Abstract
Cell death emanating from the nucleus is largely unknown. In our recent study, we determined that when temperature is lowered in the surrounding environment, apoptosis stops and bubbling cell death (BCD) occurs. The study concerns the severity of frostbite. When exposed to severe cold and strong ultraviolet (UV) irradiation, people may suffer serious damages to the skin and internal organs. This ultimately leads to limb amputations, organ failure, and death. BCD is defined as “formation of a single bubble from the nucleus per cell and release of this swelling bubble from the cell surface to extracellular space that causes cell death.” When cells are subjected to UV irradiation and/or brief cold shock (4℃ for 5 min) and then incubated at room temperature or 4℃ for time-lapse microscopy, each cell releases an enlarging nuclear gas bubble containing nitric oxide. Certain cells may simultaneously eject hundreds or thousands of exosome-like particles. Unlike apoptosis, no phosphatidylserine flip-over, mitochondrial apoptosis, damage to Golgi complex, and chromosomal DNA fragmentation are shown in BCD. When the temperature is increased back at 37℃, bubble formation stops and apoptosis restarts. Mechanistically, proapoptotic WW domain-containing oxidoreductase and p53 block the protective TNF receptor adaptor factor 2 that allows nitric oxide synthase 2 to synthesize nitric oxide and bubble formation. In this mini-review, updated knowledge in cell death and the proposed molecular mechanism for BCD are provided.
Keywords: Bubbling cell death, apoptosis, UV irradiation, cold shock, WWOX, NOS2
Cell death: Overview
Every cell is destined to die someday, somehow, and someway as it is designed or intended for. Each cell appears to have a programmed length of lifespan, unless it turns out to be cancerous or being accidentally eliminated. The lifespan is affected by neighbor cells and the conditions of life supporting or threatening surroundings, including temperature, nutrients, pH, killer proteins, oxygen, and detergents. Our current knowledge regarding cell death has been conceptually engraved with death by a “programmed” or a “nonprogrammed” manner.1–4 The programmed cell death is physiological, and occurs from embryonic stage to newborn and adult. Under this discipline, when a cell or a cell mass encounters an intrinsic trigger for death, the cells die in a timely manner so that the body can remove unwanted cells for normal development.1,2 However, cells may accidentally encounter high levels of a death trigger, for example, tumor necrosis factor (TNF), under an inflammatory signaling network for leading to death. This should be considered pathological programmed cell death. Many cancer cells have evolved to dodge the programmed death signaling to achieve survival, by either undergoing autophagy or having increased expression of antiapoptotic proteins.3–6 Necrosis is nonphysiological, in which the cell is subjected to physical-related stress, swelling, and disruption upon dipping into an imbalanced or unfriendly environment.1,2,7 Apoptosis, first discovered by Carl Vogt in 1842, is a typical pathway of programmed cell death, which depends upon the activation of caspases.8 Pyroptosis,9 anoikis,10 cornification,11 and mitotic catastrophe12 are also caspase-dependent. There are many caspase-independent pathways, namely necroptosis,13 ferroptosis,14 autophagy,2,15 pexophagy,16 entosis,17 and parthanantos.18 Autophagy, pexophagy, entosis, and parthanantos may not necessarily lead to ultimate cell death.
Is the cell really dead?
Criteria used to measure apoptosis or programmed cell death have been well documented, including determination of cell membrane blebbing, flip-over of membrane phosphatidylserine (PS) onto cell surface, reduction in cell volume, formation of apoptotic bodies, loss of mitochondrial membrane permeability, cytochrome c release, nuclear condensation, and DNA fragmentation.2 While majority of reports rely on these assays for measurement mainly using fixed cells, this raises questions regarding whether the dying cells are really dead. Fixed cells may have altered morphology and chemistry. Membrane blebbing, for example, is reversible per se. This can be readily viewed by time-lapse microscopy. Indeed, cell undergoing membrane blebbing is an expression of its struggling to escape death rather than surrendering itself to death.19 PS exposure is regarded as a very early event in apoptosis.2 Nonetheless, this event is barely detectable within the initial 30 min of apoptosis as visualized by time-lapse microscopy.19,20 Depending upon the status of cell death, the nucleus in a dying cell has a differential affinity for propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI). Time-lapse microscopy reveals clearly that an apoptotic nucleus normally picks up PI first and then DAPI with time, suggesting that alteration of nuclear membrane occurs along the line of cell death. Nonetheless, fixed cells cannot reveal the differential effects in uptaking nuclear stains to show whether they are really dead or not. Necroptosis is well defined by caspase-8 inactivation and the activation of RIPK1 and RIPK3.7 Nonetheless, the precise changes in live cell morphology and molecular event for necroptosis in a real time mode are lacking.
Cytochrome c release is dispensable for apoptosis and is not essential for leading to DNA fragmentation.21–24 Zfra (zinc finger-like protein that regulates apoptosis) is a potent inhibitor of cytochrome c release.25,26 Zfra down-regulates Bcl-2 and induces dissipation in mitochondrial membrane potential but causes no cytochrome c release, indicating a novel death pathway from the mitochondria.25,26 Overall, cell death must be defined as going beyond a point of apoptotic steps with no return. Assaying the status of being dead using fixed cells or tissues could be misleading.
Inflammation, caspase activation, and cell death
Inflammation is a highly complicated issue. Acute inflammatory response must occur rapidly in order to tackle emergency situations. For example, when skin is attacked by a bee sting, activation of the alternative complement pathway occurs immediately right on the spot.27 Autoimmune diseases, however, are accumulative results from chronic inflammation.27,28 Numerous mediators such as antibodies, complement proteins, cytokines, and intrinsic or extrinsic pathogens may get involved.27,28 Either chronic or acute inflammation may lead to mass amounts of cell death and induce the destruction of tissues and organs. Alternatively, exogenous stimuli induce death pathway activation, which leads to the generation of mass dead cell debris that renders inflammation.
Few key molecules in the cell death pathways are essential for the inflammatory responses. TNF, for example, is well known for its role in orchestrating inflammation, apoptosis, and necroptosis via three signaling links downstream the receptor and adaptor complex.29–31 Signaling by TNF leads to assembly of a complex of receptor and adaptor proteins that helps activation of the downstream caspases and nucleases. First, in the survival link TNF-mediated activation of NF-κB is needed for promoting cell survival and yet eliciting inflammatory response.29–31 Mass death of cells and tissues may occur due to excessive activation of NF-κB. Second, activation of caspase-8 is needed to initiate mitochondrial apoptosis, activation of downstream proapoptotic Bcl-2 family proteins, caspases and nucleases, and cell death. Third, suppression of caspase-8 activation may cause necroptosis or regulated necrosis.28–30 While caspase-8 is inactivated, necrosis signaling induces the activation of RIPK1 (receptor interacting protein kinase 1), RIPK3, and MLKL (mixed kinase domain-like) for necroptosis. That is, this type of cell death is caspase independent. Pyroptosis is induced by pathogenic or infectious agents, and caspase-1 and caspase-11 are involved.9
Bubbling cell death
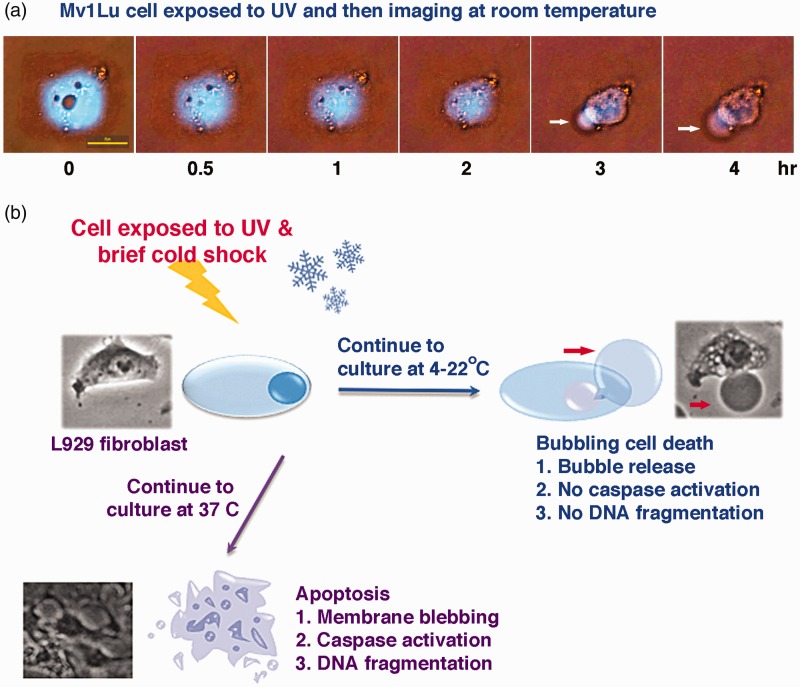
In this mini-review, new perspectives and molecular insights regarding our recent discovery of bubbling cell death (BCD) are provided. The rationale for studying BCD is that during severe winter weather (e.g., strong wind chills at below zero temperatures coupled with ultraviolet [UV] irradiation), people without full protection may suffer frostbite. The consequence is severe damage to the skin and internal organs, and this leads to limb amputation or even death.32–35 Presence of skin blisters during the progression of frostbite is a likely consequence of epidermal and dermal cell bubbling. In cell model experiments, we have reported that when the ambient temperature surrounding a dying cell is lowered from 37℃ down to 4–22℃, this dying cell via apoptosis comes to a halt and BCD is switched on.20 Based upon experimental data,20 bubbling death can be defined as “formation of a bubble from the nucleus per cell and release of this swelling bubble to the cell surface that ultimately causes cell death” (Figure 1).
Figure 1.
Bubbling cell death (BCD) and apoptosis. (a) Mink lung epithelial Mv1Lu cells were exposed to UV irradiation (480 mJ/cm2), and then subjected to time-lapse microscopy at room temperature. A bubble formation is shown (white arrow). Cyan fluorescent protein (CFP) mainly localizes in the nucleus. Three circular areas in the nucleus appear to be nucleoli. Also see Supplementary Video S1. (b) Murine L929 fibroblasts were exposed to UV irradiation and cold shock for 5 min at 4℃, and then subjected to time-lapse microscopy at 37℃. The cells underwent apoptosis. Alternatively, when UV/cold shock-treated L929 cells were undergone time-lapse microscopy at 22℃ or 4℃, BCD occurred. See the red arrow for the generated bubble. BCD is blocked by increasing temperature up to 37℃. Apoptosis is turned off by lowering the temperature down to 4–22℃. Notably, when cells are prestarved, followed by UV/cold shock, the cells undergo autophagy rather than BCD.20 (A color version of this figure is available in the online journal.)
A comparison among the essential characteristics of apoptosis, necroptosis, and BCD is shown in the Table 1. Unlike membrane blebbing, bubbling is an irreversible event. No caspase activation, internucleosomal DNA fragmentation, and flip over of membrane PS to the cell surface are observed in BCD (Table 1). Release of a single bubble per cell and hundreds or thousands of exosome-like particles is a hallmark for BCD (Table 1).20
Table 1.
Characteristics of cell death by apoptosis, necroptosis, and bubbling cell death.a
| Apoptosis | Necroptosis | Bubbling cell death | |
|---|---|---|---|
| 1. Membrane blebbing | Yes | No | Little or no |
| 2. PS surface exposure | Yes | No | No |
| 3. Plasma membrane damage | Yes | Yes | Yes |
| 4. Cytoplasmic swelling | No | Yes | +/−b |
| 5. Apoptotic bodies or cell fragmentation | Yes | No | No |
| 6. Caspase activation | Yes | No | No |
| 7. Loss of mitochondrial permeability | Yes | Yes | No |
| 8. Nuclear bubbling | No | No | Yes |
| 9. Nuclear condensation | Yes | No | +/−b |
| 10. DNA fragmentation | Yes | No | No |
| 11. Activation of DNA damage proteins | Unknown | Unknown | No |
| 12. Key initiating event | Caspase 8 activation | Caspase-8 inactivation and RIPK1 and RIPK3 activation | Nuclear accumulation of WWOX, p53, TRAF2, NOS2, ERK, Hyal-2, and C1qBP |
| 13. Relocation of nucleoli | No | Unknown | Release of nucleoli to the swelling nuclear bubble |
| 14. Cytoplasmic protein aggregation | Unknown | Unknown | Yes |
| 15. Golgi damage | Unknown | Unknown | No |
| 16. Exosome release | No | Unknown | Yes |
| 17. ATP requirement | Yes | No | No |
Cytoplasmic and nuclear condensation is not essential for bubble formation
By time-lapse microscopy, UV/cold shock induces the formation of a bubble from the nucleus in ECFP-expressing monkey kidney COS7 fibroblasts (Supplementary Video S1). Nuclear proteins are released into the enlarging bubble, and then further leaked into the extracellular space through the bubble membrane.20 No significant condensation of cytoplasm and nucleus occurs prior to bubble formation in UV/cold shock-treated COS7 cells.
Indeed, UV irradiation alone is sufficient to induce BCD.20 For example, when mink lung epithelial Mv1Lu cells were exposed to UV irradiation, condensation of cytoplasm and nucleus occurred prior to bubble formation (Figure 1(a) and Supplementary Video S2). Similar results were also found using squamous cell carcinoma SCC9 cells (data not shown). Acinus is known to participate in the caspase-3-dependent apoptotic chromatin condensation.36 Whether caspase-3 and acinus are activated and cause nuclear condensation in Mv1Lu and SCC9 cells is unknown and remains to be determined. However, many of our tested cells (more than 15 cell lines thus far) do not undergo whole cell condensation before progressing toward bubble formation.
Cold shock enhances bubble formation
BCD can be accelerated by stimulating cells with UV irradiation and a brief cold shock (5 min at 4℃). For example, exposure of mouse L929 fibroblasts to UV/cold shock, followed by imaging at room temperature, the cells underwent BCD (Figure 1(b)). BCD is accelerated by cold shock, in which it takes 10–20 min shorter in causing bubble formation. No cytoplasmic and nuclear condensation is shown in L929 cells. Depending upon the strength of UV irradiation (240–960 mJ/cm2) and the duration of cold shock, bubbling normally starts to take place in less than 30 min post exposure to UV/cold shock.
In another example, when squamous cell carcinoma SCC15 cells were subjected to UV irradiation (480 mJ/cm2) and then imaging by time-lapse microscopy at room temperature, the cells underwent bubbling and release of exosomes (Supplementary Video S3). The nuclei did not undergo condensation prior to bubble formation. In comparison, when SCC15 cells were exposed to UV/cold shock (5 min at 4℃), followed by time-lapse imaging at room temperature, the cells had an accelerated bubble formation (Supplementary Video S4). In both experiments, few SCC15 cells started exhibiting one or two bubbles plus small blebs, and then all gases appeared to be sucked into a largest bubble. That is, during BCD, there is only one enlarging big bubble progressing toward the end. In our previous report,19 we have shown that blebbing is a cellular self-protection response to fend off stress and apoptosis.
Temperature controls the switch between BCD and apoptosis
When ambient temperature rises from 4–22℃ to 37℃, BCD stops and apoptosis starts to occur.20 For example, when UV/cold shock-treated L929 cells were undergone time-lapse microscopy at 37℃, apoptosis occurred (Figure 1(b)). Alternatively, apoptosis is turned off by lowering the temperature from 37℃ down to 4–22℃, and can be turned back on upon increasing the temperature to 37℃. Because caspase activation is blocked at low temperatures, it is reasonable to assume that caspase functional activity is sensitive to temperature alterations. Perhaps, caspases undergo conformational changes at low temperatures. Notably, BCD is blocked when the cells are prestarved.20 That is, when prestarved cells are exposed to UC/cold shock, these cells preferentially undergo autophagy rather than BCD.20 The observations suggest that critical protein components are needed to be synthesized to carry out BCD.
Release of nuclear and nucleolar contents to the bubble
During BCD, the majority of the nuclear and nucleolar proteins go to the bubble, but may leak to the cytoplasm.20 The entire damaged nucleoli may dump into the bubble.20 However, certain cells release the contents of nucleoli and still retain the empty nucleolar skeleton in the damaged nuclei (data not shown). Chromosomal DNA does not appear to relocate entirely to the bubble, as DNA can be isolated from the damaged nuclei. No DNA fragmentation is shown. While UV/cold shock induces nuclear burst rapidly, no apparent activation of DNA damage-sensing proteins such as ATR, ATM, Chk1, and Chk2 is shown.20 Specific induction of DNA damage by neocarzinostatin does not result in bubbling death,20 indicating that BCD is not initiated by DNA damage.
BCD and clinical relevance with skin and organ damages by extreme cold
We were intrigued by the pathologic event of frostbite, and that has prompted us to determine how cell dies under low temperatures and UV irradiation.20 Although cases for frostbite are relatively rare compared with many types of diseases, the incidence has been considered as traumatic injury and may lead to limb amputations, severe organ damages, and death. Frostbite is a concern for people living in the Polar Regions, where severe cold and strong UV irradiation are present. Severe cold causes hypothermia, cuts down of blood flow in extremities, and renders damages to the skin and organs.32–34 In general, the injured part of the body initially shows edema, mottled, no blanching, and cyanosis. Small blebs and vesicles develop later and make injured area aching and painful. Under severe situations, damaged tissues turn black and have to be surgically removed. Frostbite bears similarities in inflammatory features to those seen in thermal burns, ischemia/reperfusion injuries, and crush injuries. In addition to traditional therapies, hyperbaric oxygen therapy may provide an effective cure for the injury.35 Whether accumulative BCD contributes to the formation of small blebs and vesicles in the damaged tissues in vivo remains to be determined. While both necroptosis and BCD are caspase-independent, their morphological changes are indeed distinct (Table 1).
UV/cold shock signals protein influx into the nucleus
When a cell is exposed to UV irradiation, the cell starts to die via apoptosis at 37℃. However, when the surrounding temperature drops, apoptosis stops and shifts to BCD. In the initial stage of BCD, UV/cold shock rapidly causes upregulation and relocation of many cytosolic proteins to the nucleus20 (Figure 2). It is not clear which subcellular compartment receives the UV energy first, and then transmits the signal to induce influx of proteins to the nucleus. Conceivably, the first scenario is that under the insult of UV/cold shock, nucleus senses the stress first and then converts the UV energy to a “rescue chemical signal” for the whole cell. The assumption is reasonable simply because the highly compact nucleus, which is rich in DNA and RNA, is able to strongly absorb UV energy.37,38 How the UV energy is converted to signal transduction under the cold temperature is unknown. The second scenario is that UV causes damage to the cell membrane first that results in signaling to the nucleus to ask for rescue. The damage to the cell membrane may result in influx of calcium and other metal ions into the cell. These scenarios are being tested. Nonetheless, unlike apoptosis, we did not detect flip over of PS onto the cell surface upon challenging cells with UV and cold shock, suggesting that the nuclear stress caused by UV/cold shock is needed to send the first signal to the cytoplasm and membrane for causing protein influx to the nucleus.
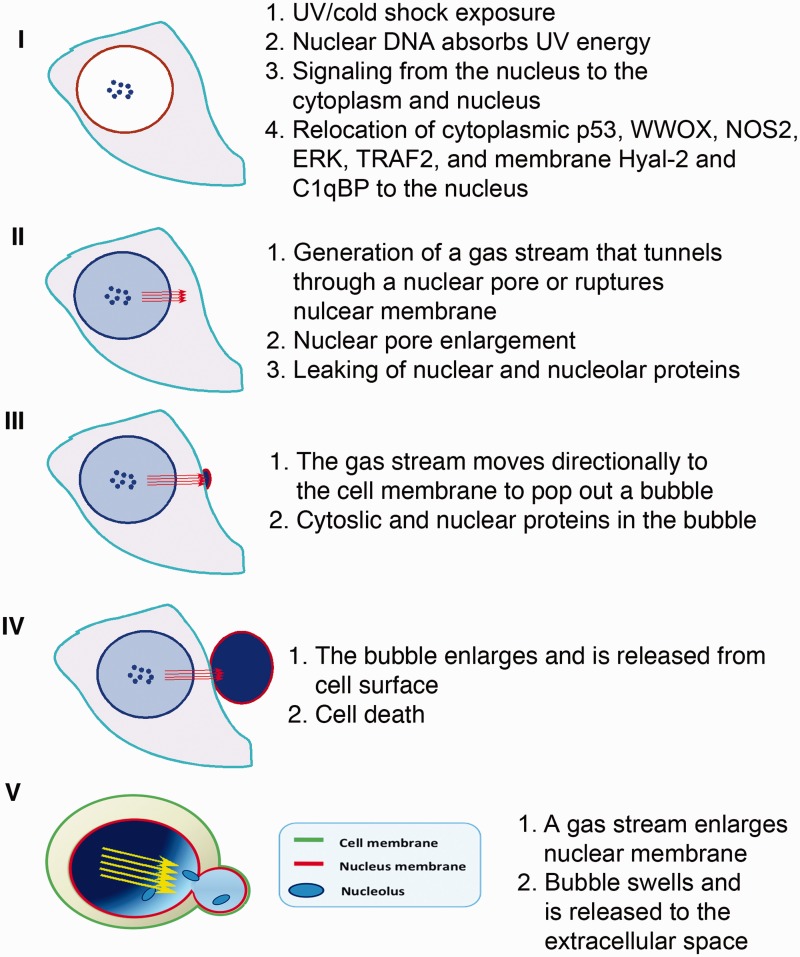
Figure 2.
Two types of bubbles. In the first type (steps I–IV), the NO-containing gas stream tunnels through a nuclear pore, and then channels via cytoplasm and cell membrane to form a swelling bubble. The bubble wall contains the cell membrane only. In the second type (step V; adapted from Chen et al.20 with permission), the gas stream pushes and swells up a nuclear bubble, which then merges with the cytoplasmic membrane. Following continual swelling, a single large bubble, which possesses nuclear and cytoplasmic membranes in the wall, pops out to the extracellular space. In most cases, dual-membrane bubbles are generated in UV/cold shock-treated cells, as determined by fluorescent immunohistochemistry. (A color version of this figure is available in the online journal.)
Role of NF-κB in UV/cold shock-induced BCD
UV energy rapidly increases the expression of many proteins such as antiapoptotic TRAF2 (TNF receptor adaptor factor 2) and NF-κB/p65 (nuclear factor κB), proapoptotic WWOX (or WOX1; WW domain-containing oxidoreductase) and p53, NOS2 (nitric oxide synthase 2), ERK (extracellular signal-regulated kinases), and others.20 Subsequent exposure of UV-treated cells to cold shock enhances the relocation of these proteins into the nucleus. These proteins are likely to counteract functionally with each other. A balance among these proteins must be achieved in deciding the bubble formation and the fate of the cell.20
NF-κB/p65 is a downstream effector of the TNF signaling, which is generally considered as prosurvival in blocking TNF-mediated cell death and thereby promoting cancer survival. However, NF-κB is potent in eliciting inflammation. Without effective inhibition by drugs, the induced inflammatory response may eventually lead to mass amounts of cell death and tissue damage.39,40 UV/cold shock rapidly induces accumulation of NF-κB/p65 in the nucleus (data not shown). Whether the event is protective against bubble formation or promoting BCD remains to be established. However, one cannot exclude the crucial role of NF-κB/p65-induced inflammation in tissues and organs caused by frostbite. It has been shown that NF-κB and heat shock proteins are involved in cold stress-mediated cardiac cell damage.40
Critical role of nitric oxide synthase NOS2 (iNOS) in the formation of a “hot air balloon” in the nucleus under the cold
The deciding factor for the formation of a nuclear bubble is probably NOS2 or iNOS. NOS2 is one of the nitric oxide synthase family proteins and is functionally active in the nucleus.41 Nuclear NOS2 is known to participate in the mitochondrial biogenesis,41 and is able to block UVA-induced apoptosis via up-regulation of Bcl-2.42 By using UVC, we showed the induction of NOS2, its nuclear translocation, and a nuclear bubble formation, especially under the cold (Figure 2).20 Perhaps, when overly accumulated in the nucleus, NOS2 is functionally active in carrying out BCD. How NOS2 works differently in the cytoplasm and nucleus during BCD is unknown and remains to be established?
Specific inhibition of NOS2 by an arginine analog, Nω-nitro-l-arginine methyl ester hydrochloride (Nω-LAME), inhibits the bubble formation and cell death. We also determined that converting UV energy to promote nuclear accumulation of NOS2 and other proteins does not require de novo generation of ATP.20 Accumulation of NOS2 in the nucleus may account for the formation of a NO-containing nuclear bubble. While the bubble formation is enhanced in low temperature, the precise temperature within each nuclear bubble, either cold or hot, is unknown. The bubble swells swiftly in less than 2 h post UV/cold shock, and is most likely a “hot air balloon” so that it can swell, release and lift in the extracellular space freely. In addition to NO, our unpublished data showed the possible presence of reactive oxygen species such as superoxide radicals in the bubbles, as antioxidants effectively retard the progress of BCD. Of course, there still remain outstanding questions regarding the time-related changes in the intracellular concentrations of NO and other gases. Whether NO and superoxide radicals are the major gases in the bubble remains to be established.
We proposed there are two types of membranes in the wall of each bubble. When a balloon starts to form in the nucleus, the intra-bubble gases expand like jet streams, push forcefully toward the nuclear envelope, and open up a nuclear pore or disrupt the nuclear envelope so that the gas streams effectively tunnel through cytoplasm to bubble up and release (steps I–IV; Figure 2).20 In this case, the wall of the bubble is composed of the cytoplasm membrane only. However, this seems to be rare. In most cases, a released balloon is composed of double membranes with cell membrane on the surface and nuclear membrane facing internally (step V; Figure 2).20
Quite frequently, BCD occurs without reductions in cell volumes prior to bubble release. In certain cells (e.g., lung Mv1Lu epithelial cell), reduction in cell volumes is needed for UV/cold shock-mediated bubble formation (Figure 1(a) and Supplementary Video S2). When bubble is released, the cell picks up nuclear stains PI and DAPI sequentially, indicating that the nuclear envelope has undergone biochemical changes with time. Later, nuclear condensation may occur. No apparent DNA fragmentation occurs at room or lower temperatures, indicating that nucleases are not activated.
Role of p53 and WWOX in the bubble formation
Tumor suppressors p53 and WWOX work together in blocking cancer growth.43–46 Both proteins are crucial in determining the membrane bubble-induced cell death.20 How they promote the formation of a nuclear bubble is intriguing and unknown. Supporting evidence shows that nuclear accumulation of p53 and WWOX is needed to activate NOS2 to induce nitric oxide generation20 (Figure 3). Knockout Wwox−/− MEF fibroblasts resist UV/cold shock-induced BCD.20 Also, dominant-negative p53 abolishes bubble formation.20 Whether WWOX or p53 stabilizes NOS2 via direct binding needs further investigation. It has been reported that p53 regulates the expression of NOS2 expression in vivo and suppresses overproduction of nitric oxide.47,48 In oral squamous cell carcinoma, p53 level is increased when NOS2 (or iNOS) expression is knocked down.49 Additionally, p53 can be activated via Ser15 phosphorylation by nitric oxide and ionizing radiation in a synergistic manner.48 In the initial stage of BCD, nuclear p53 is likely to boost the generation of nitric oxide by NOS2. This effect is probably enhanced by the functional interaction between p53 and WWOX.43–46 WWOX binds and stabilizes p53 from degradation by ubiquitination and proteosomal degradation.43–45
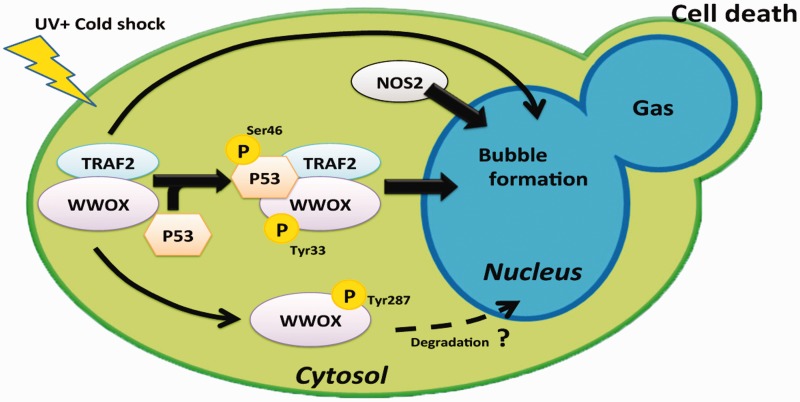
Figure 3.
Putative molecular signaling during BCD. UV/cold shock is expected to induce complex formation of pS46-p53 and pY33-WWOX.43–45 In addition, TRAF2 binds WWOX in resting cells. UV/cold shock is expected to induce the p53/WWOX/TRAF2 triad formation for nuclear translocation. p53 and WWOX assist in the bubble formation, whereas TRAF2 blocks the event. TRAF2 probably binds NOS2 to block nitric oxide generation. (A color version of this figure is available in the online journal.)
The inhibitory role of TRAF2 in BCD
TRAF2 is an inhibitor of apoptosis, and is recruited in the complex of TNF receptor and adaptor proteins1–5,50–52 TRAF2 acts to activate NF-κB for turning off TNF-induced apoptosis. TRAF2 is composed of an N-terminal RING-type zinc finger domain, a zinc finger domain, a coiled coil (TRAF-N) domain, and a MATH/TRAF (TRAF-C) domain at the C-terminus. In the frostbitten skin, we determined that there is a greater than 90% downregulation of TRAF2, along with a dramatic upregulation of WWOX in humans.20 We determined that the central zinc finger domain confers resistance to BCD.20 Whether WWOX and p53 interact with TRAF2 is being investigated. Preliminary data showed that WWOX physically binds TRAF2 (Chang et al., unpublished), whereas whether p53 directly binds TRAF2 is not known (Figure 3). During TNF signaling, TRADD (TNF receptor type 1-associated death domain protein), an adaptor protein in the TNF signaling, directly interacts with TRAF2 and other proteins in the TNF receptor and adaptor complex. Signaling from TRAF2 and NF-κB results in blocking apoptosis. To block BCD, TRAF2 must promote cell survival by activating NF-κB and inhibiting p53 from binding to the mitochondria and blocking cytochrome C release.1–5,20,45,46 Another event is that WWOX binds p53 and both proteins interact with TRAF2 to modulate BCD. This scenario is being examined.
In response to UV/cold shock, NOS2 becomes colocalized with TRAF2 (data not shown). Whether NOS2 and TRAF2 interact with each other and co-translocate to the nucleus remains to be established. Also, whether NOS2 binds p53 and WWOX is unknown. Of particular interest is that there is a significant downregulation of p53 in the Wwox knockout MEF cells, which is in agreement with our previous observations using knockdown approach.45 Notably, in the absence of WWOX, TRAF2 fails to translocate to the nucleus, suggesting that WWOX and TRAF2 relocate together to the nucleus. ERK is also rapidly phosphorylated and becomes accumulated in the nucleus during BCD (Chang et al., unpublished data). Functional significance of this regard remains to be investigated.
Exosome release during BCD
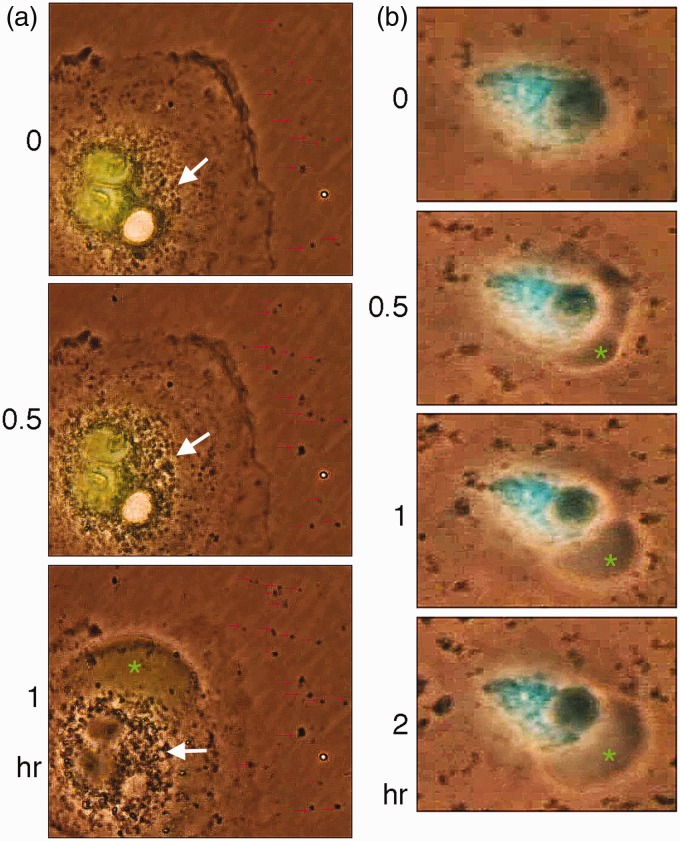
A unique feature for BCD is the rapid release of exosome-like particles by the cellular “machine gun.” There is no correlation for the timing between exosome release and bubble formation. These particles are around 100–500 nm in diameter and possess markers for exosomes such as CD63 and CD81 (data not shown), whereas their physiological functions are largely unknown. When COS7 cells, transiently overexpressing the N-terminal WW domain of WWOX, were exposed to UV/cold shock, these cells were shown to have increased numbers of exosomal particles surrounding the Golgi area, followed by release to the extracellular space (Figure 4(a) and Supplementary Video S5). Some of these particles adhered stably onto the plastic surface, and their sizes were increased with time due to addition of other release particles (see red arrows in Figure 4(a)). This indeed accounts for the size variations of the extracellular exosomal particles. COS7 cells were also transiently overexpressed with a dominant negative WW domain. Upon UV/cold shock challenge, these cells exhibited release of exosomal particles of various sizes (Supplementary Video S6). The ectopic dominant negative blocks the function of endogenous WWOX in COS7 cells,44 but fails to inhibit exosome release. Additionally, COS7 cells were transiently overexpressed a Golgi-targeting ECFP. These cells also released exosomal particles of various sizes in response to UV/cold shock (Supplementary Video S7). Together, it appears that when cells are overloaded with transiently overexpressed proteins, they tend to release large sized and irregularly shaped exosomal particles of 0.5–1 µm in diameter. This may be due to aggregation of exosomal particles in the extracellular space.
Figure 4.
UV/cold shock induces exosome release. (a) COS7 cells, expressing EGFP-WW domain or nothing, were exposed to UV irradiation at 480 mJ/cm2 and then incubated at 4℃ for 5 min. Time-lapse microscopy was then carried out at room temperature. Bubble formation from the nucleus is shown. Nuclear EGFP-WW domain leaked into the gas bubble (green star). Formation of intracellular exosomal particles (big white arrows), along with their release to the extracellular space, is shown (red arrows). Aggregation of the released exosomes with time contributes, in part, to their variations in sizes (red arrows). Also, see the Supplementary Video S5. (b) UV/cold shock also causes the release of exosomes from COS7 cells transiently overexpressed with Golgi-targeting ECFP. Note the variations in sizes of the released exosomes. Also, see the Supplementary Video S7. Adapted from Chen et al.20 with permission. (A color version of this figure is available in the online journal.)
Exosome release from live cells is considered to play crucial roles in intercellular communications and maintenance of normal cellular physiology.53,54 Exosomes are present in the body fluids such as semen, blood, urine, cerebrospinal fluid, and others. Upon fusing with cell membranes, exosomes are endocytosed and their internal contents are released to modulate cellular functions. Cancer cell-derived exosomes appear to play a critical role in tumor growth, progression, invasion, and metastasis.54 Release of exosomes by a dying cell is largely unknown and not well documented. One report showed that when a dying cell releases autoantigens in the exosomes, followed by digestion with caspase proteins, the degraded proteins facilitate the initiation of systemic autoimmunity in susceptible hosts.55 Nonetheless, the functional properties of exosomes derived from cells undergoing BCD require further investigation.
Concluding remarks and perspectives
Frostbite-mediated severe damage to tissues and organs is likely due to accelerated mass inflammatory responses in affected areas. Our recent study in BCD in vitro may represent a simulation of frostbite-induced cell death in vivo. We have shown that UV/cold shock induces a novel type of cell death, in which the death event is initiated from the nucleus, rather than from the membrane receptors or the cytoplasmic death initiators such as small double-stranded DNA in the cytoplasm. It appears that UV-induced bubbling represents formation a hot air balloon in the nucleus that is released through the cell membrane to the extracellular space. Conceivably, a big cluster of cell mass undergoing BCD will result in blister formation and cell death in the skin. The cell debris induces inflammatory responses that ultimately cause limb amputation and internal organ damages. While the underlying mechanisms are largely unknown, we have shown that blocking the activity of NOS2 could be an effective way to prevent damages caused by frostbite.
Despite the in vitro findings, there are numerous outstanding questions remained to be answered. First of all, what drives proteins relocation during BCD? The UV energy appears to be converted to chemical energy that drives protein relocation from the cytoplasm and membrane to the nucleus. The relocation is calcium-dependent manner, and is independent of the Rho/Rock signaling (unpublished observations). That is, actin-dependent bubble formation is not involved. Whether the relocated proteins are “dumped” into the bubbles from the nuclei is unknown. Indeed, it is very difficult to isolate intact bubbles. Also, proteins may leak from the bubbles to the extracellular space (Figure 4(a) and Supplementary Video S5).
Second, how does the influx of cytosolic NOS2, p53, WWOX, TRAF2, ERK, and NF-κB to the nucleus occur? The aforementioned proteins could rapidly accumulate in the nucleus upon exposure to UV (120–960 µJ/cm2) and then a brief cold shock for 5–10 min. That is, significant protein accumulation in the nucleus is found within 10 min. In resting cells, TRAF2 and WWOX form a complex in the cytoplasm (data not shown). In response to UV/cold shock, the TRAF2/WWOX complex recruits p53, and the molecular triad then relocates to the nucleus in less than 10 min. TRAF2 alone fails to undergo nuclear accumulation. It appears that cytosolic NOS2 relocates to the nucleus at the same time as with the TRAF2/WWOX/p53 triad. A likely scenario is that NOS2 binds to the TRAF2/WWOX complex and these proteins co-migrate to the nucleus. NOS2 is responsible for generating NO-containing gas, probably under the assistance of p53 and WWOX. Nonetheless, TRAF2 counteracts the event. This scenario needs further verification.
In addition to the aforementioned proteins, membrane hyaluronidase Hyal-2 and complement C1q binding protein C1qBP undergo nuclear relocation,20 whereas their role in the UV/cold shock-mediated BCD is unknown. Hyal-2 is a receptor for hyaluronan and acts as an alternative receptor for transforming growth factor beta 1 (TGF-β1).56 In a noncanonical signaling, TGF-β1 binds Hyal-2, and then recruits WWOX and Smad4 to relocate to the nucleus. Smad4-mediated gene transcription could result in cell death or proliferation.56 It would be of interest to determine whether the Hyal-2/WWOX/Smad4 signaling supports the formation of a nuclear bubble. We have shown that serum complement C1q binds to the membrane C1qBP and signals with WWOX to induce a novel type of apoptosis without PS flip over onto the cell surface.57 Whether the C1q/C1qBP/WWOX signaling recruits Smad4 and enhances bubble formation is unknown and is being determined in this laboratory.
Third, does UV/cold shock shut down the protein synthesis machinery? Heat shock proteins are involved in cold shock and cold acclimation in plants,58 fishes,59 and other organisms. Ultraviolet A (UVA) induces the expression of a 72-kDa heat shock protein in a human fibrosarcoma cell line, which is needed for cell survival.60 However, the effect of UV/cold shock on heat shock protein expression is unknown. Our preliminary data from gene chip analysis reveals that UV/cold shock down-regulates the mRNA processing, mRNA metabolic process, and RNA splicing (manuscript in preparation). The event probably generates a stress to induce transcription factors p53 and NF-κB, along with WWOX, to relocate to the nucleus for rescue. WWOX is known to regulate gene transcription together with p53, NF-κB, and other transcription factors.51–53 Interestingly, a report showed that an 18-kDa cold-inducible RNA-binding protein (CIRP) relocates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor.61 Whether this protein is needed for BCD remains to be established.
Lastly, why do exosomal sizes vary? Presumably, secreted exosomes should be fairly uniform in sizes. By re-analyzing our published video data,20 we found that the secreted exosomes can grow in sizes. This is likely due, in part, to deposition and aggregation of newly secreted exosomes with time. It is possible that exposure of adhesive proteins on the surface of exosomes leads to aggregation. Notably, intracellular ectopic protein expression in the nucleus and Golgi complex appears to increase cellular stress and enlarge the particle sizes of exosomes.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
This research was supported by the Ministry of Science and Education, Taiwan, ROC (NSC99-2320-B-006-012-MY3, 102-2320-B-006-018-, 102-2320-B-006-030-, and 102-3011-P-006-005-) (to NSC). Yu-An Chen, Yang-Wei Chen, and Wan-Hsuan Yang’s graphic artwork is appreciated.
Declaration Of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy [Review]. Curr Opin Cell Biol 2004; 16: 663–9. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wojton J, Meisen WH, Kaur B. How to train glioma cells to die: molecular challenges in cell death. J Neurooncol 2016; 126: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis [Review]. Mediators Inflamm 2015; 2015: 250762–250762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai CW, Lai FJ, Sheu HM, Lin YS, Chang TH, Jan MS, Chen SM, Hsu PC, Huang TT, Huang TC, Sheen MC, Chen ST, Chang WC, Chang NS, Hsu LJ. WWOX suppresses autophagy for inducing apoptosis in methotrexate-treated human squamous cell carcinoma. Cell Death Dis 2013; 4: e792–e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora R, Régnier-Vigouroux A. Autophagy-driven cell fate decision maker: activated microglia induce specific death of glioma cells by a blockade of basal autophagic flux and secondary apoptosis/necrosis. Autophagy 2009; 5: 419–21. [DOI] [PubMed] [Google Scholar]
- 7.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res 2003; 283: 1–16. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19: 107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005; 73: 1907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisch SM, Francis H. Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol 1994; 124: 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J Cell Biol 2008; 180: 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King KL, Cidlowski JA. Cell cycle and apoptosis: common pathways to life and death [Review]. J Cell Biochem 1995; 58: 175–80. [DOI] [PubMed] [Google Scholar]
- 13.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–9. [DOI] [PubMed] [Google Scholar]
- 14.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, III, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149: 1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deter RL, De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol 1967; 33: 437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells [Review]. Annu Rev Biochem 2000; 69: 303–42. [DOI] [PubMed] [Google Scholar]
- 17.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007; 131: 966–79. [DOI] [PubMed] [Google Scholar]
- 18.Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 2008; 1147: 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HP, Chang JY, Lin SR, Lee MH, Huang SS, Hsu LJ, Chang NS. Identification of an in vivo MEK/WOX1 complex as a master switch for apoptosis in T cell leukemia. Genes Cancer 2011; 2: 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SJ, Lin PW, Lin HP, Huang SS, Lai FJ, Sheu HM, Hsu LJ, Chang NS. UV irradiation/cold shock-mediated apoptosis is switched to bubbling cell death at low temperatures. Oncotarget 2015; 6: 8007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Ahsen O, Waterhouse NJ, Kuwana T, Newmeyer DD, Green DR. The ‘harmless’ release of cytochrome c. Cell Death Differ 2000; 7: 1192–9. [DOI] [PubMed] [Google Scholar]
- 22.Vier J, Linsinger G, Häcker G. Cytochrome c is dispensable for fas-induced caspase activation and apoptosis. Biochem Biophys Res Commun 1999; 261: 71–8. [DOI] [PubMed] [Google Scholar]
- 23.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 1998; 17: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, Yeh WC, Ohashi P, Wang X, Mak TW. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell 2005; 121: 579–91. [DOI] [PubMed] [Google Scholar]
- 25.Hsu LJ, Hong Q, Schultz L, Kuo E, Lin SR, Lee MH, Lin YS, Chang NS. Zfra is an inhibitor of Bcl-2 expression and cytochrome c release from the mitochondria. Cell Signal 2008; 20: 1303–12. [DOI] [PubMed] [Google Scholar]
- 26.Dudekula S, Lee MH, Hsu LJ, Chen SJ, Chang NS. Zfra is a small wizard in the mitochondrial apoptosis. Aging (Albany NY) 2010; 2: 1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markiewski MM, Labmris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 2007; 171: 715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton K, Dixit VM. Signaling in innate immunity and inflammation [Review]. Cold Spring Harb Perspect Biol 2012; 4: pii: a006049–pii: a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallach D. The cybernetics of TNF: old views and newer ones [Review]. Semin Cell Dev Biol 2015; 50: 105–14. [DOI] [PubMed] [Google Scholar]
- 30.Belizário J, Vieira-Cordeiro L, Enns S. Necroptotic cell death signaling and execution pathway: Lessons from knockout mice. Mediators Inflamm 2015; 2015: 128076–128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ 2015; 22: 526–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafren K. Frostbite: prevention and initial management [Review]. High Alt Med Biol 2013; 14: 9–12. [DOI] [PubMed] [Google Scholar]
- 33.Ingram BJ, Raymond TJ. Recognition and treatment of freezing and nonfreezing cold injuries [Review]. Curr Sports Med Rep 2013; 12: 125–30. [DOI] [PubMed] [Google Scholar]
- 34.Auerbach LJ, Galvez MG, De Clerck BK, Glotzbach J, Wehner MR, Chang EI, Gurtner GC, Auerbach PS. A novel mouse model for frostbite injury. Wilderness Environ Med 2013; 24: 94–104. [DOI] [PubMed] [Google Scholar]
- 35.Kemper TC, de Jong VM, Anema HA, van den Brink A, van Hulst RA. Frostbite of both first digits of the foot treated with delayed hyperbaric oxygen: a case report and review of literature [Review]. Undersea Hyperb Med 2014; 41: 65–70. [PubMed] [Google Scholar]
- 36.Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 1999; 401: 168–73. [DOI] [PubMed] [Google Scholar]
- 37.Yao DK, Chen R, Maslov K, Zhou Q, Wang LV. Optimal ultraviolet wavelength for in vivo photoacoustic imaging of cell nuclei. J Biomed Opt 2012; 17: 056004–056004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill SS, Anjum NA, Gill R, Jha M, Tuteja N. DNA damage and repair in plants under ultraviolet and ionizing radiations. ScientificWorldJournal 2015; 2015: 250158–250158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open 2015; 1(Suppl 1): e000061–e000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao FQ, Zhang ZW, Wang C, Zhang B, Yao HD, Li S, Xu SW. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones 2013; 18: 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aquilano K, Baldelli S, Ciriolo MR. Nuclear recruitment of neuronal nitric-oxide synthase by alpha-syntrophin is crucial for the induction of mitochondrial biogenesis. J Biol Chem 2014; 289: 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suschek CV, Krischel V, Bruch-Gerharz D, Berendji D, Krutmann J, Kröncke KD, Kolb-Bachofen V. Nitric oxide fully protects against UVA-induced apoptosis in tight correlation with Bcl-2 up-regulation. J Biol Chem 1999; 274: 6130–7. [DOI] [PubMed] [Google Scholar]
- 43.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276: 3361–70. [DOI] [PubMed] [Google Scholar]
- 44.Chang NS, Doherty J, Ensign A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J Biol Chem 2003; 278: 9195–202. [DOI] [PubMed] [Google Scholar]
- 45.Chang NS, Doherty J, Ensign A, Schultz L, Hsu LJ, Hong Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J Biol Chem 2005; 280: 43100–8. [DOI] [PubMed] [Google Scholar]
- 46.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22. [DOI] [PubMed] [Google Scholar]
- 47.Ambs S, Ogunfusika MO, Merriam WG, Bennett WP, Billiar TR, Harris CC. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc Natl Acad Sci U S A 1998; 95: 8823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, Billiar TR, Blumberg D. Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res 2004; 64: 8015–21. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Wang Y, Guo L, Wang L, Chen W, Shi B. The expression and correlation of iNOS and p53 in oral squamous cell carcinoma. Biomed Res Int 2015; 2015: 637853–637853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terry Powers JL, Mace KE, Parfrey H, Lee SJ, Zhang G, Riches DW. TNF receptor-1 (TNF-R1) ubiquitous scaffolding and signaling protein interacts with TNF-R1 and TRAF2 via an N-terminal docking interface. Biochemistry 2010; 49: 7821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YC, Ye H, Hsia C, Segal D, Rich RL, Liou HC, Myszka DG, Wu H. A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell 2000; 101: 777–87. [DOI] [PubMed] [Google Scholar]
- 52.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal 2013; 8: 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai CC, Chen-Han Yang CH, Chang NS. Role of exosomes in intercellular signaling, maintenance of normal physiology, and their therapeutic potentials [Commentary]. Clin Exp Pharmacol 2014; 4: 154–154. [Google Scholar]
- 54.Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology 2015; 4: e1027472–e1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schilders G, Raijmakers R, Malmegrim KC, Vande Walle L, Saelens X, Vree Egberts W, van Venrooij WJ, Vandenabeele P, Pruijn GJ. Caspase-mediated cleavage of the exosome subunit PM/Scl-75 during apoptosis. Arthritis Res Ther 2007; 9: R12–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu LJ, Schultz L, Hong Q, Van Moer K, Heath J, Li MY, Lai FJ, Lin SR, Lee MH, Lo CP, Lin YS, Chen ST, Chang NS. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J Biol Chem 2009; 284: 16049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong Q, Sze CI, Lin SR, Lee MH, He RY, Schultz L, Chang JY, Chen SJ, Boackle RJ, Hsu LJ, Chang NS. Complement C1q activates tumor suppressor WWOX to induce apoptosis in prostate cancer cells. PLoS One 2009; 4: e5755–e5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park CJ, Seo YS. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J 2015; 31: 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teigen LE, Orczewska JI, McLaughlin J, O'Brien KM. Cold acclimation increases levels of some heat shock protein and sirtuin isoforms in threespine stickleback. Comp Biochem Physiol A Mol Integr Physiol 2015; 188: 139–47. [DOI] [PubMed] [Google Scholar]
- 60.Trautinger F, Kokesch C, Klosner G, Knobler RM, Kindas-Mügge I. Expression of the 72-kD heat shock protein is induced by ultraviolet A radiation in a human fibrosarcoma cell line. Exp Dermatol 1999; 8: 187–92. [DOI] [PubMed] [Google Scholar]
- 61.De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Exp Cell Res 2007; 313: 4130–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.