Abstract
Nicotinic acid (NA) acting as the precursor of NAD+/NADH and NADP+/NADPH, participates in many biochemical processes, e.g. lipid metabolism. The main purpose of this study was to investigate the effects of dietary NA on carcass traits, meat quality, blood metabolites, and fat deposition in Chinese crossbred finishing steers. Sixteen steers with the similar body weight and at the age of 24 months were randomly allocated into control group (feeding basal diet) and NA group (feeding basal diet + 1000 mg/kg NA). All experimental cattle were fed a 90% concentrate diet and 10% forage straw in a 120-day feeding experiment. The results showed that supplemental NA in diet increased longissimus area, intramuscular fat content (17.14% vs. 9.03%), marbling score (8.08 vs. 4.30), redness (a*), and chroma (C*) values of LD muscle, but reduced carcass fat content (not including imtramuscular fat), pH24 h and moisture content of LD muscle, along with no effect on backfat thickness. Besides, NA supplementation increased serum HDL-C concentration, but decreased the serum levels of LDL-C, triglyceride, non-esterified fatty acid, total cholesterol, and glycated serum protein. In addition, NA supplementation increased G6PDH and ICDH activities of LD muscle. These results suggested that NA supplementation in diet improves the carcass characteristics and beef quality, and regulates the compositions of serum metabolites. Based on the above results, NA should be used as the feed additive in cattle industry.
Keywords: Nicotinic acid, intramuscular fat, meat quality, blood metabolites, Chinese crossbred steers
Introduction
Intramuscular fat (IMF) content is an important trait evaluating beef quality. It is associated with meat color, flavor, juiciness, and tenderness. There have been many studies about investigating the genetics of IMF variation. Quantitative trait loci (QTL) for beef IMF have been identified on nearly all bovine chromosomes (Bovine QTL database). However, these identified QTLs are responsible for less than 50% of IMF variation, suggesting that many other factors, such as environment, management, and nutrition play important roles on it. Many studies have indicated that nutritional factors such as polyunsaturated fatty acid and some vitamins can impact IMF deposition by affecting glucolipid metabolism and regulating the expressions of lipid metabolism-related genes.1–3
Nicotinic acid (NA) acting as the precursor of NAD+/NADH and NADP+/NADPH, participates in many biochemical processes, such as lipid metabolism, tissue oxidation, glycolysis, and respiratory functions.4,5 NA not only inhibits triglycerides (TG) decomposition in adipocyte and reduces serum non-esterified fatty acid (NEFA) level by interacting with niacin receptor 1 (GPR109A)6,7 but also reduces the production of TG, low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL), and enhances the level of high-density lipoprotein cholesterol (HDL-C) in liver by inhibiting the expression of DGAT2 gene.6–9 Furthermore, NA accelerates adipocyte differentiation and lipid deposition by adjusting the secretion of epinephrine, cortisone, adiponectin, and leptin.6,7,10
NA promotes the absorption of dietary fatty acid into blood through interacting with dietary fat,11,12 which results in the increase of serum non-esterified fatty acid (NEFA) and glucosamine, and finally improves milk yield and milk fat percentage in cow.12–14 NA supplementation in pharmaceutical dose reduces fat mobilization, induces amino acid changing into glucose, and decreases the β-hydroxybutyric acid and NEFA level in blood.13–15 However, the studies about the effect of dietary NA on meat quality are very limited. Real et al.16 reported that NA supplementation in low dosage (13 ∼ 55 mg/kg) improved feed conversion rate and growth performance in commercial pigs. Further, high dosage of NA not only improved the pork quality, e.g. pH, drip loss, and meat color (L*, a*, and b* value), but also reduced the carcass shrink percentage. The body weight gain and feed utilization efficiency were effectively improved in broiler chickens that were fed with diet containing 33 ∼ 66 mg/kg NA, but the weight of abdominal fat and dressing percentage of carcass were not significantly affected.17 Further study showed that 32 mg/kg of added NA in diet was the minimum dosage for improving growth and feed conversion rate in broiler chicken at the age of 21 days.18 Jiang et al.19 also found that the chicken diet added with 60 mg/kg NA increased average daily gain, improved ham muscle tenderness, as well as decreased drip loss. When supplemental NA in diet was increased to 120 mg/kg, the IMF of the chicken chest and ham muscle was enhanced, meanwhile, the percentage of abdominal fat was significantly reduced.19
The above reports suggested that supplemental NA in diet may affect the IMF content and beef quality in steers. However, there are few studies about that. Therefore, the main objective of this study was to investigate the influences of dietary NA on carcass traits, beef quality, and lipid metabolism in Chinese crossbred steers.
Materials and methods
Experiment design and animal management
The feeding experiment had been performed in Hunan Tianhuan Stockbreeding Co., Ltd. All procedures were specially approved by the ethics committee of Jiangxi Agricultural University. Sixteen crossbred steers (Augus × Chinese indigenous cattle) with similar body weight (575.50 ± 31.50 kg) and at the same age of 24 months, were randomly allocated into control group (feeding basal diet) and NA group (feeding basal diet + 1000 mg/kg NA). The experimental cattle were tethered in individual pens and provided a 90% concentrate diet and 10% forage straw by free feed intake (Table 1). For NA group, 1000 mg/kg NA was added to the concentrate diet and mixed well. The diets were provided twice per day at 7:00 and 16:00. Fresh water was offered at anytime through automatic drinker. The feeding experiment had been lasted for 120 days.
Table 1.
Compositions and nutrition ingredients of the basal diet (based on dry matter)
| Item | Content (%) |
|---|---|
| Diet ingredient | |
| Corn | 41.4 |
| Roasted barley | 11.2 |
| Dried distiller’s grain | 10.3 |
| Wheat bran | 19.8 |
| Roasted soybean | 5.4 |
| Limestone | 0.9 |
| Premix* | 1.0 |
| Rice straw | 10.0 |
| Chemical composition | |
| Dry matter | 88.4 |
| Crude protein | 12.8 |
| Crude fat | 4.2 |
| Acid detergent fiber | 10.8 |
| Neutral detergent fiber | 25.3 |
| Ash | 3.5 |
Premix supplied in per kilogram: Vitamin A, 250,000 IU; Vitamin D3, 30,000 IU; Vitamin E, 800 IU; Cu, 1 g; Fe, 5 g; Mn, 4 g; Zn, 3 g; Se, 10 mg; I, 50 mg; Co, 10 mg.
Sample collection and carcass trait
Blood samples were collected via jugular venipuncture in 24 h before slaughter. After coagulation at room temperature, the serum samples were separated by centrifuging the clots at 4℃ and 3000 rpm for 20 min. All serum samples were stored at −80℃ until analyzed.
After 120-day formal feeding experiment, all steers were measured the final body weight and slaughtered at a commercial slaughterhouse following the normal procedures. Hot carcass and internal organs including heart, liver, lung, spleen, and tripe were weighed within 30-min postmortem. At the same time, the longissimus dorsi (LD) muscle between the 12th and 13th rib were harvested and immediately frozen in liquid nitrogen for subsequent analyses of lipogenic-related enzyme activities. An intact LD muscle sample between the 12th and 13th ribs was dissected from the right side of the carcass to determine pH, meat color, marbling, shear force, and the chemical composition. And then, the carcass was chilled for 24 h at 2℃. The left side of the carcass was segmented, and lean meat, fat tissues, bone, and other tissues including fascia and tendon were weighted. The subcutaneous backfat thickness was determined by a vernier caliper. The mean value in triplicate in each carcass was treated as the measured value. The ribeye area was obtained by tracing the transverse section of each LD muscle on a sulfate paper, and then calculated by volume-curve method using the Leica Qwin software.
Measurement of meat quality and chemical composition
Postmortem pH45 min and pH24 h were determined by a Delta 320 pH Meter (Mettler Toledo, Switzerland) with the probe inserting into the center of LD muscle. Meat color values of lightness (L*), redness (a*), and yellowness (b*) were measured at 24 h after slaughter using a colorimeter (WSC-S, Shanghai, China) with a illuminant D65, 10° observer and CIELAB system. The chroma (C*) and Hue angle H° were expressed as C* = [(a*2 + b*2)]1/2 and H° = arctan (b*/a*), respectively. Marbling score of LD muscle was assessed at 24 h postmortem according to the Japan marbling grading standards (8–12: abundant, 5–7: moderate, 3–4: mean, 2: a little, and 1: trace).
To determine the shear force of LD muscle, steaks (3 cm × 4 cm × 5 cm) were separated from the muscle sample, and placed in the valve bags, and then cooked in an electric-heated thermostatic water bath until the internal temperature reached up to 70℃. After the meat temperature dropped to 20℃ at room temperature, six beef strips of 3 × 1 × 1 cm (length × width × height) were parallelly prepared along the muscle fiber direction and cut with a Warner-Bratzler shear device (C-LM4, Haerbin, China) by running perpendicular along the muscle fiber direction. The shear force was expressed as the mean of six shear force values (kgf).
Biochemical compositions of LD muscle were measured following the procedures of AOAC methods (AOAC 2000). Briefly, moisture and dry matter were quantified by stoving a wet muscle at 105℃ to constant weight. Crude protein of meat was measured by the Kjeldahl nitrogen method. IMF content was determined with diethyl ether extraction by using a Soxhlet apparatus.
Determination of blood biochemical parameters
Serum TG, TC, LDL-C, and HDL-C level were determined by diagnostic kits of Determiner-C TG, Determiner-L TCII, Determiner-L LDL-C, and Determiner-L HDL-C (Kyowa Medex, Japan), respectively, according to the manufacturer’s manuals. Glycation serum protein (GSP) level was determined by the Fructosamine Assay kit (Chengdu, China). A routine glucose oxidase method was used to measure the glucose concentration. NEFA level in serum was determined by a colorimetric assay kit (Jiangcheng, Nanjing, China). All measurements were operated in an AU5421 Automatic Biochemistry Analyzer (Backman-Kelt, USA) at the First Affiliated Hospital of Nanchang University.
Measuring the activities of lipogenic enzymes
To test the activities of lipogenic enzymes, crude enzyme liquid was prepared by homogenizing and centrifuging the mixture of 100 mg tissue sample with 1000 mL physiological saline. The activities of glucose-6-phosphate dehydrogenase (G6PDH), isocitrate dehydrogenase (ICDH), and malate dehydrogenase (MDH) were determined by colorimetric assay kits (Comin BIO-TEK, Suzhou, China). For G6PDH and ICDH, the enzyme activities were defined as nanomoles of NADPH that was produced per minute and per milligram protein. For MDH, nanomoles of NADH that was spent per minute and per milligram protein was set as its activity. Bovine serum albumin was selected as the standard in the measurement of soluble protein content in crude enzyme liquid.
Statistical analysis
The significance of each comparison of phenotypic values between control and NA group were tested by one-way analysis of variance (ANOVA). All statistics were performed with SPSS 17.00 software (SPSS Inc., Chicago, IL). The significance threshold was set at P ≤ 0.05, and the trends were determined at P ≤ 0.10. The results presented in the tables and the figures are the means and standard error of means (SEM).
Results
Effect of supplemental NA on carcass traits
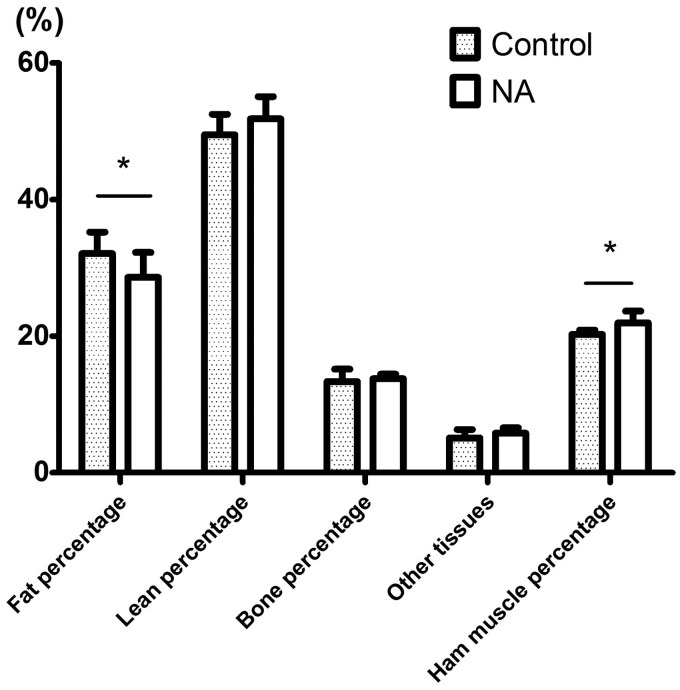
Steers fed NA diet have significantly higher slaughter weight than those fed control diet (703.25 kg vs. 652.73 kg, P < 0.05), but no significant difference was observed in hot carcass weight and dressing percentage between two groups (Table 2). As shown in Figure 1, dietary NA increased longissimus area and ham muscle percentage by 6.03 cm2 and 2.34% (P < 0.05), respectively, and reduced carcass fat percentage by 6.54% (P < 0.05). However, there was no significant difference in backfat thickness, carcass lean percentage, and bone percentage between two groups (P > 0.15).
Table 2.
Effects of supplemental NA on carcass characteristics in finishing steers
| Item | Control group | NA group | SEM* | P-value |
|---|---|---|---|---|
| Slaughter wt. (kg) | 652.73 | 703.25 | 13.116 | 0.054 |
| Hot carcass wt. (kg) | 388.64 | 412.63 | 7.983 | 0.142 |
| Dressing percentage (%) | 59.51 | 58.75 | 0.552 | 0.513 |
| Backfat thickness (cm) | 3.40 | 3.20 | 0.150 | 0.512 |
| Longissimus area (cm2) | 90.90 | 96.93 | 1.436 | 0.026 |
SEM: standard error of the means, n = 8 steers per treatment.
Figure 1.
Effect of supplemental NA on carcass traits in Chinese crossbred finishing steers. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01
Effect of dietary NA on meat quality
Supplemental NA did not affect pH45 min (P > 0.05), but significantly decreased the pH24 of LD muscle (5.49 vs. 5.59, P < 0.01, Figure 2). Marbling score was significantly improved by supplemental NA (8.080 vs. 4.300, P < 0.001, Figure 3), but we did not observe the significant difference in shear force between two groups. NA supplementation did affect the meat color values with regarding to higher redness (a*) (22.00 vs. 16.65, P < 0.05) and chroma (C*) (22.31 vs. 17.02, P < 0.05, Figure 4), but did not affect the yellowness (b*) and hue angle (H*) (P > 0.05). Although meat lightness (L*) was also decreased by dietary NA (36.94 vs. 38.95), the difference did not achieve the significance level (P = 0.077).
Figure 2.
Effect of supplemental NA on meat quality. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01
Figure 3.
Effect of dietary NA on intramuscular fat content (IMF) in Chinese crossbred steers during the finishing period. N1, N2, N3, and N4 were LD samples from NA group; C1, C2, C3, and C4 were LD samples from control group. (A color version of this figure is available in the online journal.)
Figure 4.
Effect of dietary NA on meat color. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01. L*: Lightness; a*: Redness; b*: Yellowness; C*: Chroma; H*: Hue angle
More interestingly, supplemental NA significantly increased IMF content (17.14 vs. 9.03, P < 0.001, Figures 3 and 5) and decreased the moisture of LD muscle (60.89 vs. 67.00, P < 0.007). But we did not detect significant effect of NA on crude protein content (P > 0.05, Figure 5).
Figure 5.
Effect of supplemental NA on meat chemical compositions. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01
IMF: intramuscular fat; CP: crude protein
Effect of dietary NA on blood biochemical parameters
Compared with that in steers fed control diet, the lipid metabolites including TG, NEFA, TC, and LDL-C were significantly reduced in steers fed NA diet (P < 0.05, Figure 6), but serum HDL-C level in NA group was significantly increased (2.84 vs. 2.40, P < 0.05, Figure 6). Although serum glucose level was not significantly affected by supplemental NA, GSP content was significantly reduced by NA treatment (1.58 vs. 1.75, P < 0.01). More interestingly, dietary NA reduced more than half of serum NEFA in NA group (0.055 vs. 0.128, P < 0.01).
Figure 6.
Effect of supplemental NA on blood biochemical parameters. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01
GLU: glucose; GSP: glycation serum protein; TG: triglycerides; NEFA: non-esterified fatty acid; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol
Effect of dietary NA on the activities of lipogenic enzymes of LD muscle
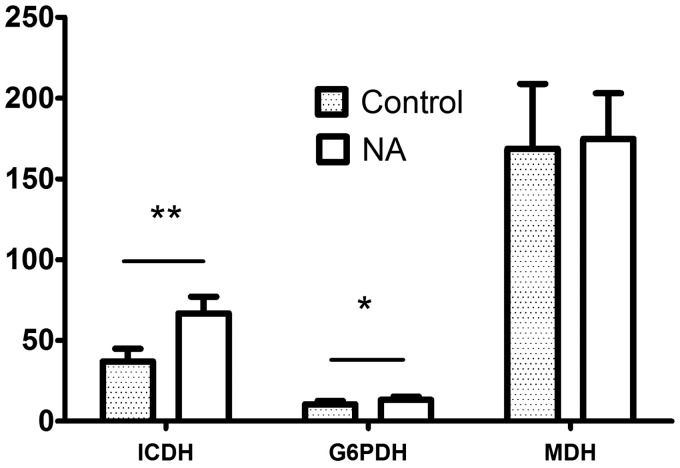
Supplemental NA affected the activities of lipogenic enzymes of LD muscle (Figure 7). Dietary NA almost doubled the ICDH activity (66.73 vs. 38.66, P < 0.001), and also significantly increased the activity of G6PDH in LD muscle of treated group (13.23 vs. 10.05, P < 0.05). However, there was no significant difference on MDH activity between two groups (177.77 vs. 167.94, P > 0.05) (Figure 7).
Figure 7.
Effect of supplemental NA on the activities of lipogenic enzymes. * represents the significance level of P < 0.05 and ** indicates the significance level of P < 0.01
ICDH: isocitrate dehydrogenase; G6PDH: glucose-6-phosphate dehydrogenase; MDH: malate dehydrogenase
Discussion
NA can be converted to NAD+ and NADP+, which participate in the processes of lipid metabolism, tissue oxidation, respiratory functions, and glycolysis.4,5 But, there have been few reports about its effects on beef quality. Thus, in current study, we evaluated the effects of NA supplementation in diet on carcass traits, meat quality, and blood metabolites in Chinese crossbred steers.
Supplemental NA significantly increased the slaughter weight of Chinese finishing steers, which was consistent with the results reported in pigs and chicken.16–19 We also found that supplemental NA significantly reduced carcass fat percentage (not including IMF). This was in agreement with the results reported by Jiang et al.,19 in which chicken abdominal fat percentage was significantly reduced by feeding 120 mg/kg NA. The similar conditions were also observed in humans, where Chinese dyslipidemia patients treated with NA showed significant reductions in liver fat content (P < 0.001) and visceral fat content (P < 0.05) through inhibiting DGAT2 gene expression.9 However, Celik et al.20 discovered that supplementation of 50 mg/L NA through drinking water did not affect the carcass weight, carcass yield, and abdominal fat weight of broiler chicks. These contradictory results might be caused by different breeds of experimental animals and different way of feeding NA. Dietary NA increased the longissimus area in current study. This should be due to the increased fat deposition in muscle. This hypothesis was verified by Fujimori et al.,10 in which NA showed a positive effect on adipogenesis and TG deposition in adipocytes.
The change of meat pH value is a key indicator to evaluate the degree of glycogenolysis by glycosome after slaughter.21 Beef with pH24 h > 5.5 is regarded as the result of less glycogen concentration in muscle and the consequent inability to accumulate enough lactic acid content in muscle.22 Current results showed that NA supplementation decreased the pH24 h of LD muscle in finishing steers (P < 0.05). The reduced pH24 h may be attributed to the result of the increased glycogen concentration in muscle or the improvement of the activities of glycogen decomposition enzymes by NA treatment. But we did not observe the effect of dietary NA on pH45 min. This suggested that the change of biochemical components in beef was not significant in a short time after slaughter.
Dietary NA increased the marbling score and IMF content of LD muscle (P < 0.01). This result may be related to the promoting effect of NA on differentiation of adipocyte and formation of lipid droplets in preadipocytes.10 Similar results were also shown in Real et al.16 in which supplemental NA in commercial pigs increased the marbling score of LD muscle compared with the control group. Besides, current results showed that supplemental NA reduced the moisture of LD muscle (P < 0.01), which may be due to the fact that high IMF content reduced the water holding capacity of muscle.23 Interestingly, dietary NA did not increase the backfat thickness. From these results, we suggested that dietary NA can improve the meat quality and increase the meat production (increased loin eye area, P < 0.05), but does not affect subcutaneous fat deposition. Previous study also showed that NA supplementation might keep IMF content but did not increase the undesirable abdominal and subcutaneous fat in chicken.19
Meat color is an important commercial characterization that influences the consumers’ purchasing decision. The intensity of meat visual color is largely due to the relative proportions of oxymyoglobin (bright red, the ferrous oxymetmyoglobin form of myoglobin), myoglobin (dark red), and metmyoglobin (gray-brown).24 To keep the stability of meat color, the myoglobin should sustain its ferrous oxygenated form (Fe2+) rather than oxygenated ferric (Fe3+) metmyoglobin.24 NA supplementation increased the redness (a*) and chroma (C*) of LD muscle, which meant that NA increased the proportion of oxymyoglobin. Meat color has been found to be closely associated with a higher content of oxidative fibers in muscles. Studies have shown that niacin could induce type II muscle fibers switching to type I muscle fibers.25,26 Type I muscle fibers are redder in color, and have higher oxidative capacity and glycolytic enzyme activity.27
In this study, NA reduced the concentrations of serum TG, NEFA, GSP, TC, and LDL-C, but increased the concentration of HDL-C. As the matter of fact, NA has been used to treat cardiovascular disease in humans for a long time because of its effect on regulating serum lipids.28–30 Similar results have also been reported in previous studies in cattle.13–15 Compared with the control group, NEFA level in NA group was decreased more than 50%. Serum NEFA is mainly from the synthesis in liver and the TG decomposition in adipocyte. So the possible mechanisms for this reduction of serum NEFA level are (a) NA administration inhibited the TG decomposition in adipocyte and (b) dietary NA reduced the NEFA synthesis in liver.
NADPH is one of the most important enzymes for de novo fatty acid synthesis. The enzymes including ICDH, G6PDH, and MDH that are involved in the synthesis of NADPH were chosen to determine the activities. As a restricted enzyme of pentose phosphate pathway and because of its effect on facilitating gluconeogenesis, the activity of G6PDH is positively connected with the deposition of IMF in beef, and thus could be treated as an index reflecting the marbling.31 Supplemental NA increased the activities of G6PDH and ICDH, which might be a good explanation for the improvement of IMF and the marbling score of LD muscle in this study. The result was also concordance with the report from in vitro cell culture, in which NA increased the NADPH level and the expressions of G6PDH and G3PDH.32 These results suggested that the increased IMF deposition and meat quality in current study might be due to the increased activities of NADPH synthetase such as G6PDH and ICDH for de novo fatty acid synthesis.
In conclusion, NA supplementation in diet up-regulated the activities of G6PDH and ICDH, increased serum HDL-C level, longissimus area, meat color, IMF content, and marbling score of LD muscle, but reduced carcass fat content (not including IMF), pH24 h and moisture content, and decreased LDL-C and other serum metabolite levels. These results suggested that NA plays an important role in modifying the bovine lipid metabolism. Considering the rich resource and low price, supplementation of NA in diet should be a promising way to improve the beef quality in cattle industry.
Acknowledgements
This study was supported by the Natural Science Foundation of China (31402100) and National Beef Cattle Industry Technology & System (CARS-Beef Cattle System: CARS-38). We also thank Hunan Tianhuan Stockbreeding Co., Ltd for their help on cattle feeding experiment.
Authors’ contributions
Z-QY was responsible for the experimental design, experiments, statistical analyses, and the manuscript writing; L-BB conducted the feeding experiment; X-HZ, C-YW, SZ, J-MG, C-BF, L-JX, and C-JL carried out the data collection; M-RQ, the project leader, was responsible for the administration and review of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, Jiang Z, Poulos SP, Sainz RD, Smith S, Spurlock M, Novakofski J, Fernyhough ME, Bergen WG. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci 2009; 87: 1218–46. [DOI] [PubMed] [Google Scholar]
- 2.Smith SB, Kawachi H, Choi CB, Choi CW, Wu G, Sawyer JE. Cellular regulation of bovine intramuscular adipose tissue development and composition. J Anim Sci 2009; 87(14 Suppl): E72–82. [DOI] [PubMed] [Google Scholar]
- 3.Du M, Yin J, Zhu MJ. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci 2010; 86: 103–9. [DOI] [PubMed] [Google Scholar]
- 4.Thornton JH, Schultz LH. Effects of administration of nicotinic acid on glucose, insulin, and glucose tolerance in ruminants. J Dairy Sci 1980; 63: 262–8. [DOI] [PubMed] [Google Scholar]
- 5.Chilliard Y. Dietary fat and adipose tissue metabolism in ruminants, pigs, and rodents: a review. J Dairy Sci 1993; 76: 3897–931. [DOI] [PubMed] [Google Scholar]
- 6.Kang I, Kim SW, Youn JH. Effects of nicotinic acid on gene expression: potential mechanisms and implications for wanted and unwanted effects of the lipid-lowering drug. J Clin Endocrinol Metab 2011; 96: 3048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukasova M, Hanson J, Tunaru S, Offermanns S. Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol Sci 2011; 32: 700–7. [DOI] [PubMed] [Google Scholar]
- 8.Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res 2004; 45: 1835–45. [DOI] [PubMed] [Google Scholar]
- 9.Hu M, Chu WC, Yamashita S, Yeung DK, Shi L, Wang D, Masuda D, Yang Y, Tomlinson B. Liver fat reduction with niacin is influenced by DGAT-2 polymorphisms in hypertriglyceridemic patients. J Lipid Res 2012; 53: 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimori K, Amano F. Niacin promotes adipogenesis by reducing production of anti-adipogenic PGF2alpha through suppression of C/EBPbeta-activated COX-2 expression. Prostaglandins Other Lipid Mediat 2011; 94: 96–103. [DOI] [PubMed] [Google Scholar]
- 11.Christensen RA, Clark JH, Drackley JK, Blum SA. Fatty acid flow to the duodenum and in milk from cows fed diets that contained fat and nicotinic acid. J Dairy Sci 1998; 81: 1078–88. [DOI] [PubMed] [Google Scholar]
- 12.Erickson PS, Trusk AM, Murphy MR. Effects of niacin source on epinephrine stimulation of plasma nonesterified fatty acid and glucose concentrations, on diet digestibility and on rumen protozoal numbers in lactating dairy cows. J Nutr 1990; 120: 1648–53. [DOI] [PubMed] [Google Scholar]
- 13.Titgemeyer EC, Spivey KS, Mamedova LK, Bradford BJ. Effects of pharmacological amounts of nicotinic acid on lipolysis and feed intake in cattle. Int J Dairy Sci 2011; 6: 134–41. [Google Scholar]
- 14.Yuan K, Shaver RD, Bertics SJ, Espineira M, Grummer RR. Effect of rumen-protected niacin on lipid metabolism, oxidative stress, and performance of transition dairy cows. J Dairy Sci 2012; 95: 2673–9. [DOI] [PubMed] [Google Scholar]
- 15.Pescara JB, Pires JA, Grummer RR. Antilipolytic and lipolytic effects of administering free or ruminally protected nicotinic acid to feed-restricted Holstein cows. J Dairy Sci 2010; 93: 5385–96. [DOI] [PubMed] [Google Scholar]
- 16.Real DE, Nelssen JL, Unruh JA, Tokach MD, Goodband RD, Dritz SS, DeRouchey JM, Alonso E. Effects of increasing dietary niacin on growth performance and meat quality in finishing pigs reared in two different environments. J Anim Sci 2002; 80: 3203–10. [DOI] [PubMed] [Google Scholar]
- 17.Waldroup PW, Hellwig HM, Spencer GK, Smith NK, Fancher BI, Jackson ME, Johnson ZB, Goodwin TL. The effects of increased levels of niacin supplementation on growth rate and carcass composition of broiler chickens. Poult Sci 1985; 64: 1777–84. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz N, Harms RH, Linda SB. Niacin requirement of broiler chickens fed a corn-soybean meal diet from 1 to 21 days of age. Poult Sci 1990; 69: 433–9. [DOI] [PubMed] [Google Scholar]
- 19.Jiang RR, Zhao GP, Chen JL, Zheng MQ, Zhao JP, Li P, Hu J, Wen J. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J Anim Physiol Anim Nutr (Berl) 2011; 95: 137–45. [DOI] [PubMed] [Google Scholar]
- 20.Celik L, Ozturkcan O, Inal TC, Canacankatan N, Kayrin L. Effects of L-carnitine and niacin supplied by drinking water on fattening performance, carcass quality and plasma L-carnitine concentration of broiler chicks. Arch Tierernahr 2003; 57: 127–36. [DOI] [PubMed] [Google Scholar]
- 21.Villarroel M, Maria GA, Sanudo C, Olleta JL, Gebresenbet G. Effect of transport time on sensorial aspects of beef meat quality. Meat Sci 2003; 63: 353–7. [DOI] [PubMed] [Google Scholar]
- 22.Mach N, Bach A, Velarde A, Devant M. Association between animal, transportation, slaughterhouse practices, and meat pH in beef. Meat Sci 2008; 78: 232–8. [DOI] [PubMed] [Google Scholar]
- 23.Byrne DV, Bredie WL, Bak LS, Bertelsen G, Martens H, Martens M. Sensory and chemical analysis of cooked porcine meat patties in relation to warmed-over flavour and pre-slaughter stress. Meat Sci 2001; 59: 229–49. [DOI] [PubMed] [Google Scholar]
- 24.Carlez A, Veciana-Nogues T, Cheftel JC. Changes in colour and myoglobin of minced beef meat due to high pressure processing. LWT-Food Sci Technol 1995; 28: 528–38. [Google Scholar]
- 25.Khan M, Couturier A, Kubens JF, Most E, Mooren FC, Kruger K, Ringseis R, Eder K. Niacin supplementation induces type II to type I muscle fiber transition in skeletal muscle of sheep. Acta Vet Scand 2013; 55: 85–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringseis R, Rosenbaum S, Gessner DK, Herges L, Kubens JF, Mooren FC, Kruger K, Eder K. Supplementing obese Zucker rats with niacin induces the transition of glycolytic to oxidative skeletal muscle fibers. J Nutr 2013; 143: 125–31. [DOI] [PubMed] [Google Scholar]
- 27.Klont RE, Brocks L, Eikelenboom G. Muscle fibre type and meat quality. Meat Sci 1998;49S1:S219–29. [PubMed]
- 28.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys 1955; 54: 558–9. [DOI] [PubMed] [Google Scholar]
- 29.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 2005; 258: 94–114. [DOI] [PubMed] [Google Scholar]
- 30.Kamanna VS, Kashyap ML. Mechanism of action of niacin. T Am J Cardiol 2008; 101: 20B–26B. [DOI] [PubMed] [Google Scholar]
- 31.Bonnet M, Faulconnier Y, Leroux C, Jurie C, Cassar-Malek I, Bauchart D, Boulesteix P, Pethick D, Hocquette JF, Chilliard Y. Glucose-6-phosphate dehydrogenase and leptin are related to marbling differences among Limousin and Angus or Japanese Black x Angus steers. J Anim Sci 2007; 85: 2882–94. [DOI] [PubMed] [Google Scholar]
- 32.Yan Q, Briehl M, Crowley CL, Payne CM, Bernstein H, Bernstein C. The NAD+ precursors, nicotinic acid and nicotinamide upregulate glyceraldehyde-3-phosphate dehydrogenase and glucose-6-phosphate dehydrogenase mRNA in Jurkat cells. Biochem Biophys Res Commun 1999; 255: 133–6. [DOI] [PubMed] [Google Scholar]