Abstract
The retinal dopaminergic system is involved in the myopic shift following form deprivation. Citicoline has been demonstrated to stimulate the dopaminergic system in the brain and retina. Furthermore, citicoline has been used in many neurogenic diseases, such as senile cognitive impairment, stroke and Parkinson's disease as well as in amblyopia and glaucoma. Our aim was to investigate the effect of citicoline on the refractive state and retinal dopamine level in form deprivation myopia of guinea pigs. Guinea pigs, at an age of four weeks, were randomly divided into normal control, deprivation, deprived + citicoline and deprived + vehicle groups. Form deprivation myopia was induced by a translucent eye shield covering the right eye. Citicoline was injected intraperitoneally twice a day (500 mg/kg, 9 am and 9 pm) for 10 days. In vitro, retinal explants were cultured with citicoline for 24 h, with a final citicoline concentration of 100 µmol/L. The ocular refractive parameters and retinal dopamine content were measured. After occlusion for 10 days, the form-deprived eyes became myopic with an increase in axial length and a decrease in retinal dopamine content. The intraperitoneal injection of citicoline reduced the myopic degree (from −3.25 ± 0.77D to −0.62 ± 0.47D, P < 0.001) and partially raised retinal dopamine levels (from 0.55 ± 0.21 ng to 0.81 ± 0.24 ng, P < 0.01) in the form-deprived eyes. After 24 h of culturing retinal explants with citicoline, retinal dopamine content increased significantly (from 0.42 ± 0.14 ng to 0.62 ± 0.21 ng, P < 0.05). These results demonstrated that an intraperitoneal injection of citicoline could retard the myopic shift induced by form deprivation in guinea pigs, which was mediated by an increase in the retinal dopamine levels.
Keywords: Citicoline, dopamine, form deprivation myopia, guinea pig, therapy, retina
Introduction
Myopia is the most important reason for visual impairment throughout the world, affecting about one-half of the world’s population.1,2 In East Asia, its prevalence and incidence continues to increase significantly in teenagers;3,4 thus, myopia has become a major public health issue. In recent years, a rising trend in the United States,5 Europe,6 and Australia7 has also been observed. Although we have the capacity to correct the myopic refractive error, myopia progression is often accompanied by some serious ocular disorders, such as retinosis, retinal detachment, macular degeneration and hemorrhage, and glaucoma.8 These complications can cause irreversible vision loss, and even blindness. Therefore, it is very urgent to find the most effective treatment for preventing myopia progression, while minimizing side effects.
At present, there are some promising optical devices and pharmacological interventions for retarding myopia progression in the clinic, such as contact lenses, orthokeratology, multifocal spectacles, and muscarinic acetylcholine receptor (mAChR) antagonists.9 Much of the evidence coming from randomized controlled trials has shown that the most effective treatment to slow myopia progression thus far is topical use of mAChR antagonist medications.10–12 Atropine instilled once a day has been demonstrated to result in approximately 90% average reduction of myopia progression in children with low-to-moderate myopia.10 In addition, this effect is greater in Asian children than in White children by meta-analysis.11 Pirenzepine, another anti-muscarinic medication, can slow myopia by 44%.12 However, these mAChR antagonists can cause some side effects during the period of treatment, such as near blur, photophobia, glare, and recurrent allergic blepharitis. Although pirenzepine gel has less of an effect on pupil size and accommodation than atropine eye drops, the adverse reactions of these mAChR antagonists are unacceptable for most patients. Thus, their clinical application is limited and not practical.
Dopamine is known as a major neurotransmitter in the retina and postretinal visual pathways. Retinal dopamine is involved in the signaling cascade regulating eye growth,13,14 as evidenced by its reduced levels in myopic eyes in various species, such as chickens,15 tree shrews,15 rabbits,16 and guinea pigs.17 Dopamine synthesis and release are stimulated by light and display a circadian rhythm that is higher during the daytime in light and lower at night in darkness. Form deprivation mainly causes a decrease of retinal dopamine content during the daytime.13 Many studies have shown that local administration of apomorphine (a non-selective dopamine receptor agonist) to simulate high retinal dopamine levels can retard the myopic shift induced by form deprivation in rhesus monkeys and guinea pigs.17,18 In addition, systemic levodopa (a precursor of dopamine)19 or intravitreal dopamine16 can inhibit the development of form deprivation myopia (FDM) in guinea pigs and rabbits. However, their adverse effects are still a large problem for myopic therapy, especially when these medications are used long-term. Recent epidemiological evidence suggests that children who spend more time outdoors are less likely to become myopic.20,21 The protective effect of outdoor activity on myopia progression seems to be partly mediated by the light-stimulated synthesis and release of retinal dopamine. Considering the important role of retinal dopamine in myopia development, it is imperative to explore safe and effective dopaminergic drugs for clinically preventing myopia progression as much as possible.
Cytidine 5'-diphosphocholine (CDP-choline) is an indispensable intermediate in the biosynthetic pathway of structural phospholipids in the cytomembrane. Citicoline, as a drug formulation of exogenous CDP-choline, is distributed throughout the body and easily penetrates brain cells across the blood–brain barrier to provide neuroprotection. It has been widely demonstrated that citicoline can activate the biosynthesis of phospholipids in neuronal membranes and can stimulate cerebral metabolism and some brain neurotransmitter systems, including the dopaminergic system.22 When used as a long-term medication, it is a safe and well-tolerated drug that has no significant systemic side effects, and it has been used in the pharmacotherapy of senile cognitive impairment,23 addictive disorders,24 stroke, head trauma and Parkinson's disease25 as well as in amblyopia26 and glaucoma.27,28 At present, citicoline is available in the United States as a dietary supplement for neurological disorders such as stroke and dementia.25 Rejdak et al.29 reported that citicoline-treated rabbits displayed a significantly higher retinal dopamine concentration compared with vehicle-treated controls. Moreover, citicoline treatment could also promote the release of dopamine in the striatum.30 Therefore, it is conceivable that citicoline could enhance dopaminergic neurotransmission in the retina to slow myopia progression. In this study, our aim was to investigate the effect of citicoline on the refractive state and retinal dopamine level in the FDM of guinea pigs.
Materials and methods
Animals
All animal experiments were carried out in compliance with the guidelines of the Association for Research in Vision and Ophthalmology Statement for the use of animals in ophthalmic and vision research and approved by the Animal Ethics Committee of Xiangya Medical College, Central South University (Changsha, China). Pigmented guinea pigs (Cavia porcellus, at age of 4 weeks) were obtained from the Animal Center of Xiangya Medical College. A total of 73 animals were studied, and both sexes were used.
In vivo, 53 guinea pigs were randomly divided into the following four groups: (1) normal control (n = 8), (2) deprivation (n = 15), (3) deprived + citicoline (n = 15), and (4) deprived + vehicle (n = 15). The other 20 animals were randomly assigned for in vitro studies of retinal explant culture, as follows: (1) deprivation (n = 10) and (2) deprived + citicoline (n = 10). Guinea pigs with abdominal hemorrhage, infection or death were excluded (Table 1).
Table 1.
The reasons for excluding guinea pigs in vivo and in vitro.
| Abdominal hemorrhage (n) | Infection (n) | Death (n) | Remains (n) | |
|---|---|---|---|---|
| Deprivation in vivo | 1 | 14 | ||
| Deprived + citicoline in vivo | 1 | 2 | 12 | |
| Deprived + vechile in vivo | 1 | 1 | 2 | 11 |
| Deprived + citicoline in vitro | 1 | 9 |
Induction of FDM
FDM was induced by a translucent eye shield covering the right eye of the guinea pig, as described in our previous study.19 Then, animals were placed in a wire mesh cage and were maintained on a daily 12 h light/12 h darkness cycle (light on at 7 am and off at 7 pm). Light was emitted by LED fluorescent lamps (20 W) fixed to the roof and the walls around the cage (100 lux at eye level in the light). After 10 days, the eye shield was removed and ocular biometric measurements were performed. The radius of corneal curvature was measured with a keratometer (Topcon, OM-4, Japan). An A-scan ultrasonic examination (Cinescan A/B, Quantel Medical, French) was conducted to determine the anterior segment depth, lens thickness, vitreous chamber depth, and axial length. After the pupils were dilated with 1% tropicamide, ocular refraction was detected with a streak retinoscope in the darkroom. The technicians who performed the analysis were blinded with respect to the experimental intervention. Measurements were conducted for each animal three times, and the mean value was used for analysis. In the study, animals were euthanized by cervical dislocation at 2 pm. All manipulations of retinal tissue were performed in the darkroom with dim red light (15 W Kodak 1A safelight filter).
Retinal explant culture in the deprived eyes
After animals were euthanized, their eye bulbs were immediately dissected, and the cornea, lens, and vitreous were removed. Then, the whole neural retina was gradually detached from the retinal pigment epithelium and choroid using short gentle motions, transferred onto a 0.4 mm Millicell filter (Millipore) and placed with the ganglion cell layer facing upward. Each retina was gently flattened with two end-blunted glass pipettes. Under a hood, the retina-containing Millicell filter was placed onto a filter in the incubation chamber filled with the Neurobasal-A medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 2 mmol/L L-glutamine, 10 U/mL penicillin, and 10 µg/mL streptomycin. All explants were placed on a shaker in the 37℃ incubator and cultured for 24 h at 5% CO2.
Citicoline treatment in vivo and in vitro
Citicoline (Sigma-Aldrich) solution was made by dissolving citicoline in sterilized injection-water at a concentration of 500 mg/mL. In vivo, animals were injected intraperitoneally with citicoline solution (500 mg/kg) twice daily (9 am and 9 pm) for 10 days. The sterilized injection-water was used as the vehicle for the intraperitoneal injection. In vitro, retinal explants were cultured with citicoline for 24 h, with a final citicoline concentration of 100 µmol/L in the incubation medium.
Measurement of retinal dopamine content
Retinal dopamine content was determined by high-pressure liquid chromatography with electrochemical detection (HPLC-ECD, Thermo Fisher Scientific, Inc.). After animals were euthanized, the whole neural retinas were carefully isolated, weighed, snap frozen in liquid nitrogen and stored at −80℃. Thawed retinas were homogenized in 500 µL of the prechilled eluent with 10 ng of the internal standard dihydroxybenzylamine (Sigma-Aldrich) and then centrifuged for 20 min at 4℃, 12000 rpm. A volume of 20 µL of the supernatant from each sample was directly loaded into the HPLC system. Chromatographic conditions used the following materials and parameters: a Hypersil™ ODS-2 column (C18, 5 µm, 250 × 4.6 mm), a mobile phase consisting of 20 mmol/L sodium citrate and methanol (90:7, v/v), a flow rate of 1.0 mL/min, a column temperature at 35℃, and a working voltage of 750 mV. The dopamine content (ng) per milligram retina (wet weight) was then determined.
Statistical analysis
All data were presented as means ± SEM. Parametric analysis was performed with SPSS Statistics 19.0. In vivo, ocular biometric parameters and retinal dopamine content were analyzed by one-way ANOVA among different groups. In vitro, the comparison of retinal dopamine content was made by a paired sample t-test. Differences among all means were regarded as statistically significant when probability values (P) were < 0.05.
Results
Effect of intraperitoneal citicoline on ocular refractive development and retinal dopamine in the right eye
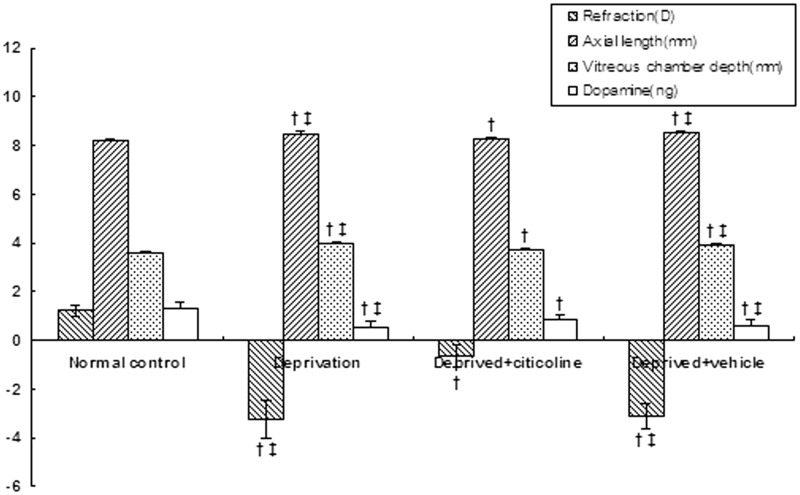
After the right eyes were occluded for 10 days, the form-deprived eyes became myopic (−3.25 ± 0.77D), with a myopic shift of −4.47D compared with the normal control (1.22 ± 0.21D). There was a statistically significant difference in ocular refraction between the deprivation group and normal control (P < 0.001). The increases in axial length (from 8.20 ± 0.04 mm to 8.50 ± 0.08 mm, P < 0.001) and vitreous chamber depth (from 3.60 ± 0.04 mm to 3.90 ± 0.08 mm, P < 0.001) were also statistically significant compared with the normal control. Citicoline treatment significantly reduced the myopic degree (−0.62 ± 0.47D, P < 0.001) and inhibited the increase in axial length (8.32 ± 0.06 mm, P < 0.001) and vitreous chamber depth (3.72 ± 0.07 mm, P < 0.001) in the form-deprived eyes. However, there were still statistically significant differences in ocular refraction (P < 0.001), axial length (P < 0.001), and vitreous chamber depth (P < 0.01) between the citicoline group and normal control. No statistically significant differences were observed in corneal radius of curvature, anterior segment depth or lens thickness among all groups (P > 0.05) (Figure 1).
Figure 1.
After covering the right eyes for 10 days, the deprived eyes became myopic with the decrease of retinal dopamine. Intraperitoneal injection of citicoline significantly inhibited the myopic shift (P < 0.01) and raised retinal dopamine levels (P < 0.01). †P < 0.05 vs. normal control; ‡P < 0.05 vs. the deprived + citicoline group
Ten days of form deprivation caused a significant decrease in retinal dopamine content in right eyes (0.55 ± 0.21 ng) when compared with the normal control (1.29 ± 0.27 ng, P < 0.001). After citicoline treatment, a significant increase in retinal dopamine was observed in the deprived eyes (0.81 ± 0.24 ng, P < 0.01). However, there was a significant difference between the deprived + citicoline group and the normal control (P < 0.001). The sterilized injection-water treatment, as a vehicle, had no significant effects on retinal dopamine or ocular biometric parameters (P > 0.05) (Figure 1).
Effect of intraperitoneal citicoline on ocular refractive development and retinal dopamine in the left eyes
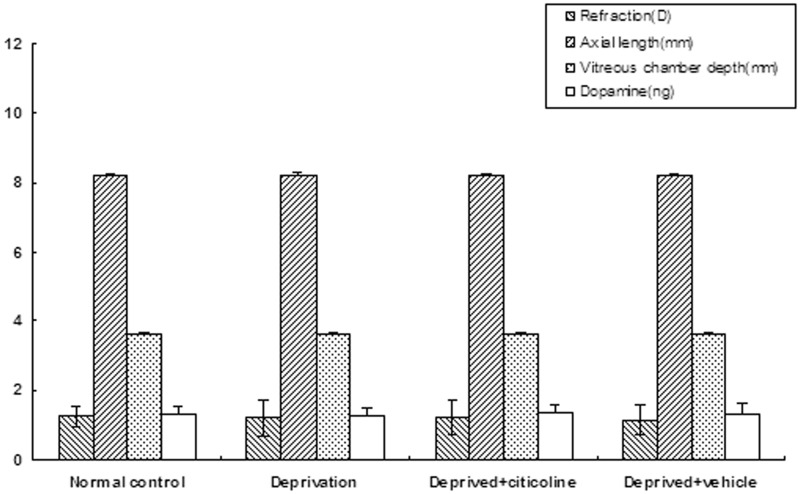
In the left eyes, there were no statistically significant differences in ocular biometric parameters among all groups, such as refraction, axial length, corneal radius of curvature, anterior segment depth, lens thickness, and vitreous chamber depth (P > 0.05). The retinal dopamine content displayed a slight increasing trend (from 1.27 ± 0.20 ng to 1.37 ± 0.24 ng) after citicoline treatment. However, this difference in retinal dopamine content was not statistically significant (P > 0.05) (Figure 2).
Figure 2.
In the left eyes, among all groups, no significant differences were found in ocular biometric parameters or retinal dopamine content (P > 0.05)
The effect of citicoline on retinal dopamine of the deprived eyes in vitro
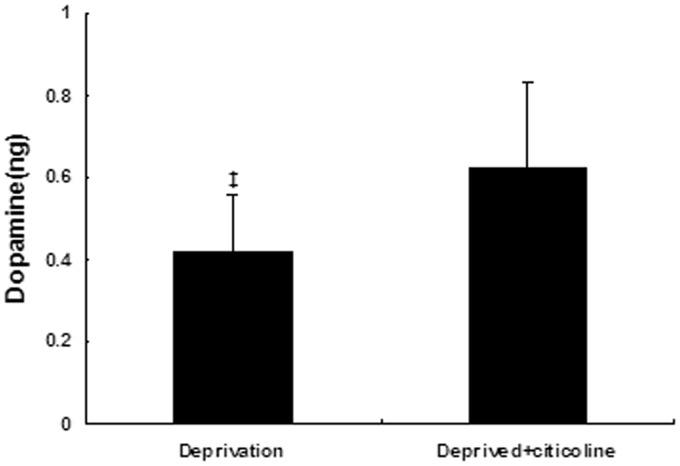
After 24 h of explant culture, retinal dopamine content was 0.42 ± 0.14 ng in the deprived eyes. The citicoline treatment caused a significant increase in retinal dopamine content (0.62 ± 0.21 ng) compared with the deprivation group (P < 0.05) (Figure 3).
Figure 3.
Citicoline treatment caused a significant increase in retinal explant dopamine content in the deprived eyes in vitro (P < 0.05). ‡P < 0.05 vs. the deprived + citicoline group
Discussion and conclusions
This study was the first to confirm the inhibitory action of citicoline on myopia progression, despite the use of an animal model. Our data showed that the intraperitoneal injection of citicoline could compensate for the decrease of retinal dopamine levels and attenuate the myopic shift following form deprivation in guinea pigs. Moreover, it was also found that exogenous citicoline caused an increase of dopamine content in retinal explant culture of the form-deprived eyes.
In the present study, the decrease of myopic degree induced by citicoline is approximately 2.63D (from −3.25D to −0.62D) in the deprived eyes. This inhibitory effect looks more effective than that of levodopa (approximately 2.12D, from −3.62D to −1.5D),19 a finding which requires further investigation. However, citicoline treatment cannot completely suppress the myopic development following form deprivation because there is a statistically significant difference between the deprived + citicoline group and its normal control. The partial inhibitory effect of citicoline may be associated with an insufficient dose of citicoline and multiple myopic signal factors in the retina including dopamine,13,14 retinoic acid,31,32 and nitric oxide.33,34 Accumulating evidence indicates that a 500 mg/kg dose of citicoline could cause effective neuroprotection from retinal injury induced by kainic acid and brain lesions induced by 6-hydroxydopamine in rats.35–37 In our study, guinea pigs were injected intraperitoneally with citicoline (500 mg/kg, twice a day). Although the dosage was enough to slow myopia progression, it may not be optimal for inhibiting the myopic shift following form deprivation in guinea pigs.
After administration, citicoline undergoes a quick transformation to cytidine and choline, which are believed to enter brain cells and retinal neurons separately. Furthermore, citicoline enhances phosphatidylcholine synthesis and stimulates the dopaminergic system in the retina, as confirmed by our study. According to our results, the inhibitory effect of citicoline on myopia progression was associated with the increase of retinal dopamine content in guinea pigs. In addition, other neurochemical mechanisms might also be involved in the inhibitory effect of citicoline on myopia progression. Giménez et al.38 found that chronic citicoline treatment caused an increase of dopamine receptor densities and promoted a partial recovery of dopamine receptor function that was normally reduced with aging in the mice striatum. It has been shown that form deprivation upregulated the content and activity of retinal nitric oxide synthase in guinea pigs.33,34 In addition to its neuroprotective effect on dopaminergic neurons,39 citicoline could also reduce nitric oxide synthase levels and attenuate nitric oxide function in the neuronal damage of the retina40 and spinal cord.41 Thus, retinal nitric oxide may be involved in the citicoline inhibition of myopia progression. Rejdak et al.29 reported that intraperitoneal injection of citicoline increased retinal dopamine content in normal albino rabbits. In our study, there is only a trend of increasing retinal dopamine content in the undeprived eyes, but no significant difference when compared with the normal control. The discrepancy in results is likely from differences in species and drug dosage, and the possibility that citicoline could preferentially elevate lower retinal dopamine levels; these possibilities require more research in the future.
In summary, the intraperitoneal injection of citicoline effectively retarded the myopic shift induced by form deprivation in guinea pigs, which was associated with the reinforcement of the retinal dopaminergic pathway. Therefore, citicoline treatment is a very promising research strategy for slowing myopia progression in the future.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30600694). We thank John Massion for a critical reading of the manuscript.
Author contributions
All authors took part in the design of studies, data analysis and review of the manuscript. JM and CF conducted the experiments, and JM wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthal Physiol Opt 2012; 32: 3–16. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet 2012; 379: 1739–48. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Li L, Chen L, Lee J, Wu J, Yang A, Chen C, Xu D, Lam DS, Sharma A, Griffiths S, Gao Y, Congdon N. Population density and refractive error among Chinese children. Invest Ophthalmol Vis Sci 2010; 51: 4969–76. [DOI] [PubMed] [Google Scholar]
- 4.Asakuma T, Yasuda M, Ninomiya T, Noda Y, Arakawa S, Hashimoto S, Ohno-Matsui K, Kiyohara Y, Ishibashi T. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study. Ophthalmology 2012; 119: 1760–5. [DOI] [PubMed] [Google Scholar]
- 5.Vitale S, Sperduto RD, Ferris FLI. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol 2009; 127: 1632–39. [DOI] [PubMed] [Google Scholar]
- 6.Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E, Buitendijk GH, Cougnard-Grégoire A, Creuzot-Garcher C, Erke MG, Hogg R, Höhn R, Hysi P, Khawaja AP, Korobelnik JF, Ried J, Vingerling JR, Bron A, Dartigues JF, Fletcher A, Hofman A, Kuijpers RW, Luben RN, Oxele K, Topouzis F, von Hanno T, Mirshahi A, Foster PJ, van Duijn CM, Pfeiffer N, Delcourt C, Klaver CC, Rahi J, Hammond CJ. European Eye Epidemiology (E(3)) Consortium. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology 2015; 122: 1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology 2013; 120: 1482–91. [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Pan CW, Ohno-Matsui K, Lin X, Cheung GC, Gazzard G, Koh V, Hamzah H, Tai ES, Lim SC, Mitchell P, Young TL, Aung T, Wong TY, Saw SM. Myopia-related fundus changes in Singapore adults with high myopia. Am J Ophthalmol 2013; 155: 991–9. [DOI] [PubMed] [Google Scholar]
- 9.Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev 2011;(12):CD004916. [DOI] [PMC free article] [PubMed]
- 10.Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther 2011; 27: 361–8. [DOI] [PubMed] [Google Scholar]
- 11.Li SM, Wu SS, Kang MT, Liu Y, Jia SM, Li SY, Zhan SY, Liu LR, Li H, Chen W, Yang Z, Sun YY, Wang N, Millodot M. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci 2014; 91: 342–50. [DOI] [PubMed] [Google Scholar]
- 12.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K. U.S. Pirenzepine Study Group. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS 2008; 12: 332–9. [DOI] [PubMed] [Google Scholar]
- 13.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114: 106–19. [DOI] [PubMed] [Google Scholar]
- 14.Nebbioso M, Plateroti AM, Pucci B, Pescosolido N. Role of the dopaminergic system in the development of myopia in children and adolescents. J Child Neurol 2014; 29: 1739–46. [DOI] [PubMed] [Google Scholar]
- 15.McBrien NA, Cottriall CL, Annies R. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis Neurosci 2001; 18: 571–80. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Chen X, Ge J, Cui D, Wu J, Tang F, Tan J, Zhong X, Gao Q. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther 2008; 24: 543–50. [DOI] [PubMed] [Google Scholar]
- 17.Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis 2011; 17: 2824–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci 1991; 32: 1674–7. [PubMed] [Google Scholar]
- 19.Junfeng M, Shuangzhen L, Wenjuan Q, Fengyun L, Xiaoying W, Qian T. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci 2010; 87: 53–60. [DOI] [PubMed] [Google Scholar]
- 20.Li SM, Li H, Li SY, Liu LR, Kang MT, Wang YP, Zhang F, Zhan SY, Gopinath B, Mitchell P, Wang N. Anyang Childhood Eye Study Group. Time outdoors and myopia progression over 2 years in chinese children: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci 2015; 56: 4734–40. [DOI] [PubMed] [Google Scholar]
- 21.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 22.Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review. Methods Find Exp Clin Pharmacol 2006; 28: 1–56. [PubMed] [Google Scholar]
- 23.Cotroneo AM, Castagna A, Putignano S, Lacava R, Fantò F, Monteleone F, Rocca F, Malara A, Gareri P. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging 2013; 8: 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wignall ND, Brown ES. Citicoline in addictive disorders: a review of the literature. Am J Drug Alcohol Abuse 2014; 40: 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saver JL. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis 2008; 5: 167–77. [PubMed] [Google Scholar]
- 26.Pescosolido N, Stefanucci A, Buomprisco G, Fazio S. Amblyopia treatment strategies and new drug therapies. J Pediatr Ophthalmol Strabismus 2014; 51: 78–86. [DOI] [PubMed] [Google Scholar]
- 27.Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, Ricci B, Quaranta L, Manni G. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res 2008; 173: 541–54. [DOI] [PubMed] [Google Scholar]
- 28.Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5'-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol 2005; 110: 91–102. [DOI] [PubMed] [Google Scholar]
- 29.Rejdak R, Toczołowski J, Solski J, Duma D, Grieb P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res 2002; 34: 146–9. [DOI] [PubMed] [Google Scholar]
- 30.Agut J, Ortiz JA, Wurtman RJ. Cytidine (5')diphosphocholine modulates dopamine K(+)-evoked release in striatum measured by microdialysis. Ann N Y Acad Sci 2000; 920: 332–5. [DOI] [PubMed] [Google Scholar]
- 31.Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci 2006; 47: 1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao JF, Liu SZ, Dou XQ. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int J Ophthalmol 2012; 5: 670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen D, Song W, Liu S, Tan X, Liu F. Upregulated expression of N-methyl-D-aspartate receptor 1 and nitric oxide synthase during form-deprivation myopia in guinea pigs. Int J Clin Exp Pathol 2015; 8: 3819–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Liu Q, Yang X, Yang H, Wang XM, Zeng JW. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res 2007; 1186: 155–63. [DOI] [PubMed] [Google Scholar]
- 35.Park CH, Kim YS, Noh HS, Cheon EW, Yang YA, Yoo JM, Choi WS, Cho GJ. Neuroprotective effect of citicoline against KA-induced neurotoxicity in the rat retina. Exp Eye Res 2005; 81: 350–8. [DOI] [PubMed] [Google Scholar]
- 36.Park CH, Kim YS, Cheon EW, Noh HS, Cho CH, Chung IY, Yoo JM, Kang SS, Choi WS, Cho GJ. Action of citicoline on rat retinal expression of extracellular-signal-regulated kinase (ERK1/2). Brain Res 2006; 1081: 203–10. [DOI] [PubMed] [Google Scholar]
- 37.Barrachina M, Domínguez I, Ambrosio S, Secades J, Lozano R, Ferrer I. Neuroprotective effect of citicoline in 6-hydroxydopamine-lesioned rats and in 6-hydroxydopamine-treated SH-SY5Y human neuroblastoma cells. J Neurol Sci 2003; 215: 105–10. [DOI] [PubMed] [Google Scholar]
- 38.Giménez R, Raïch J, Aguilar J. Changes in brain striatum dopamine and acetylcholine receptors induced by chronic CDP-choline treatment of aging mice. Br J Pharmacol 1991; 104: 575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radad K, Gille G, Xiaojing J, Durany N, Rausch WD. CDP-choline reduces dopaminergic cell loss induced by MPP(+) and glutamate in primary mesencephalic cell culture. Int J Neurosci 2007; 117: 985–98. [DOI] [PubMed] [Google Scholar]
- 40.Han YS, Chung IY, Park JM, Yu JM. Neuroprotective effect of citicoline on retinal cell damage induced by kainic acid in rats. Korean J Ophthalmol 2005; 19: 219–26. [DOI] [PubMed] [Google Scholar]
- 41.Yücel N, Cayli SR, Ateş O, Karadağ N, Firat S, Turköz Y. Evaluation of the neuroprotective effects of citicoline after experimental spinal cord injury: improved behavioral and neuroanatomical recovery. Neurochem Res 2006; 31: 767–75. [DOI] [PubMed] [Google Scholar]