Abstract
Osteosarcoma is the most frequent type of primary bone tumors among children and adolescents. During the past years, little progress has been made regarding prognosis of osteosarcoma patients, especially for those with metastatic disease. Genomic instability and gene alterations are common, but current data do not reveal a consistent and repeatable pattern of osteosarcoma development, thus paralleling the tumor's high heterogeneity. Critical signal transduction pathways have been implicated in osteosarcoma pathobiology and are being evaluated as therapeutic targets, including receptor activator for nuclear factor-κB (RANK), Wnt, Notch, phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin, and mechanotransduction pathways. Herein, we recapitulate and discuss recent advances in the context of molecular mechanisms and signaling networks that contribute to osteosarcoma progression and metastasis, towards patient-tailored and novel-targeted treatments.
Keywords: Osteosarcoma, signal transduction, activator protein-1, nuclear factor-κB, mammalian target of rapamycin, mechanotransduction
Introduction
Osteosarcoma (OS) is the most frequent primary bone-derived tumor among children and adolescents. OS affects the metaphysis of long bones and approximately 15–20% of patients will present metastases at the time of diagnosis, especially at the lung and bones. Controversies have been raised in the context of treatment strategies, since little progress has been made in order to improve prognosis of OS patients. Current treatment involves preoperative chemotherapy, followed by surgical resection of the primary tumor, as well as detectable metastatic tumors, along with adjuvant chemotherapy after surgery. A multiagent regimen with combined methotrexate, doxorubicin, and cisplatin seems to improve patient outcome, while at the same time, five-year survival of young adolescents is about 60–65% in Europe and North America.1 However, prognosis of metastatic disease remains almost the same during the past 30 years, approximately 20%.2
OS presents heterogeneity among different types, and deregulation of several oncogenic and tumor-suppressing pathways has been acknowledged to drive cell transformation and metastasis. In accordance, molecular mechanisms of OS development include aberrant activation of critical signaling networks, which are essential for cellular homeostasis.3 Therefore, it is of high importance to decipher molecular mechanisms and activated signaling cascades in favor of patient-tailored therapeutic strategies, development of new agents, and alternative therapeutic routes. In the present minireview, we discuss recent studies regarding OS pathogenic signal transduction mechanisms and focus on certain pathways that emerge as important aspects of OS pathogenesis and can modulate treatment responses.
Essentials of bone remodeling and OS development
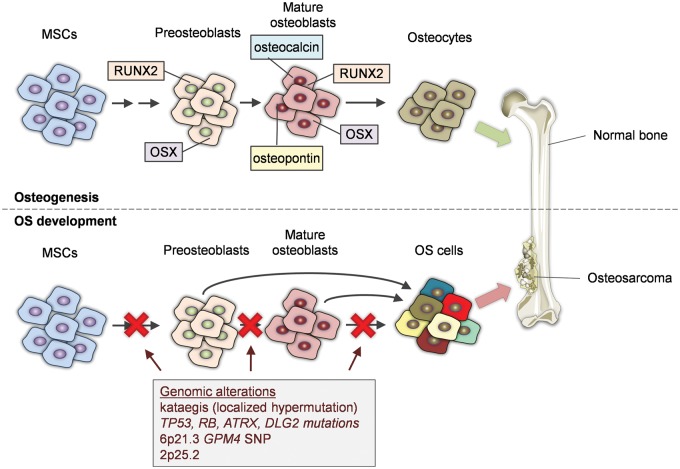
Bone remodeling is a dynamic process which occurs under mechanical and metabolic stimulation throughout human life. It involves resorption of old bone and formation of new in a coordinated manner and balance.4 This balance depends on homeostatic regulation of two main types of bone cells. Osteoblasts are generated from mesenchymal stem cells, which in turn give rise to preosteoblasts (Figure 1).2 Mature osteoblasts express cell-specific markers such as RUNX-related transcription factor 2, osterix, osteopontin, bone sialoprotein, and osteocalcin. Osteoblasts are responsible for the formation of bone osteoid generating mature osteocytes. Osteoclasts, on the other hand, are responsible for bone resorption and their function depends on paracrine cues generated from osteoblasts, including receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG), as well as circulating hormones.2 Osteoclasts express the receptor activator for nuclear factor-κB (RANK), which upon RANKL binding activate downstream molecules, such as tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6), and transcription factors, such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1).4
Figure 1.
Key events during osteosarcoma development. The figure depicts normal osteogenesis from MSCs to osteocytes versus OS development accompanied by critical genomic alterations, which drive cell transformation. ATRX: transcriptional regulator ATRX; DLG2: disks large homolog 2; GRM4: glutamate receptor metabotropic 4; MSCs: mesenchymal stem cells; OS: osteosarcoma; RB1: retinoblastoma 1; RUNX2: RUNX-related transcription factor 2; SNP: single nucleotide polymorphism; TP53: tumor protein p53
OS has been associated with several risk factors and heritable syndromes such as rapid bone turnover during puberty, taller stature, radiation, Paget's disease, and Li-Fraumeni syndrome.2 Genomic imbalance and chromosomal abnormalities are key features during OS development depicting frequent TP53 and RB mutations (Figure 1).5 In order to reveal widely accepted molecular signatures related to OS development, a genome-wide association study carried out in 941 patients and 3291 healthy controls revealed two significant loci. The first was detected at 6p21.3 with ars1906953 single nucleotide polymorphism on the GRM4 gene encoding for glutamate receptor metabotropic 4. This shows that cyclic adenosine monophosphate (cAMP) pathway, where GRM4 is associated with the pathway's inactivation, is important for OS development. The second locus at 2p25.2 with ars7591996 single nucleotide polymorphism was not associated with an active transcription factor binding site.6 Another study was conducted in order to assess a consensus of somatic mutations in 34 pediatric OSs. Whole genome sequencing of DNA revealed a pattern of hypermutations related to structural variations, termed kataegis. TP53 mutations were identified in the majority (>90%) of the tumors and other frequent mutations were located in RB1, ATRX, and DLG2 genes (Figure 1). This means that TP53 alterations represent oncogenic driver mutations in OS. The fact that most of these mutations are structural variations raises the possibility that genomic instability may precede TP53 inhibition and be the underlying cause of OS development (Figure 1). However, variations between studies require validation in a larger cohort.7 Accordingly, there is an ongoing project examining nearly 100 pediatric OS patient cases screening for gene expression, chromosome copy number changes and loss of heterozygosity, epigenetic and transcriptome profiling (https://ocg.cancer.gov/programs/target).
RANKL/RANK pathway
As previously described, RANK signaling is critical for bone homeostasis regulating differentiation of osteoclasts through paracrine and homotypic interactions with its ligand RANKL, which is produced by osteoblasts. This particular signaling pathway is important in several pathologic mechanisms and has been already implicated in OS.8–11 RANK is expressed in human OS cell lines and human tissues, and upon RANKL stimulation induces activation of downstream signaling molecules, extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and IκB (Figure 2).10 RANKL increased expression has also been associated with poor response of patients to preoperative chemotherapy and lower cancer-free survival.12 On the other hand, RANK shows multifold expression in tumor tissues of a transgenic mouse model of OS. Homotypic RANK signaling does not impact cell proliferation but induces cell motility and anchorage-independent growth in primary OS cells derived from the mouse model.13
Figure 2.
Signal transduction pathways in osteosarcoma development. The figure illustrates the main signal transduction cascades and putative molecular crosstalk implicated in OS progression, including RANKL/RANK, MAPKs, PI3K/Akt/mTOR, cAMP/PKA, Wnt/β-catenin, Notch, mechanoresponsive pathways, which are funneled through respective transcription factor activation. AP-1: activator protein-1; CREB: cAMP response element-binding protein; CSL: CBF1/ suppressor of hairless/Lag-1; ERK1/2: extracellular signal-regulated kinase 1/2; Fzs: frizzleds; Gαs: Gs alpha subunit; HES-1: hairy/enhancer of split 1; HEY-2: hairy/enhancer-of-split related with YRPW motif protein 2; ICD: intracellular domain of Notch; IκBα: nuclear factor of κB inhibitor: alpha; IKK: IκB kinase; ILK: integrin-linked kinase; JNK: c-Jun N-terminal kinase; LRP5/6: low-density lipoprotein receptor-related protein 5/6; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor-κB; NKD2: naked cuticle homolog 2; OPG: osteoprotegerin; OS: osteosarcoma; PI3K: phosphatidylinositol 3-kinase; PKA: protein kinase A; PTEN: tumor-suppressor phosphatase and tensin homolog; PTH: parathyroid hormone; PTHR: parathyroid hormone receptor; RANK: receptor activator for nuclear factor-κB; RANKL: receptor activator of nuclear factor-κB ligand; RTKs: receptor tyrosine kinases; TRAF6: TNF receptor associated factor 6; TCF/LEF: T cell factor/lymphoid enhancer factor; WIF1: Wnt inhibitory factor 1
In corroboration with these findings, in a genomic unstable mouse model modeling human OS, a deletion at 11qE1 containing Prkar1a gene was identified in 38% of tumors. Protein kinase type I, α regulatory subunit (Prkar1a) is a subunit of protein kinase A (PKA) which becomes activated as a cAMP-dependent substrate. This deletion characterizes this specific tumor subclass and is accompanied by deregulation of PKA signaling and overexpression of RANKL. Therefore, Prkar1a is a tumor suppressor being downregulated in OS, thus driving RANKL overexpression.9 Recent data show that deletions of the RB and TP53 genes, along with Prkar1a gene lead to faster development of tumors in vivo and increased activity of PKA and its downstream mediator cAMP response element–binding protein (Figure 2), accompanied by higher expression levels of RANKL compared to wild-type Prkar1a mice. Further experiments proved that RANKL-mediated signaling promotes OS development, whereas specific inhibition of Rank only in osteoclasts abrogated the effect.14
It is also known that enhanced secretion of osteoclast-induced cytokines through activation of RANK downstream modulator NF-κB transcription factor is a feature in OS (Figure 2).2 Deletion of Rank in the previous study was associated with lower expression levels of RANK and RANKL in tumor cells, as well as lower levels of NF-κB-p65. This downregulation resulted in upregulation of tumor-suppressor phosphatase and tensin homolog (PTEN), which in turn negatively regulated phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway (Figure 2).14
OS also exhibits osteolytic lesions generated from increased activity of osteoclasts. This is the outcome of a vicious cycle between osteoclasts, bone stromal cells/osteoblasts, and cancer cells.4,8 OPG is a soluble protein belonging to the TNF receptor superfamily, which prevents binding of RANKL to RANK. It is conceivable that RANKL/OPG ratio increases during OS development in favor of osteolysis (Figure 2). Administration of murine OPG in OS rodent models diminished tumor growth and osteolytic lesions during OS development and increased survival of the animal models. OPG seems to exert an indirect effect on tumor progression via inhibition of RANKL increased activity.8 Additional preclinical models also establish RANKL blockade as a way of attenuating osteolytic activity.15,16 Promising results from the discussed preclinical models regarding RANKL inhibition against OS progression are being evaluated at the clinic and a recent report describes complete metabolic remission of an OS patient with RANK and RANKL overexpression that was treated with combination of sorafenib, a tyrosine kinase inhibitor, and RANKL inhibitor denosumab.17
Wnt pathway
Wnt is a highly conserved pathway playing a notable role in cellular differentiation and homeostasis. Wnt ligands bind to their receptors, Frizzleds and LRP-5/6, thereby activating downstream signaling cascades including the canonical Wnt/β-catenin/T cell factor/lymphoid enhancer factor pathway and the non-canonical pathways. Especially in bone biology, Wnt is essential for bone morphogenesis during embryogenesis, bone mass regulation, and bone regeneration.18 Furthermore, Wnt aberrant activation has been implicated in OS development. Increased activity of the canonical Wnt pathway has been observed in primary human OSs and corresponding sarcoma cell lines.19 This increased activation seems to occur through an autocrine mechanism that positively affects cell proliferation through upregulation of the cell cycle gene CDC25A.20 Increased activation is translated in overexpression of several Wnt ligands and receptors in OS tissues and cell lines (Figure 2).18 Expression of LRP5 has been associated with tumor metastasis and lower cancer-free survival in OS human tissues and Wnt10b ligand with decreased overall survival.21,22 In addition, stable transfection of Saos-2 OS cells with a dominant negative, soluble LRP5 plasmid, showed inhibition of cell invasion, downregulation of mesenchymal markers (N-cadherin, Slug, Twist, matrix metalloproteinases 2 and 4), and upregulation of epithelial markers (E-cadherin), implying that LRP5 in particular promotes epithelial-to-mesenchymal transition (EMT) and metastasis, whereas LRP inhibition reverses these effects.23
Moving to intracellular Wnt components, β-catenin presents cytoplasmic and nuclear accumulation approximately to 70% of OS human tissues, associated expression levels in tumors of high grade, as well as to LM8 murine OS cell line which exhibits high metastatic potential to the lung (Figure 2).24–26
Wnt contribution to OS development and metastasis is reinforced by in vitro and in vivo studies investigating the role of Wnt antagonists (Figure 2). Xenografts injected with 143b OS cells stably overexpressing the Wnt antagonist Dickkopf-3 protein (DKK-3/REIC) present decreased tumor growth and lung metastases. Likewise, in vitro functional assays reveal that DKK3 overexpressing cells demonstrate lower migration, anchorage-independent growth, cell viability, and EMT inhibition.27 Another Wnt antagonist, Wnt inhibitory factor 1 (WIF1), demonstrates epigenetic-related downregulation in OS tissues and cell lines via promoter hypermethylation in its gene promoter. Re-expression of WIF1 was associated with lower β-catenin expression levels and decreased tumor growth in vivo and in vitro.28,29 However, a second study reports that WIF1 downregulation is not attributed to DNA methylation rather than to histone deacetylases leading to lower acetylation levels, an event accompanied by trimethylation of specific repressive histone marks H3K4/H3K27.30 Microarray analysis in a dual non-metastatic and metastatic mouse model of OS identified downregulation of an additional Wnt antagonist, naked cuticle homolog 2 (NKD2) (Figure 2). Overexpression of NKD2 in OS cells diminished cell proliferation, migration, and invasion, and at the same time decreased in vivo tumor growth. Downregulation of NKD2 was also evident in human metastatic and recurrent OS tissues.31
Activation of canonical Wnt is also associated with cancer stem cells leading to recurrence of OS tumors. In particular, activation of Wnt/β-catenin is associated not only with presence of cancer stem cell population but also with cell transition to a cancer stem-like phenotype in OS cells following treatment with chemotherapy, thereby implying that this mechanism confers drug resistance to cancer cells.32,33
Non-canonical Wnt signaling involves β-catenin-independent pathways such as Wnt/Ca2+, Wnt/planar cell polarity (PCP), and Wnt/PKA pathways.18 These Wnt pathways are mediated through frizzleds and alternative co-receptors named receptor tyrosine kinase-like orphan receptors (RORs) and receptor-like tyrosine kinase.18 Wnt-5a is a specific non-canonical Wnt ligand that activates ROR-2. Both proteins have been found to be highly expressed in OS tissues and, in particular, elevated expression has been associated with detrimental characteristics of the tumors.34 Furthermore, in vitro experiments have demonstrated that Wnt-5a and ROR-2 activate Src family kinases (SFKs) in order to express matrix metalloproteinase-13 (MMP-13), a molecular mechanism that ultimately leads to increased invasion in OS in a cell-autonomous manner.35 Wnt-5a also enhances cell migration of MG-63 cells via activation of PI3K/Akt pathway.36
Receptor tyrosine kinases and downstream pathways
Several membrane receptor tyrosine kinases (RTKs) become upregulated in OS as a response to increased expression of their respective ligands and represent potential targets with monoclonal antibodies (Figure 2).2 Vascular endothelial growth factor overexpression in OS has been associated with reduced overall survival, platelet-derived growth factor (PDGF)-AA overexpression with tumor progression, but increased expression of PDGF-alpha receptor (PDGFαR) is not a prognosticator in OS.37–39 Overexpression of erbB2 (HER-2/neu) has been also associated during a meta-analysis with poor prognosis of OS patients.40 Insulin-like growth factor-I has long been known now to stimulate cell proliferation in MG-63 cells and inhibition of the respective Insulin-like growth factor-I receptor to abrogate this effect.41 Numerous studies test the effect of RTKs inhibitors. While preclinical results are promising towards this end, clinical outcome is not quite encouraging due to molecular mechanisms of acquired resistance from the patients.42
Downstream intracellular kinase pathways are also important for transmitting signals generated from membrane receptors and have been implicated in OS pathogenesis. We have already described the engagement of integrin-linked kinase (ILK) in the development of chondrosarcomas.43 ILK is a serine/threonine kinase that mediates cell-to-extracellular matrix adhesion as a downstream substrate of β1 and β3 integrins.43 ILK is upregulated in OSs and this overexpression has been associated with the presence of distant metastases, poor overall and cancer-free survival.44 In vitro findings suggested that ILK downregulation was accompanied by limited invasion, migration, angiogenesis, and induced apoptosis.44 Another central intracellular molecule is Src tyrosine kinase, which belongs to the Src family of kinases and is the first proto-oncogene ever detected. Src is upregulated in several tumor types and is already therapeutically targeted.45 In OS, Src overexpression is associated with increased cell proliferation in vitro and depletion of Src phosphorylation with decreased development of tumor mass in vivo.46–48 However, a second study announced that while the Src inhibitor dasatinib abrogated OS cell adhesion and migration in vitro, there was no similar effect on tumor growth, apoptosis, and pulmonary metastasis in vivo, indicating the controversial effect of Src on OS progression.49
Mitogen-activated protein kinase (MAPK) pathway is responsible for proliferative and oncogenic potential in numerous malignant phenotypes and OS. MAPK signaling cascades become activated in OS and integrate upregulation of upstream membrane receptors, as well as crosstalk with other sidestream signaling pathways, eventually mediating induction of downstream transcription factors (Figure 2).50
A representative example is AP-1 transcription complex, which is generated by heterodimerization of c-Jun with c-Fos proteins. Important genes for regulation of osteoblast homeostasis harbor AP-1 binding sites within their promoter regions, such as encoding type I collagen, osteopontin, and osteocalcin.51 C-Jun is phosphorylated and thereby activated by phosphorylated c-Jun N-terminal kinases (JNKs) on Ser/Thr sites.51 JNK/AP-1 signaling network is upregulated in bone tumors such as chondrosarcoma, but also is activated in OS human tissues compared to normal bone tissue, whereas c-Jun activation seems prominent in high-grade tumors (Figure 2).51,52 In vivo transgenic models show that there is a critical c-Fos threshold necessary for OS development.53 Furthermore, activation of the α chain of the nascent polypeptide-associated complex, which functions as a bone-specific AP-1 co-activator, implies that c-Jun homodimers are prominent in OS development.51 AP-1 is also critical during invasion and metastasis in OS. JNK and AP-1 are prominently upregulated in murine OS cells presenting high metastatic potential. In vitro inhibition of AP-1 with a dominant-negative mutant of c-Jun significantly blocked cell migration and invasion, as well as in vivo metastasis.54
PI3K/Akt/mTOR pathway
The PI3K/Akt/mTOR pathway is important for cellular homeostasis, therefore regulating cell growth, autophagy, proliferation, and other important aspects of cellular physiology. This cascade of protein kinases has been implicated in various tumor types and there is also an increasing body of evidence featuring PI3K/Akt/mTOR contribution to OS tumor progression and putative therapeutic targeting (Figure 2).55
Deregulation of PI3K/Akt/mTOR pathway exerts pleiotropic effects on cancer cell properties. It affects proliferation, invasion and metastasis, tumorigenesis, apoptosis, cell cycle regulation, angiogenesis, and resistance to chemical agents.55 A recent paper by Perry et al. highlights this particular signaling pathway as a core mechanism in OS development. Whole-genome sequencing, whole-exome sequencing, and RNA-sequencing in 59 tumor versus normal pairs revealed that PI3K/Akt/mTOR is the most common pathway altered in OS. Alterations observed included mutations on PTEN, TSC2, PI3KCA, PDPK1, AKT1, and EIF4B genes, whereas in vivo genome-wide, pooled shRNA screen analysis suggested that mTOR and Pi3kca are essential for OS proliferation and survival.56 To this end, a forward genetics screen was employed using the conditional Sleeping Beauty transposon mutagenesis system in wild-type mice and mice with Trp53 loss. Analysis of common insertion sites demonstrated, among others, PTEN as one of the most frequently mutated genes in Trp53-Sleeping Beauty mutated OS tumors and also mutations of the Akt2 gene. Ingenuity Pathway Analysis also identified enrichment in the PI3K/Akt/mTOR pathway.57 A genome-wide gene expression analysis revealed active Akt signaling among 20 different high-grade OS cell lines and Akt inhibition was able to abrogate proliferation in cell lines with wild-type Kras.58 In addition, mTOR inhibitor, rapamycin, attenuates OS cell proliferation,59 but only dual inhibition of PI3K and mTOR in primary murine and human OS cell cultures was able to induce apoptosis compared to targeting of either PI3K or mTOR alone.60
Chemical compounds against these molecules are also evaluated at the clinic. Activity of the mTOR pathway, mTORC2 in particular, seems to be the reason that patients develop resistance to the kinase inhibitor, sorafenib. Preclinical models demonstrated encouraging data of dual sorafenib and everolimus administration against this escape mechanism.61 Therefore, combination regimen of the mTOR inhibitor everolimus and sorafenib was evaluated in patients with unresectable OS. Although 45% of the patients presented six months of progression-free survival, the target of 50% was not reached.62
Notch pathway
Notch pathway is an evolutionary conserved pathway that is critical for normal bone development, affecting both osteoblasts and osteoclasts.63 Notch involves the Jagged and Delta-like ligands, along with Notch1, Notch2, Notch3, and Notch4 receptors.63 Upon ligand binding, Notch receptor undergoes proteolytic cleavage and the intracellular domain of Notch transfers to the nucleus functioning as a co-activator of CSL transcription factor target genes, which are basic helix-loop-helix transcription factors of the Hairy/Enhancer of Split (HES) and the HES-related repressor proteins families (Figure 2).63
Besides hematologic and epithelial cancer, Notch has been implicated in the development of OS. Notch1 receptor, Jagged-1 ligand, along with Notch target transcription factors HES-1 and HEY-2 have been found upregulated in OS primary tumors. Inhibition of Notch proteolytic cleavage by using γ-secretase inhibitors also reduces cell proliferation in SaOs-2 OS cells and Notch inhibition also reduces tumor development in nude xenografts in vivo.64 Reduced proliferation is an outcome of cell cycle regulation since Notch inhibition has proved to promote G1 arrest, downregulate cell cycle promoters, and upregulate p21.65 In particular, Notch alterations seem to affect invasion and metastasis in OS development.63 Notch inhibition, also by γ-secretase blockage, reduces invasiveness in OS cells in a dose-dependent manner. Transfection of OS187 cells with a dominant-negative Mastermind-Like vector for Notch inhibition presented limited pulmonary metastases compared to control.63
Moreover, a recent report provides evidence that Notch is not only altered during OS development but represents a driver of transformation in bone cells.66 Restricted expression of intracellular domain of Notch in mice osteoblasts induced bone tumor formation, exhibiting histopathologic features typical of an aggressive OS tumor.66 Cell lines were isolated from these mice, were immortalized and finally injected into nude mice creating new OS-like tumors between 14 and 28 days.66 On the other hand, OS cells themselves demonstrate basic levels of Notch-ligands expression. Therefore, Notch signaling in OS seems to be an outcome of paracrine induction from the tumor's microenvironment, mainly from pericytes and endothelial cells.67
Bone morphogenetic protein signaling
More than 20 bone morphogenetic proteins (BMPs) have been identified to date, which belong to the transforming growth factor-β superfamily of cytokines.68 BMPs serve different cellular functions but have been mostly associated with osteogenesis and, in particular, differentiation of bone and cartilage.68 BMPs are ligands which upon binding to types I and II BMP receptors (BMPRs; transmembrane Ser/Thr kinases) activate downstream signaling networks, mainly Smad 1/5/8, MAPK, and JNK pathways.68 BMP signaling components have been associated with OS development and prognosis. In vitro investigation demonstrates that most OS cell lines express a variety of BMP ligands.68,69 However, differential expression of BMP ligands across OS tissues do not yet reproduce a repeatable pattern of detectable differences between OS histologic subtypes.68 Certain studies suggest that overexpression of BMP family components and receptors is associated with a metastatic phenotype and poor prognosis in OS tumors.70–72 On the other hand, a different study in high-grade OS tumors revealed limited BMP signaling activity that was linked to lower survival.73 Overall, BMPs deregulation seems to be a feature in OS development and progression, but their precise role has not been defined yet, as well as their potential use as OS prognosticators.
Mechanotransduction and OS development
The process through which bone cells sense extracellular mechanical cues and convert them into intracellular biochemical events in order to elicit a response is called mechanotransduction. Mechanotransduction is essential for the preservation of bone homeostasis, especially during bone remodeling processes. Deregulated response of bone cells against mechanical stimulation has been implicated in several bone disorders such as spondyloarthritides, chondrosarcoma, osteoporosis, and obviously OS.74 Focal adhesions and focal adhesion-related molecules are responsible for signaling-mediated responses upon mechanical stimulation (Figure 2). This process seems to elicit time-dependent, differential biochemical events in OS cells. In particular, U2OS cell line subjected to uniaxial mechanical stretching demonstrates rapid growth of focal adhesion almost instantly after initialization of stretching. However, several minutes of stretching show focal adhesion disassembly perpendicular to stretch and loss of cell polarity. Focal adhesion kinase activation and SFK are essential for promotion and inhibition of rapid growth, respectively. For delayed disassembly, apart from cell reorientation, calpain-2, SFK, and focal adhesion kinase are required. Taken together, the above findings imply that orientation of the forces applied is critical in OS, since delayed disassembly of focal adhesion occurred only in cells where mechanical stretching was enforced perpendicular to their long axes.75
Furthermore, an oncoprotein of the extracellular matrix termed Tenascin-C (TN-C) is overexpressed in tumors and especially the TN-C FNIIIA1 variant has been found upregulated in OS tissues and also promotes cell migration via mTOR pathway in MG-63 cells upon mechanical stimulation.76 TN-C demonstrates 10-fold increased mRNA and protein expression after application of mechanical strain in OS cells, a mechanism mediated by Rho-associated protein kinase-I/II.77 Distinct kinds of mechanical forces such as laminar shear stress induce apoptosis in MG-63 cells compared, for example, to oscillatory shear stress.78 Furthermore, cyclic stretch induces remodeling of stress fibers and activation of MAPKs JNK, ERK, and p38 according to mechanical rates.79
Modulation of properties of OS cells has been also associated with chief mechanosensor molecules termed polycystins.80 Polycystin-1 (PC1) and polycystin-2 (PC2) initiate mechano-responsive signaling pathways and in this way regulate osteoblast gene expression upon mechanical stress.81 Polycystins have been also associated with cancer and have recently emerged as novel prognostic tools in colorectal tumors.82,83 Unpublished data from our laboratory indicate that modulation of PC1 activity affects cell migration and proliferation in MG-63 cells. In addition, stable downregulation of Polycystic Kidney Disease 1 gene in MG-63 cells enhances cell proliferation and affects proper cell differentiation, alterations mediated through impaired intracellular calcium-cAMP regulation, and activation of PKA.80 Significance of mechano-regulated calcium intracellular concentration is also illustrated by the fact that store-operated calcium affects cell polarity and migration in U2OS cells, where calcium-selective channels are implicated, among them TRPC1, which belongs to the same family of transient receptor potential (TRP) channels where PC2 also belongs.84
Conclusions and future perspectives
The fact that prognosis of OS metastatic disease remains relatively unaltered during the past decades brings patient-tailored and targeted therapy to forefront. Tyrosine kinase receptor and intracellular kinase inhibitors are being evaluated in preclinical models and clinical trials in order to reveal efficient therapeutic targets and tools to overcome drug resistance.2,85
Although a genetically unstable tumor, there is emerging evidence that development of OS is also epigenetically regulated, therefore impairing proper function of tumor-suppressing genes in bone homeostasis.86 DNA hypermethylation, aberrant histone chemical modifications, and oncogenic/tumor suppressing microRNAs promote progression and metastasis, therefore anti-epigenetic agents are being tested at the clinic.86
Another aspect dynamically involved in OS pathobiology is the role of the microenvironment's immune cells and putative immune-targeted therapies.87 Crosstalk between bone cells and cells of the immune system needs further clarification.2 However, an activator of the immune system, liposomal muramyl tripeptide phosphatidylethanolamine (L-MTP-PE), is approved within the European Union, in combination with chemotherapy, as adjuvant treatment since it prolongs overall survival.2,87 Monoclonal antibodies targeting immune checkpoint mechanisms show remarkable efficiency in several types of malignancy and are also evaluated in OS preclinical models and clinical trials.2,88–90
Nevertheless, even with novel therapeutic targets, drug resistance is an emerging aspect of inefficient OS treatment. Combination regimens are being continuously designed in order to overcome acquired resistance to chemotherapy. Molecular mechanisms are poorly elucidated and therefore identifying signal transduction cascades which integrate the impact of deregulated epigenetic and microenvironment components is of critical importance for the development of new targets as well as combinatorial treatment strategies.
Acknowledgments
This work was supported in part by ‘IKY fellowships of excellence for postgraduate studies in Greece-Siemens Program’ awarded to CA.
Authors' contributions
All authors participated in the writing and review of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol 2015; 33: 3029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 2014; 14: 722–35. [DOI] [PubMed] [Google Scholar]
- 3.Papachristou DJ, Papavassiliou AG. Osteosarcoma and chondrosarcoma: new signaling pathways as targets for novel therapeutic interventions. Int J Biochem Cell Biol 2007; 39: 857–62. [DOI] [PubMed] [Google Scholar]
- 4.Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med Res Rev 2012; 32: 611–36. [DOI] [PubMed] [Google Scholar]
- 5.Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF. The molecular pathogenesis of osteosarcoma: a review. Sarcoma 2011; 2011: 959248–959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage SA, Mirabello L, Wang Z, Gastier-Foster JM, Gorlick R, Khanna C, Flanagan AM, Tirabosco R, Andrulis IL, Wunder JS, Gokgoz N, Patiño-Garcia A, Sierrasesúmaga L, Lecanda F, Kurucu N, Ilhan IE, Sari N, Serra M, Hattinger C, Picci P, Spector LG, Barkauskas DA, Marina N, de Toledo SR, Petrilli AS, Amary MF, Halai D, Thomas DM, Douglass C, Meltzer PS, Jacobs K, Chung CC, Berndt SI, Purdue MP, Caporaso NE, Tucker M, Rothman N, Landi MT, Silverman DT, Kraft P, Hunter DJ, Malats N, Kogevinas M, Wacholder S, Troisi R, Helman L, Fraumeni JF, Jr, Yeager M, Hoover RN, Chanock SJ. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet 2013; 45: 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, Parker M, Rusch M, Nagahawatte P, Wu J, Mao S, Boggs K, Mulder H, Yergeau D, Lu C, Ding L, Edmonson M, Qu C, Wang J, Li Y, Navid F, Daw NC, Mardis ER, Wilson RK, Downing JR, Zhang J, Dyer MA. St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep 2014; 7: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V, Blanchard F, Gouin F, Pitard B, Heymann D, Redini F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res 2007; 67: 7308–18. [DOI] [PubMed] [Google Scholar]
- 9.Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J, Kashyap M, Hu P, Maiuri T, Narala SR, Stambolic V, Squire J, Penninger J, Sanchez O, Triche TJ, Wood GA, Kirschner LS, Khokha R. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 2010; 120: 3310–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori K, Le Goff B, Berreur M, Riet A, Moreau A, Blanchard F, Chevalier C, Guisle-Marsollier I, Léger J, Guicheux J, Masson M, Gouin F, Rédini F, Heymann D. Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. J Pathol 2007; 211: 555–62. [DOI] [PubMed] [Google Scholar]
- 11.Wittrant Y, Lamoureux F, Mori K, Riet A, Kamijo A, Heymann D, Redini F. RANKL directly induces bone morphogenetic protein-2 expression in RANK-expressing POS-1 osteosarcoma cells. Int J Oncol 2006; 28: 261–9. [PubMed] [Google Scholar]
- 12.Lee JA, Jung JS, Kim DH, Lim JS, Kim MS, Kong CB, Song WS, Cho WH, Jeon DG, Lee SY, Koh JS. RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr Blood Cancer 2011; 56: 738–43. [DOI] [PubMed] [Google Scholar]
- 13.Beristain AG, Narala SR, Di Grappa MA, Khokha R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci 2012; 125: 943–55. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Di Grappa MA, Molyneux SD, McKee TD, Waterhouse P, Penninger JM, Khokha R. RANKL blockade prevents and treats aggressive osteosarcomas. Sci Transl Med 2015; 7: 317ra197–317ra197. [DOI] [PubMed] [Google Scholar]
- 15.Bago-Horvath Z, Schmid K, Rossler F, Nagy-Bojarszky K, Funovics P, Sulzbacher I. Impact of RANK signalling on survival and chemotherapy response in osteosarcoma. Pathology 2014; 46: 411–5. [DOI] [PubMed] [Google Scholar]
- 16.Ohba T, Cole HA, Cates JM, Slosky DA, Haro H, Ando T, Schwartz HS, Schoenecker JG. Bisphosphonates inhibit osteosarcoma-mediated osteolysis via attenuation of tumor expression of MCP-1 and RANKL. J Bone Miner Res 2014; 29: 1431–45. [DOI] [PubMed] [Google Scholar]
- 17.Cathomas R, Rothermundt C, Bode B, Fuchs B, von Moos R, Schwitter M. RANK ligand blockade with denosumab in combination with sorafenib in chemorefractory osteosarcoma: a possible step forward? Oncology 2015; 88: 257–60. [DOI] [PubMed] [Google Scholar]
- 18.Cai Y, Cai T, Chen Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem 2014; 115: 625–31. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Zhao M, Tian A, Zhang X, Yao Z, Ma X. Aberrant activation of Wnt/beta-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015; 6: 17570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayakumar S, Liu G, Rus IA, Yao S, Chen Y, Akiri G, Grumolato L, Aaronson SA. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer Cell 2011; 19: 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, MacDonald T, Toretsky JA, Uren A. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer 2008; 51: 349–55. [DOI] [PubMed] [Google Scholar]
- 22.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 2004; 109: 106–11. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Zi X, Koontz Z, Kim A, Xie J, Gorlick R, Holcombe RF, Hoang BH. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J Orthop Res 2007; 25: 964–71. [DOI] [PubMed] [Google Scholar]
- 24.Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer 2002; 102: 338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis 2003; 20: 525–9. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, Gitelis S, O'Keefe RJ, Konttinen YT, Yin G, Li TF. Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun 2013; 431: 274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CH, Guo Y, Ghaffar S, McQueen P, Pourmorady J, Christ A, Rooney K, Ji T, Eskander R, Zi X, Hoang BH. Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma 2013; 2013: 147541–147541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 2009; 119: 837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin EM, Guo Y, Tu K, Xie J, Zi X, Hoang BH. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol Cancer Ther 2010; 9: 731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker EK, Taylor S, Gupte A, Chalk AM, Bhattacharya S, Green AC, Martin TJ, Strbenac D, Robinson MD, Purton LE, Walkley CR. Wnt inhibitory factor 1 (WIF1) is a marker of osteoblastic differentiation stage and is not silenced by DNA methylation in osteosarcoma. Bone 2015; 73: 223–32. [DOI] [PubMed] [Google Scholar]
- 31.Zhao S, Kurenbekova L, Gao Y, Roos A, Creighton CJ, Rao P, Hicks J, Man TK, Lau C, Brown AM, Jones SN, Lazar AJ, Ingram D, Lev D, Donehower LA, Yustein JT. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene 2015; 34: 5069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins-Neves SR, Corver WE, Paiva-Oliveira DI, van den Akker BE, Briaire-de-Bruijn IH, Bovée JV, Gomes CM, Cleton-Jansen AM. Osteosarcoma stem cells have active Wnt/beta-catenin and overexpress SOX2 and KLF4. J Cell Physiol 2016; 231: 876–86. [DOI] [PubMed] [Google Scholar]
- 33.Martins-Neves SR, Paiva-Oliveira DI, Wijers-Koster PM, Abrunhosa AJ, Fontes-Ribeiro C, Bovée JV, Cleton-Jansen AM, Gomes CM. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/beta-catenin signaling. Cancer Lett 2016; 370: 286–95. [DOI] [PubMed] [Google Scholar]
- 34.Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D, Yan CM, Wang DJ, Sun JY. Expression of WNT-5a and ROR2 correlates with disease severity in osteosarcoma. Mol Med Rep 2012; 5: 1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enomoto M, Hayakawa S, Itsukushima S, Ren DY, Matsuo M, Tamada K, Oneyama C, Okada M, Takumi T, Nishita M, Minami Y. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 2009; 28: 3197–208. [DOI] [PubMed] [Google Scholar]
- 36.Zhang A, He S, Sun X, Ding L, Bao X, Wang N. Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int 2014; 14: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulzbacher I, Birner P, Dominkus M, Pichlhofer B, Mazal PR. Expression of platelet-derived growth factor-alpha receptor in human osteosarcoma is not a predictor of outcome. Pathology 2010; 42: 664–8. [DOI] [PubMed] [Google Scholar]
- 38.Sulzbacher I, Birner P, Trieb K, Traxler M, Lang S, Chott A. Expression of platelet-derived growth factor-AA is associated with tumor progression in osteosarcoma. Mod Pathol 2003; 16: 66–71. [DOI] [PubMed] [Google Scholar]
- 39.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol 2014; 35: 155–60. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Ma YH, Sun ZZ, Rui YJ, Yin QD, Song S, Wei XM, Liu J, Liu XG, Hu KJ. Effect of c-erbB2 overexpression on prognosis in osteosarcoma: evidence from eight studies. Tumour Biol 2014; 35: 8939–43. [DOI] [PubMed] [Google Scholar]
- 41.Pollak MN, Polychronakos C, Richard M. Insulin-like growth factor I: a potent mitogen for human osteogenic sarcoma. J Natl Cancer Inst 1990; 82: 301–5. [DOI] [PubMed] [Google Scholar]
- 42.Segaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol 2015; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papachristou DJ, Gkretsi V, Rao UN, Papachristou GI, Papaefthymiou OA, Basdra EK, Wu C, Papavassiliou AG. Expression of integrin-linked kinase and its binding partners in chondrosarcoma: association with prognostic significance. Eur J Cancer 2008; 44: 2518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee SH, Han I, Lee MR, Cho HS, Oh JH, Kim HS. Role of integrin-linked kinase in osteosarcoma progression. J Orthop Res 2013; 31: 1668–75. [DOI] [PubMed] [Google Scholar]
- 45.Gargalionis AN, Karamouzis MV, Papavassiliou AG. The molecular rationale of Src inhibition in colorectal carcinomas. Int J Cancer 2014; 134: 2019–29. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, Zhou Z, Shan L, Zeng H, Hua Y, Cai Z. The importance of Src signaling in sarcoma. Oncol Lett 2015; 10: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, Pledger WJ, Jove R. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res 2007; 67: 2800–8. [DOI] [PubMed] [Google Scholar]
- 48.Spreafico A, Schenone S, Serchi T, Orlandini M, Angelucci A, Magrini D, Bernardini G, Collodel G, Di Stefano A, Tintori C, Bologna M, Manetti F, Botta M, Santucci A. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. FASEB J 2008; 22: 1560–71. [DOI] [PubMed] [Google Scholar]
- 49.Hingorani P, Zhang W, Gorlick R, Kolb EA. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin Cancer Res 2009; 15: 3416–22. [DOI] [PubMed] [Google Scholar]
- 50.Chandhanayingyong C, Kim Y, Staples JR, Hahn C, Lee FY. MAPK/ERK Signaling in osteosarcomas, ewing sarcomas and chondrosarcomas: therapeutic implications and future directions. Sarcoma 2012; 2012: 404810–404810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papachristou DJ, Batistatou A, Sykiotis GP, Varakis I, Papavassiliou AG. Activation of the JNK-AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone 2003; 32: 364–71. [DOI] [PubMed] [Google Scholar]
- 52.Papachristou DJ, Papachristou GI, Papaefthimiou OA, Agnantis NJ, Basdra EK, Papavassiliou AG. The MAPK-AP-1/-Runx2 signalling axes are implicated in chondrosarcoma pathobiology either independently or via up- regulation of VEGF. Histopathology 2005; 47: 565–74. [DOI] [PubMed] [Google Scholar]
- 53.Wang ZQ, Liang J, Schellander K, Wagner EF, Grigoriadis AE. c-fos-induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos. Cancer Res 1995; 55: 6244–51. [PubMed] [Google Scholar]
- 54.Leaner VD, Chick JF, Donninger H, Linniola I, Mendoza A, Khanna C, Birrer MJ. Inhibition of AP-1 transcriptional activity blocks the migration, invasion, and experimental metastasis of murine osteosarcoma. Am J Pathol 2009; 174: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta 2015; 444: 182–92. [DOI] [PubMed] [Google Scholar]
- 56.Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS, Helman E, Taylor-Weiner A, McKenna A, DeLuca DS, Lawrence MS, Ambrogio L, Sougnez C, Sivachenko A, Walensky LD, Wagle N, Mora J, de Torres C, Lavarino C, Dos Santos Aguiar S, Yunes JA, Brandalise SR, Mercado-Celis GE, Melendez-Zajgla J, Cárdenas-Cardós R, Velasco-Hidalgo L, Roberts CW, Garraway LA, Rodriguez-Galindo C, Gabriel SB, Lander ES, Golub TR, Orkin SH, Getz G, Janeway KA. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci USA 2014; 111: E5564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, Manlove LA, LaRue RS, Temiz NA, Molyneux SD, Choi K, Holly KJ, Sarver AL, Scott MC, Forster CL, Modiano JF, Khanna C, Hewitt SM, Khokha R, Yang Y, Gorlick R, Dyer MA, Largaespada DA. A sleeping beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet 2015; 47: 615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuijjer ML, van den Akker BE, Hilhorst R, Mommersteeg M, Buddingh EP, Serra M, Bürger H, Hogendoorn PC, Cleton-Jansen AM. Kinome and mRNA expression profiling of high-grade osteosarcoma cell lines implies Akt signaling as possible target for therapy. BMC Med Genomics 2014; 7: 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao S, Lu N, Chai Y, Yu X. Rapamycin inhibits tumor growth of human osteosarcomas. J BUON 2015; 20: 588–94. [PubMed] [Google Scholar]
- 60.Gupte A, Baker EK, Wan SS, Stewart E, Loh A, Shelat AA, Gould CM, Chalk AM, Taylor S, Lackovic K, Karlström Å, Mutsaers AJ, Desai J, Madhamshettiwar PB, Zannettino AC, Burns C, Huang DC, Dyer MA, Simpson KJ, Walkley CR. Systematic screening identifies dual PI3K and mTOR inhibition as a conserved therapeutic vulnerability in osteosarcoma. Clin Cancer Res 2015; 21: 3216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pignochino Y, Dell'Aglio C, Basiricò M, Capozzi F, Soster M, Marchiò S, Bruno S, Gammaitoni L, Sangiolo D, Torchiaro E, D'Ambrosio L, Fagioli F, Ferrari S, Alberghini M, Picci P, Aglietta M, Grignani G. The combination of sorafenib and everolimus abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res 2013; 19: 2117–31. [DOI] [PubMed] [Google Scholar]
- 62.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, Capozzi F, Biagini R, Gambarotti M, Fagioli F, Casali PG, Picci P, Ferrari S, Aglietta M. Italian Sarcoma Group. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol 2015; 16: 98–107. [DOI] [PubMed] [Google Scholar]
- 63.Hughes DP. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res 2009; 152: 479–96. [DOI] [PubMed] [Google Scholar]
- 64.Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet 2009; 18: 1464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer 2009; 100: 1957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, Gannon F, Lee BH. Notch activation as a driver of osteogenic sarcoma. Cancer Cell 2014; 26: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McManus MM, Weiss KR, Hughes DP. Understanding the role of Notch in osteosarcoma. Adv Exp Med Biol 2014; 804: 67–92. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen A, Scott MA, Dry SM, James AW. Roles of bone morphogenetic protein signaling in osteosarcoma. Int Orthop 2014; 38: 2313–22. [DOI] [PubMed] [Google Scholar]
- 69.Gobbi G, Sangiorgi L, Lenzi L, Casadei R, Canaider S, Strippoli P, Lucarelli E, Ghedini I, Donati D, Fabbri N, Warzecha J, Yeoung C, Helman LJ, Picci P, Carinci P. Seven BMPs and all their receptors are simultaneously expressed in osteosarcoma cells. Int J Oncol 2002; 20: 143–7. [PubMed] [Google Scholar]
- 70.Yoshikawa H, Takaoka K, Hamada H, Ono K. Clinical significance of bone morphogenetic activity in osteosarcoma. A study of 20 cases. Cancer 1985; 56: 1682–7. [DOI] [PubMed] [Google Scholar]
- 71.Yoshikawa H, Takaoka K, Masuhara K, Ono K, Sakamoto Y. Prognostic significance of bone morphogenetic activity in osteosarcoma tissue. Cancer 1988; 61: 569–73. [DOI] [PubMed] [Google Scholar]
- 72.Guo W, Gorlick R, Ladanyi M, Meyers PA, Huvos AG, Bertino JR, Healey JH. Expression of bone morphogenetic proteins and receptors in sarcomas. Clin Orthop Relat Res 1999; 365: 175–83. [DOI] [PubMed] [Google Scholar]
- 73.Mohseny AB, Cai Y, Kuijjer M, Xiao W, van den Akker B, de Andrea CE, Jacobs R, ten Dijke P, Hogendoorn PC, Cleton-Jansen AM. The activities of Smad and Gli mediated signalling pathways in high-grade conventional osteosarcoma. Eur J Cancer 2012; 48: 3429–38. [DOI] [PubMed] [Google Scholar]
- 74.Spyropoulou A, Karamesinis K, Basdra EK. Mechanotransduction pathways in bone pathobiology. Biochim Biophys Acta 2015; 1852: 1700–8. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Pasapera AM, Koretsky AP, Waterman CM. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc Natl Acad Sci USA 2013; 110: E2352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng L, Zhang D, Zhang Y, Wen Y, Wang Y. mTOR signal transduction pathways contribute to TN-C FNIII A1 overexpression by mechanical stress in osteosarcoma cells. Mol Cells 2014; 37: 118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Man Y, Ding Y, Ma B, Qiu X, Fan Q, Zheng L. Mechanical strain and growth factors regulate expression of tenascin-C by OS cells additively. Oncol Res 2013; 20: 509–16. [DOI] [PubMed] [Google Scholar]
- 78.Lien SC, Chang SF, Lee PL, Wei SY, Chang MD, Chang JY, Chiu JJ. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta 2013; 1833: 3124–33. [DOI] [PubMed] [Google Scholar]
- 79.Hsu HJ, Lee CF, Locke A, Vanderzyl SQ, Kaunas R. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One 2010; 5: e12470–e12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu N, Zhou H, Xiao Z. Downregulation of PKD1 by shRNA results in defective osteogenic differentiation via cAMP/PKA pathway in human MG-63 cells. J Cell Biochem 2012; 113: 967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalagiorgou G, Piperi C, Georgopoulou U, Adamopoulos C, Basdra EK, Papavassiliou AG. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol Life Sci 2013; 70: 167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gargalionis AN, Korkolopoulou P, Farmaki E, Piperi C, Dalagiorgou G, Adamopoulos C, Levidou G, Saetta A, Fragkou P, Tsioli P, Kiaris H, Zizi-Serbetzoglou A, Karavokyros I, Papavassiliou KA, Tsavaris N, Patsouris E, Basdra EK, Papavassiliou AG. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int J Cancer 2015; 136: 1515–27. [DOI] [PubMed] [Google Scholar]
- 83.Gargalionis AN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Polycystins: mechanosensors with diagnostic and prognostic potential in cancer. Trends Mol Med 2016; 22: 7–9. [DOI] [PubMed] [Google Scholar]
- 84.Huang YW, Chang SJ, I-Chen Harn H, Huang HT, Lin HH, Shen MR, Tang MJ, Chiu WT. Mechanosensitive store-operated calcium entry regulates the formation of cell polarity. J Cell Physiol 2015; 230: 2086–97. [DOI] [PubMed] [Google Scholar]
- 85.Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med 2014; 17: 301–7. [PubMed] [Google Scholar]
- 86.Li B, Ye Z. Epigenetic alterations in osteosarcoma: promising targets. Mol Biol Rep 2014; 41: 3303–15. [DOI] [PubMed] [Google Scholar]
- 87.Uehara T, Fujiwara T, Takeda K, Kunisada T, Ozaki T, Udono H. Immunotherapy for Bone and Soft Tissue Sarcomas. Biomed Res Int 2015; 2015: 820813–820813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–22. [DOI] [PubMed] [Google Scholar]
- 90.Lussier DM, O'Neill L, Nieves LM, McAfee MS, Holechek SA, Collins AW, Dickman P, Jacobsen J, Hingorani P, Blattman JN. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother 2015; 38: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]