Abstract
Histone deacetylase 6 is a multifunctional lysine deacetylase that is recently emerging as a central facilitator of response to stress and may play an important role in cancer cell proliferation. The histone deacetylase 6-inhibitor tubacin has been shown to slow the growth of metastatic prostate cancer cells and sensitize cancer cells to chemotherapeutic agents. However, the proteins histone deacetylase 6 interacts with, and thus its role in cancer cells, remains poorly characterized. Histone deacetylase 6 deacetylase activity has recently been shown to be required for efficient basal autophagic flux. Autophagy is often dysregulated in cancer cells and may confer stress resistance and allow for cell maintenance and a high proliferation rate. Tubacin may therefore slow cancer cell proliferation by decreasing autophagic flux. We characterized the histone deacetylase 6-interacting proteins in LNCaP metastatic prostate cancer cells and found that histone deacetylase 6 interacts with proteins involved in several cellular processes, including autophagy. Based on our interaction screen, we assessed the impact of the histone deacetylase 6-inhibitor tubacin on autophagic flux in two metastatic prostate cancer cell lines and found that tubacin does not influence autophagic flux. Histone deacetylase 6 therefore influences cell proliferation through an autophagy-independent mechanism.
Keywords: Autophagy, metastatic prostate cancer, HDAC6, tubacin
Introduction
Histone deacetylase 6 (HDAC6) is a cytoplasmic lysine deacetylase that has a role in maintaining homeostasis in response to cellular stressors.1 HDAC6 facilitates aggregation and turnover of misfolded proteins and is protective during misfolded-protein stress;2,3 its ubiquitin-binding domain is required for heat shock factor 1 (HSF1) activation and downstream induction of cytoprotective chaperone proteins;4 and its deacetylase activity is required for efficient fusion of autophagosomes and lysosomes for autophagic flux.5 Despite this critical role in homeostasis, how HDAC6 is regulated, the proteins it interacts with, its substrates and cell-type specific functions are only recently coming to light.6–8
The cytoprotective functions of autophagy suggest this process would be important in maintaining cell viability under conditions of heightened stress, such as during rapid proliferation or nutrient limitation. This is particularly relevant to cancer because the tumor microenvironment is an inherently stressful niche characterized by low nutrient concentrations, hypoxia, and damaging reactive oxygen species (ROS). Furthermore, metastatic cancers are likely evolved to require autophagy in response to selective pressures exerted throughout the process of dissemination.9–11 Consequently, autophagy is under investigation as a therapeutic target in cancer cells with the goal of inhibiting its cytoprotective function to slow growth or sensitize cancer cells to established therapeutic agents.12,13
Consistent with its in role in cell maintenance and stress response, HDAC6 inhibition (HDAC6i) or depletion has been shown to slow cancer cell growth and migration and sensitize cancer cells to chemotherapeutic agents.14–16 A cytoprotective role in cancer cells is also supported by associative clinical data showing a tendency toward increased expression of HDAC6 in patient tumors17 (The Cancer Genome Atlas datasets18,19). The HDAC6 inhibitor tubacin20 has been shown to slow growth and sensitize LNCaP metastatic prostate cancer cells to chemotherapeutic agents.14
We hypothesized that growth retardation and a synergistic decrease in cell viability in combination with other therapeutic agents may be a result of decreased autophagic flux in response to HDAC6i. To test this hypothesis, we first characterized the proteins that interact with HDAC6 in LNCaP cells to gain insight into the protein’s role in metastatic prostate cancer cells. We found HDAC6 to interact with proteins associated with autophagy and subsequently tested whether HDAC6i influences autophagic flux21 in two metastatic prostate cancer cell lines.
Materials and methods
Chemicals and reagents
Tubacin (N1-[4-[(2R,4R,6S)-4-[[(4,5-diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N8-hydroxyoctanediamide) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and resuspended in dimethyl sulfoxide (DMSO) (EMD Millipore, Darmstadt, Germany). Ammonium chloride (NH4Cl) (Sigma-Aldrich) was prepared in sterile water. Mitochondrial dye MitoTracker Deep Red FM was purchased from Life Technologies (Carlsbad, CA, USA) and resuspended in DMSO. Primary antibodies for p62 (Santa Cruz Biotechnology), LC3A/B (Cell Signaling Technology, Danvers, MA, USA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology), cytochrome c (CYCS) (Santa Cruz Biotechnology), ubiquitin (Ub) (Santa Cruz Biotechnology), acetylated-α-tubulin (Sigma, St. Louis, MO, USA), α-tubulin (Sigma) and β-actin (Sigma) were used in accordance with the manufacturer’s protocol. HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for Western Blotting detection and AlexaFluor-conjugated secondary antibodies for immunofluorescence/confocal imaging (Life Technologies) were used in accordance with the manufacturer’s protocol.
Cell lines and culture conditions
PC3 and LNCaP prostate cancer cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1640 media with L-glutamine supplemented with fetal bovine serum (FBS, 50 ml FBS/500 ml media) at 37℃ 5% CO2. Cell lines were validated by Idexx Radil (Columbia, MO, USA) on December 24, 2012. Cells were treated under the indicated conditions prior to harvest. Treatment reagents were used at the following concentrations: 8 µM tubacin, 30 mM NH4Cl. DMSO or other appropriate carrier was used as needed for control treatments.
Protein preparation and Western blot analysis
Protein lysates were prepared in radioimmunoprecipitation assay (RIPA) protein lysis buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 20 mM TRIS pH 8.0) supplemented with protease inhibitor cocktail (Thermo Scientific, Waltham, MA, USA). Lysates were cleared of insoluble material by centrifugation (10 min, 13,000 r/min) at 4℃ and quantitated by the DCA Protein Assay (BioRad, Hercules, CA, USA). Equal amounts of protein were separated by SDS-PAGE and blotted to a PVDF membrane (BioRad) using NuPAGE reagents and equipment in accordance with the manufacturer’s protocol (Life Technologies). Membranes were blocked and probed for the indicated proteins following standard protocols. Membranes were stripped and reprobed as needed. Membranes were incubated in SuperSignal West Femto Reagent (Thermo Scientific) and developed on the AlphaInnotech FluorChem 8900 system for visualization (San Jose, CA, USA). Densitometric analyses were performed on the native membrane image using AlphaInnotech FluorChem 8900 software. For each membrane, the relative densitometric value of each replicate for a given probe was normalized to the corresponding relative level of the normalizing protein (β-actin). DMSO (control) was set to 1.
Immunofluorescent staining
Cells were grown and treated on glass coverslips. Mitochondria were stained using MitoTracker Deep Red FM probe following the manufacturer’s protocol. The MitoTracker probe was added at 200 nM during the final 30 min of cell treatment and then cells were prepared for immunostaining. Briefly, cells were rinsed in PBS and fixed/permeabilized in ice-cold 100% methanol for 10 min at −20℃. Cells were placed in blocking buffer (1X PBS, 5% BSA, 0.3% Triton X-100). Cells were probed with primary antibodies in antibody dilution buffer (1X PBS, 1% BSA, 0.3% Triton X-100) overnight at 4℃. After incubation with secondary antibodies cells were rinsed three times in PBS, with the final rinse containing DAPI nuclear stain. Coverslips were mounted on glass slides using ProLong Gold Antifade Reagent (Life Technologies) and allowed to set. Slides were visualized on a Zeiss LSM Meta Confocal Microscope (Oberkochen, Germany) at the Center for Genome Research and Biocomputing (CGRB) at Oregon State University. Colocalization quantification was done using Coloc 2 (Fiji distribution for ImageJ2).22
Quantitative real-time PCR
Total RNA was harvested by TRIzol reagent in accordance with the manufacturer’s protocol (Life Technologies). cDNA was prepared from 1 µg RNA using the SuperScript III kit from Life Technologies. Approximately 50 ng cDNA was amplified by Fast SYBR Green Reagent in accordance with the manufacturer’s protocol (Life Technologies) on a 7900HT Real Time PCR Machine (Applied Biosciences, Life Technologies).
Primers: GAPDH (sense 5′-CGAGATCCCTCCAAAATCAA-3′, antisense 5′-TTCACACCCATGACGAACAT-3′); p62 (sense 5′-CATCGGAGGATCCGAGTGTG-3′, antisense 5′-TTCTTTTCCCTCCGTGCTCC-3′); HDAC6 (sense 5′-CAGCACAGTCTTATGGATGG-3′, antisense 5′-CGGTGGATGGAGAAATAGAG-3′). Relative expression was determined by the Comparative Ct Method ([delta] [delta] Ct = [delta]Ct,sample - [delta]Ct,reference). Graphing was done using GraphPad Prism Software (La Jolla, CA, USA). Graphs depicts mean + SEM for two independent experiments.
HDAC6 immunoprecipitation and liquid chromatography tandem mass spectrometry
LNCaP cell lysate (3 mg) was immunoprecipitated with HDAC6 antibody in 1% Triton X-100, 137 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl pH 8.0 overnight at 4℃. The following day, Protein G agarose (Life Technologies) was added and incubated for an additional 2 h. Immunocomplexes were isolated by low-speed centrifugation and washed twice in 50 mM ammonium bicarbonate. The last wash was removed and immunocomplexes were resuspended in 50 µl 0.5 M urea, 5 mM DTT, 50 mM Tris pH 8.0. Samples were then sonicated in a water bath (2X 1 min), vortexed and spun down. Each sample was digested with 500 ng Trypsin Gold (Promega, Madison, WI, USA) at 37℃.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) was carried out as described previously.23 High-confidence protein identifications were made under the following conditions: Protein Threshold 99.0%, Minimum # of Peptides = 2, Peptide Threshold 5% FDR.
Results
Identification of HDAC6-interacting proteins in LNCaP cells
HDAC6 has been shown to have a significant role in many cellular processes in various cell types.17,24,25 However, the proteins that interact with HDAC6 in metastatic prostate cancer cells have not been fully characterized. To address this issue we characterized the proteins that HDAC6 interacts with in an unbiased manner. We identified 52 high-confidence proteins as the major HDAC6-interacting proteins in LNCaP cells (Table 1). As expected, we identified many well-characterized HDAC6 interacting proteins, including components of the cytoskeletal network26,27 and heat shock proteins.27,28 We also identified more recently reported HDAC6-interacting proteins such as myosin-97 (Table 1).
Table 1.
HDAC6-interacting proteins identified by mass spectrometry
| HDAC6-interacting proteins in LNCaP cells | Accession | EUSC | Assignment |
|---|---|---|---|
| 40S ribosomal protein S15 | 4506687 | 2 | AS (31) |
| 40S ribosomal protein S15a | 14165469 | 2 | AS (31) |
| 40S ribosomal protein S17-like | 312284072 | 3 | AS (31) |
| 40S ribosomal protein S18 | 11968182 | 2 | AS (31) |
| 40S ribosomal protein S3 isoform 1 | 15718687 | 2 | AS (31) |
| 40S ribosomal protein S5 | 13904870 | 2 | AS (31) |
| 40S ribosomal protein S7 | 4506741 | 5 | AS (31) |
| 40S ribosomal protein SA | 59859885 | 2 | AS (31) |
| 60 kDa heat shock protein, mitochondrial | 31542947 | 9 | MP (32) |
| 60S acidic ribosomal protein P2 | 4506671 | 3 | AS (31) |
| 60S ribosomal protein L21 | 18104948 | 2 | AS (31) |
| 60S ribosomal protein L3 isoform a | 4506649 | 2 | AS (31) |
| Actin, cytoplasmic 2 | 316659409 | 7 | AM (5) |
| alpha-enolase isoform 1 | 4503571 | 6 | AS (35) |
| ATP synthase subunit alpha, mitochondrial isoform c | 382546190 | 3 | MP (32) |
| ATP synthase subunit beta, mitochondrial precursor | 32189394 | 6 | MP (32) |
| ATP-dependent RNA helicase DDX3X isoform 2 | 301171467 | 4 | |
| D-3-phosphoglycerate dehydrogenase | 23308577 | 4 | |
| E3 ubiquitin-protein ligase TRIM21 | 15208660 | 4 | |
| Elongation factor 1-alpha 1 | 4503471 | 2 | |
| Elongation factor 2 | 4503483 | 3 | |
| Eukaryotic initiation factor 4A-I isoform 1 | 4503529 | 3 | |
| Fatty acid synthase | 41872631 | 10 | AS (35) |
| FK506-binding protein 15 | 150010552 | 8 | |
| Glyceraldehyde-3-phosphate dehydrogenase isoform 2 | 378404908 | 3 | AS (35) |
| Heat shock 70 kDa protein 1A/1B | 167466173 | 2 | MP (32) |
| Heat shock cognate 71 kDa protein isoform 2 | 24234686 | 4 | H6S (7),AM (33) |
| Heat shock protein HSP 90-beta isoform a | 20149594 | 5 | H6S (28) |
| Heterogeneous nuclear ribonucleoprotein K isoform b | 14165435 | 3 | |
| Heterogeneous nuclear ribonucleoprotein R isoform 1 | 156151394 | 16 | |
| Heterogeneous nuclear ribonucleoprotein U isoform b | 14141161 | 4 | |
| Histone deacetylase 6 | 13128864 | 6 | AM (5) |
| Histone H2B type 1-B | 10800140 | 2 | |
| Keratin, type I cytoskeletal 18 | 40354195 | 5 | |
| Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial precursor | 392494079 | 2 | MP (32) |
| myc box-dependent-interacting protein 1 isoform 1 | 21536400 | 27 | |
| Myosin light polypeptide 6 isoform 1 | 17986258 | 2 | |
| Myosin-10 isoform 1 | 365192532 | 2 | AM (50) |
| Myosin-9 | 12667788 | 5 | H6S (7) |
| Non-POU domain-containing octamer-binding protein isoform 1 | 224028244 | 2 | |
| Nucleolin | 55956788 | 2 | |
| Nucleophosmin isoform 1 | 10835063 | 6 | |
| PRKC apoptosis WT1 regulator protein | 55769533 | 2 | |
| protein-L-isoaspartate(D-aspartate) O-methyltransferase isoform 1 | 226530908 | 7 | |
| Pyruvate kinase isozymes M1/M2 isoform e | 332164779 | 2 | AS (34,35) |
| RNA-binding protein FUS isoform 3 | 283135173 | 3 | |
| Stress-70 protein, mitochondrial precursor | 24234688 | 4 | MP (32) |
| T-complex protein 1 subunit epsilon | 24307939 | 5 | |
| transformation/transcription domain-associated protein isoform 1 | 347360922 | 2 | |
| Tubulin alpha-1B chain | 57013276 | 10 | H6S (26), AM (3) |
| Tubulin beta chain | 29788785 | 20 | H6S (26), AM (3) |
| Tubulin beta-4B chain | 5174735 | 5 | H6S (26), AM (3) |
EUSC: exclusive unique spectrum count; H6S: HDAC6 substrate; AS: autophagy substrate; MP: mitochondrial protein; AM: autophagy mediator.
Thirty of the HDAC6-associated proteins we identified were found to be involved in autophagy. We identified proteins that are characterized macroautophagy substrates and mediators, including mitochondria-associated proteins, ribosomes, actin and tubulin (Table 1).3,29–32 HDAC6 interaction with tubulin was confirmed by co-immunoprecipitation and mitochondrial association was assessed by confocal microscopy (Figure S1).
We also identified proteins associated with chaperone-mediated autophagy. Chaperone-mediated autophagy (CMA) delivers a subset of cytosolic proteins to the lysosome via heat shock cognate 71 kDa protein (hsc70) and the lysosomal receptor LAMP2A.33 Hsc70 has recently been reported as an HDAC6 substrate.7 Our LC-MS/MS screen for HDAC6 interacting proteins also identified hsc70 as a prominent HDAC6-interactor in LNCaP cells (Table 1). In addition to hsc70, we also identified multiple recently characterized CMA substrates involved in regulation of cellular energy production, including α-enolase (ENO1), pyruvate kinase (PKM) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)34,35 (Table 1).
Tubacin does not inhibit autophagy in metastatic prostate cancer cells
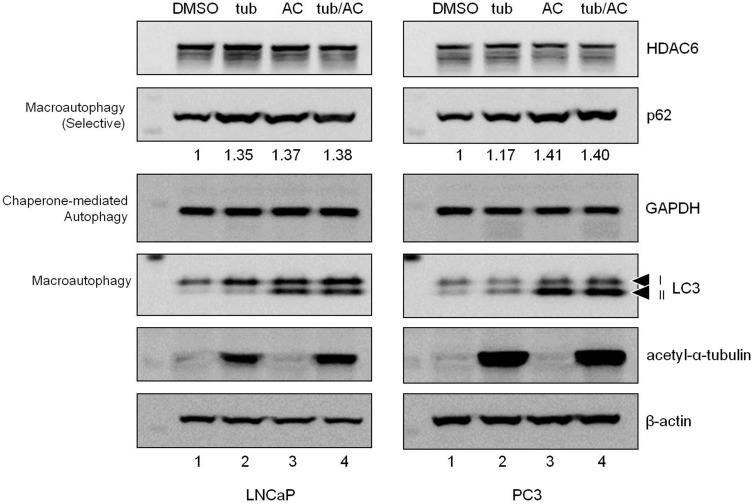
The interaction of HDAC6 with proteins and substrates involved in autophagy in LNCaP cells suggests that tubacin may slow LNCaP growth by interfering with autophagic flux. To test this hypothesis we treated LNCaP and PC3 metastatic prostate cancer cells with tubacin and monitored autophagic flux (Figure 1). Ammonium chloride (AC) was used to assess delivery of substrates to the lysosome. The selective autophagy adaptor protein p62 (sequestosome 1, SQSTM1) was monitored in addition to LC3-II turnover to assess macroautophagy.21,36
Figure 1.
Tubacin does not influence autophagy in metastatic prostate cancer cells. LNCaP and PC3 cells were treated with control (DMSO, Lane 1), tubacin (tub, Lane 2), ammonium chloride (AC, Lane 3), or tubacin and AC (tub/AC, Lane 4) for 24 h prior to harvesting for protein analysis. To assess autophagic flux, membranes were probed for the selective autophagy adaptor and substrate p62, the chaperone mediated autophagy substrate GAPDH, and autophagosome marker and autophagy substrate LC3. One representative experiment from at least three independent experiments is pictured. p62 levels (relative to DMSO control) are indicated. p62 was normalized to the loading control β-actin
Tubacin did not influence overall HDAC6 protein level in either cell-type and AC treatment indicated that HDAC6 is not turned over through the lysosomal pathway (Figure 1). Tubacin did lead to an increase in p62 protein (Figure 1), suggesting impaired p62-selective flux. We did not observe an increase in p62 comparing AC treatment to the tubacin/AC combination treatment (Figure 1), indicating that the increase in p62 observed during tubacin treatment is not the result of an increase in gene expression. However, the p62 response in PC3 cells was not consistent (Figure S2). We observed on several occasions that tubacin enhanced p62 degradation (decrease in p62 protein with no associated decrease in gene expression). When assessing macroautophagy based on LC3-II levels, tubacin did not significantly impair or enhance flux under any treatment conditions (Figure 1 Lane 2 and Figure S2).
Tubacin did not influence CMA in either cell type as determined by GAPDH levels (Figure 1). However, we did not observe accumulation of GAPDH in AC-treated cells, suggesting no significant turnover of GAPDH under these experimental conditions.
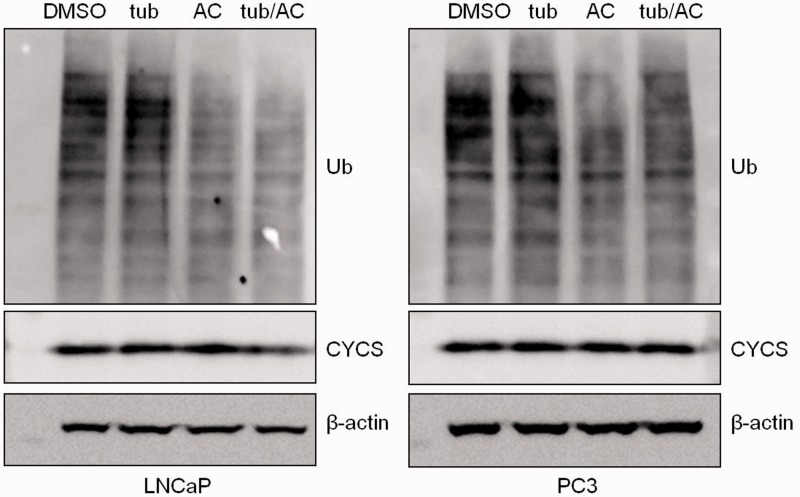
The observed influence of tubacin on p62 levels suggests that although macroautophagy is occurring unimpeded, p62 substrate degradation may be specifically altered. To test this hypothesis we assayed turnover of global poly-ubiquitinated protein levels and mitochondria (Figure 2). Tubacin did not alter global poly-ubiquitinated proteins or mitochondria levels (as assessed by cytochrome c (CYCS)) despite increased p62 levels (Figure 2). No change in mitochondrial levels was also observed in circumstances where tubacin increased p62 degradation (Figure S2).
Figure 2.
Tubacin does not influence global ubiquitinated protein levels or mitochondrial turnover. Known p62 and HDAC6 substrates were assessed to determine whether altered p62 levels influenced substrate degradation. PC3 and LNCaP cells were treated as in Figure 1. Membranes were probed for poly-ubiquitin (Ub) and mitochondrial marker cytochrome c (CYCS). One representative blot from at least three independent experiments is shown (same samples depicted in Figure 1)
Tubacin leads to the appearance of HDAC6 puncta in PC3 cells
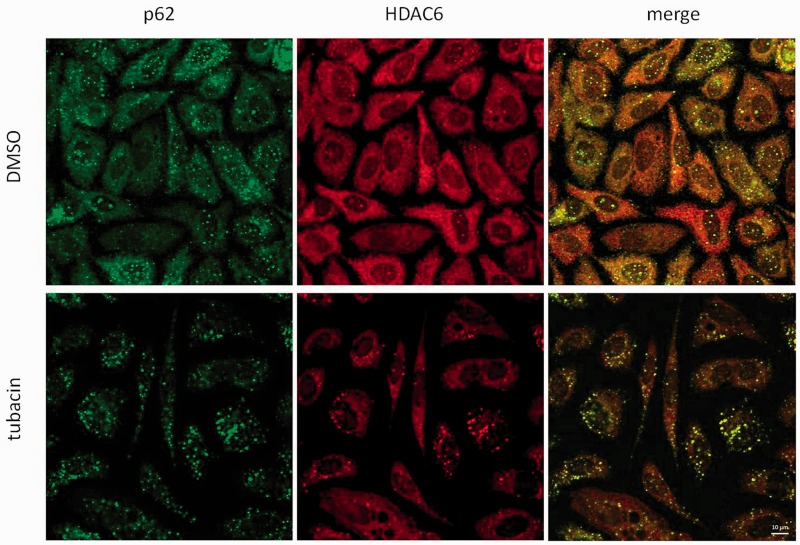
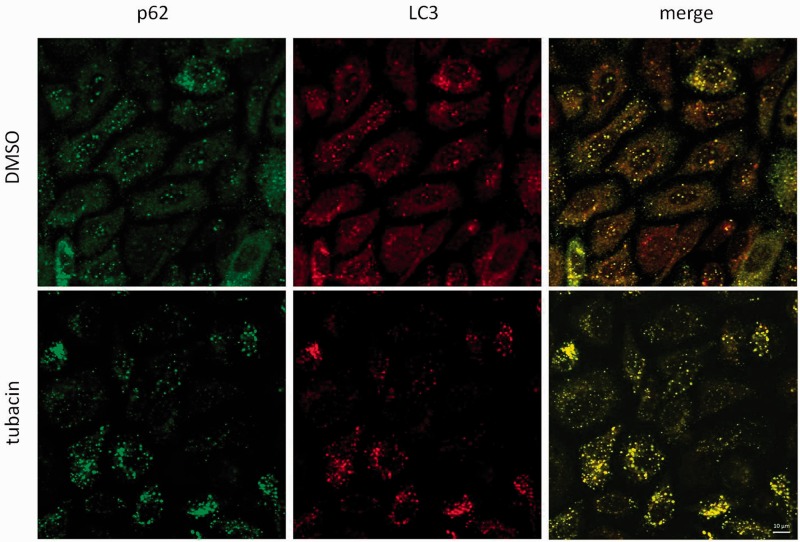
Accumulation or degradation of p62 in response to tubacin suggests direct regulation of p62 stability independent of autophagy. p62 is also a known HDAC6-interacting protein.37 We observed colocalization of p62 and HDAC6 under basal conditions in PC3 cells (Figure 3). Tubacin treatment led to an increase in p62 and HDAC6 puncta (Figure 3). Tubacin also led to an increase in association between p62 and HDAC6 foci: HDAC6-only foci were not observed in tubacin-treated cells, and colocalization analysis noted an increase in association as assessed by Pearson’s R and Spearman’s rank correlation (Figure S3). Analysis of LC3 by immunofluorescence showed an increase in LC3 puncta, indicating an increase in autophagosome formation in response to tubacin (Figure 4). LC3 puncta were also found to colocalize with p62 foci in tubacin-treated cells.
Figure 3.
Tubacin leads to an increase in HDAC6 foci in PC3 cells. PC3 cells were treated with control (DMSO) or tubacin for 24 h prior to processing for HDAC6 (red) and p62 (green) visualization by confocal microscopy. HDAC6 formed cytoplasmic foci in response to tubacin. HDAC6 foci strongly colocalized with p62 foci. Scale bar is 10 µm
Figure 4.
Tubacin leads to the appearance of LC3 puncta in PC3 cells. PC3 cells were treated with control (DMSO) or tubacin for 24 h prior to processing for p62 (green) and LC3 (red) visualization by confocal microscopy. Tubacin led to an increase in LC3 puncta in PC3 cells. LC3 puncta colocalized with p62 foci. Scale bar is 10 µm
Discussion
A number of recent studies have provided evidence supporting the further development of HDAC6 as a cancer therapeutic target. Selective inhibition of HDAC6 deacetylase activity slows cancer cell proliferation and sensitizes cancer cells to chemotherapeutic agents in a preclinical model of leukemia16 and similar results have been found in prostate cancer cells in vitro.14 The observation that HDAC6 knockout mice are viable and are largely normal38 also suggests that systemic HDAC6 inhibition will have limited off target adverse effects, a desirable characteristic of any clinical target. Despite these promising results, how HDAC6i slows prostate cancer cell proliferation is poorly understood.
In this investigation, we characterized the HDAC6-interacting proteins in LNCaP metastatic prostate cancer cells to gain insight into the role of HDAC6 in prostate cancer cells and how HDAC6i slows cancer cell proliferation. We found HDAC6 to interact with many proteins associated with autophagy (Table 1) and subsequently tested the hypothesis that HDAC6i slows proliferation by impairing basal autophagic flux. Our results show that tubacin does not impair macroautophagy in metastatic prostate cancer cells, suggesting flux-inhibition is not responsible for slowed cancer growth associated with tubacin treatment.
Autophagic flux independent of HDAC6 tubulin deacetylase activity was not expected given its known role in mediating efficient autophagosome-lysosome fusion.5 HDAC6 was found to associate with proteins and substrates known to be involved in, or degraded by, autophagy (Table 1, Figure S1 and Figure 3) and the presence of HDAC6 at the site of autophagosomes can also be inferred from complete overlap of both LC3 and HDAC6 puncta with p62 foci in tubacin-treated cells (Figures 3 and 4). Despite this association, HDAC6 activity is dispensable for autophagic flux in metastatic prostate cancer cells. This may suggest a unique role for HDAC6 in prostate cell or prostate cancer cell biology relative to other cell types.
Cancer cells undergo vast changes in metabolism and intracellular signaling pathways during transformation to enable rapid proliferation and enhanced survival.39 Metastatic prostate cancer cells are particularly prone to constitutive activation of the PI3K/AKT/mTOR signaling axis,40,41 a key regulator of autophagy,42 and both LNCaP and PC3 cells lines that were used in our experiments contain mutations in PTEN, a negative regulator of the PI3K signaling axis.43,44 This may suggest that these cell lines, and perhaps metastatic prostate cancers in general, evolve to allow autophagic flux independent of well-established regulators.
In addition to monitoring LC3-II levels, the autophagy adaptor and substrate p62 was assessed to evaluate flux. We unexpectedly found that tubacin influences p62 stability independent of autophagic flux (Figure 1 and Figure S2). HDAC6 and p62 physically interact in mouse embryonic fibroblasts (MEFs) and p62 has been proposed to influence HDAC6 activity.37 We also found a close association between these two proteins in metastatic prostate cancer cells (Figure 3); however, we found p62 levels to be dependent on HDAC6 activity (Figure 1 and Figure S2), suggesting a co-regulatory relationship. The observation that the outcome of HDAC6i on p62 protein level is variable also suggests the involvement of other factors - HDAC6 and p62 are both multifunctional proteins known to interact with an array of other proteins.7,24,45–47 Variable p62 levels may also be a result of overlapping function with other autophagy adaptors and shared substrates (e.g. neighbor of BRCA1 gene 1 (NBR1) or optineurin (OPTN)48,49). Further work will be needed to understand how HDAC6 may regulate p62 stability.
Localization analyses suggested that tubacin stimulates autophagy as assessed by an increase in LC3 puncta that co-localized with p62 (Figure 4). However, we observed no accumulation of LC3-II protein in the flux assay (Figure 1, Lanes 3 and 4), confirming no increase in autophagic activity. Autophagy-independent p62 activity may explain why p62 degradation was observed to be independent of LC3-II level (Figure 1 and Figure S2) and why we did not observe global changes in two p62 substrates (Figure 2). An alternative hypothesis also taking into account the appearance of HDAC6 foci may suggest that tubacin leads to more efficient trafficking across the cytoskeletal network as a result of increased microtubule acetylation.8,50–52 Enhanced trafficking may lead to the accumulation of HDAC6 and p62 at degradation sites, leading to the appearance of discrete foci without significantly affecting overall autophagic vesicle fusion. This would support a model where tubacin does not actively cause HDAC6 re-localization, but instead passively leads to deposition of HDAC6 at sites of protein degradation. This suggests that p62/HDAC6 foci represent either protein bodies that are de-ubiquitinated and degraded,53 or are sites of ubiquitin-independent basal protein turnover.54 It is unlikely that HDAC6 foci represent stress granules or P bodies as HDAC6 activity is required for their formation6 and we did not observe obvious signs of cell stress. Further work will be needed to evaluate these various interpretations.
Our unbiased LC-MS/MS screen for HDAC6-interacting proteins suggested a role for HDAC6 in CMA (Table 1). A test of whether tubacin influenced CMA in metastatic prostate cancer cells using GAPDH as a reporter indicated no major role (Figure 1). However, inhibition of lysosomal degradation by AC also revealed no increase in GAPDH, indicating no significant turnover under our experimental conditions (lysosomal turnover of GAPDH has been detected within 2 h of inhibition of degradation in liver35). This may reflect cell-type specific metabolism and suggests HDAC6 may influence cellular energetics not through altering degradation of metabolic enzymes, but instead through direct regulation. This will be an interesting area of future research in understanding how HDAC6i may slow cancer growth.
In this investigation, we showed that tubacin does not impede basal autophagic flux; however, this does not necessarily mean HDAC6 deacetylase activity is dispensable for flux in other circumstances. HDAC6 is known to have a context-dependent role in autophagic flux,5 suggesting HDAC6 may be required in a stimulus-dependent manner.55 This will be particularly relevant in future work exploring whether HDAC6 has a role in flux following exposure to chemotherapeutic agents.14
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Carmen Wong for providing feedback during manuscript drafting. This work was supported by the National Cancer Institute (CA090890), National Institutes of Health (1S10RR107903-01), National Institute of Environmental Health Sciences (P30 ES000210) and Oregon Agricultural Experimental Station. The authors wish to acknowledge the Confocal Microscopy Facility of the Center for Genome Research and Biocomputing and the Environmental and Health Sciences Center at Oregon State University.
Author contributions
GWW, SW and YF generated the data. GWW, SW, YF, CSM, DEW, RHD, VIP and EH contributed to overall study design, evaluation of data and manuscript preparation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Matthias P, Yoshida M, Khochbin S. HDAC6 a new cellular stress surveillance factor. Cell Cycle 2008; 7: 7–10. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003; 115: 727–38. [DOI] [PubMed] [Google Scholar]
- 3.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 2005; 280: 40282–92. [DOI] [PubMed] [Google Scholar]
- 4.Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc'h C, Matthias P, Khochbin S. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev 2007; 21: 2172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. Embo J 2010 3; 29: 969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev 2007; 21: 3381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Liu S, Liu N, Zhang Y, Liu M, Li D, Seto E, Yao TP, Shui W, Zhou J. Proteomic identification and functional characterization of MYH9, Hsc70, and DNAJA1 as novel substrates of HDAC6 deacetylase activity. Protein Cell 2014; 6: 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci 2007; 27: 3571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell 2008; 19: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, Taichman RS. TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia 2013; 15: 1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol 2009; 22: 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 2011 Sep; 10: 1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12: 401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namdar M, Perez G, Ngo L, Marks PA. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc Natl Acad Sci USA 2010; 107: 20003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, Mandawat A, Atadja P, Bradner JE, Bhalla K. HDAC6 inhibition enhances 17-AAG–mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood 2008; 112: 1886–93. [DOI] [PubMed] [Google Scholar]
- 16.Aldana-Masangkay GI, Rodriguez-Gonzalez A, Lin T, Ikeda AK, Hsieh YT, Kim YM, Lomenick B, Okemoto K, Landaw EM, Wang D, Mazitschek R, Bradner JE, Sakamoto KM. Tubacin suppresses proliferation and induces apoptosis of acute lymphoblastic leukemia cells. Leuk Lymphoma 2011; 52: 1544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol 2010; 2011: 875824–875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signaling 2013; 6: l1–l1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 2003; 100: 4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA Jr., Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4: 151–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods 2012; 9: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson GW, Wickramasekara S, Palomera-Sanchez Z, Black C, Maier CS, Williams DE, Dashwood RH, Ho E. SUV39H1/H3K9me3 attenuates sulforaphane-induced apoptotic signaling in PC3 prostate cancer cells. Oncogenesis 2014; 3: e131–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene 2007; 26: 5468–76. [DOI] [PubMed] [Google Scholar]
- 25.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol 2008; 18: 291–7. [DOI] [PubMed] [Google Scholar]
- 26.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature 2002; 417: 455–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 2007; 27: 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular Cell 2005; 18: 601–7. [DOI] [PubMed] [Google Scholar]
- 29.Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 2014; 15: 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in Parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol 2010; 189: 671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 2008; 112: 1493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 2008; 134: 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res 2013; 24: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H, Zha Z, Liu Y, Li Z, Xu Y, Wang G, Huang Y, Xiong Y, Guan KL, Lei QY. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell 2011; 42: 719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab 2014; 20: 417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140: 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Seibenhener ML, Calderilla-Barbosa L, Diaz-Meco MT, Moscat J, Jiang J, et al. SQSTM1/p62 interacts with HDAC6 and regulates deacetylase activity. PloS One 2013; 8: e76016–e76016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Molecular Cell Biol 2008; 28: 1688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 40.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005; 24: 7455–64. [DOI] [PubMed] [Google Scholar]
- 41.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene 2005; 24: 7465–74. [DOI] [PubMed] [Google Scholar]
- 42.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 2010; 584: 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res 1998; 58: 2720–3. [PubMed] [Google Scholar]
- 44.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1998; 95: 15587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell 2011; 44: 134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009; 137: 1001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci 2012; 37: 230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, Bilusic I, Theurillat JP, Overvatn A, Ishii T, Elazar Z, Komatsu M, Dikic I, Johansen T. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 2009; 33: 505–16. [DOI] [PubMed] [Google Scholar]
- 49.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in Parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA 2014; 111: E4439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 2006; 16: 2166–72. [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Owens GC, Makarenkova H, Edelman DB. HDAC6 regulates mitochondrial transport in hippocampal neurons. PloS One 2010; 5: e10848–e10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J Biol Chem 2010; 285: 11219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao R, Nanduri P, Rao Y, Panichelli RS, Ito A, Yoshida M, Yao TP. Proteasomes activate aggresome disassembly and clearance by producing unanchored ubiquitin chains. Mol Cell 2013; 51: 819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe Y, Tanaka M. p62/SQSTM1 in autophagic clearance of a non-ubiquitylated substrate. J Cell Sci 2011; 124(Pt 16): 2692–701. [DOI] [PubMed] [Google Scholar]
- 55.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010; 40: 280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.