Abstract
Inhibitors of DNA binding/inhibitors of differentiation (Id) protein family have been shown to be involved in carcinogenesis. However, the roles of Id during lung adenocarcinoma (ADC) progression remain unclear. Eighty-eight ADC samples were evaluated for Id-1,2,3 level and angiogenesis (CD 34 and VEGF microvessel density) by immunohistochemistry and morphometry. The impact of these markers was tested on follow-up until death or recurrence. A significant difference between tumor and normal tissue was found for Id-1,2,3 expression (P < 0.01). In addition, high levels of nuclear Id-1 were associated with higher angiogenesis in the tumor stroma (P < 0.01). Equally significant was the association between patients in T1-stage and low cytoplasmic Id-2, as well as patients in stage-IIb and low Id-3. High cytoplasm Id-3 expression was also directly associated to lymph nodes metastasis (P = 0.05). Patients at stages I to III, with low Id-1 and Id-3 cytoplasm histoscores showed significant long metastasis-free survival time than those with high Id-1 or Id-3 expression (P = 0.04). Furthermore, high MVD-CD34 and MVD-VEGF expression were associated with short recurrence-free survival compared to low MVD-CD34 and MVD-VEGF expressions (P = 0.04). Cox model analyses controlled for age, lymph node metastasis, and adjuvant treatments showed that nuclear Id-1, cytoplasmic Id-3, and MVD-CD34 were significantly associated with survival time. Median score for nuclear Id-1 and cytoplasmic Id-3 divided patients in two groups, being that those with increased Id-1 and Id-3 presented higher risk of death. Ids showed an independent prognostic value in patients with lung ADC, regardless of disease stage. Id-1 and Id-3 should be considered new target candidates in the development of personalized therapy in lung ADC.
Keywords: Lung adenocarcinoma, inhibitors of DNA binding/inhibitors of differentiation, angiogenesis, immunohistochemistry, morphometry, prognosis
Introduction
A small number of patients with lung adenocarcinoma (ADC) have tumors appropriately localized to be considered treatable by surgical resection, and among those whose tumors are successfully resected, approximately 30–45% survive five years.1–3 Evidently some ADC have developed occult spread beyond the lung even when they appear to have been completely removed. If we could identify those tumors destined to recur, we could personalize the treatment with molecular targeted drugs and perhaps eradicate any residual tumor. Thus, there is of great interest to identify which tumors are likely to recur and shorten patient’s survival,4,5 and for the personalized treatment to be effective, we must identify precociously these tumors.
In this regard, our group has studied molecular or other markers in the primary and tumor stroma which could be related to tumor recurrence and shortened survival.6–8 Because of cell cycle regulation and angiogenesis have been thought to be important in tumor recurrence, invasion and metastasis,9,10 a group of inhibitor of DNA binding (Id) proteins have been targeted as potentially tumor markers.11–15 Inhibitor of DNA binding (Id) proteins are members of a family of basic helix-loop-helix (bHLH) transcription factors lacking the DNA-binding domain.16 Id acts as dominant-negative regulators of bHLH proteins by forming transcriptionally inactive Id-bHLH protein complexes.16 Different from other bHLH transcription factors, they lack the DNA-binding domain. Required expression of Id genes in a variety of cell types promotes proliferation16 and, under appropriate physiological conditions, also drives apoptosis.17 In addition, Id function is required for the G1 to S-phase transition of the cell cycle.18 In common with several other positive regulators of G1 to S-phase transition, Id genes function as cooperating oncogenes in the immortalization of primary cells17 and also induce disordered cell growth in fibroblast and epithelial cell lines.19 More recent data have shown that Id function is required for vascularization and invasiveness of tumor growth in vivo.20 Id proteins have been shown to be deregulated in many different cancer types and have been implicated in different steps in tumorgenesis, differentiation, and metastasis.21–23 Id protein family consists of four members namely Id-1 to Id-4.24,25 Although they belong to the same family, their localization in chromosomes, pattern of expression and function have marked differences.26 For example, expressions of Id-1, Id-2, and Id-3 are increased in colorectal ADC specimens when compared to normal tissues,27 whereas Id-4 when hypermethylated shows decreased expression and is associated with poor prognosis in colorectal ADC.28 Id-1 is a prognostic marker of cervical carcinoma, and when greatly expressed, is associated with poor prognosis.15 Id-1, Id-2, and Id-3 also participate in early stages of hepatocarcinogenesis, but not in progression; reduced expression of Id-1 is also associated with better prognosis in hepatocellular carcinoma.29 Id-1 when over-expressed in gastric cancers correlated with tumor progression,13 while Id-4 is hypermethylated, and frequently reduced.30 Id-1 and Id-3 are crucial to metastasis of gastric cancer, since their metastatic potential are significantly decreased in Id-1 and -3 double-knock down cells.31 Up-regulation of Id-1 and Id-2 have also been reported in pancreatic cancer and could be early markers of malignant transformation.32 Because of these multiple functions, Id-1 has been related to prognosis of other cancers, such as prostate,11, breast,12 gastric,13 esophageal,14 uterine cervical cancers,15 and lung.33,34
Vascular endothelial growth factor (VEGF) is one of the key mediators of angiogenesis, a step considered essential for tumor growth and metastasis. Its overexpression is associated with poor prognosis in various tumors types,35 among them, lung carcinomas. It is also known that Id-1 plays an important role in angiogenesis. There are several studies demonstrating the association between VEGF, microvessel density (MVD), and Id-1 expression in angiogenesis and so, on tumor progression and metastases.15,36
In the above studies, staining for Ids and angiogenesis has also been significantly associated with survival, but there has been uncertainty about its relationship with ADC histological type and what is the best way to report the staining results for Ids. More important, to our knowledge, Id-1 to Id-3 have never been evaluated in the tissue from lung ADC patients. To date, only data regarding to Id-1 expression in non-small cell cancer and small cell cancer have been reported.33,34,37,38 To demonstrate the importance of tumor Ids and angiogenesis and to explore the quantitative relationship between these proteins with patients’ outcome, we focused our study on Id-1 to Id-3 expression in 88 cases of localized ADC of the lung.
Patients and methods
Patients
Formalin-fixed paraffin-embedded (FFPE) specimens were retrospectively obtained from 88 patients with stage I–IIIb ADCs who underwent curative surgical resection at Clinical Hospital of University of Sao Paulo, between 2003 and 2011. Tumor pathological stages were determined according to the TNM classification of the American Joint Committee on Cancer, 7th edition.39 Their preoperative clinical stages were T1-4N0-2M0 and the mean follow-up was 27 months. No patient received adjuvant chemotherapy at diagnosis. Patients in stages II to III were treated with surgery followed by chemotherapy, whereas patients showing N2 disease were considered for postoperative radiation therapy, according to pathologic findings within the tumor sample resected. Relevant clinical information were extracted from medical records and are summarized in Table 1.
Table 1.
Clinical characteristics of all patients
| Patients (total) | 88 |
|---|---|
| Age (yrs) | 67 (29–87)a |
| Gender | 42 women, 45 men |
| T stage | |
| T1 | 26 |
| T2 | 53 |
| T3 | 6 |
| T4 | 3 |
| N stage | |
| N0 | 58 |
| N1 | 18 |
| N2 | 6 |
| Mediast lymph node | 6 |
| Stage | |
| Ia | 10 |
| Ib | 39 |
| IIa | 11 |
| IIb | 20 |
| IIIa | 7 |
| IIIb | 1 |
| Follow-up (months) | 27 (0–70)b |
| Censored patients for survival analysis to date of last follow-up | 47 |
Values represent the median (minimum and maximum).
The study was approved by the local Ethics Committee.
Immunohistochemistry
To avoid artifacts due to a poorly selective antibodies, serial slide sections from FFPE tissues were paraffin coated and cold stored at 4℃ during a median time period from 2003 to 2011. We also validated the specificity of the antibodies through Western blots of tissue extracts that contain the Id1-3 protein. Sections (4 µm) of FFPE tissue samples from pulmonary ADC were dried overnight at 37℃ on a silanized-slide (Dako, Carpinteria, CA). The protocol of universal Dako-Labelled Streptavidin-Biotin kit (Dako) was followed for each sample. Samples were deparaffinized in xylene at room temperature for 30 min, rehydrated with graded ethanol, and washed in phosphate-buffered saline (PBS). The samples were then placed in 10 mmol/L citrate buffer (pH 6.0) and boiled in a microwave for 10 min for epitope retrieval. Endogenous peroxidase activity was quenched by incubating tissue sections in 3% H2O2 for 10 min. Primary rabbit antibodies for Id-1, Id-2, and Id-3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), mouse CD34 (Dako, Glostrup, Denmark), and rabbit anti-VEGF-related antigen (Zymed, San Francisco, CA) were used overnight at 4℃ at dilutions of 1:50, 1:100, 1:100, 1:500, and 1:200, respectively. The slides were washed and biotinylated with the secondary antibody (Dako) for 30 min; streptavidin-conjugated horseradish peroxidase (Dako) was added on slides for 30 min. Then, the slides were washed and treated with the chromogen 3,3′-diaminobenzidine (Dako) for 5 min, rinsed in PBS, and counterstained with Mayer's hematoxylin, dehydrated in graded ethanol, cleared in xylene and cover-slipped with a mounting medium, Entellan New (Merck, Darmstadt, Germany). For the negative controls, the primary antibodies for Id1-3, CD34, and VEGF were suppressed and the corresponding preimmune animal serums (rabbit, mouse and rabbit, respectively) (Dako) were used instead.
Assessment of immunohistochemical score
Previous staining scores37,40,41 were modified by us considering both the intensity and the number of stained cells. Staining scores were established in a semi-quantitative method by two independent observers who were blinded for patient’s clinical data. The evaluation was done in 500 cells per slide. The histological score taken into account the intensity of nuclear and cytoplasmic Ids staining and the number of cells obtained in each staining grade, as follows: 0 (colorless), 1 (diffuse brown), 2 (brown with or without occasional clumps), 3 (brownish unevenly distributed), and 4 (uniform deep brown). A final score, designated Histoscore, was calculated by Σ(I0–4 × Pi0–500), where I and Pi represent the intensity and number of cells stained at each color intensity, respectively. The cutoff used was 578 and 497, 233 and 578, 72.5 and 590 for nuclear and cytoplasmic expression of Id 1–3, respectively.
Assessment of angiogenesis
Angiogenesis was assessed by MVD with mouse CD34 and rabbit VEGF-related antibodies. Blood vessels clearly stained were taken into account. The MVD-CD34 and MVD-VEGF were quantified by image analysis on tumor stroma by means of the Image Pro-Plus 6.0 software. Tumor stroma was analyzed at a magnification of 200x in 10 fields with the highest vascular density. MVD was determined as the percentage of vessel obtained. The median percentage used as the cut off point was 55% and 24% for MVD-VEGF and MVD-CD-34, respectively.
Statistical analysis
Statistical analyses were done by using SPSS 18.0 software. Student’s t-test, chi-square test, and Fisher’s exact test were employed to evaluate the relationship between Id 1–3 expression (independent variables), CD34 and VEGF expression with clinicopathologic characteristics (dependent variables) including age, sex, diagnosis, T stage, and N stage. Paired 2-tailed Student’s t-tests were used to compare the two variables. To evaluate the prediction of metastasis, univariate and multivariate logistic regression models were conducted. Survival analysis, disease free survival (DFS) and overall survival (OS) were calculated from the date of surgery or treatment beginning until disease recurrence or death. Patients follow-up was calculated from the surgery or treatment beginning until death, loss of follow-up or, the last contact with the patient. To estimate the DFS times of low and high Id1-3 expression groups (the median of the histoscores was selected as a cut off point), we used the Kaplan–Meier analysis. Log rank test was used to compare the survival distribution of the two Id groups. Furthermore, Cox proportional hazards model was used for multivariate analyses of OS. The correlations between Id 1–3 histoscores and MVD were performed through Spearman's coefficient of correlation for descriptive analysis. Differences were considered significant when P was less than 0.05.
Results
Proliferation and inhibition of differentiation in lung cancer are associated with Id1-3 expression and cancer stage
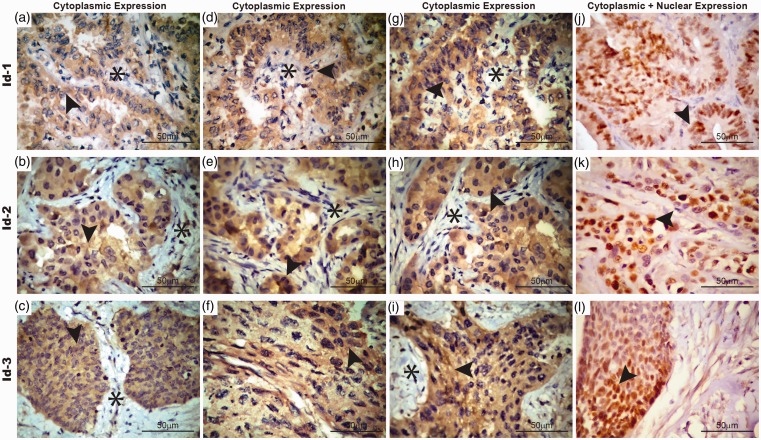
Most patients presented ADC with >3 cm (≥T2) and almost half of them had lymph node invasion. The Id1-3 staining was diffuse and heterogeneously located in the cytoplasm and nuclei of cancer cells in different intensity levels (Figure 1). However, it was always negative in tumor stroma (Figure 1). Some tumor cells had nuclear expression of Id1-3 (Figure 1). In addition, Id-2 and Id-3 cytoplasm expression were higher than nuclear (P < 0.01) in tumor cells. No difference was observed between nuclear and cytoplasm Id-1 expression in tumor cells.
Figure 1.
Cytoplasmic and nuclear immunoexpression of Id 1-3 in adenocarcinoma (a,b,c). Low expression of cytoplasm Id 1-3 (d,e,f). Intermediate expression of cytoplasmic Id1-3 (g,h,i). High expression of cytoplasmic Id1-3 note the absence signal for Id-1, Id-2, and Id-3 in tumor stroma. (j, k, l) Nuclear expression of Id-1, Id-2, and Id-3. Arrowheads indicate cytoplasmic and nuclear expression of adenocarcinoma cells; asterisks indicate stromal tissue. Bar 50 µm. (A color version of this figure is available in the online journal.)
Table 2 summarizes the morphometric results. We observed a significant difference between tumor and non-tumor tissue for nucleus and cytoplasm, indicating a distinct profile of Ids expression. We observed a significant direct correlation between the expression of Id-1 and Id-3 (R = 0.62, P = 0.01). Furthermore, Id-2 and Id-3 tumor cells expression were related to tumor stage group (Table 3: T1 stage vs. Id-2<578; stage IIb vs. Id-3 <590).
Table 2.
Immunohistochemical stain histoscore of Id-1, Id-2, Id-3, MVD-VEGF, and MVD-CD34
| Nucleus | Cytoplasm | P value | |
|---|---|---|---|
| Id-1 intensity | |||
| Tumor | 64.79 ± 29.42 | 547.26 ± 288.52 | <0.01 |
| Normal tissue | 29.44 ± 45.01 | 62.30 ± 49.78 | <0.01 |
| Id-2 intensity | |||
| Tumor | 337.68 ± 32.36 | 575.07 ± 276.91 | <0.01 |
| Normal tissue | 43.59 ± 47.61 | 122.72 ± 101.82 | <0.01 |
| Id-3 intensity | |||
| Tumor | 131.91 ± 19.94 | 646.78 ± 217.66 | <0.01 |
| Normal tissue | 32.99 ± 24.16 | 135.27 ± 82.51 | <0.01 |
| MVD-VEGF | |||
| Tumor | 56.38 ± 17.54 | <0.01 | |
| MVD-CD34 | |||
| Tumor | 9.07 ± 4.15 | <0.01 |
MVD: microvessel density.
Distribution of morphometric results for the nucleus and cytoplasm Id 1-3 histoscores in 500 cells and cytoplasmic MVD-CD34 and MVD-VEGF tumor stroma. Paired-samples t test was used to compare tumor nucleus and cytoplasm histoscore, as well as, tumor and normal tissue histoscore; P value = 0.05.
Table 3.
The association between clinicopathologic characteristics and Id 1-3 expression and MVD-VEGF and MVD-CD34
| Id-1 |
Id-2 |
Id-3 |
MVD-VEGF |
MVD-CD34 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | ≤497 | >497 | ≤578 | >578 | ≤590 | >590 | ≤55 | >55 | ≤24.3 | >24.3 |
| ≤67 yr | 18 (52.9%) | 17 (45.9%) | 18 (50%) | 18 (45%) | 15 (44.1%) | 19 (55.9%) | 20 (55.9%) | 16 (45.7%) | 16 (40.4%) | 24 (61.5%) |
| >67 yr | 16 (47.1%) | 20 (54.1%) | 18 (50%) | 22 (55.5%) | 14 (37.8%) | 23 (62.2%) | 15 (42.9%) | 19 (54.3%) | 20 (55.6%) | 15 (38.5%) |
| Gender | ||||||||||
| Women | 13 (46.4%) | 22 (51.2%) | 12 (33.3%) | 24 (66.7%) | 19 (55.9%) | 18 (46.2%) | 15 (44.1%) | 19 (55.9%) | 13 (32.5%) | 27 (67.5%) |
| Men | 15 (53.6%) | 21 (48.8%) | 18 (45.0%) | 22 (55%) | 14 (37.8%) | 23 (62.2%) | 14 (37.8%) | 23 (62.2%) | 16 (45.7%) | 19 (54.3%) |
| T stage | ||||||||||
| T1 | 7 (20.6%) | 5 (14.3%) | 8 (23.9%)* | 4 (10.3%) | 7 (21.2%) | 4 (11.1%) | 5 (13.9%) | 7 (21.9%) | 6 (15.8%) | 6 (17.1%) |
| T2 | 24 (70.6%) | 24 (68.6%) | 24 (68.6%) | 29 (74.4%) | 23 (69.7% | 26 (72.2%) | 26 (72.2%) | 21 (65.6%) | 30 (78.9%) | 22 (62.9%) |
| T3 | 3 (8.8%) | 3 (8.6%) | 3 (8.6%0) | 3 (7.7%) | 2 (6.1%) | 4 (11.1%) | 4 (11.1%) | 2 (6.3%) | 2 (5.3%) | 4 (11.4%) |
| T4 | 0 (0.0%) | 3 (8.6%) | 0 (0%) | 3 (7.7%) | 1 (3.0) | 2 (5.6%) | 1 (2.8%) | 2 (6.3%) | 0 (0.0%) | 3 (8.6%) |
| N stage | ||||||||||
| N0 | 20 (60.6%) | 21 (67.7%) | 20 (58.8%) | 24 (70.6%) | 17 (56.7%) | 25 (75.8% | 22 (64.7%) | 19 (67.9%) | 24 (68.6%) | 20 (62.5%) |
| N1 | 10 (30.3%) | 7 (22.6%) | 11 (32.4% | 7 (20.6% | 11 (36.7% | 5 (15.2%) | 9 (26.5) | 7 (25%) | 7 (20.0%) | 10 (31.3%) |
| N2 | 3 (9.1%) | 3 (9.7%) | 3 (8.8%) | 3 (8.8%) | 2 (6.7%) | 3 (9.1%) | 3 (60.0%) | 2 (7.1%) | 4 (11.4%) | 2 (6.3%) |
| Stage | ||||||||||
| Ia | 7 (17.9%) | 2 (5.1%) | 7 (17.1%) | 2 (4.8%) | 6 (15.4%) | 2 (5.1%) | 4 (10.3%) | 5 (12.8%) | 5 (12.2%) | 4 (9.5%) |
| Ib | 16 (41%) | 19 (48.7%) | 16 (39.0%) | 23 (54.8%) | 13 (33.3%) | 24 (61.5% | 19 (48.7%) | 17 (43.6%) | 26 (63.4%) ‡ | 13 (31.0%) |
| IIa | 0 (0%) | 1 (2.6%) | 1 (2.4%) | 0 (0%) | 0 (10.0%) | 1 (2.6%) | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | 1 (2.4%) |
| IIb | 9 (23.1%) | 10 (25.6% | 9 (22.0%) | 11 (26.2%) | 13 (33%)† | 6 (15.4%)† | 10 (25.6%) | 8 (20.5%) | 6 (14.6%) | 13 (31.0%) |
| IIIa | 4 (10.3%) | 3 (7.7%) | 4 (9.8%) | 3 (7.1%) | 2 (5.1%) | 4 (10.3%) | 3 (7.7%) | 3 (7.7%) | 4 (9.8%) | 3 (7.1%) |
| IIIb | 0 (0%) | 1 (2.6%) | 0 (0%) | 1 (2.4%) | 0 (0.0%) | 1 (2.6%) | 1 (2.6%) | 0 (0%) | 0 (0.0%) | 1 (2.4%) |
| Lymph | ||||||||||
| N = 0 | 26 (89.7%) | 24 (88.9%) | 26 (89.7%) | 29 (90.6%) | 24 (92.3%) | 28 (90.3%) | 30 (90.9%) | 20 (90.9%) | 26 (86.7%) | 28 (93.3%) |
| N = 1 | 1 (3.4%) | 1 (3.7%) | 1 (3.4%) | 1 (3.1%) | 1 (3.8%) | 1 (3.2%) | 1 (3.0%) | 1 (4.5%) | 1 (3.3%) | 1 (3.3%) |
| N = 2 | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 1 (3.1%) | 1 (3.8%) | 0 (0.0%) | 1 (3.0%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) |
| N = 3 | 2 (6.9%) | 1 (3.7%) | 2 (6.9%) | 1 (3.1%) | 0 (0.0%) | 2 (6.5%) | 1 (3.0%) | 1 (4.5%) | 2 (6.7%) | 1 (3.3%) |
MVD: microvessel density.
Distribution of the morphometric results of Id1-3 histoscores, MVD-CD34 and MVD-VEGF tumor staining according to patients’ clinicopathologic features. Chi-square test was used to associate clinical and morphological variables; P value = 0.05.
Linear-by-linear association (R) = 3.3, P value = 0.05.
Likelihood ratio (R) = 14.42, P value = 0.02.
Likelihood ratio (R) = 19.77, P value = 0.003.
Angiogenesis shows a critical role in Id1-3-staining tumor cells
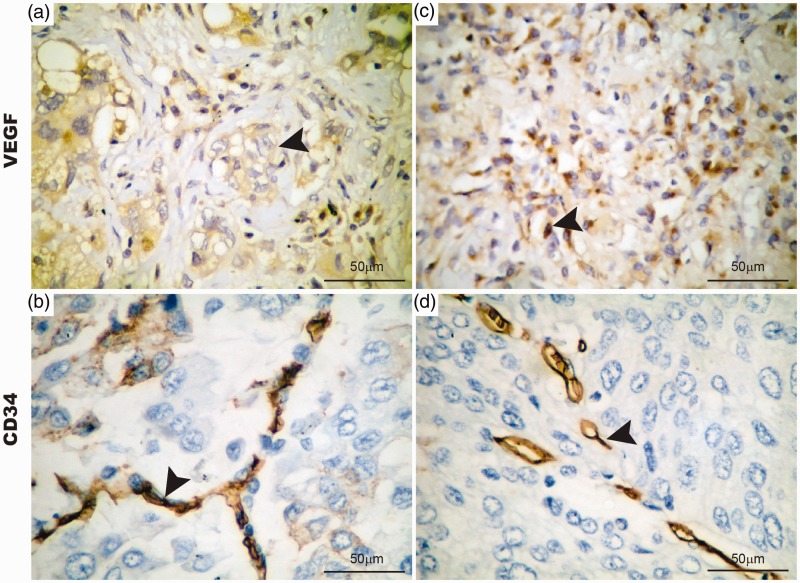
Id-1, CD34, and VEGF-related antigens were strongly expressed in endothelial cells in tumor stroma (Figure 2). Endothelium Id-1 and MVC-CD34 represented 38.11 ± 4.54% and 32.45 ± 10.92 of the total area of the tumor microenvironment (P < 0.01), while MVD-VEGF represented 56.38 ± 17.54%.
Figure 2.
Cytoplasmic immunoexpression of VEGF and CD34 in stroma vessels of adenocarcinomas (a,c). Glomerular-like microvessels (b,d) and linear-like microvessels vessels are positive for cytoplasmic VEGF and CD34 in stroma vessels. Arrowheads indicate stroma vessels on adenocarcinoma stroma. Bar 50 µm. (A color version of this figure is available in the online journal.)
The multivariate model demonstrated that Id-1 histoscore correlated with MVC-CD34 (R2 = 0.62; P = 0.02) and MVC-VEGF (R2 = 0.56; P = 0.003). MVD-CD34 and MVD-VEGF expressions showed strong association with Id-2 cytoplasmic expression (R2 = 0.53; P < 0.01 and R2 = 0.24; P = 0.03, respectively). Id-3 cytoplasmic expression showed a trend to be associated with MVD-CD34. Furthermore, 61% of weak Id-2 expression correlated with MVD-VEGF in tumor stroma (P = 0.03). Tumor with overexpression of Id-1,2 showed higher fractions of angiogenesis in the stroma.
We also found that MVD-CD34 and MVD-VEGF tumor staining were correlated to some clinicopathologic features. Significant association was found between stage Ib vs. MVD-CD34 (P < 0.01; Table 3).
DFS with Id1-3 expression
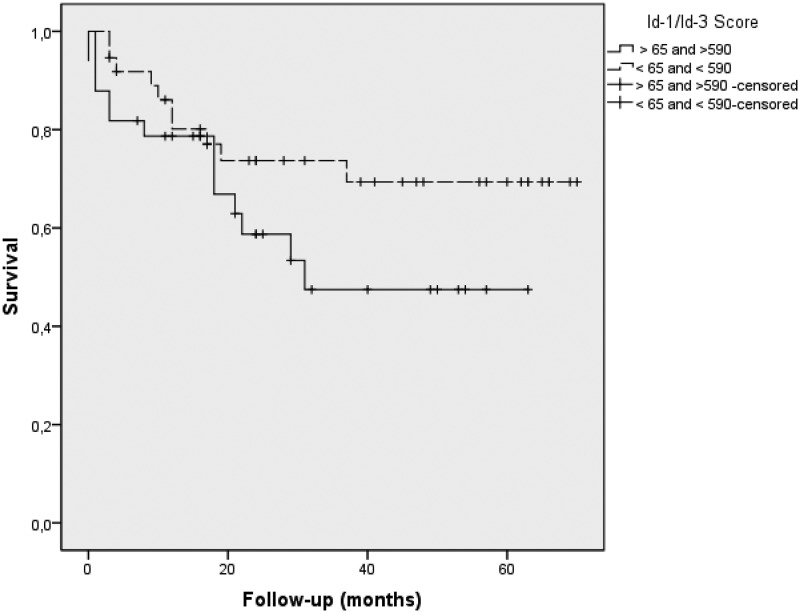
ADC patients were divided into two groups based on Id1-3 median histoscores. In the first step, according to Kaplan–Meier analysis, low nuclear Id-1 and cytoplasmic Id-3 histoscores showed significant long metastasis-free survival time than those with high nuclear Id-1 or cytoplasmic Id-3 expression (log rank: 1.28 and 1.61, P = 0.04, respectively). Furthermore, high MVD-CD34 and MVD-VEGF expression were associated with short recurrence-free survival compared to low MVD-CD34 and MVD-VEGF expression (P = 0.04), suggesting these variables are related to prognosis. In fact, Cox model analyses controlled for age, lymph node metastasis, and adjuvant treatments showed that nuclear Id-1, cytoplasmic Id-3, and MVD-CD34 were associated with survival. The median score for nuclear Id-1 and cytoplasmic Id-3 divided patients in two groups with distinctive prognosis: those with Id-1 >65 and Id-3 >590 had a higher risk of death (1.65, 1.28 and 2.56, respectively). Finally, we examined the importance of Id1-3 to survival in just the 80 of our patients who had pathological stage I and II tumors. Once again, nuclear Id-1 and cytoplasmic Id-3 were significantly related to survival time by the Cox model (P < 0.01). In this subset, 50% of the patients had Id-1 and Id-3 values >65 and 590, respectively, and 50% had Id-1 and Id-3 values <65 and <590, respectively. Although there is no biologically distinctive cut point in Id-1 and Id-3, we chose this median value of 65 and 590 as a practical way to examine the stage I and II patients’ outcome. We found that it separated these stage I and II patients into two groups with distinctly different average survival times as illustrated by Kaplan–Meier plots in Figure 3. The group with <Id-1/Id-3 appears as the top curve, and their median survival time was not reached during our follow-up. Their mean survival time, however, was quite long (52.8 months). By contrast, those with Id-1/Id-3 (bottom curve) had a median survival time of just 37.6 months after surgery (P < 0.01 by log-rank test).
Table 4.
Survival analysis with stratification of the variables in optimal upper and lower binary cut-off limits by the Kaplan–Meier method and the differences by means of the log-rank test
| Overall survival (months) |
||||
|---|---|---|---|---|
| Variables | Mean | Standard error | Chi-square (log rank) | P value |
| Age (yrs) | ||||
| ≤67 | 48.64 | 4.6 | ||
| >67 | 37.19 | 5.3 | 2.98 | 0.08 |
| Gender | ||||
| Women | 41.42 | 5.3 | ||
| Men | 44.51 | 4.8 | 0.03 | 0.8 |
| Stage | ||||
| Ia | 63 | 0.15 | ||
| Ib | 66 | 0.6 | 16.24 | 0.006 |
| IIa | 70 | 0.5 | ||
| IIb | 51 | 0.14 | ||
| IIIA | 8 | 0.7 | ||
| IIIB | ||||
| T stage | ||||
| T1 | 42.77 | 9.02 | ||
| T2 | 46.24 | 4.51 | 8.85 | 0.03 |
| T3 | 28.00 | 11.90 | ||
| T4 | 10.00 | 1.10 | ||
| N metastasis | ||||
| N0 | 49.47 | 4.67 | ||
| N1 | 44.05 | 8.24 | 10.96 | 0.004 |
| N2 | 17.00 | 7.74 | ||
| Mediastinal metastasis | ||||
| N = 0 | 48.68 | 4.22 | ||
| N = 1 | 13.00 | 12.00 | 14.49 | 0.002 |
| N = 2 | 1.000 | 0.00 | ||
| N = 3 | 25.00 | 13.11 | ||
| Id-1 | ||||
| ≤497 | 35.09 | 4.72 | 1.28 | 0.04 |
| >497 | 46.38 | 4.93 | ||
| Id-2 | ||||
| ≤578 | 33.16 | 4.51 | ||
| >578 | 48.16 | 4.70 | 2.89 | 0.08 |
| Id-3 | ||||
| ≤590 | 35.31 | 4.75 | ||
| >590 | 47.12 | 4.85 | 1.61 | 0.04 |
| MVD-VEGF | ||||
| ≤55 | 41.17 | 4.90 | ||
| >55 | 44.79 | 4.80 | 0.18 | 0.06 |
| MVD-CD34 | ||||
| ≤24.3 | 34.67 | 4.58 | 2.27 | 0.05 |
| >24.3 | 48.44 | 4.61 | ||
Table 5.
Cox proportional hazard model analysis of survival time (chi-square = 18.042, P = 0.01)
| β coefficient | Standard error | Wald | P value | Risk of β coefficient | 95.0% CI for exp (B) |
||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age >67 yrs | −1.20 | 0.54 | 4.75 | 0.02 | 0.30 | 0.10 | 0.88 |
| Metastases | |||||||
| N0 | 9.69 | 0.001 | |||||
| N1 | −1.98 | 0.65 | 9.20 | 0.002 | 0.14 | 0.03 | 0.49 |
| N2 | −2.13 | 0.88 | 5.86 | 0.016 | 0.12 | 0.02 | 0.66 |
| Adjuvant treatment (CT or RT) | −2.67 | 0.71 | 9.81 | 0.01 | 0.28 | 0.23 | 0.89 |
| Nuclear Id-1 >497 | −0.43 | 0.53 | 0.66 | 0.04 | 1.65 | 0.22 | 1.84 |
| Cytoplasmic Id-3 >590 | −0.25 | 0.57 | 0.19 | 0.06 | 1.28 | 0.41 | 3.95 |
| MVD-CD34 >24.3 | −0.94 | 0.57 | 2.72 | 0.05 | 2.56 | 0.84 | 7.82 |
MVD: microvessel density.
Cox model analyses controlled for age, lymph node metastasis, and adjuvant treatment showed that nuclear Id-1, cytoplasmic Id-3, and MVD-CD34 were independent predictors of survival in patients resected for pulmonary adenocarcinoma.
Figure 3.
Kaplan–Meier plots of survival probability versus follow-up time in months in those patients with pathological stage I and II disease. The group with <65 Id-1 and 590 Id-3 appears as the top curve, and the group with > 65 Id-1 and 590 Id-3 appears as the bottom curve
Discussion
In the present study, for the first time, we explored the quantitative relationship between Id-1 to Id-3 family and prognosis, as well as the relationship between this family and angiogenesis, in ADC of the lung, by examining samples from 88 patients. We scored the sign of Ids family, and assessed quantitatively angiogenesis by density MVD-CD34+ and microvessel endothelial activity MVD-VEGF+ in lung samples. It was found that high Ids H-score and high level of angiogenesis group presented a poor prognosis compared to the low H-score and low level of angiogenesis group. The almost general co-expression of Ids and angiogenesis supports their collaboration in vivo and require further investigation of these markers as a therapeutic target.
In the first part of the study, we compared the levels of Id-1 in 88 normal human lung tissue samples with matched ADC tumor samples by using immunohistochemistry (IHC). As we observed that the majority of tumor cells expressed nuclear Ids, we applied a modified H-score, where the extension of nuclear intensity of staining was scored as percentage of positive cells and the intensity of staining was assessed compared with a known external positive control. Actually, there has been uncertainty about staining for Ids and its relationship with different histological type33,34,37,38) and how best to record staining for Ids. Yang et al.42 employed a semi-quantitative score in breast carcinoma classified by plus into four groups: +, 0–10%; ++, 10–20%; +++, 20–30%; ++++, 30–40%. The intensity of ‘+, ++’ were labeled as ‘low expression’, and ‘+++, ++++’ were labeled as ‘high expression’. The ‘hot spot’ method was employed by Tsui et al.43 in evaluation of the staining results of gastric ADC. They graded extent and intensity of cytoplasmic staining by an arbitrary scale ranged from 0 to 3, representing negative (0), weak (1), moderate (2), and strong (3) staining, respectively. They also scored nuclear staining by the percentage of nuclei positively stained in the whole section with at least 200 nuclei counted. McCarty et al.41 consider both the intensity and the percentage of cells stained in each of five intensity categories: 0 (no staining), 1 (weak staining), 2 (distinct staining), 3 (strong staining), and 4 (very strong staining). Another important matter in these reports that deserve discussion is nearly as many ways to report IHC for Ids and angiogenesis as studies. Some used binary cut points (low and high expression, strong and weak expression) to define a positive or negative tumor. Others have scored in low and high, strong and weak, or expressed the staining in relation to stromal staining or other proteins (see Rothschild et al.38). Yet others have expressed Ids and MVD quantitatively.15,36 In the present work, the Ids and MVD median of the score and percentage, respectively, were used as the cut off point. Compared to the literature, our method is fast, of low cost and more objective because removes the subjectivity of the pathologist.
In the current work, we found that Id-2 and Id-3 cytoplasmic expression were significantly higher than nuclear in tumor cells, whereas nuclear Id-1 expression was higher in tumor cells. As we used Santa Cruz polyclonal antibodies against Id proteins, this has been proven to be a limitation when addressing the expression patterns. Actually, the cytoplasmic expression of Id proteins has been extensively criticized since when using specific monoclonal antibodies against Id proteins, the expression is clearly restricted to the nucleus.44 For that reason, to avoid artifacts due to a poorly selective antibodies, serial slide sections from FFPE tissues were paraffin coated and cold stored at 4℃ during a median time period from 2003 to 2011. We also validated the specificity of the antibodies through Western blots of tissue extracts that contain the Id1-3 protein. Nuclear and cytoplasmic sign findings also deserve a comment, since contradictory results have been published on Ids expression in tumor tissue. Yang et al.42 investigated the expression of Id-1, Id-2, Id-3, and Id-4 proteins in breast carcinoma and 22 normal specimens by immunohistochemical and described only cytoplasmic sign, although examining carefully the histologic illustrations in the paper, we can observe clear evidence of nuclear staining. Indeed, Id proteins act in the cytoplasm binding with the transcription factors to block their passing from the nuclear membrane into the nuclear. Thus, the transcription factors cannot bind with DNA and Id proteins and this may explain why the Id proteins express only in the cytoplasm in some malignant cells. Nuclear and cytoplasmic Id-1 immunoexpression are also evident in tumors illustrated by Ponz-Sarvis et al.37 who found significantly higher Id-1 protein levels in lung tumors compared with normal tissues and in squamous carcinomas compared with ADC s. Our findings about nuclear and cytoplasmic Ids sign coincide with Rothschild et al.38 work, who described Id-1 expression in the nucleus of 70% of squamous cell carcinomas and 50% of non-squamous cell carcinomas, although vascular endothelium of tumor and non-tumor tissue was negative.
By IHC staining, we similarly found that sign of all three proteins was significantly increased in the cytoplasm and nucleus of the malignant cells comparing to the marginal normal tissue. Whereas Id-2 and Id-3 were expressed in higher levels in the cytoplasm of the malignant cells, nuclear and cytoplasmic Id-1 were not statistically different. The cytoplasmic immunostaining of Id-2 and Id-3 protein within ADC cells was similar to some other cancer cells such as prostate,11 breast,12 gastric,13 and uterine cervical.15 A similar increase was also found in levels of Id-2 and Id-3 by Kamalian et al.34 on 26 pairs of lung tissues from small cell carcinoma (SCC). They also found that all three Id proteins were overexpressed in cytoplasm of the malignant cells, whereas in nuclei of SCLC cells, Id-1 expression was significantly reduced. In another words, the increased cytoplasmic expression of Id-2 and Id-3 significantly correlated with the malignant features of lung specimens. On the other hand, we also observed that the expression of Id-3 in the nuclei of the ADC cells was reduced. Therefore, not only the level of Id-3 expression but also the pattern of its spreading was associated with the malignant changes of the lung epithelial cells. Further investigation is needed to comprehend the biological significance of both the increased level and the different localization of Id-3 in malignant changes of the ADC cells. For Id-2 localization it has been reported that it is different among different cancers. For example, in prostate cancer, Id-2 is expressed in both cytoplasm and nuclei,11 whereas in pancreatic carcinoma, it expressed only in the cytoplasm.32 However, in squamous cell carcinoma14 and cervical cancer,15 its expression was confined to nuclei only. In this work, we found that Id-2 is expressed predominantly in the cytoplasm of the ADC cells. It is not clear whether the different distribution patterns observed in different types of cancers relate to different roles played by increased Id-2. Similar differences in distribution patterns among different cancer types were also observed in Id-3, which was cytoplasmic in prostate cancer11 but nuclear in squamous cell carcinoma14 and cervical cancer.15 The results in this work showed that Id-1 in both cytoplasm and nuclei of the ADC cells was significantly increased compared to marginal normal lung, but the intensity of nuclear expression was similar to that in cytoplasm, indicating that similar amount of Id-1 protein concentrated in nuclei and cytoplasm probably function associated as a cancer promoter.
In agreement with Ponz-Sarvis et al.,37 our analyses showed a significantly higher expression of Id-1 protein in ADC tissue compared with normal lung tissue. Unexpectedly, Bhattacharya et al.33 showed significantly lower Id-1 mRNA expression levels in ADC and matched normal but no IHC staining was performed to confirm the presence of the protein. Kamalian et al.34 conducted an investigation in malignant cell lines from small cell lung cancer and found that Id-1 gene was up-regulated. Although a potential post translational regulation of Id-1 cannot be refused, no other studies on Id-1 mRNA levels in non-small cell lung cancer samples are available but several reports in other tumors have shown Id-1 overexpression in neoplastic tissue at the mRNA level.45
Interestingly, we have also found that high levels of tumor with Id-1,2 were associated with higher fractions of angiogenesis in the tumor stroma. The highly overlapping expression of Id-1, Id-2 and angiogenesis claiming for a functional role of these Ids in signaling pathways such as the via PI3K/Akt and NF-κB/MMP-2 signaling pathways.46 The statistically significant correlation of Id-1 and Id-2 with cytoplasmic Ids expression in all samples of our study supports this statement.
Our results have impact on clinicopathological characteristics of the patients. Id-2 and Id-3 protein were up-regulated in larger tumors, while Id-1 protein expression tended to be lower. Interestingly, a higher Id-3 protein expression in lung ADC suggested metastatic potential of the malignant cells to lymph nodes. Kaplan–Meier survival curves showed an inverse correlation between Id-1 and Id-3 proteins, and DFS and OS. Angiogenesis also showed a negative relationship with DFS and OS. In fact, Cox model analyses controlled for age and lymph node metastasis showed that Id-1, Id-3, and angiogenesis were independent variables associated with survival time, and allowed the division of patients into two groups with distinctive prognosis. Those with Id-1 >497 or Id-3 >590 and angiogenesis >24.3% had a higher risk of death. Taken together, these data showed that the differentially expressed Id proteins overlapped to angiogenesis were involved in the neoplastic transformation and progression of lung ADC. It suggests that Id protein may serve in the long-term survival expectation in this study. Our findings warrant further studies to investigate the exact mechanism by which Id proteins are involved in lung ADC and pathogenesis. We conclude that Id-1,3 are extensively expressed in lung ADC tumors and it may represent an independent novel prognostic factor among patients with ADC histology. This result deserves further investigation of Id-1,3 expression in prospectively designed clinical trials. Moreover, Id-1,3 contributes to ADC cell proliferation and angiogenesis and may be crucial for adjuvant treatment indication. Thus, Id-1,3 represents new potential drug target candidate to be evaluated in the development of new antineoplastic agents for personalized therapy in lung ADC.
Acknowledgments
This work was supported by the National Council for Scientific and Technological Development (CNPq471939/2010-2 and 483005/2012-6) and Foundation for Research of the State of São Paulo (FAPESP2011/52095-0 and 2013/14277-4).
Author contributions
LA, TT, MA, RT, and TT generated the data. EP, FV, AF, and VC contributed to overall study design, evaluation of data, and manuscript preparation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Riquet M, Bagan P, Lepimpecbarthes F, Banu E, Scotte F, Foucault C, Dujon A, Danel C. Completely resected non-small cell lung cancer: reconsidering prognostic value and significance of N2 metastases. Ann Thorac Surg 2007; 84: 1818–24. [DOI] [PubMed] [Google Scholar]
- 2.Ratto GB, Costa R, Maineri P, Alloisio A, Bruzzi P, Dozin B. Is there a subset of patients with preoperatively diagnosed N2 non-small cell lung cancer who might benefit from surgical resection? J Thorac Cardiovasc Surg 2009; 138: 849–58. [DOI] [PubMed] [Google Scholar]
- 3.Rush WR, Asamuar H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, Members of IASLC Staging Committee J Thorac Oncol 2009; 4: 568–77. [DOI] [PubMed] [Google Scholar]
- 4.Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, Travis WD, Moreira AL. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010; 34: 1155–62. [DOI] [PubMed] [Google Scholar]
- 5.Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, Adusumilli PS, Travis WD. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences following limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015; 10: 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sá VK, Carvalho L, Gomes A, Alarcão A, Silva MR, Couceiro P, Sousa V, Soares FA, Capelozzi VL. Role of the extracellular matrix in variations of invasive pathways in lung cancers. Braz J Med Biol Res 2013; 46: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Sá VK, Olivieri E, Parra ER, Ab'Saber AM, Takagaki T, Soares FA, Carraro D, Carvalho L, Capelozzi VL. Hyaluronidase splice variants are associated with histology and outcome in adenocarcinoma and squamous cell carcinoma of the lung. Hum Pathol 2012; 43: 675–83. [DOI] [PubMed] [Google Scholar]
- 8.Souza P, Rizzardi F, Noleto G, Atanazio M, Bianchi O, Parra ER, Teodoro WR, Carrasco S, Velosa AP, Fernezlian S, Ab'saber AM, Antonângelo L, Takagaki T, Schainberg CG, Yoshinari NH, Capelozzi VL. Refractory remodeling of the microenvironment by abnormal type V collagen, apoptosis, and immune response in non-small cell lung cancer. Hum Pathol 2010; 41: 239–48. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. What is the evidence that tumours are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 10.Gugger M, Kappeler A, Vonlanthen S, Altermatt HJ, Ris HB, Lardinois D, Borner MM, Heighway J, Betticher DC. Alterations of cell cycle regulators are less frequent in advanced non-small cell lung cancer than in resectable tumours. Lung Cancer 2001; 33: 229–39. [DOI] [PubMed] [Google Scholar]
- 11.Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Id proteins expression in prostate cancer: high-level expression of Id-4 in primary prostate cancer is associated with development of metastases. Mod Pathol 2006; 19: 931–41. [DOI] [PubMed] [Google Scholar]
- 12.Jang KS, Han HX, Paik SS, Brown PH, Kong G. Id-1 overexpression in invasive ductal carcinoma cells is significantly associated. With intratumoral microvessel density in ER-negative/node positive breast cancer. Cancer Lett 2006; 244: 203–10. [DOI] [PubMed] [Google Scholar]
- 13.Jang TJ, Jung KH, Choi EA. Id-1 gene downregulation by sulindac sulfide and its upregulation during tumor development in gastric cancer. Int J Cancer 2006; 118: 1356–63. [DOI] [PubMed] [Google Scholar]
- 14.Yuen HF, Chan YP, Chan KK, Chu YY, Wong ML, Law SY, Srivastava G, Wong YC, Wang X, Chan KW. Id-1 and Id-2 are markers for metastasis and prognosis in oesophageal squamous cell carcinoma. Br J Cancer 2007; 97: 1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maw MK, Fujimoto J, Tamaya T. Expression of the inhibitor of DNA-binding (ID)-1 protein as an angiogenic mediator in tumour advancement of uterine cervical cancers. Br J Cancer 2008; 99: 1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton JD, Deed RW, Craggs G, Sablitzky F. Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol 1998; 8: 58–65. [PubMed] [Google Scholar]
- 17.Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol 1998; 18: 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone MV, Pepperkok R, Peverali FA, Philipson L. Id proteins control growth induction in mammalian cells. Proc Natl Acad Sci U S A 1994; 91: 4985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desprez P-Y, Lin CQ, Thomasset N, Sympson CJ, Bissel MJ, Campisi J. A novel pathway for mammary epithelial cell invasion induced by the helix-loophelix protein Id-1. Mol Cell Biol 1998; 18: 4577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumourxenografts. Nature 1999; 401: 670–7. [DOI] [PubMed] [Google Scholar]
- 21.Benezra R, Rafii S, Lyden D. The Id proteins and angiogenesis. Oncogene 2001; 20: 8334–41. [DOI] [PubMed] [Google Scholar]
- 22.Hasskarl J, Munger K. Id proteins-tumor markers or oncogenes? Cancer Biol Ther 2002; 1: 91–6. [DOI] [PubMed] [Google Scholar]
- 23.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell 2003; 3: 525–30. [DOI] [PubMed] [Google Scholar]
- 24.Riechmann V, Sablitzky F. Mutually exclusive expression of two dominant-negative helix-loop-helix (dnHLH) genes, Id4 and Id3, in the developing brain of the mouse suggests distinct regulatory roles of these dnHLH proteins during cellular proliferation and differentiation of the nervous system. Cell Growth Differ 1995; 6: 837–43. [PubMed] [Google Scholar]
- 25.Riechmann V, van Cruchten I, Sablitzky F. The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2 and Id3. Nucleic Acids Res 1994; 22: 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer 2005; 5: 603–14. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JW, Deed RW, Inoue T, Balzi M, Becciolini A, Faraoni P, Potten CS, Norton JD. Expression of Id helix-loop-helix proteins in colorectal adenocarcinoma correlates with p53 expression and mitotic index. Cancer Res 2001; 61: 8803–10. [PubMed] [Google Scholar]
- 28.Umetani N, Takeuchi H, Fujimoto A, Shinozaki M, Bilchik AJ, Hoon DS. Epigenetic inactivation of ID4 in colorectal carcinomas correlates with poor differentiation and unfavorable prognosis. Clin Cancer Res 2004; 10: 7475–83. [DOI] [PubMed] [Google Scholar]
- 29.Damdinsuren B, Nagano H, Kondo M, Yamamoto H, Hiraoka N, Yamamoto T, Marubashi S, Miyamoto A, Umeshita K, Dono K, Nakamori S, Wakasa K, Sakon M, Monden M. Expression of Id proteins in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Int J Oncol 2005; 26: 319–27. [PubMed] [Google Scholar]
- 30.Chan AS, Tsui WY, Chen X, Chu KM, Chan TL, Chan AS, Li R, So S, Yuen ST, Leung SY. Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. Oncogene 2003; 22: 6946–53. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya T, Okaji Y, Tsuno NH, Sakurai D, Tsuchiya N, Kawai K, Yazawa K, Asakage M, Yamada J, Yoneyama S, Kitayama J, Osada T, Watanabe T, Tokunaga K, Takahashi K, Nagawa H. Targeting Id1 and Id3 inhibits peritoneal metastasis of gastric cancer. Cancer Sci 2005; 96: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruyama H1, Kleeff J, Wildi S, Friess H, Büchler MW, Israel MA, Korc M. Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am J Pathol 1999; 155: 815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya R, Kowalski J, Larson AR, Brock M, Alani RM. Id1 promotes tumor cell migration in nonsmall cell lung cancers. J Oncol 2010; 2010: 856105–856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamalian L, Gosney JR, Forootan SS, Foster CS, Bao ZZ, Beesley C, Ke Y. Increased expression of Id family proteins in small cell lung cancer and its prognostic significance. Clin Cancer Res 2008; 14: 2318–25. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol 2013; 128: 391–6. [DOI] [PubMed] [Google Scholar]
- 36.Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T, Wei F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys Sin (Shanghai) 2011; 43: 796–804. [DOI] [PubMed] [Google Scholar]
- 37.Ponz-Sarvise M, Nguewa PA, Pajares MJ, Agorreta J, Lozano MD, Redrado M, Pio R, Behrens C, Wistuba II, García-Franco CE, García-Foncillas JG, Montuenga LM, Calvo A, Gil-Bazo I. Inhibitor of differentiation-1 as a novel prognostic factor in NSCLC patients with adenocarcinoma histology and its potential contribution to therapy resistance. Clin Cancer Res 2011; 17: 4155–66. [DOI] [PubMed] [Google Scholar]
- 38.Rothschild SI, Kappelerc A, Ratschiller D, Betticherd DC, Tschana MP, Guggerc M, Gautschia O. The stem cell gene “inhibitor of differentiation 1” (ID1) is frequently expressed in non-small cell lung cancer. Lung Cancer 2011; 71: 306–11. [DOI] [PubMed] [Google Scholar]
- 39.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. International Association for the Study of Lung Cancer International Staging C, Participating I. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forth coming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 40.Irigoyen M(1), Pajares MJ, Agorreta J, Ponz-Sarvisé M, Salvo E, Lozano MD, Pío R, Gil-Bazo I, Rouzaut A. TGFBI expression is associated with a better response to chemotherapy in NSCLC. Mol Cancer 2010; 9: 130–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 1985; 109: 716–21. [PubMed] [Google Scholar]
- 42.Yang H-Y, Liu H-L, Ke J, Wu HE, Zhu H, Jliu J-R, Liu L-X, Jiang H-C. Expression and prognostic value of Id protein family in human breast carcinoma. Oncol Rep 2010; 23: 321–8. [PubMed] [Google Scholar]
- 43.Tsui WY, Chen X, Chu KM, Chan TL, Chan AS, Li R, So S, Yuen ST, Leung SY. Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. Oncogene 2003; 22: 6946–53. [DOI] [PubMed] [Google Scholar]
- 44.Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y, Chao S, Cheong W, Ke Y, Al-Ahmadie H, Gerald WL, Brogi E, Benezra R. Reassessment of Id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-Id1 antibody. Cancer Res 2006; 66: 10870–7. [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Xu X, Han B, Zhou R. Inhibitor of DNA binding-1 overexpression in prostate cancer: relevance to tumor differentiation. Pathol Oncol Res 2009; 15: 91–6. [DOI] [PubMed] [Google Scholar]
- 46.Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S, Fu S. Id1 enhances human ovarian cancer endothelial progenitor cell angiogenesis via PI3K/Akt and NF-κB/MMP-2 signaling pathways. J Transl Med 2013; 29: 132–132. [DOI] [PMC free article] [PubMed] [Google Scholar]