Abstract
Varying degrees of renal injury could lead to different changes in urinary protein composition. We want to find urinary candidate peptide biomarkers in type 2 diabetic patients with different extents of kidney injury. Two sets of patients were recruited. Discovery set: weak cationic-exchange magnetic beads coupled with matrix-assisted laser desorption ionization time-of-flight mass spectrometry were used to profile the low-molecular weight peptidome in urine samples from type 2 diabetes patients with normoalbuminura and microalbuminuria. The differently expressed urinary peptides were screened by ClinProTools2.1 bioinformatics software and identified through nano-liquid chromatography–tandem mass spectrometry. Verification set: the above screened urinary peptides were validated by use matrix-assisted laser desorption ionization time-of-flight mass spectrometry on another group of type 2 diabetes patients with different extents use of kidney injury. In the screening and identification stages, seven urinary peptides were selected as the most promising biomarker candidates, and they were identified as fragments of vitronectin precursor, isoform 1 of fibrinogen alpha chain precursor, prothrombin precursor and inter-alpha-trypsin inhibitor heavy chain H4. The diagnostic efficacy of these urinary peptides was evaluated by area under the receiver operating characteristic curve, and they were 0.767, 0.768, 0.868, 0.910, 0.860, 0.843, and 0.865, respectively. In the verification stage, m/z 1743.9, 2154, 2175.5, and 2184.9 were decreased as albumin-to-creatinine (Alb/Cre) increased and m/z 2231.1, 2430.8, and 2756.1 were elevated as Alb/Cre rose. These small molecule peptides are related to type 2 diabetes kidney damage, and they may play an important role in monitoring type 2 diabetes.
Keywords: Urinary peptides, type 2 diabetes mellitus, diabetes nephropathy, magnetic bead processing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry
Introduction
Diabetes mellitus is a chronic metabolic disease, and its prevalence is increasing dramatically. The number of patients with type 2 diabetes (DM2) will be at least 350 million worldwide by the year 2030.1 Renal injury in patients with DM2 has become the most common cause of end-stage renal disease and a major healthcare problem not only in developed countries but also in developing countries. In clinical practice, diabetes renal injury is diagnosed by renal biopsy or the presence of albuminuria. However, renal biopsy is rarely used for its invasion. Even though microalbuminuria has been used as an increasing risk for developing diabetes nephropathy (DN) for 30 years, more and more recent reports indicate that the changes in microalbuminuria could not predict nephropathy progression based on only a small percentage of patients with microalbuminuria progress to proteinuria and progressive renal functional decline is already present in one-third of patients that progress into microalbuminuria.2–6 Also, glomerular filtration rate reduction can be an indicator for DN but only at a late stage of disease, when success of treatment is severely compromised by the presence of advanced structural damage.7
The peptides in our body fluids are the products of protein synthesis, processing, and degradation. The most widely known peptides biomarkers used in the clinic are brain natriuretic peptide, C-peptide, and collagen N-terminal telopeptides.8,9 Therefore, the peptides in our body fluids carry great potential for disease diagnosis. Studies have also demonstrated urine to be highly enriched with small peptides.10 As a result of the combined action of renal glomeruli filtration and renal tubules resorption, normal urine contains only trace amounts of small molecular weight proteins or peptides. However, it is this phenomenon that reduces the possibility for high abundance proteins to mask potential biomarkers. Once protein is present in the urine, it can indicate that renal injury is not in its early stages. DN is characterized by morphological and ultrastructural changes in the kidney including glomerular basement membrane thickening, mesangial expansion, and tubulointerstitial fibrosis.11 If we fail to detect DN at an early stage, it would ultimately result in proteinuria which mediates the progression of renal failure by its nephrotoxic and proinflammatory actions.12 Therefore, it is possible to speculate that changes in the composition of urinary peptides or lower weight proteins could provide information for early diagnosis of DN. Thus, the characterization of urinary peptides is of great clinical interest and significance.
Applying proteomics to researching the potential urinary biomarkers gives promising early diagnosis of disease. Urine has increasingly emerged as the focus of research for proteomic-based, biomarker evaluation for the ease of collection and pre-analytical handling as well as the stability of proteins or peptides in urine due to the fact that urine stores for hours in the bladder.13,14 The combination of analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and bead-based separation approach make it possible to look for biomarkers of small molecular urinary peptides and can avoid additional manipulation (e.g. tryptic digests).15,16 A major advantage of urinary proteomics analysis is that several hundred polypeptides can be examined simultaneously. Further, the analysis is not biased by the fact that only known polypeptides can be examined due to the need of specific reagents. Every polypeptide that lies within the detection limit of the analysis can be visualized and, subsequently, identified.
In our study, bead-based MALDI-TOF MS was used to screen and identify the potential urinary peptides for early detection and ongoing monitoring of diabetes kidney injury.
Materials and methods
Study population
Firstly, the study protocol was approved by the local ethics committee of Beijing Shijitan Hospital, Capital Medical University, and the experiments were undertaken with the understanding and written informed consent of each subject, according to ethical principles, including the World Medical Association Declaration of Helsinki. Then, patients with DM2 were recruited at Beijing Shijitan Hospital between March 2013 and May 2013. Patients with DM2 were defined through the use of oral glucose-lowering treatment, a fasting plasma glucose >7.0 mmol/L (126 mg/dL) or non-fasting plasma glucose > 11.0 mmol/L (>200 mg/dL).
Our first set, the discovery set, includes 58 DM2 patients. These patients were divided into two groups according to the urinary albumin excretion (UAE): microalbuminuria group (DMM, n = 28) with albumin-to-creatinine (Alb/Cre) ratio between 30 and 300 mg/g; normoalbuminuria group (DMN, n = 30) with Alb/Cre ratio below 30 mg/g. The DMM were those that had renal injury, and the DMN group had none.
Our second set, the verification set, is comprised of 86 DM2 patients who were divided into three groups on the basis of UAE: first group (VMN, n = 52) with Alb/Cre ratio below 30 mg/g; second group (VMM1, n = 26) with Alb/Cre ratio between 30 and 100 mg/g; third group (VMM2, n = 8) with Alb/Cre ratio between 100 and 300 mg/g.
Details of the clinical characteristics of selected subjects are shown in Table 1. The clinical data between different groups were compared by SPSS17.0. P values were two-sided and P < 0.05 was considered statistically significant. The Chi-square test was used for ordinal data (gender). The distributions of continuous data were tested for normality. If distributions were normal, data were compared with independent-samples T test for two groups and ANOVA-one for three groups, otherwise, data were compared with Mann–Whitney test for two groups and nonparametric tests (Kruskal–Wallis test).
Table 1.
Demographics and clinical characteristics of type 2 diabetes mellitus
| Training cohorts |
Validation cohorts |
||||||
|---|---|---|---|---|---|---|---|
| DMN (n = 30) | DMM (n = 28) | P value | VMN (n = 52) | VMM1 (n = 26) | VMM2 (n = 8) | P value | |
| Gender (M/W) | 22/8 | 15/13 | 0.10 | 37/21 | 9/11 | 6/2 | 0.226 |
| Duration (year) | 4.90 ± 4.80 | 11.80 ± 7.10 | 0.006 | 5.83 ± 6.09 | 6.70 ± 3.85 | 9.63 ± 6.48 | 0.01 |
| Age (year) | 53.10 ± 9.90 | 67.40 ± 10.60 | <0.001 | 59.79 ± 12.07 | 66.90 ± 9.71 | 68.75 ± 13.24 | 0.02 |
| FPG (mmol/L) | 9.19 ± 1.82 | 9.40 ± 4.20 | 0.3 | 9.04 ± 2.03 | 8.00 ± 2.21 | 9.22 ± 2.09 | 0.125 |
| HbA1c% | 7.7 ± 1.4 | 7.8 ± 1.5 | 0.8 | 7.61 ± 1.22 | 7.25 ± 0.86 | 7.34 ± 0.70 | 0.416 |
| HbA1c (mmol/mol) | 60 ± 15 | 61 ± 16 | 0.8 | 59.68 ± 13.33 | 55.73 ± 9.39 | 556.69 ± 7.66 | 0.416 |
| CHOL (mmol/L) | 5.46 ± 1.10 | 4.80 ± 0.95 | 0.02 | 5.09 ± 1.14 | 4.89 ± 0.67 | 4.56 ± 1.44 | 0.371 |
| HDL (mmol/L) | 1.38 ± 0.56 | 1.15 ± 0.18 | 0.03 | 2.99 ± 0.94 | 2.78 ± 0.73 | 3.15 ± 0.89 | 0.542 |
| LDL (mmol/L) | 3.41 ± 0.90 | 2.87 ± 0.79 | 0.03 | 1.33 ± 0.42 | 1.15 ± 0.19 | 1.15 ± 0.16 | 0.088 |
| TRIG (mmol/L) | 1.51 ± 0.59 | 2.06 ± 1.43 | 0.2 | 1.50 ± 0.84 | 2.01 ± 1.15 | 2.24 ± 2.01 | 0.061 |
| A/Cr (mg/g) | 11.20 ± 6.80 | 116.7 ± 111.6 | <0.001 | 12.30 ± 6.78 | 57.30 ± 23.15 | 265.17 ± 106.45 | <0.001 |
Data are presented as mean ±SD unless otherwise indicated. Comparisons of clinical data between the two groups were made with SPSS17.0. P values were two-sided and P < 0.05 was considered statistically significant. The Chi-square test was used for ordinal data (gender). The distributions of continuous data were tested for normality. If distributions were normal, data were compared with independent-samples T test and if distributions were not normal, data were compared with Mann–Whitney test. DMN, type 2 diabetes with normoalbuminura; DMM, type 2 diabetes with microalbuminuria; M/W, men/women; FPG, fasting plasma glucose; CHOL, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; TRIG, triglyceride; A/Cr, albumin/creatine in urine.
Urine samples collection and preparation
Midstream clean-catch urine samples were collected from DM2 patients in the morning and tested for the presence of leukocyturia and/or hematuria. Then, urine samples were centrifuged at 400g for 5 min to remove cell debris and casts, and finally supernatants were divided in aliquots and frozen at −80℃.
Fractionation of urine peptides
We use weak cationic-exchange magnetic beads (MB-WCX) to separate and purify urinary peptides through binding, washing, and elution according to the manufactures' instructions (Bruker Daltonics). First, 10 μL MB-WCX and 95 μL MB-WCX binding solution, 10 μL urine sample were added in a polypropylene tube, mixed thoroughly, and incubated for 5 min. The tube was placed on a magnetic bead separator (Bruker Daltonics) for 1 min to make the bead be collected on the tube wall. The supernatant was removed and 100 μL bead washing solution was added. The washing step was repeated for three times. Then, another 10 μL MB-WCX eluting solution was added and the magnetic beads were collected on the tube wall by the separator for 15 min. Finally, the clear supernatant was transferred into a fresh tube, and 5 μL MB-WCX stabilizing solution was added, the well mixed eluate was then stored at −20 ℃.
MALDI-TOF MS anchor chip spotting and data acquisition
The eluate was diluted 1:10 in matrix solution containing α-cyano-4-hydroxycinnamic acid (Bruker Daltonics). Then 1 μL of the resulting mixture was spotted onto the AnchorChip target (Bruker Daltonics), allowed to air dry and ionized by a nitrogen laser (λ = 337 nm) operating at 25 Hz. There were 11 peptides that were used as external standard preparation and the average molecular weight deviation was no more than 100 μg/g. The standard preparation would be calibrated in every eight samples. Then, mass calibration was performed. For each MALDI spot, 400 spectra were acquired in analysis (50 laser shot at 8 different spot positions). And the average of eight spots represented one urine sample.
Data generation process
The peak m/z values or intensities in the mass of 1000–10,000 Da of all signals with a signal-to-noise ratio > 5 were determined. We used ClinProTools2.1 software to normalize spectra (using total ion count), subtract baseline and determine peak m/z values and intensities. To align the spectra, a mass shift of no more than 0.1% was determined. The peak area was used as quantitative standardization.
Peptide sequence
A nano-liquid chromatography-tandem mass spectrometry, which consisted of an Aquity UPLC system (Waters) and a LTQ Obitrap XL mass spectrometer (Thermo Fisher) equipped with a nano-ESI source was used to identify the sequences of differential expression peptides. Firstly the peptide solutions were loaded to a C18 trap column (symmetry® 180 μm × 20 mm × 5 μm, nano Acquity™) with the flow rate of 15 μL/min for 3 min. Then the desalted peptides were analyzed by C18 analytical column (symmetry® 75 μm × 150 μm × 3.5 μm, nano Acquity™) at a flow rate of 400 nL/min. The mobile phases A (5% acetonitrile, 0.1% formic acid, Sigma-Aldrich) and B (95% acetonitrile, 0.1% formic acid) were used for analytical columns. Gradient elution profile was as follows: 5%B–45%B–80%B–80%B–5%B–5%B in 60 min. The MS instrument was operated in a data-dependent model. The range of full scan was 400–2000 m/z with a mass resolution of 100,000 (m/z 400). The 10 most intense monoisotope ions were the precursors for collision induced to two consecutive scans per precursor ion followed by 90 s of dynamic exclusion.
Bioinformatics and identification of urine biomarkers
The obtained spectra were analyzed with BioworksBrowser3.3.1 SP1 (Thermo Fisher) and the resulting mass lists were matched against the IPI Human database (v3.45) using Sequest search. Parameters were set as follows: Delton≥0.1; Charge2+, Xcorr2.0; charge3+, Xcorr2.5; peptide probability<=1e−003; parent ion masses tolerance: 50 ppm; fragment ion masses tolerance: 1 Da; enzyme: no enzyme; variable modification: oxidation of methionine.
Results
Urinary peptidome profiling—first set (discovery set)
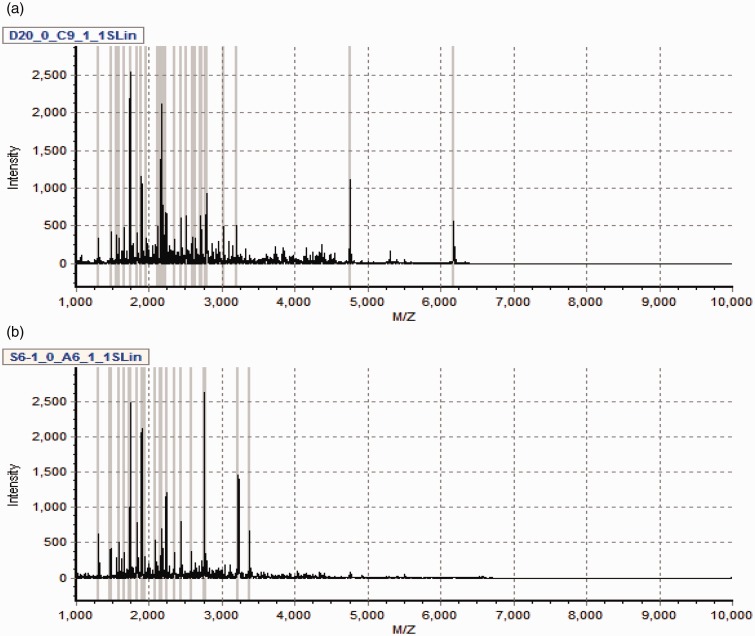
The profile of spectral peaks in the 1000–10,000 Da range was exhibited in the urine samples from 58 volunteers (DMM and DMN). The analysis of MALDI-TOF spectra are shown in Figure 1.
Figure 1.
Typical urinary sample mass spectrum from MALDI-TOF MS after purified by weak cation exchange magnetic beads. (a) From one sample of type 2 diabetic patients with normoalbuminuria. (b) From one sample of type 2 diabetic patients with microalbuminuria. (A color version of this figure is available in the online journal.)
Statistical data analysis of the first set (discovery set)
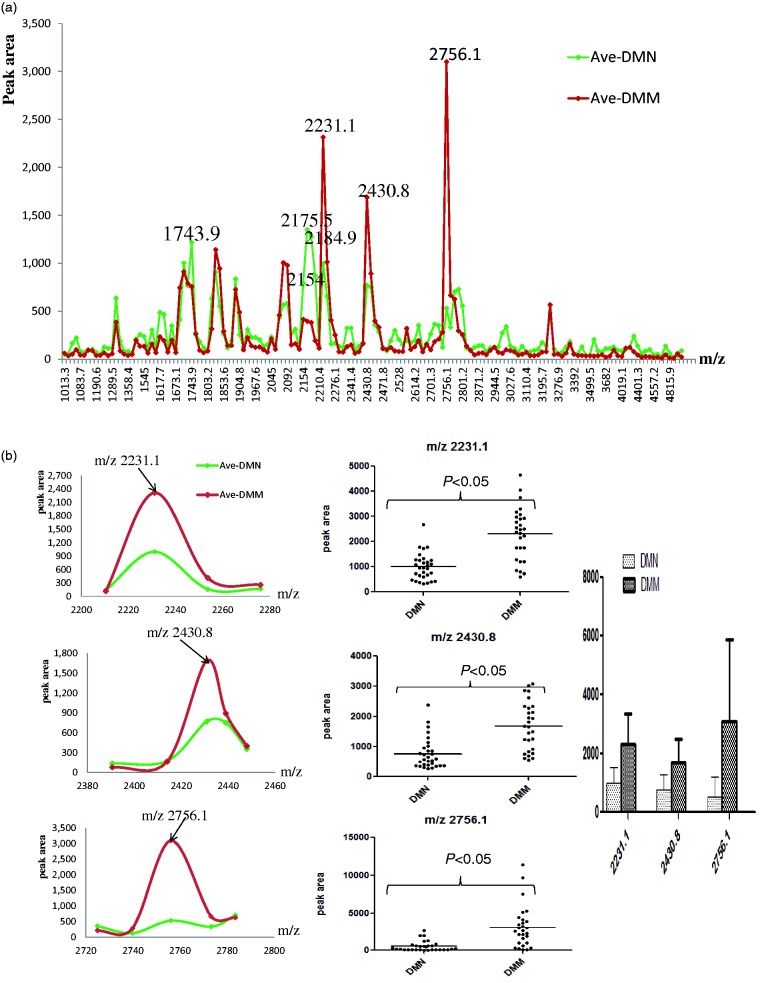
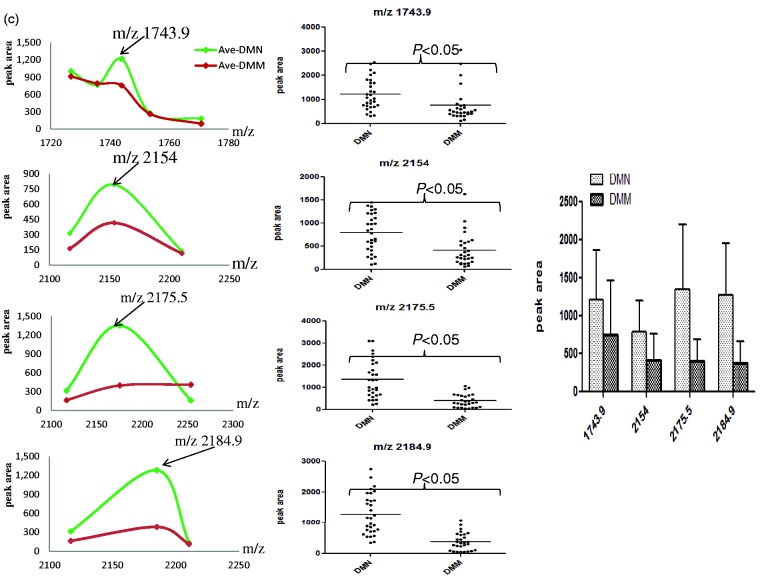
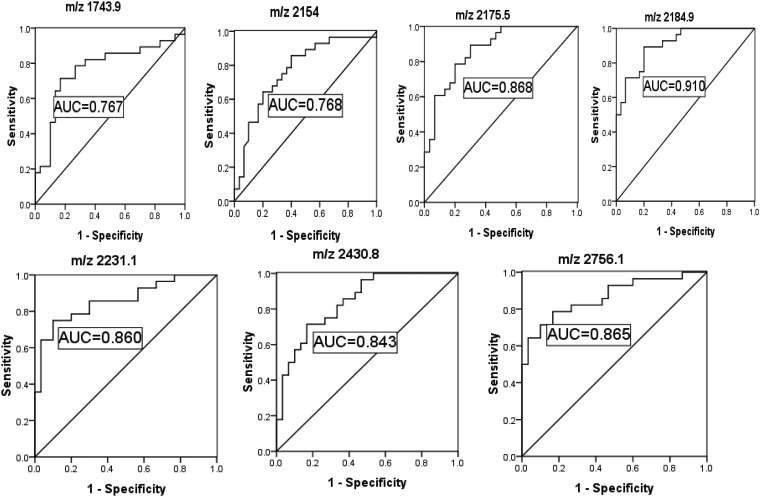
Using ClinprotTools2.1, a total of 156 distinguishable peaks were detected in the 1000–10,000 m/z range, with 109 peaks having differential expression and statistical significance P < 0.05. To avoid bias, we picked seven relative higher peaks for further analysis (Figure 2a) of which three were up-regulated (Figure 2b) and four were down-regulated (Figure 2c) in the DMM groups compared to DMN groups. To evaluate the diagnostic efficacy of these peptides, the receiver operating characteristic curve (ROC) analysis was performed to calculate the sensitivities, specificities, and accuracies at different cut-off points for differentiating DMM patients from control subjects. In the ROC curves, m/z 2184.9 had excellent area under the curve (AUC = 0.910) which indicates a highly accurate diagnostic test, m/z 2231.1, 2430.8, 2756.1, and 2175.5 had limited clinical utility AUC of 0.86, 0.843, 0.865, 0.805, and 0.868, respectively, while m/z 1743.9 and 2154 had an AUC of 0.767 and 0.768 that suggest low diagnostic accuracy (Figure 3).
Figure 2.
The features of the seven selected peaks in two groups of type 2 diabetic patients. (a) The distribution of average peak area from two groups. (b) The average values of three up-regulated peaks in two groups (left and right) and their distribution in all samples (middle). (c) The average value of four decreased peaks in two groups (left and right) and their distribution in all samples (middle). (A color version of this figure is available in the online journal.)
Figure 3.
Receiver operator characteristics (ROC) curves generated with the m/z 1743.9, 2154, 2175.5, 2184.9, and 2231.1, 2430.8, 2756.1 used to distinguish type 2 diabetic patients with microalbuminuria from type 2 diabetic patients with normoalbuminuria. The areas under the curve (AUC) were 0.767, 0.768, 0.868, 0.910, 0.860, 0.843, and 0.865 for the above mentioned peaks, respectively
The second set (verification set)—verification of the discovery set
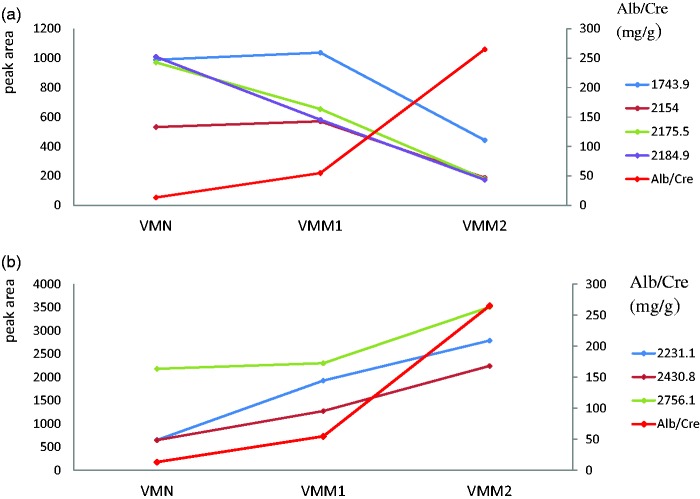
We verified the detected peaks that were generated from the discovery set in another group DM2 patients with different extents of UAE (VMN, VMM1 and VMM2). The three up-regulated peaks were increased with UAE (Figure 4a) and the four down-regulated peaks were decreased with UAE (Figure 4b).
Figure 4.
The changing trend of the detected peaks screened from discovery set with the UAE in verification set. (a) The four down-regulated peaks were decline with UAE in type 2 diabetic patients with different extent of albumin-to-creatinine ratio. (b) The three up-regulated peaks were increased with UAE in type 2 diabetic patients with different extents of albumin-to-creatinine ratio. (A color version of this figure is available in the online journal.)
Identification of the potential urinary peptides for DMM
With this bead-based proteomic technology, we found seven potential biomarkers for DM2 with microalbuminuria. The peptide sequence of the seven differential peaks was identified by a nano-liquid chromatography–tandem mass spectrometry, and the Sequest search reported the protein (shown in Table 2). Unfortunately, m/z 2430.8 was not identified.
Table 2.
Identified peptides sequence of the selected peaks
| m/z | Molecular weight | Amino sequences | Protein name |
|---|---|---|---|
| 2756.1 | 2756.22 | S.SYSKQFTSSTSYNRGDSTFESKSY.K | Isoform 1 of fibrinogen alpha chain precursor |
| 2430.8 | Identification failure | ||
| 2231.1 | 2231.15 | Q.TPVLKPEEEAPAPEVGASKPEG.I | Vitronectin precursor |
| 1743.9 | 1744.67 | K.MADEAGSEADHEGTHST.K | Fibrinogen alpha chain precursor |
| 2154 | 2152.05 | SLEDKTERELLESYIDGR | Prothrombin |
| 2175.5 | 2174.13 | Q.TPVLKPEEEAPAPEVGASKPE.G | Vitronectin precursor |
| 2184.9 | 2181.92 | S.RQLGLPGPPDVPDHAAYHPF.R | Inter-alpha-trypsin inhibitor heavy chain H4 |
Discussion
In this study, we screened and identified seven urinary peptides that were associated with DM2 kidney injury. Compared with blood samples, it is easier and less invasive to collect urine sample (without the requirement of venipuncture) and most importantly, urinary proteome is very stable. The comparative data analysis discovered the peptide pattern of patients with microalbuminuria represented several differences to the normal pattern and the differently expressed peaks had significant statistical difference. The peaks m/z 2231.1, 2430.8, and 2756.1 were found increased and m/z 1743.9, 2154, 2175.5, and 2184.9 decreased in DM2 patients with microalbuminuria when compared with these patients with normoalbuminuria. These urinary peptides are fragments of proteins and they might serve as better markers of diabetes kidney injury because the corresponding proteins, due to their larger size, can only be found in urine once advanced renal damage has occurred. On the other hand, these smaller urinary peptides should pass more easily through glomerular filter and, hence, could serve as early indicators of renal damage.
The standard model holds that the development of proteinuria is the result of changes in glomerular perm selectivity. For urinary albumin, coauthor Comper and his colleagues claim the normally low urinary excretion of immunoreactive albumin does not result from a low rate of glomerular filtration of this protein but, rather, from a high rate of reabsorption of filtered albumin by the tubule.17–19 The reabsorbed albumin is subsequently either returned to the circulation undegraded18 or excreted as peptide fragments in the urine.19 Large quantities of peptides (1–4 g) are excreted in human urine each day.20 Many conventional assays and specific assays based on the presence of intact protein fail to detect the degraded component in urine. Therefore, we will underestimate the total amount of protein present and will not indicate the onset of pepiduria. However, this is another characteristic marker of renal disease in diabetes.
By use of nano-liquid chromatography–tandem mass spectrometry, the corresponding amino acid sequences of these peaks were identified and, unfortunately, one of these peaks m/z 2430.8 was not identified. Through searching for the protein database, we deduced m/z 2756.1 and 1743.9 were different fragments that come from isoform 1 of fibrinogen alpha chain precursor, m/z 2231.1 and 2175.5 were fragments of protein vitronectin precursor, m/z 2154 was one part of prothrombin and m/z 2184.9 was fragment of inter-alpha-trypsin inhibitor heavy chain H4.
Fibrinogen and prothrombin play an important role in blood clotting mechanism and prothrombin translated into thrombin under the action of prothrombin activator, fibrinogen translate into fibrin monomer under the action of thrombin. The urinary peptides that we identified in our study were the fragments of the product from above mentioned processes. In DM2 patients, hyperglycemia leads to an increase in oxidative stress by exacerbating glucose oxidation and mitochondrial generation of reactive oxygen species which injure vascular intimal membrane and make the patients in the pathological state of blood coagulation and fibrinolysis.21–23 The urinary peptide m/z 2184.9 was identified as fragment of inter-trypsin inhibitor heavy chain H4 which is a disease-associated fragment including cancer and inflammatory disease that had been discovered in the plasma.24–27 Vitronectin is a multifunctional glycoprotein present in plasma and extracellular matrix of various tissues. Vitronectin deposition has been detected in a large variety of diseases involving fibrosis and necrosis, including glomerulonephritis.28,29 The most common kidney lesions in people with diabetes are those that affect the glomeruli including glomerular basement membrane thickening, mesangial expansion.11 Numerous studies have documented major alterations in extracellular matrix proteins in these pathological processes.30–32
Therefore, the above-mentioned pathological process can be summed as follows. DM2 patients with kidney injury group had longer duration of diabetes history than DMN group which can lead to multi-systems metabolic disorders. During hyperglycemia there is increased glucose influx (and catabolism) into endothelial cells and this started the detrimental molecular pathways that lead to endothelial dysfunction and activation of the coagulation system.33 So the metabolism of fibrinogen and thrombin will produce corresponding changes. Hypoxic environment will lead to increased production of oxygen free radicals, which in turn damage the cells, forming a vicious cycle. Cell injury can stimulate the proliferation of fibrous tissue which leads to changes in the extracellular matrix components and prompting deposition of vitronectin in glomerular basement membrane.
To verify the results from the first part of the study more detail, we divided the verification set into three groups based on Alb/Cre ratio. Even in narrow scope of Alb/Cre, these urinary peptides were differently expressed.
In our previous study, we had studied the different peptidome components between DM2 without microalbuminuria patients and healthy control.34 In this study, we further focused on the urine peptidome of DM2 with microalbuminuria patients and DM2 without microalbuminuria patients. Our study in this paper is a continuous work of our previous research. We hoped that the already existed data could be re-evaluated after years in order to find out the discovered peptides have better prognostic value than albumin in the progression of diabetic nephropathy to macroalbuminuria and/or decreased GFR.
From the above mentioned urinary peptides that involved in different pathophysiologic processes, we can conclude that diabetes kidney injury is a multifactorial progressive disease where the pathogens of the disease are extremely complex involving many different cells, molecules, and factors. Though the pathogens of DN are complex and still not fully elucidated, some clues could be found in our study.
Acknowledgments
This work was supported by National High Technology Research and Development Program of China (2014AA020901) and Beijing Key Laboratory of Urinary Cellular Molecular Diagnostics (Z151100001615060).
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. GZF, YD, and MZ conceived and designed the experiments. GZF, YD, and LNC performed the experiments. GZF and LNC analyzed the data. GZF, YD, and MZ wrote the manuscript. GZF and YD contributed equally to the work and should be regarded as co-first authors.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health 2005; 4: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir MR, Bakris GL. Editorial perspective. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol 2010; 31: 469–70. [DOI] [PubMed] [Google Scholar]
- 3.Lambers Heerspink HJ, de ZD. Debate: PRO position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol 2010; 31: 458–61. [DOI] [PubMed] [Google Scholar]
- 4.Glassock RJ. Debate: CON position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol 2010; 31: 462–5. [DOI] [PubMed] [Google Scholar]
- 5.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348: 2285–93. [DOI] [PubMed] [Google Scholar]
- 6.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007; 18: 1353–61. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–9. [DOI] [PubMed] [Google Scholar]
- 8.Norden AG, Rodriguez-Cutillas P, Unwin RJ. Clinical urinary peptidomics: learning to walk before we can run. Clin Chem 2007; 53: 375–6. [DOI] [PubMed] [Google Scholar]
- 9.Worsfold M, Powell DE, Jones TJ, Davie MW. Assessment of urinary bone markers for monitoring treatment of osteoporosis. Clin Chem 2004; 50: 2263–70. [DOI] [PubMed] [Google Scholar]
- 10.Wittke S, Fliser D, Haubitz M, Bartel S, Krebs R, Hausadel F, Hillmann M, Golovko I, Koester P, Haller H, Kaiser T, Mischak H, Weissinger EM. Determination of peptides and proteins in human urine with capillary electrophoresis–mass spectrometry, a suitable tool for the establishment of new diagnostic markers. J Chromatogr 2003; 1013: 173–81. [DOI] [PubMed] [Google Scholar]
- 11.Phillips AO, Steadman R. Diabetes nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol 2002; 17: 247–52. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int 2003; 63: 809–25. [DOI] [PubMed] [Google Scholar]
- 13.Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, Hermjakob H, Apweiler R, Haab BB, Simpson RJ, Eddes JS, Kapp EA, Moritz RL, Chan DW, Rai AJ, Admon A, Aebersold R, Eng J, Hancock WS, Hefta SA, Meyer H, Paik YK, Yoo JS, Ping P, Pounds J, Adkins J, Qian X, Wang R, Wasinger V, Wu CY, Zhao X, Zeng R, Archakov A, Tsugita A, Beer I, Pandey A, Pisano M, Andrews P, Tammen H, Speicher DW, Hanash SM. Overview of the HUPO Plasma Proteome Project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and publicly-available database. Proteomics 2005; 5: 3226–45. [DOI] [PubMed] [Google Scholar]
- 14.Kolch W, Neususs C, Pelzing M, Mischak H. Capillary electrophoresis-mass spectrometry as a powerful tool in clinical diagnosis and biomarker discovery. Mass Spectrom Rev 2005; 24: 959–77. [DOI] [PubMed] [Google Scholar]
- 15.Decramer S, Gonzalez de Peredo A, Breuil B, Mischak H, Monsarrat B, Bascands JL, Schanstra JP. Urine in clinical proteomics. Mol Cell Proteomics 2008; 7: 1850–62. [DOI] [PubMed] [Google Scholar]
- 16.Chu L, Fu G, Meng Q, Zhou H, Zhang M. Identification of urinary biomarkers for type 2 diabetes using bead-based proteomic approach. Diabetes Res Clin Pract 2013; 101: 187–93. [DOI] [PubMed] [Google Scholar]
- 17.Greive KA, Nikolic-Paterson DJ, Guimarães MA, Nikolovski J, Pratt LM, Mu W, Atkins RC, Comper WD. Glomerular permselectivity factors are not responsible for the increase in fractional clearance of albumin in rat glomerulonephritis. Am J Pathol 2001; 159: 1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo L, Bakris G, Comper W. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis 2002; 39: 899–919. [DOI] [PubMed] [Google Scholar]
- 19.Osicka TM, Comper WD. Protein degradation during renal passage in normal kidneys is inhibited in experimental albuminuria. Clin Sci (Lond) 1997; 93: 65–72. [DOI] [PubMed] [Google Scholar]
- 20.Sterling K. The turnover rate of serum albumin in man as measured by I131-tagged albumin. J Clin Invest 1951; 30: 1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwill AG, Frisbee JC. Oxidant stress and skeletal muscle microvasculopathy in the metabolic syndrome. Vasc Pharmacol 2012; 57: 150–9. [DOI] [PubMed] [Google Scholar]
- 22.Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol 2012; 57: 139–49. [DOI] [PubMed] [Google Scholar]
- 23.Lapolla A, Fedele D, Traldi P. Glyco-oxidation in diabetes and related diseases. Clin Chim Acta 2005; 357: 236–50. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Patel M, Rosenzweig CN, Chan-Li Y, Sokoll LJ, Fung ET, Choi-Miura NH, Goggins M, Chan DW, Zhang Z. Quantification of fragments of human serum Inter-alphatrypsin Inhibitor heavy chain 4 by a surface-enhanced laser desorption/ionization based immunoassay. Clin Chem 2006; 52: 1045–53. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Bast RC, Yu Y, Li J, Sokoll LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY, Berchuck A, Van Haaften-Day C, Hacker NF, de Bruijn HW, van der Zee AG, Jacobs IJ, Fung ET, Chan DW. Three biomarkers identified from serum proteomic changes for the detection of early stage ovarian cancer. Cancer Res 2004; 64: 5882–90. [DOI] [PubMed] [Google Scholar]
- 26.Whicher J, Biasucci L, Rifai N. Inflammation, the acute phase response and atherosclerosis. Clin Chem Lab Med 1999; 37: 495–503. [DOI] [PubMed] [Google Scholar]
- 27.Romette J, di Costanzo-Dufetel J, Charrel M. Inflammatory syndrome and changes in plasma proteins. Pathol Biol (Paris) 1986; 34: 1006–12. [PubMed] [Google Scholar]
- 28.Seiffert D. Constitutive and regulated expression of vitronectin. Histol Histopathol 1997; 12: 787–97. [PubMed] [Google Scholar]
- 29.Okada M, Yoshioka K, Takemura T, Akano N, Aya N, Murakami K, Maki S. Immunohistochemical localization of C3d fragment of complement and S-protein (vitronectin) in normal and diseased human kidneys: association with the C5b-9 complex and vitronectin receptor. Virchows Arch A Pathol Anat Histopathol 1993; 422: 367–73. [DOI] [PubMed] [Google Scholar]
- 30.Desjardins M, Bendayan M. Ultrastructural distribution of glomerular basement membrane components in experimental diabetes. Diabetes Res 1990; 14: 65–73. [PubMed] [Google Scholar]
- 31.Adler S. Structure-function relationships associated with extracellular matrix alterations in diabetes glomerulopathy. J Am Soc Nephrol 1994; 5: 1165–72. [DOI] [PubMed] [Google Scholar]
- 32.Olgemöller B, Schleicher E. Alterations of glomerular matrix proteins in the pathogenesis of diabetes nephropathy. Clin Investig 1993; 71: S13–9. [DOI] [PubMed] [Google Scholar]
- 33.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107: 1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Fu G, Lei T. Two urinary peptides associated closely with type 2 diabetes mellitus. PloS One 2015; 10: e0122950. [DOI] [PMC free article] [PubMed] [Google Scholar]