absorbance at 550 nm (SpectraMax 340PC384 microplate reader; Molecular Devices, LLC, Sunnyvale, CA, USA). In direct cell counting, the cells were detached using trypsin (Gibco; Thermo Fisher Scientific, Inc.), stained with trypan blue (Sigma-Aldrich) and counted using a haemocytometer (Neubauer-improved; Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). The result was expressed as a percentage relative to the cell number of the control group.
Cell cycle analysis
MB49 (24 h assay, 2.2×105 cells/well; 48 h assay, 1.4×105 cells/well) and BFTC 905 (24 h assay, 2.2×105 cells/well; 48 h assay, 1.4×105 cells/well) cells were seeded in 6-well plates (Nunc; Thermo Fisher Scientific, Inc.). Following a 24 h incubation, RPR extract was added (0, 0.5, 1, 2, 3, 3.5 mg/ml). After 24 and 48 h, cells were trypsinized, centrifuged at 640 × g and 25°C for 5 min (Model 3740; Kubota, Tokyo, Japan), and fixed with ice-cold 75% ethanol overnight at 4°C. Subsequently, the ethanol was removed and the cells were stained with a DNA staining solution, containing 1 mg/ml propidium iodide (Sigma-Aldrich) and 10 mg/ml ribonuclease A (Sigma-Aldrich) dissolved in phosphate-buffered saline for 30 min at room temperature. The DNA content of the stained cells was measured using a FACScan™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The cell doublets were removed by gating the left region of FL2-W/FL2-A plot for analysis. Cell cycle data from flow cytometry was analyzed using ModFit LT™ software (version 3.0; Verity Software House, Inc., Topsham, ME, USA).
Mouse orthotopic bladder tumor model
In total, 16 female C57BL/6 mice aged 5 weeks were provided by the National Laboratory Animal Center (Taipei, Taiwan). Animals were maintained at the animal care facility of the Biotechnology and Health Hall, National Chiayi University (Chiayi, Taiwan) for 1 week prior to the start of the present study. The mice were housed in polycarbonate cages, provided with food and water ad libitum and maintained on a 12-h light-dark cycle at 22±2°C. MB49 cells stably transformed with the luciferase gene to produce MB49-Luc cells were provided by Dr. Hsiao-Sheng Liu of National Cheng Kung University (Tainan, Taiwan); MB49-Luc cells grew more quickly than MB49 cells. The implantation of murine bladder cancer MB49-Luc cells into C57BL/6 mice was performed as previously described (26–28). Following MB49-Luc cell inoculation (day 1), the mice were randomly assigned to two groups (8 mice/group); one group was intravesically treated with RPMI-1640 medium (control mice) and the other group received 2.5 mg/mouse RPR extract intravesically on days 4, 6, 8, 10, 12, 14, 16, 18 and 20 (RPR-treated mice). On day 21, the mice were sacrificed and the bladder tumor volumes were measured prior to formalin (Sigma-Aldrich) fixation of the tumor. The present study was approved by the Institutional Animal Care and Use Committee of National Chiayi University (Approval No. 103031).
Statistical analysis
Data are presented as the mean ± standard error of the mean. The data were statistically evaluated by performing one-way analysis of variance with SigmaPlot software (version 11.0; Systat Software, Inc., San Jose, CA, USA). P<0.05 indicated a statistically significant difference.
Results
Major components of RPR extract and Cortex Moutan extract
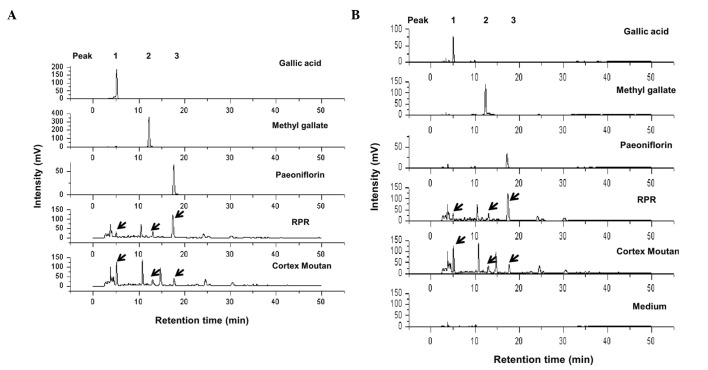
Paeoniae Radix and Cortex Moutan belong to the Paeoniaceae family and contain numerous similar compounds. Previously, Cortex Moutan extract was observed to exert effective anti-bladder tumor activity (3); therefore, using the same extraction preparation procedure, the present study prepared RPR and Cortex Moutan extract and determined their components using HPLC. Fig. 1A demonstrates that gallic acid, methyl gallate and paeoniflorin were identified in RPR and Cortex Moutan extracts. RPMI-1640 medium was used as a control, and no peaks for gallic acid, methyl gallate and paeoniflorin were observed (Fig. 1B). Compared with alcohol (Fig. 1A), RMPI-1640 as a solvent resulted in some minor noise signals in the standards (Fig. 1B). The concentration ratio of gallic acid is increased in Cortex Moutan compared with RPR extract. By contrast, the concentration ratio of paeoniflorin is increased in RPR compared with Cortex Moutan extract. The different percentage of gallic acid, methyl gallate and paeoniflorin in RPR and Cortex Moutan extracts indicates that the anti-tumor and adverse effects of these two extracts may be different.
Figure 1.
High-performance liquid chromatography analysis of RPR and Cortex Moutan extracts. RPR and Cortex Moutan extracts were prepared using RPMI-1640 medium. The solvents for the standards are (A) ethanol (gallic acid and methyl gallate), methanol (paeoniflorin) and (B) RPMI-1640 medium. Peak 1, gallic acid; Peak 2, methyl gallate; Peak 3, paeoniflorin. Arrows indicate the peaks of gallic acid, methyl gallate and paeoniflorin in the two extracts. RPR, Radix Paeoniae Rubra.
Cytotoxicity of RPR extract in BFTC 905, MB49 and SV-HUC-1 cells
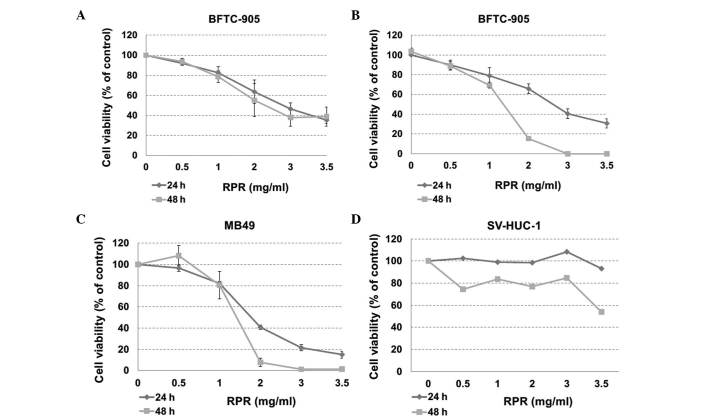
The present study calculated the cytotoxicity of RPR extract to cancer cells using an MTT assay. In human bladder cancer BFTC 905 cells, the half maximal inhibitory concentration (IC50) of RPR extract was ~2.3 and 2.8 mg/ml at 48 and 24 h treatment times, respectively (Fig. 2A). The cells were observed to have died at 48 h by microscopic observation; therefore, the present study analyzed the cytotoxicity again using direct cell counting. The cells died following 3.0 and 3.5 mg/ml RPR treatment for 48 h. The IC50 was ~1.4 and 2.8 mg/ml at 48 and 24 h treatment in BFTC 905 cells (Fig. 2B). In mouse bladder cancer MB49 cells, the IC50 of RPR extract was ~1.4 and 1.8 mg/ml at 48 and 24 h treatment (Fig. 2C). In human normal urothelial SV-HUC-1 cells, the IC50 was >3.5 mg/ml at 24 h and ~3.5 mg/ml at 48 h (Fig. 2D). According to these data and our previous study (3), RPR extract demonstrates a similar anti-cancer effect and a lower cytotoxicity to normal cells than Cortex Moutan extract; the IC50 of Cortex Moutan was >3.5 mg/ml at 24 h and 1.6 mg/ml at 48 h in SV-HUC-1 cells (3).
Figure 2.
Cytotoxicity of RPR extract in bladder cancer and normal urothelial cells. The cytotoxicity was analyzed subsequent to RPR treatment for 24 and 48 h. (A) Cytotoxicity of RPR extract in human bladder papillary transitional cell carcinoma BFTC 905 cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (B-D) Cytotoxicity of RPR extract in (B) BFTC 905, (C) mice bladder carcinoma MB49 and (D) human SV40-transformed uroepithelium SV-HUC-1 cells using direct cell counting. Control cells were treated with the same volume of medium but without RPR. The cell number for the control was regarded as 100%. Data are represented as the mean ± standard error of the mean. The experiment was repeated three times. RPR, Radix Paeoniae Rubra; SV, simian virus.
RPR extract clearly increases the number of cells in the subG1 population of the cell cycle
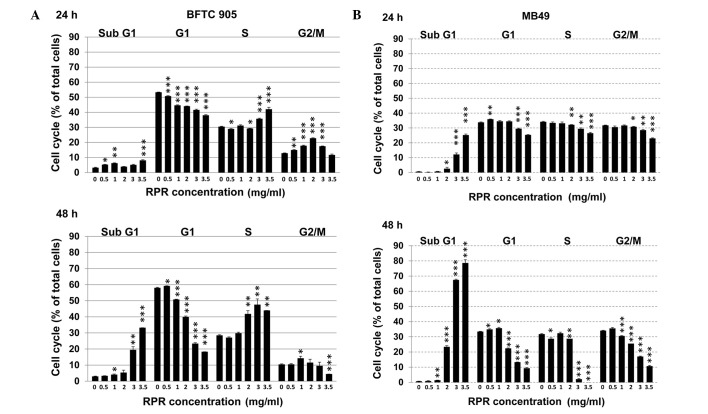
Cell cycle analysis was performed in BFTC 905 and MB49 cells following RPR extract treatment. RPR dose-dependently increased the sub-G1 cell population while decreasing the G1 cell population in BFTC 905 cells (Fig. 3A). RPR also increased the sub-G1 cell population while decreasing the G1 and S cell populations in MB49 cells (Fig. 3B). These results suggest that RPR induces cell death in cells in G1 and S phases, which is concurrent with an increase of the subG1 cell population.
Figure 3.
RPR extract induces cell cycle alterations. Cell cycle distribution was analyzed following RPR treatment. (A) Human bladder papillary transitional cell carcinoma BFTC 905 and (B) mice bladder carcinoma MB49 cells were treated with RPMI-1640 medium alone (0 mg/ml RPR) or RPR extract dissolved in RPMI-1640 medium (0.5, 1, 2, 3 and 3.5 mg/ml) for 24 h and 48 h, and subsequently collected for cell cycle analysis. Data are represented as the mean ± standard error of the mean. The experiment was repeated three times. *P<0.05; **P<0.01; ***P<0.001. RPR, Radix Paeoniae Rubra.
RPR extract inhibits tumor growth in a mouse orthotopic bladder tumor model
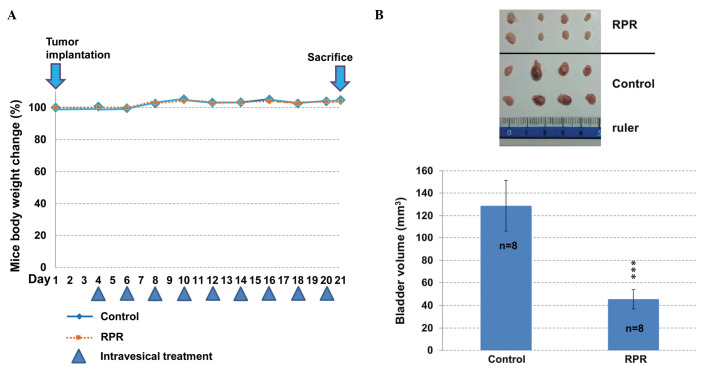
Following tumor implantation at day 1, intravesical application of RPR extract was performed between day 4 and day 20 once every 2 days. The body weight of the mice was not significantly altered between the control and RPR-treated mice (Fig. 4A). The bladder tumor size was calculated following sacrifice on day 21. RPR-treated mice had a significantly decreased bladder tumor volume (P=0.001; Fig. 4B). The blood biochemistry data of the mice demonstrated no significant difference between the two groups (Table I). These results suggest that intravesical RPR treatment decreases bladder tumor size without exerting adverse affects in the liver and kidneys.
Figure 4.
Anti-tumor effect of RPR extract in a mouse orthotopic bladder tumor model. (A) Mouse body weight and drug schedule. MB49 cells stably transformed with the luciferase gene were implanted in the mice on day 1. Following tumor implantation, RPR extract was applied intravesically nine times. Mouse body weights were recorded prior to every intravesical application. (B) Following euthanization of the mice, the bladders were collected. Intravesical application of RPR extract decreased the bladder tumor growth of the RPR-treated mice. Bladder volume (mm3) = (length × wide2) / 2. Data are represented as the mean ± standard error of the mean. ***P=0.001.
Table I.
Blood biochemical parameters following intravesical application of RPR extract in 8 mouse orthotopic bladder tumor models.
| Biochemical parameter | Control | RPR-treated | P-value |
|---|---|---|---|
| SGOT, U/l | 115.0±17.61 | 196.0±35.23 | 0.059 |
| SGPT, U/l | 24.50±1.74 | 31.40±2.73 | 0.051 |
| BUN, mg/dl | 28.50±0.84 | 26.30±2.39 | 0.400 |
| Creatinine, mg/dl | 0.500±0.01 | 0.500±0.02 | 1.000 |
Data are represented as the mean ± standard error of the mean. There were no significant differences between control and RPR-treated groups. RPR, Radix Paeoniae Rubra; SGOT, serum glutamate-oxaloacetate transaminase; SPGT, serum glutamate-pyruvate transaminase; BUN, blood urea nitrogen.
Discussion
The present study provides the first evidence that RPR extract has anti-bladder cancer activity and reduces mouse bladder tumor size using intravesical therapy. Compared with Cortex Moutan extract (3), RPR exhibits similar cytotoxicity in mice bladder carcinoma MB49 cells and less cytotoxicity in normal bladder SV-HUC-1 cells. Following mouse intravesical therapy with RPR nine times, RPR also had a similar effect in reducing bladder tumor volume as Cortex Moutan did. Similarly to Cortex Moutan extract, there was no physiological harm to the mice or damage to the liver or kidneys using blood biochemical parameter analysis. The present data suggest that, for intravesical therapy of bladder cancer, RPR extract may be a more effective therapeutic agent than Cortex Moutan extract.
In the present component analysis using HPLC, there were 4 major peaks observed within the RPR extract. In addition to gallic acid, methyl gallate and paeoniflorin, the peak between gallic acid and methyl gallate was the 4th major peak (at ~11 min). In Cortex Moutan extract, there was also a 5th major peak (at ~15 min). The components of the 4th and 5th peak in the present analysis were not known without the standards; however, they are not paeonol, since this elutes at ~30–40 min under the same HPLC conditions (29). Paeonol and paeoniflorin are two of the major compounds present in Cortex Moutan extract (26,30). In RPR extract, paeoniflorin is increased compared with Cortex Moutan extract, which suggests that, in RPR extract, paeoniflorin may exhibit an anti-cancer effect that has a lower cytotoxicity in normal bladder cells. It has been reported that paeoniflorin exerts its antiproliferative activity through cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway in human non-small cell lung cancer A549 cells (27). Furthermore, paeoniflorin induces apoptosis by downregulating B-cell lymphoma (Bcl)-2 and upregulating Bcl-2-like protein 4 (Bax) and caspase-3 in a human cervical cancer cell line (28). Paeoniflorin also induces apoptosis in hepatocellular carcinoma cells by downregulating prostaglandin E2 receptor subtype EP2 expression and increasing the Bax-to-Bcl-2 ratio (31). In addition, paeoniflorin promotes anti-cancer drug activity by inhibiting nuclear factor κB activation (32,33).
According to the present HPLC analysis and the observations of a previous study (26), gallic acid and methyl gallate are two important components in RPR extract. There are numerous studies that have investigated the anti-cancer effect of gallic acid (34–37), and its chemopreventive effect (38,39). In addition, there are certain studies concerning the anti-cancer effect of methyl gallate (40,41). Therefore, the three major components that exist in RPR extract (gallic acid, methyl gallate and paeoniflorin) may work in combination to contribute to the anti-tumor activity of RPR extract. In TCM theory, the appropriate combination of various components provides a synergic activity or reduces the adverse effect of other compounds (42). The present study concerning RPR and the previous study concerning Cortex Moutan (3), demonstrate that these compounds produce effective anti-bladder tumor activity without evident side-effects in vivo. RPR and Cortex Moutan extracts exhibit more effective results in bladder tumor therapy by intravesical application compared with pure compounds of RPR and Cortex Moutan (23–25). This suggests that RPR and Cortex Moutan extracts may be novel alternative intravesical therapy agents for the treatment of bladder cancer.
Mouse orthotopic bladder tumor is a beneficial model for human bladder tumors, and it aids in the demonstration of treatment via the intravesical pathway, which is the most extensive treatment for superficial bladder tumors (43). In previous studies, an intravesical application of Cortex Moutan provides a beneficial therapeutic effect, but it is cytotoxic to human normal urothelial SV-HUC-1 cells (3). RPR extract contains a mixture of bioactive compounds, and demonstrates an effect in bladder cancer intravesical therapy with a lower cytotoxicity in SV-HUC-1 cells.
In conclusion, the present data elucidate the anti-bladder cancer effect of RPR extract in vitro and in vivo, and suggest that RPR extract may provide an alternative therapeutic strategy for the intravesical treatment of superficial bladder cancer.
Acknowledgements
The present study was supported by grants from the National Science Council of Taiwan (Taipei, Taiwan; grant no. NSC101-2320-B-415-002-MY3) and the Chiayi Christian Hospital (Chiayi, Taiwan; grant no. R103-017).
Glossary
Abbreviations
- BCG
Bacillus Calmette-Guérin
- RPR
Radix Paeoniae Rubra
- UC
urothelial carcinoma
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TCM
traditional Chinese medicine
References
- 1.Huang YQ, Ma X, Wang J, et al. Therapeutic efficacy and safety of Paeoniae Radix Rubra formulae in relieving hyperbilirubinemia induced by viral hepatitis: A meta-analysis. Front Pharmacol. 2016;7:63. doi: 10.3389/fphar.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang R, Xiong AZ, Teng ZQ, Yang QW, Shi YH, Yang L. Radix Paeoniae Rubra and Radix Paeoniae Alba attenuate CCl4-induced acute liver injury: An ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) based metabolomic approach for the pharmacodynamic study of Traditional Chinese Medicines (TCMs) Int J Mol Sci. 2012;13:14634–14647. doi: 10.3390/ijms131114634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MY, Lee YR, Chiang SY, Li YZ, Chen YS, Hsu CD, Liu YW. Cortex Moutan induces bladder cancer cell death via apoptosis and retards tumor growth in mouse bladders. Evid Based Complement Alternat Med. 2013;2013:207279. doi: 10.1155/2013/207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front Pharmacol. 2011;2:10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng H, Yan C, Xiao T, Yuan D, Xu J. Total glucosides of Paeonia lactiflora Pall inhibit vascular endothelial growth factor-induced angiogenesis. J Ethnopharmacol. 2010;127:781–785. doi: 10.1016/j.jep.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Chen CX, Shen YH. Effects of total glucosides from paeony (Paeonia lactiflora Pall) roots on experimental atherosclerosis in rats. J Ethnopharmacol. 2011;135:469–475. doi: 10.1016/j.jep.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Yang L, Xiong A, Lu L, Wang R, Wang Z. Metabolomics study of anti-inflammatory action of Radix Paeoniae Rubra and Radix Paeoniae Alba by ultraperformance liquid chromatography-mass spectrometry. Zhongguo Zhong Yao Za Zhi. 2011;36:694–697. (In Chinese) [PubMed] [Google Scholar]
- 8.Juan YC, Chang CC, Tsai WJ, Lin YL, Hsu YS, Liu HK. Pharmacological evaluation of insulin mimetic novel suppressors of PEPCK gene transcription from Paeoniae Rubra Radix. J Ethnopharmacol. 2011;137:592–600. doi: 10.1016/j.jep.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Zhong W, Liu J, Liu H, Zhu B. Study on anti-tumor effect of total glycosides from Radix paeoniae rubra in S180 tumor-bearing mice. Afr J Tradit Complement Altern Med. 2013;10:580–585. doi: 10.4314/ajtcam.v10i3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang WK, Weng YJ, Chang MH, Lin CC, Chen YS, Hsu HH, Tsai FJ, Tsai CH, Kuo WH, Lu CY, Huang CY. Proliferative effects of chishao on injured peripheral neurons. Am J Chin Med. 2010;38:735–743. doi: 10.1142/S0192415X10008202. [DOI] [PubMed] [Google Scholar]
- 11.Liu EH, Qi LW, Li B, Peng YB, Li P, Li CY, Cao J. High-speed separation and characterization of major constituents in Radix Paeoniae Rubra by fast high-performance liquid chromatography coupled with diode-array detection and time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:119–130. doi: 10.1002/rcm.3848. [DOI] [PubMed] [Google Scholar]
- 12.Li SL, Song JZ, Choi FF, Qiao CF, Zhou Y, Han QB, Xu HX. Chemical profiling of Radix Paeoniae evaluated by ultra-performance liquid chromatography/photo-diode-array/quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2009;49:253–266. doi: 10.1016/j.jpba.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Koo YK, Kim JM, Koo JY, Kang SS, Bae K, Kim YS, Chung JH, Yun-Choi HS. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Pharmazie. 2010;65:624–628. [PubMed] [Google Scholar]
- 14.Xu HY, Chen ZW, Wu YM. Antitumor activity of total paeony glycoside against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Med Oncol. 2012;29:1137–1147. doi: 10.1007/s12032-011-9909-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Yang CL, Or TC, Chen G, Zhou J, Li JC, Lau AS. Differential effects of Radix Paeoniae Rubra (Chishao) on cytokine and chemokine expression inducible by mycobacteria. Chin Med. 2011;6:14. doi: 10.1186/1749-8546-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou TT, Wu CH, Hsu JD, Chyau CC, Lee HJ, Wang CJ. Paeonia lactiflora Pall inhibits bladder cancer growth involving phosphorylation of Chk2 in vitro and in vivo. J Ethnopharmacol. 2011;135:162–172. doi: 10.1016/j.jep.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;42(s218):12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 18.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. European Association of Urology (EAU): EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–314. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 19.Manoharan M. Intravesical therapy for urothelial carcinoma of the bladder. Indian J Urol. 2011;27:252–261. doi: 10.4103/0970-1591.82846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witjes JA, Palou J, Soloway M, Lamm D, Brausi M, Spermon JR, Persad R, Buckley R, Akaza H, Colombel M, Bӧhle A. Clinical practice recommendations for the prevention and management of intravesical therapy-associated adverse events. Euro Urol. 2008;7(Suppl):667–674. doi: 10.1016/j.eursup.2008.08.001. [DOI] [Google Scholar]
- 21.Gee J, Sabichi AL, Grossman HB. Chemoprevention of superficial bladder cancer. Crit Rev Oncol Hematol. 2002;43:277–286. doi: 10.1016/S1040-8428(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 22.Chan E, Patel A, Heston W, Larchian W. Mouse orthotopic models for bladder cancer research. BJU Int. 2009;104:1286–1291. doi: 10.1111/j.1464-410X.2009.08577.x. [DOI] [PubMed] [Google Scholar]
- 23.Shen CH, Shee JJ, Wu JY, Lin YW, Wu JD, Liu YW. Combretastatin A-4 inhibits cell growth and metastasis in bladder cancer cells and retards tumour growth in a murine orthotopic bladder tumour model. Br J Pharmacol. 2010;160:2008–2027. doi: 10.1111/j.1476-5381.2010.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SY, Lee YR, Huang CC, Li YZ, Chang YS, Yang CY, Wu JD, Liu YW. Curcumin-induced heme oxygenase-1 expression plays a negative role for its anti-cancer effect in bladder cancers. Food Chem Toxicol. 2012;50:3530–3536. doi: 10.1016/j.fct.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 25.Wu JY, Tsai KW, Li YZ, Chang YS, Lai YC, Laio YH, Wu JD, Liu YW. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid Based Complement Alternat Med. 2013;2013:579751. doi: 10.1155/2013/579751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Seo CS, Lee KS, Kim HJ, Chang HW, Jung JS, Song DK, Son JK. Protective constituents against sepsis in mice from the root cortex of Paeonia suffruticosa. Arch Pharm Res. 2004;27:1123–1126. doi: 10.1007/BF02975116. [DOI] [PubMed] [Google Scholar]
- 27.Hung JY, Yang CJ, Tsai YM, Huang HW, Huang MS. Antiproliferative activity of paeoniflorin is through cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol Physiol. 2008;35:141–147. doi: 10.1111/j.1440-1681.2008.04935.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Zhang S. Modulating Bcl-2 family proteins and caspase-3 in induction of apoptosis by paeoniflorin in human cervical cancer cells. Phytother Res. 2011;25:1551–1557. doi: 10.1002/ptr.3534. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Yang L, Tian R, Wang Z, Liu Z, Xie P, Feng Q. Species differentiation and quality assessment of Radix Paeoniae Rubra (Chi-shao) by means of high-performance liquid chromatographic fingerprint. J Chromatogr A. 2009;1216:2163–2168. doi: 10.1016/j.chroma.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid Based Complement Alternat Med. 2009;6:57–63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu S, Sun W, Wei W, Wang D, Jin J, Wu J, Chen J, Wu H, Wang Q. Involvement of the prostaglandin E receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis. Anticancer Drugs. 2013;24:140–149. doi: 10.1097/CAD.0b013e32835a4dac. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Li W, Wang T, Shu Y, Liu P. Paeoniflorin suppress NF-kappaB activation through modulation of I kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human gastric carcinoma cells. Biomed Pharmacother. 2008;62:659–666. doi: 10.1016/j.biopha.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Fang S, Zhu W, Zhang Y, Shu Y, Liu P. Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-κB activation. Mol Med Rep. 2012;5:351–356. doi: 10.3892/mmr.2011.652. [DOI] [PubMed] [Google Scholar]
- 34.Ou TT, Wang CJ, Lee YS, Wu CH, Lee HJ. Gallic acid induces G2/M phase cell cycle arrest via regulating 14-3-3β release from Cdc25C and Chk2 activation in human bladder transitional carcinoma cells. Mol Nutr Food Res. 2010;54:1781–1790. doi: 10.1002/mnfr.201000096. [DOI] [PubMed] [Google Scholar]
- 35.Kuo CL, Lai KC, Ma YS, Weng SW, Lin JP, Chung JG. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-κB, Ras and matrix metalloproteinase-2 and −9. Oncol Rep. 2014;32:355–361. doi: 10.3892/or.2014.3209. [DOI] [PubMed] [Google Scholar]
- 36.Liang W, Li X, Li Y, Li C, Gao B, Gan H, Li S, Shen J, Kang J, Ding S, et al. Gallic acid induces apoptosis and inhibits cell migration by upregulating miR-518b in SW1353 human chondrosarcoma cells. Int J Oncol. 2014;44:91–98. doi: 10.3892/ijo.2013.2155. [DOI] [PubMed] [Google Scholar]
- 37.Ho HH, Chang CS, Ho WC, Liao SY, Lin WL, Wang CJ. Gallic acid inhibits gastric cancer cells metastasis and invasive growth via increased expression of RhoB, downregulation of AKT/small GTPase signals and inhibition of NF-κB activity. Toxicol Appl Pharmacol. 2013;266:76–85. doi: 10.1016/j.taap.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Verma S, Singh A, Mishra A. Gallic acid: Molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35:473–485. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Kim JK, Kim DW, Hwang HS, Eum WS, Park J, Han KH, Oh JS, Choi SY. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochim Biophys Acta. 2013;1830:4017–4029. doi: 10.1016/j.bbagen.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Lee H, Lee H, Kwon Y, Lee JH, Kim J, Shin MK, Kim SH, Bae H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. J Immunol. 2010;185:6698–6705. doi: 10.4049/jimmunol.1001373. [DOI] [PubMed] [Google Scholar]
- 42.Che CT, Wang ZJ, Chow MSS, Lam CWK. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules. 2013;18:5125–5141. doi: 10.3390/molecules18055125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barocas DA, Clark PE. Bladder cancer. Curr Opin Oncol. 2008;20:307–314. doi: 10.1097/CCO.0b013e3282f8b03e. [DOI] [PubMed] [Google Scholar]