Abstract
Cells with DNA damage undergo apoptosis or cellular senescence if the damage cannot be repaired. Recent studies highlight that cellular senescence plays a major role in aging. However, age-associated diseases, including emphysema and neurodegenerative disorders, are caused by apoptosis of lung alveolar epithelial cells and neurons, respectively. Therefore, enhanced apoptosis also promotes aging and shortens the life span depending on the cell type. Recently, we reported that ku70−/−bax−/− and ku70−/−bax+/− mice showed significantly extended life span in comparison with ku70−/−bax+/+ mice. Ku70 is essential for non-homologous end joining pathway for DNA double strand break repair, and Bax plays an important role in apoptosis. Our study suggests that Bax-induced apoptosis has a significant impact on shortening the life span of ku70−/− mice, which are defective in one of DNA repair pathways. The lung alveolar space gradually enlarges during aging, both in mouse and human, and this age-dependent change results in the decrease of respiration capacity during aging that can lead to emphysema in more severe cases. We found that emphysema occurred in ku70−/− mice at the age of three-months old, and that Bax deficiency was able to suppress it. These results suggest that Bax-mediated apoptosis induces emphysema in ku70−/− mice. We also found that the number of cells, including bronchiolar epithelial cells and type 2 alveolar epithelial cells, shows a higher DNA double strand break damage response in ku70 KO mouse lung than in wild type. Recent studies suggest that non-homologous end joining activity decreases with increased age in mouse and rat model. Together, we hypothesize that the decline of Ku70-dependent DNA repair activity in lung alveolar epithelial cells is one of the causes of age-dependent decline of lung function resulting from excess Bax-mediated apoptosis of lung alveolar epithelial cells (and their progenitor cells).
Keywords: Bax, Ku70, apoptosis, aging, life span, DNA damage, DNA repair, non-homologous end joining, emphysema, lung alveolar epithelial cells
Introduction
There are many causes of death of aged animals including cancer, diabetes, neuronal disorders, infectious diseases, and other life-threatening diseases. We speculate that there are common age-dependent progressive changes in animal body that makes ageing as a risk factor for these different types of age-associated life-threatening diseases. As explained below, lung alveolar space is known to enlarge gradually according to age, probably due to the imbalance of cell death and cell division of lung alveolar epithelial cells. In this mini review, we will discuss the role of apoptosis in age-associated diseases, by focusing on Bax-induced apoptosis in emphysema. Our discussion is based on our recent study using mouse genetics as explained in detail below.
Ku70 knockout (KO) mouse and Ku70-Bax double KO mouse
Bax is a 21 kDa protein that is known to play an essential role in programmed cell death (PCD) or apoptosis.1,2 Bax belongs to the Bcl-2 family of proteins, which are an evolutionarily conserved family of proteins controlling cell death.1,2 Bax resides in the cytosol in healthy cells, but Bax translocates from the cytosol to mitochondria in response to apoptotic stresses (including DNA damage, protein misfolding, ER stresses, etc.)3 and Bax triggers cytochrome c release from the mitochondria, which in turn activates the caspase cascade, inducing cell death.2 Ku70 is a 70 kDa protein that plays an essential role in the non-homologous end joining (NHEJ) pathway of DNA damage repair.4
Previously we found that Ku70 has anti-apoptotic activity through the suppression of mitochondrial translocation of Bax.5–7 Numerous studies also confirmed Ku70’s anti-apoptotic activity as a Bax inhibitor in various types of cells.6–20 In addition, it has been shown that Ku70-deficient cells are sensitive to apoptotic stresses that are not limited to DNA damaging stresses,5,7,21 supporting the hypothesis that Ku70 has anti-apoptotic activity independent from its previously known DNA damage repair activity. Furthermore, the Bax inhibiting peptide (BIP) designed from the Bax-binding domain of Ku705,22 has been used to rescue damaged cells from Bax-mediated cell death in cell culture6,23–26 as well as in animal models.27–30 Taken together, these studies suggest that Ku70 has a biological activity as an inhibitor of Bax in addition to its well-known role in DNA repair.
To further examine the physiological significance of Ku70-dependent inhibition of Bax-induced cell death, we generated ku70−/−bax−/− and ku70−/−bax+/− mice and compared their phenotype with Ku70 single KO (ku70−/−) mice, which are known to have an accelerated aging phenotype.31 We speculated that accelerated aging of ku70−/− mice might be, at least in part, due to the increased Bax-induced apoptosis because of the absence of Ku70’s inhibition against Bax. After 10 years of effort to develop mouse colonies and analyze the life span of these mutant mice, we found that Bax deficiency was able to extend the life span of Ku70 KO mice (median life span of ku70−/−, ku70−/−bax−/−, and ku70−/−bax+/− were 26 (n=55), 37.5 (n = 46, P < 0.001), and 38 (n = 23, P < 0.01) weeks, respectively), suggesting that Bax-mediated apoptosis plays a role in inducing premature death in ku70−/− mice.32 This result supports the hypothesis that the absence of Ku70 and the lack of its Bax inhibitory function may lead to Bax hyperactivation, which accelerates the development of age-associated diseases that shorten the life span of ku70−/− mice. In addition, the increased accumulation of DNA damage due to the absence of Ku70 can trigger the DNA damage response to indirectly initiate apoptosis through p53-dependent Bax activation. We suspect that both of these mechanisms of Bax activation are contributing to the premature death observed in ku70−/− mice. Although the restoration of the abnormal aging phenotype in ku70−/− mice by Bax deficiency does not fully prove the role of Ku70 as a Bax inhibitor (since other mechanisms can explain this phenotype), the fact that Bax deficiency was able to extend the life span of Ku70 KO mice implies very important roles for Ku70 and Bax in the development of age-associated life-threatening diseases. In this article, we will discuss the previously unrecognized role of Ku70 and Bax to regulate the progression of age-dependent enlargement of lung alveolar space that causes the decrease of respiration activity of aged animals.33–36
Cell death or cellular senescence?
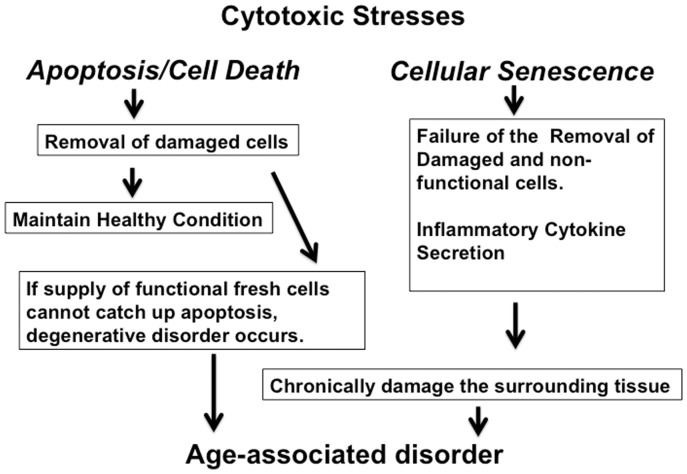
Cell death and cellular senescence are two major responses to irreparable DNA damage, and these responses prevent the proliferation of mutated cells. Since apoptosis removes unwanted damaged cells (including cells with potentially cancerous mutations), apoptosis is considered to be beneficial for longevity.37 On the other hand, the presence of senescent cells is deleterious to surrounding cells since senescent cells secrete inflammatory cytokines that induce chronic inflammation and lead to other deleterious local tissue changes such as fibrosis.38 Therefore, cellular senescence, rather than apoptosis, is considered to be the causative cellular event that induces organismal aging. In fact, a recent study showed that removal of senescent cells by genetic engineering was able to extend the life span of mice.39 However, our evidence demonstrates that age-dependent degenerative diseases occur in part due to apoptosis of essential cells. Thus, apoptosis can have both positive and negative impacts on longevity (Figure 1).
Figure 1.
Roles of apoptosis and cellular senescence in aging
A previous study showed that the deletion of the DNA damage response gene cdkn1a was able to extend the lifespan of mice with dysfunctional telomeres,40 which suggests that DNA damage responses, including apoptosis and cellular senescence, have significant influences on the life span determination of mutant mice with genomic instability. Importantly, Maslov et al.41 reported that remaining permanent DNA damages or significantly increased mutation rates were not detected in mutant mice with defects in DNA repair that show abnormal aging phenotype. This study further suggests that the DNA damage response, rather than DNA damage (or mutations) itself, may play an important role in aging-associated diseases that are accelerated in DNA repair defective mice.41 Cellular senescence seems to have a certain role in the induction of age-associated diseases in defective DNA repair mutant mice.42 However, the suppression of cellular senescence by p21 knockdown in ku80 null mice43 or the removal of senescent cells in mice with dysfunctional telomeres44 did not result in a significant life span extension in mice, although there was a delay in the development of age-associated disorders.44 Very recently, approximately 20% life span extension of wild type mouse (i.e. DNA repair pathways and telomere lengths are normal) was achieved by the removal of senescent cells in mice using genetic engineering which induces apoptosis in senescent cells,39 supporting the hypothesis that cellular senescence plays an important role in the progression of aging and age-associated life-threatening diseases.
Based on our recent findings showing that Bax deficiency extended the life span of ku70−/− mice, we propose that Bax-mediated apoptosis has a significant impact on survival and life span determination in mice with defective DNA repair. Previous studies have shown that ku70−/−, ku80−/− and ku70−/−, ku80−/− mice exhibit similar abnormal aging phenotypes, including shortened life spans. Since Ku70 protein levels become very low in ku80−/− cells,31,45 ku80−/− mice are expected to be similar to ku70−/− mice and have an increased DNA damage response as well as a lower threshold to initiate Bax-mediated apoptosis. Therefore, we speculate that Bax-induced apoptosis has a significant role to shorten the life span of ku80 KO mice as well. Cellular senescence is known to be induced by two major pathways, p53- or retinoblastoma (RB) protein-dependent cell cycle arrest pathways.46 In general, DNA damage triggers p53-dependent cellular response, i.e. apoptosis or cellular senescence, if DNA damage cannot be repaired.46 The main mediators of p53-dependent DNA damage response are Bax (apoptosis) and p21 (cell cycle arrest and cellular senescence), respectively.46 Interestingly, the deletion of p21 was not able to extend the survival of ku80−/− mice, despite the significant decrease of cellular senescence was confirmed by cultured fibroblast experiments.43 This previous study suggests that p21-dependent cellular senescence may not cause life span shortening, though p21-independent cellular senescence may still induce aging-associated dysfunction in ku80−/− and p21−/− mice. Importantly, our recent study shows that Bax deficiency is able to extend the survival and life span of ku70−/− mice. Previous studies showed that cell death inhibition of DNA-damaged cells increases the emergence of senescent cells.38 If this is the case, it is expected that ku70-bax double KO mice would have more cellular senescence than ku70 single KO mice because cell death is suppressed by bax deletion. If cellular senescence was the cause of a shortened life span in mice rather than cell death, bax deletion would be predicted to shorten the life span of ku70−/− mouse by increasing cellular senescence. The result, however, was the opposite. Overall, our results suggest that promoting cell survival by the inhibition of Bax may have a greater beneficial impact on extending the survival period of prematurely aging animals despite the potential risk of increased cellular senescence by the inhibition apoptosis.
Ku70 KO mice showed age-associated changes much earlier than wild type mice, such as kyphosis and alopecia.31 Interestingly, Bax deficiency slowed down these phenotypes in ku70−/− mice.32 This result suggests that the absence of Bax-induced apoptosis can slow down the progression of organismal aging in ku70−/− mice. Ku70 KO mice lost subcutaneous fat at the age of six-months old, whereas Bax-deficient ku70 KO mice retained this fat layer.32 The maintenance of this fat tissue in Bax-deficient ku70 KO mice suggests that Bax deficiency improved overall health condition of ku70−/− mice as discussed in detail in our recent report.32
How does Bax deficiency extend the life span in ku70 KO mice?
If Bax-induced apoptosis enhances aging in Ku70 KO mice, the next important issue is to understand the cell types that are the targets of excess Bax-induced apoptosis. Ku70 KO mice have immune deficiency and this defect is likely to be one of the causes of their shortened life span.31 However, Bax deletion does not improve this immune deficiency because Bax deletion cannot overcome the decreased lymphocyte development due to the absence of NHEJ DNA repair pathway that is required for T-cell receptor maturation and IgG gene arrangement in B-cells.32 Therefore, an improvement of immune function is not likely the reason for life span extension in this case.
Ku70 KO mice have a defect in brain development due to increased neuronal cell death during embryogenesis,47 and we confirmed that the brain weight of ku70−/− mice was approximately 50% of wild type mice at three months of age.32 We also found that complete Bax deletion (i.e. ku70−/−bax−/− mice) was able to suppress excessive neuronal apoptosis in ku70−/− mice, and brain weight was significantly restored similar to wild type.32 Therefore, restoration of brain function may be one of the reasons why ku70−/−bax−/− mice have longer life span than ku70−/−. However, there seems to be another potentially more important reason why Bax deficiency was able to extend the life span of ku70 KO mice. To be noted, ku70−/−bax+/− mice as well as ku70−/−bax−/− mice showed the similar extension of survival compared to ku70−/− mice.32 Interestingly, brain weights of ku70−/−bax+/− mice were similar to those of ku70−/− mice and the number of survived neurons in the brain were also similar to that of ku70−/− mice.32 Probably, the remaining Bax protein (bax+/− cells express approximately 50% Bax protein in comparison with wild type (bax+/+) cells32) induced excess neuronal cell death in ku70−/− mouse. These results imply that there are reasons other than suppression of neuronal apoptosis that help ku70−/−bax+/− mice live longer than ku70−/− mice. As explained in the next paragraph, we found that the lung structure was damaged progressively during aging in ku70−/− mice, and this abnormality was commonly restored in ku70−/−bax−/− and ku70−/−bax+/− mice. This observation suggests that the lung is another important target organ (maybe more important than the brain) affected by Bax-induced cell death to shorten the life span of ku70−/− mice.
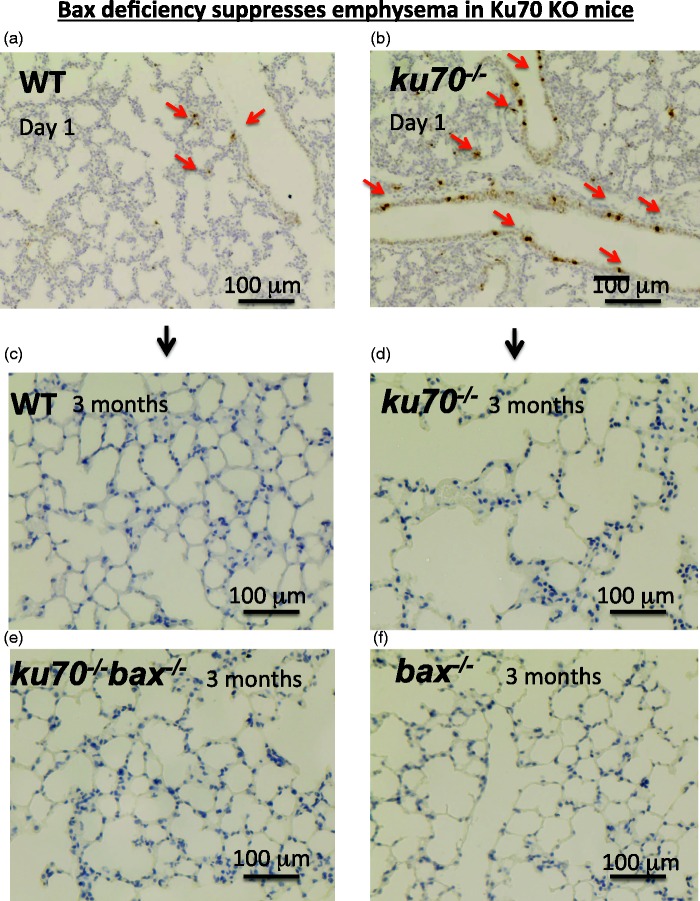
As reported in our recent study,32 we found that ku70−/− mice showed accelerated age-dependent enlargement of lung alveolar spaces, known as emphysema (example histology are shown in Figure 2). As shown in Figure 2, the lung alveolar sizes and structures of wild type and ku70−/− mice are similar to each other at an early age. However, the lung alveolar size of ku70−/− mice became significantly larger than wild type mice with age. These histological results suggest that the expansion of alveolar space of ku70−/− mice is an age-dependent process of progressive alveolar degeneration, rather than defects in the lung development. Emphysema and the consequential chronic obstructive pulmonary disease (COPD) lead to declines in respiratory activity48 and triggers secondary life-threatening diseases, such as pulmonary artery hypertension and heart failure.49 Importantly, Bax deficiency (both bax−/− and bax+/−) was able to suppress the incidence of emphysema in ku70−/− mice (Figure 2).32 These observations suggest that Bax-mediated cell death induces emphysema in ku70−/− mice. The age-dependent alveolar space enlargement is known to occur in mice (including wild type mice) and humans.33–36 The imbalance of apoptosis and the division of alveolar cells (e.g. epithelial cells, endothelial cells, and fibroblasts) is likely the mechanism of this degenerative disease,50,51but the exact details of this imbalance are not well understood. Among several apoptosis-inducing proteins, our recent finding suggests that Bax plays a major role in age-dependent emphysema. If this is the case, then bax−/− mice is expected to have a decreased incidence or delayed age-dependent emphysema compared to wild type mice. However, age-dependent changes of lung function of bax−/− mice have not been well characterized, and thus future study investigating this issue in detail is clearly necessary to understand the mechanism of age-dependent degeneration of the lung. The bax−/− mice do not show a significantly extended life span in comparison with wild type mice.52 Since global bax gene knockout results in the survival of both essential cells as well as unwanted cells, the beneficial effects of Bax deficiency such as suppression of emphysema may be cancelled by the negative effects caused by the absence of clearance of unwanted cells, including senescent and/or mutated cells. Further study using conditional bax gene knockout in specific cell types in the lung (such as lung alveolar epithelial cells) will be necessary to determine the role of Bax-induced cell death in the age-dependent enlargement of lung alveolar space.
Figure 2.
Ku70-deficient mice show accelerated age-dependent emphysema, and Bax deficiency suppressed emphysema in Ku70-deficient mice. Lung histology of wild type, ku70−/−, bax−/−, and ku70−/−bax−/− mice are shown. Lung structures of newborn wt (a) and ku70−/− (b) mice are similar, indicating that lung development during embryogenesis was not affected by Ku70 deficiency. However, at the age of three-months old, alveolar space of ku70−/− mice (d) became much larger than wt mice (c) showing that Ku70 deficiency causes emphysema. Bax deficiency was able to suppress enlargement of alveolar space (e) suggesting that Bax-induced apoptosis causes emphysema in ku70−/− mice. Bax KO (bax−/−) (f) and wild type mice (c) showed the similar lung alveolar size at the age of three months old. In panel A and B, sections were stained by phospho-H2AX antibody detecting DNA double strand break (DSB) response (Red Arrows, brown staining). Number of cells with DNA DSB damage is higher in ku70−/− lung (b) than wt lung (a). Experiments were performed as previously reported in Ngo et al.32 (A color version of this figure is available in the online journal.)
Alveolar epithelial cells may depend on Ku70-dependent DNA repair pathway
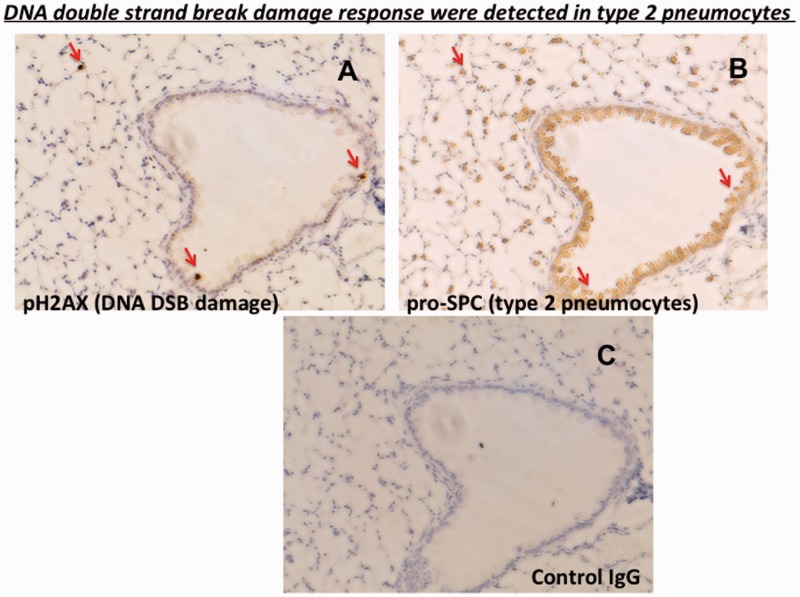
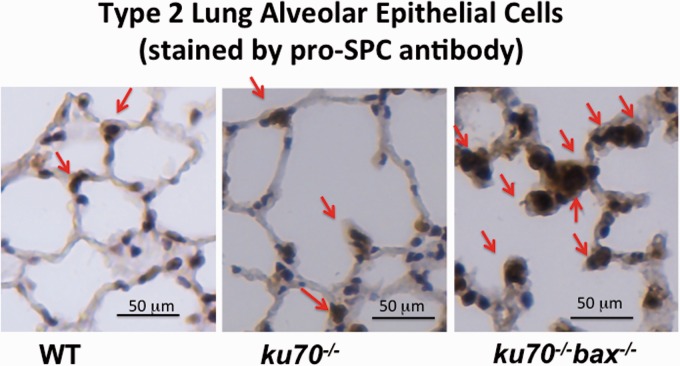
The NHEJ and homologous recombination (HR) pathways are major mechanisms of DNA double strand break (DSB) repair.53 An important distinction among these two pathways is that non-dividing cells such as stem (or progenitor) cells at a quiescent state can only use the NHEJ pathway, and not the HR pathway, since the HR pathway requires cell cycle progression.53 Interestingly, DNA DSB damage responses, such as H2AX phosphorylation, were detected in bronchiolar and alveolar cells in ku70−/− mice at higher frequency than in wild type mice (Figure 2).32 Ku70 is known to be essential for NHEJ, and thus ku70−/− has an apparent defect in NHEJ. Our observations suggest that the NHEJ DNA repair pathway has a significant role in repairing DNA damage in certain cell types in the bronchia and alveoli. As shown in Figure 3, some (but not all) of the phosphorylated H2AX-positive cells (i.e. DNA DSB damage response positive cells) were double stained by pro-SPC, which is the cell surface maker for type 2 alveolar epithelial (AT2) cells, suggesting that at least AT2 cells have a higher dependency on NHEJ to repair DNA DSB. Interestingly, clusters of multiple AT2 cells were often observed in ku70−/−bax−/− mice that were not seen in wild type and ku70−/− mice (Figure 4). These results imply that (1) AT2 cells require Ku70 (and Ku70-dependent DNA DSB repair pathway, i.e. NHEJ pathway) to repair DNA DSB damage and that (2) the absence of Bax-mediated apoptosis allows AT2 cells to survive after DNA damage. Probably, these cells enter cell division to utilize the HR pathway, and that is why clusters of multiple AT2 cells were formed in the lung of ku70−/−bax−/− mice. AT2 cells as well as bronchiolar cells are known to function as progenitor cells, producing type 1 lung alveolar (AT1) cells that maintain the structure of lung alveoli.54–56 Therefore, the increased death of AT2 and bronchiolar cells due to the DNA damage in ku70−/− may cause a shortage of progenitor cells producing AT1 that results in the acceleration of age-dependent enlargement of lung alveoli.
Figure 3.
DNA double strand break (DSB) responses were detected in type 2 alveolar epithelial cells (pneumocytes) and bronchiolar epithelial cells. Serial sections (5 µm sections) of the lung from three-months old ku70−/− mice were stained by antibodies detecting phosphor-H2AX (DNA DSB response marker) and pro-SPC (type 2 alveolar epithelial cell marker). IgG from normal rabbit serum was used as a negative control. Red arrows show pro-SPC-positive lung cells that were also phosphor-H2AX-positive. Experiments were performed as previously reported in Ngo et al.32 (A color version of this figure is available in the online journal.)
Figure 4.
Cell clusters of multiple type 2 alveolar epithelial cells exist in Bax-deficient Ku70 KO mice. Lung sections of wt, ku70−/−, and ku70−/−bax−/− mice were stained by pro-SPC antibody detecting type 2 alveolar epithelial (AT2) cells. AT2 cell does not form multiple cell clusters in wt and ku70−/− mice, but AT2 cell clusters were often detected in ku70−/−bax−/− mice. Experiments were performed as previously reported in Ngo et al.32 (A color version of this figure is available in the online journal.)
Why these lung cells do not use cell cycle-dependent HR pathway to repair DNA DSB instead of choosing to die by Bax-mediated apoptosis is unknown. The HR pathway is more accurate than the NHEJ pathway to repair DNA damage53; therefore, it is surprising that these progenitor cells depend on the error prone NHEJ pathway. One speculation is that these cells may have a strict restriction of cell division even when these cells receive DNA DSB damage, and that is why these cells depend on the NHEJ pathway to repair DNA damage. This restriction of cell division may be a safety mechanism to maintain efficient gas exchange area (lung alveolar space) by limiting cell doubling of alveolar epithelial cells, and to suppress expansion of cancerous mutated cells. In fact, abnormally increased numbers of AT2 cells were detected in some of Bax-deficient ku70−/− mice.32 However, if this is the case, this safety mechanism may be one of the causes of age-dependent alveolar enlargement since NHEJ activity in the lung seems to decline with the progression of age, as we will discuss in the next paragraph, and thus lung progenitor cells may be forced to undergo apoptosis due to the accumulation of DNA damage, rather than enter the cell cycle to use the HR pathway.
Ku70-dependent NHEJ DNA repair activity decreases as the lungs age
Lee et al.57 reported that the NHEJ activity was significantly decreased by aging in rat lung (whole lung homogenates were used to measure the NHEJ activity). Caloric restriction (CR), which is considered to be beneficial for healthy aging, significantly suppressed the decline of NHEJ activity in these lungs, and CR increased the levels of Ku70 in the lung.57 Messenger RNA expression of genes involved in NHEJ (e.g. XRCC4 and XRCC5) has been also reported to be significantly decreased in the lungs of aged mice (24-months old) in comparison with young mice (three-months old),58 suggesting that the decrease in NHEJ activity occurs indeed as a result of aging. Together with our recent findings, we hypothesize that the age-dependent decrease in Ku70-dependent NHEJ activity in the lung (especially in alveolar epithelial progenitor cells such as AT2) is among the major contributors in age-associated emphysema. Notably, a recent study showed that Ku70 protein levels (in total lung homogenate) were decreased by cigarette smoke in mouse lung with emphysema,59 supporting our hypothesis that the decrease of Ku70 expression promotes emphysema.
Conclusion
Our recent study demonstrated that Bax deficiency was able to extend the life span of ku70 null mice.32 To our knowledge, this is the first report showing a significant role of an apoptosis-inducing gene in the induction of premature death in mutant mice with defects in DNA repair. We propose that the pro-apoptotic protein Bax has a significant role in age-dependent diseases, especially emphysema. Our studies investigating the phenotype of ku70−/− mice also revealed that Ku70 is essential in order to maintain normal lung alveoli structures during aging. It is not well understood how the DNA damage response influences the development of age-associated emphysema; therefore, further study is clearly needed to better understand the role of Ku70 in the maintenance of homeostasis in the pulmonary organs.
Authors’ contribution
SM, JP, and JN contributed the writing sentences of this manuscript based on the experimental results produced by themselves as well as by AB, IP-F, KW, and MM. All authors contributed to the development of the proposed hypothesis about the biology of aging and the interpretation of the experimental results.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) Grant R01AG031903 (to SM). The study was also supported in part by the Flow Cytometry and Confocal Microscopy core facilities in the Comprehensive Cancer Center of Case Western Reserve University and University Hospital P30CA43703.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999; 59(7 Suppl): 1693s–700s. [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004; 116: 205–19. [DOI] [PubMed] [Google Scholar]
- 3.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 1997; 139: 1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downs JA, Jackson SP. A means to a DNA end: the many roles of Ku. Nat Rev Mol Cell Biol 2004; 5: 367–78. [DOI] [PubMed] [Google Scholar]
- 5.Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, et al. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Transac 2007; 35(Pt 4): 797–801. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Yokota T, Gama V, Yoshida T, Gomez JA, Ishikawa K, et al. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ 2007; 14: 2058–67. [DOI] [PubMed] [Google Scholar]
- 7.Gama V, Gomez JA, Mayo LD, Jackson MW, Danielpour D, Song K, et al. Hdm2 is a ubiquitin ligase of Ku70-Akt promotes cell survival by inhibiting Hdm2-dependent Ku70 destabilization. Cell Death Differ 2009; 16: 758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 2004; 13: 627–38. [DOI] [PubMed] [Google Scholar]
- 9.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004; 305: 390–2. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A 2005; 102: 4842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plesca D, Mazumder S, Gama V, Matsuyama S, Almasan A. A C-terminal fragment of cyclin E, generated by caspase-mediated cleavage, is degraded in the absence of a recognizable phosphodegron. J Biol Chem 2008; 283: 30796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima K, Muranaka C, Kobayashi J, Sakamoto S, Komatsu K, Matsuura S, et al. NBS1 regulates a novel apoptotic pathway through Bax activation. DNA Repair (Amst) 2008; 7: 1705–16. [DOI] [PubMed] [Google Scholar]
- 13.Anekonda TS, Adamus G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res Notes 2008; 1: 122–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pucci S, Paola M, Fabiola S, David BA, Luigi SG. Interleukin-6 affects cell death escaping mechanisms acting on Bax-Ku70-Clusterin interactions in human colon cancer progression. Cell Cycle 2009; 8: 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008; 28: 6384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trougakos IP, Lourda M, Antonelou MH, Kletsas D, Gorgoulis VG, Papassideri IS, et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin Cancer Res 2009; 15: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Woods NT, Piluso LG, Lee HH, Chen J, Bhalla KN, et al. p53 acetylation is crucial for its transcription-independent proapoptotic functions. J Biol Chem 2009; 284: 11171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vishnudas VK, Miller JB. Ku70 regulates Bax-mediated pathogenesis in laminin-alpha2-deficient human muscle cells and mouse models of congenital muscular dystrophy. Hum Mol Genet 2009; 18: 4467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou H, Volonte D, Galbiati F. Interaction of caveolin-1 with Ku70 inhibits Bax-mediated apoptosis. PloS One 2012; 7: e39379–e39379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JJ, Gu QH, Li M, Yang HP, Cao LM, Hu CP. Role of Ku70 and Bax in epigallocatechin-3-gallate-induced apoptosis of A549 cells in vivo. Oncol Lett 2013; 5: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Kim D, Han JS, Jeong CS, Chung BS, Kang CD, et al. Ku autoantigen affects the susceptibility to anticancer drugs. Cancer research 1999; 59: 4012–7. [PubMed] [Google Scholar]
- 22.Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun 2004; 321: 961–6. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Kobayashi N, Gutierrez AS, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, et al. Prolonged survival of mice with acute liver failure with transplantation of monkey hepatocytes cultured with an antiapoptotic pentapeptide V5. Transplantation 2006; 81: 427–37. [DOI] [PubMed] [Google Scholar]
- 24.Chen YN, Yamada H, Mao W, Matsuyama S, Aihara M, Araie M. Hypoxia-induced retinal ganglion cell death and the neuroprotective effects of beta-adrenergic antagonists. Brain Res 2007; 1148: 28–37. [DOI] [PubMed] [Google Scholar]
- 25.Soto P, Smith LC. BH4 peptide derived from Bcl-xL and Bax-inhibitor peptide suppresses apoptotic mitochondrial changes in heat stressed bovine oocytes. Mol Reprod Dev 2009; 76: 637–46. [DOI] [PubMed] [Google Scholar]
- 26.Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, et al. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Molecular Cell 2012; 46: 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Q, Patil K, Sharma SC. The role of Bax-inhibiting peptide in retinal ganglion cell apoptosis after optic nerve transection. Neurosci Lett 2004; 372: 17–21. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Han W, Du X, Zhu C, Carlsson Y, Mallard C, et al. Neuroprotective effect of Bax-inhibiting peptide on neonatal brain injury. Stroke 2010; 41: 2050–5. [DOI] [PubMed] [Google Scholar]
- 29.Han B, Wang Q, Cui G, Shen X, Zhu Z. Post-treatment of Bax-inhibiting peptide reduces neuronal death and behavioral deficits following global cerebral ischemia. Neurochem Int 2011; 58: 224–33. [DOI] [PubMed] [Google Scholar]
- 30.Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, Lee HG. Inhibition of Bax protects neuronal cells from oligomeric abeta neurotoxicity. Cell Death Dis 2012; 3: e309–e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Vogel H, Holcomb VB, Gu Y, Hasty P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol 2007; 27: 8205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo J, Matsuyama M, Kim C, Poventud-Fuentes I, Bates A, Siedlak SL, et al. Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases. Cell Death Dis 2015; 6: e1706–e1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest 1992; 101: 800–9. [DOI] [PubMed] [Google Scholar]
- 34.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest 1992; 101: 793–9. [DOI] [PubMed] [Google Scholar]
- 35.MacNee W. Aging, inflammation, and emphysema. Am J Respir Crit Care Med 2011; 184: 1327–9. [DOI] [PubMed] [Google Scholar]
- 36.Calvi CL, Podowski M, D'Alessio FR, Metzger SL, Misono K, Poonyagariyagorn H, et al. Critical transition in tissue homeostasis accompanies murine lung senescence. PloS One 2011; 6: e20712–e20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh Y, Lee KA, Kim WH, Han BG, Vijg J, Park SC. Aging alters the apoptotic response to genotoxic stress. Nat Med 2002; 8: 3–4. [DOI] [PubMed] [Google Scholar]
- 38.Campisi J. Aging, cellular senescence, and cancer. Ann Rev Physiol 2013; 75: 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016; 530: 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet 2007; 39: 99–105. [DOI] [PubMed] [Google Scholar]
- 41.Maslov AY, Ganapathi S, Westerhof M, Quispe-Tintaya W, White RR, Van Houten B, et al. DNA damage in normally and prematurely aged mice. Aging Cell 2013; 12: 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peeper DS. Ageing: old cells under attack. Nature 2011; 479: 186–7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao B, Benson EK, Qiao R, Wang X, Kim S, Manfredi JJ, et al. Cellular senescence and organismal ageing in the absence of p21(CIP1/WAF1) in ku80(-/-) mice. EMBO Rep 2009; 10: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koike M, Koike A. The Ku70-binding site of Ku80 is required for the stabilization of Ku70 in the cytoplasm, for the nuclear translocation of Ku80, and for Ku80-dependent DNA repair. Exp Cell Res 2005; 305: 266–76. [DOI] [PubMed] [Google Scholar]
- 46.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol 2012; 13: 579–90. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y, Sekiguchi J, Gao Y, Dikkes P, Frank K, Ferguson D, et al. Defective embryonic neurogenesis in Ku-deficient but not DNA-dependent protein kinase catalytic subunit-deficient mice. Proc Natl Acad Sci U S A 2000; 97: 2668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voelkel NF, Gomez-Arroyo J, Mizuno S. COPD/emphysema: the vascular story. Pulm Circ 2011; 1: 320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 2012; 40: 1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106: 1311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 2012; 122: 2749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci U S A 2007; 104: 5229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012; 47: 497–510. [DOI] [PubMed] [Google Scholar]
- 54.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest 2012; 122: 2724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014; 507: 190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JE, Heo JI, Park SH, Kim JH, Kho YJ, Kang HJ, et al. Calorie restriction (CR) reduces age-dependent decline of non-homologous end joining (NHEJ) activity in rat tissues. Exp Gerontol 2011; 46: 891–6. [DOI] [PubMed] [Google Scholar]
- 58.Zahn JM, Poosala S, Owen AB, Ingram DK, Lustig A, Carter A, et al. AGEMAP: a gene expression database for aging in mice. PLoS Genet 2007; 3: e201–e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao H, Sundar IK, Gorbunova V, Rahman I. P21-PARP-1 pathway is involved in cigarette smoke-induced lung DNA damage and cellular senescence. PloS One 2013; 8: e80007–e80007. [DOI] [PMC free article] [PubMed] [Google Scholar]