Abstract
Nonproliferative diabetic retinopathy (DR) is characterized by multiple degenerative changes that could be potentially corrected by stem cell therapies. Most studies so far have attempted to alleviate typical abnormalities of early retinopathy, including vascular hyperpermeability, capillary closure and pericyte dropout. Success was reported with adult stem cells (vascular progenitors or adipose stem cells), as well as induced pluripotent stem cells from cord blood. The cells were able to associate with damaged vessels in both pericyte and endothelial lining positions in models of DR and ischemia-reperfusion. In some diabetic models, functional amelioration of vasculature and electroretinograms was noted. Another approach for endogenous progenitor cell therapy is to normalize dysfunctional diabetic bone marrow and residing endothelial progenitors using NO donors, PPAR-δ and -γ agonists, or inhibition of TGF-β. A potentially important strategy would be to reduce neuropathy by stem cell inoculations, either naïve (e.g., paracrine-acting adipose stem cells) or secreting specific neuroprotectants, such as ciliary neurotrophic factor or brain-derived neurotrophic factor that showed benefit in amyotrophic lateral sclerosis and Parkinson’s disease. Recent advances in stem cell therapies for diabetic retinal microangiopathy may form the basis of first clinical trials in the near future. Additionally, stem cell therapies may prove beneficial for diabetic corneal disease (diabetic keratopathy) with pronounced epithelial stem cell dysfunction.
Keywords: Diabetic retinopathy, stem cell, neuroprotection, endothelial progenitor cell, diabetic keratopathy
Introduction
Diabetic retinopathy (DR) has been usually considered as a vascular disease or, particularly, an endotheliopathy manifested by ischemia-induced pathologic alterations in the retinal microvasculature. In recent years, however, it has become more obvious that diabetes-associated neurodegenerative changes take place before endothelial alterations, suggesting that diabetic retinopathy should be regarded as a neurovascular degenerative disease.
A continuous hyperglycemic condition leads to dysfunction and loss of endothelial cells (EC), pericytes and vascular smooth muscle cells, causing hypoxia. This is characteristic of both type I (insulin-dependent) and type II (non-insulin-dependent) diabetes. Specifically, pericyte and EC ability for self-renewal is impaired in diabetes, and their turnover potential is finally depleted,1,2 after which acellular capillaries become non-perfused, and adjacent retina turns hypoxic. This hypoxic environment upregulates vascular endothelial growth factor (VEGF), which contributes to elevated vascular permeability3,4 that can result in diabetic macular edema and, eventually, in loss of visual function.5 Neurodegeneration, which includes neuronal apoptosis and glial cell reactivity, occurs in diabetes before the alterations of ECs are observed.6–8 In fact, persistent hyperglycemia during early retinopathy can lead to ganglion cell depletion7 and consequently, to changes in the retinal electrical activity prior to a noticeable endotheliopathy.9 Neuroretinal hypoxia stimulates the translocation of hypoxia-inducible factor-1α (HIF-1α),10 which induces the expression of hypoxia-regulated cytokines and growth factors, such as VEGF. VEGF-driven increased proliferation of ECs initially causes intraretinal microvascular abnormalities (IRMA) in areas lacking functional capillaries11 and may also be responsible for the development of microaneurysms in which ECs proliferate in the absence of pericytes that normally keep ECs dormant. Non-proliferative DR (NPDR) is manifested by capillary non-perfusion, leakage, microaneurysms and IRMAs (Figure 1). EC proliferation and migration from vessels can eventually result in the development of preretinal neovascularization characteristic of proliferative DR (PDR). More than 3.5% of type I diabetic patients lose vision as a consequence of the disease,12 which makes DR one of the leading causes of blindness in the world. At present, there are few effective treatments of DR focused on prevention, including tight glycemic and cholesterol control that may maintain sustained protection from disease progression.13–16 When the disease progresses to sight-threatening macular edema or PDR, laser photocoagulation can help to regress disease by destroying peripheral retina and reducing oxygen demand.17,18 However, photocoagulation may bring about complications resulting in deteriorating visual acuity, retinal thickening and field loss.19
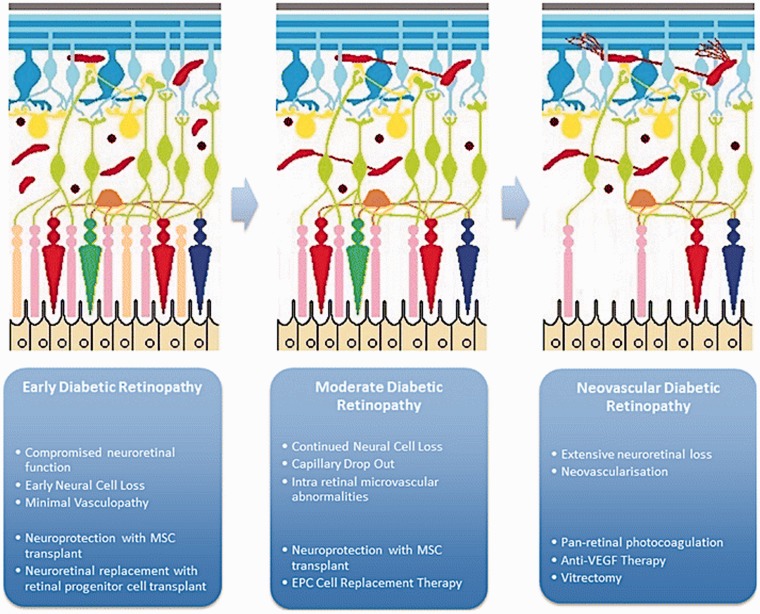
Figure 1.
Maximizing the potential efficacy of stem cell therapies will depend on the timing of their use. MSC-derived neuroprotection and neuroretinal cell replacement may help if given early in disease. EPC-derived vascular regeneration may offer benefit right up until end stage disease. Reproduced with permission from Megaw and Dhillon. 23 (A color version of this figure is available in the online journal.)
To date, no treatment has yet been developed to support regeneration of the damaged retinal vasculature as a result of long-term hyperglycemia. Cell-based therapies may be a feasible option for both preventing neurovascular damage and promoting regeneration of damaged retina, as evidenced by recent studies with several types of stem cells (Figure 1). Novel approaches developed recently (for reviews see literatures20–23) to treat early and moderate DR are mainly based on the ability of mesenchymal stem cells (MSC) to produce neuroprotective and neurotrophic factors, and the potential of endothelial progenitor cells (EPC) to repair vasculature, or the ability of adipose stromal cells (ASC) to accomplish both of the above functions.
Mesenchymal stem cells and their role in diabetes
These are multipotent stromal cells usually isolated from bone marrow, although they can be found in most tissues.24 In vitro cultured MSCs are known to express such surface markers as CD105, CD44, CD90, CD166, CD54 and stromal antigen 125 but lack surface markers that are characteristic for hematopoietic cells (CD45, CD11a and CD14).26 MSCs have recently become possible candidates for use in disease treatment and tissue replacement due to several factors. These include relatively simple donor biopsies that can be expanded in vitro and administered intravenously, allowing an autologous treatment. Also, MSCs secrete neuroprotective growth factors such as fibroblast growth factor-2 (FGF-2) and ciliary neurotrophic factor (CNTF),27 and they proved to be safe in human trials so far.
The ability of MSCs to maintain and restore the neural retina damaged in degenerative diseases was demonstrated for age-related macular degeneration (AMD) and retinitis pigmentosa (RP). When injected locally or systemically, engrafted MSCs were reported to provide visual protection and a delay in degeneration.28 This could be due to stimulation of resident neural progenitors to regenerate neuroretinal tissue,27 paracrine supply of neuroprotectants29–31 or their possible differentiation into photoreceptors and retinal pigment epithelium in these disease models.32–35
MSCs have a potential as candidates for the treatment of diabetes, although mechanisms of their action in alleviating organ damage (immunomodulatory, neuroprotective, or regenerative) remain disputable. MSCs have immunomodulatory effect as they inhibit differentiation of monocytes into dendritic cells in vivo.36 In addition, MSCs can increase levels of the anti-inflammatory cytokine interleukin (IL)-10 and downregulate levels of the pro-inflammatory IL-12 and interferon-γ.37–40 The benefit of MSCs for treating diabetes and protecting vascular cells is thought to be due to their production of trophic and immunomodulatory factors.40,41 This hypothesis was partially corroborated by the finding that normal MSCs transiently alleviated hyperglycemia in NOD mice, whereas NOD MSCs did not.42 It should be noted that MSCs derived from bone marrow of non-obese diabetic (NOD) mice have decreased capacity for adhesion and migration43 and reduced ability for retinal differentiation,44 as well as more pro-inflammatory cytokine profile.
In early diabetes, the loss of pericytes and neuroretinal damage appear to be caused by oxidative stress.2 MSCs were shown to absorb reactive oxygen species (ROS) via expression of sulfoxide reductase A, which may suggest a mechanism for their neuroprotective effect.45 When delivered intravitreally, MSCs could protect the neuroretina in degenerative retinal animal models by secreting neurotrophic factors that may prevent apoptosis, stimulate angiogenesis, and promote resident neural progenitors to regenerate neuroretinal tissue.27,29–31
It is presently unclear whether dysfunctional tissue recovery is mostly due to trophic factors secreted by MSCs that facilitate survival of degenerating tissue and the endogenous stem cells or to the transdifferentiation of transplanted cells that functionally integrate into the diseased tissue. In this respect, it is worth mentioning that MSCs can self-renew and differentiate into tissues of mesodermal origin.37,38 However, some data also suggest their ability to turn into other cell types, such as glial and neural cells,46–49 or pancreatic50–52 and hepatocyte-like cells.53,54 Despite considerable debate regarding the ability of MSC-derived cells to express markers’ characteristic for fully differentiated ecto- and endodermal lineages, there is growing evidence supporting MSCs potential for generating cell types of multiple lineages suitable for cell therapy in various degenerative and metabolic conditions, including diabetes (reviewed in literatures32,55,56). This issue needs further investigation in connection with the retinal changes in degenerative diseases including DR.
Although the exact mechanisms by which MSCs provide neuroprotection for damaged retina still remain somewhat unclear, advantages of MSCs are obvious, and clinical trials may soon be underway examining their effect on visual function in ischemic and diabetic retinopathies. At the same time, their autologous use in diabetic patients may be hampered because of their dysfunctional state. A promising approach for future studies would be normalization of their functions using gene therapy, antioxidants, etc.
Endothelial progenitor cells
For a long time, conventional view was that retinal vasculature develops after birth. This view was contested by a breakthrough study that isolated a population of circulating cells capable to differentiate into endothelial cells and to play a role in adult neovascularization.57 The presence of circulating cells involved in endothelial repair was supported by the finding that bone marrow transplantation leads to donor-derived endothelial cells in the vessel wall.58 These rare cells, called endothelial progenitor cells (EPCs), are able to migrate to areas of ischemia and incorporate into sites of active angiogenesis.57 In fact, retinal ischemia generated by retinal vein occlusion was shown to promote the re-endothelialization of acellular capillaries leading to retinal re-vascularization.59
In diabetes, IRMAs contain many endothelial cells and are thought to be a result of the ischemic retina trying to stimulate angiogenesis. While there is a general decline of retinal angiogenesis in diabetes, the slow and gradual character of the disease allows for reparative angiogenesis to occur. If retinal angiogenesis could be targeted successfully, there is a possibility that it could eliminate ischemia that drives DR.
Impaired EPC and HSPC mobilization in diabetes
EPCs represent heterogeneous groups of cells ranging from mostly proangiogenic hematopoietic cells to subsets of hematopoietic stem and progenitor cells (HSPCs).22,60,61 Circulating EPCs were first identified when human CD34+ cells (a HSPC marker) or mouse flk1+ (endothelial marker also known as VEGF receptor 2, or VEGFR2) cells from the peripheral blood were found to acquire endothelial-like properties in vitro and promote neovascularization in response to ischemia.57 EPCs are usually defined in humans as peripheral mononuclear cells that are positive for the stem cell markers (CD34, VEGFR2 and/or CD133), and can repair damaged vasculature by directly differentiating into endothelial cells (re-endothelialization), or by paracrine actions of EPCs that stimulate resident progenitor cells (neovascularization).62,63
Numerous studies showed diabetes-associated changes in EPCs, including a decrease in circulating EPCs,64 and defects in proliferation and vascular tube formation in vitro.65,66 The number of circulating EPCs is reduced in patients with both types of diabetes,67,68 which is usually associated with diabetic complications.69 Additionally, the number of CD34+ cells is decreased in the peripheral blood70 and their reaction to granulocyte colony stimulating factor (G-CSF) is compromised in diabetic individuals.71–73 The impaired mobilization of EPCs and HSPCs in diabetes suggests that the bone marrow is also affected by the disease.74
Analogous observations were made in diabetic animal studies that revealed decreased numbers of circulating EPCs and reduced mobilization in response to ischemia75 or wound injury.76 Similarly to humans, mice with short (5–8 weeks) duration of streptozotocin (STZ)-induced type I diabetes have impaired HSPC mobilization in response to G-CSF, which is correlated with an increase in HSC numbers in the bone marrow.71
With regard to mechanisms of these alterations, it was found that diabetic animals have decreased level of a signaling molecule, C-X-C motif chemokine 12 (CXCL12, also called SDF-1) in local tissues and reduced activation of a mobilization-promoting endothelial nitric oxide synthase (eNOS) pathway in the bone marrow.
Interestingly, mobilization defects depend on microenvironment and take place only in nondiabetic-to-diabetic bone marrow transplantation and not in diabetic-to-nondiabetic transplants. Studies of the stem cell niche revealed that decreased number of osteoblasts and altered innervation are among factors contributing to mobilization defects. There is an extensive network of nerves in the bone marrow, including sympathetic nervous system (SNS) that is needed to mobilize HSPC.77 Also, inhibition of osteoblast activity and suppression of CXCL12 in the bone by SNS mediate, in part, G-CSF-induced mobilization of HSPCs.77 Circulating HSPCs were found to display circadian oscillations caused by circadian secretion of noradrenaline by the SNS into the BM, which downregulates CXCL12 via adrenergic receptor on MSCs.78,79 Involvement of the bone marrow sympathetic nerves in the regulation of EPC release was studied in a rat model of type II diabetes,80 which revealed a reduction in sympathetic nerve terminals followed by decreased circadian release of EPCs and accumulation of slowly proliferating EPCs in the bone marrow.
These studies strongly implicate dysfunctional stem cell niche in the bone marrow in diabetes and emphasize possible structural and functional changes in the bone marrow induced by diabetes.
EPCs potential for cell replacement
There are two main subtypes of EPCs isolated from the peripheral blood that share certain surface markers, such as CD34, CD131, and KDR.81,82 One of the subtypes, endothelial colony forming cells (ECFCs), is directly involved in vascular repair by incorporating into mature blood vessels and forming an endothelial layer of the damaged vasculature.83,84 They can also secrete paracrine factors required for vessel repair.85 The other subtype, endothelial cell colony forming units (CFU-EPCs) can affect vascular repair only in a paracrine manner, i.e. by releasing growth factors that stimulate resident endothelial progenitors in blood vessel walls.86
The use of a mixed cell population to enhance EPCs reparative capacity in a mouse model of limb ischemia yielded some promising results.87 However, they were not corroborated by subsequent clinical studies88 suggesting the potential for CFU-EPCs to promote inflammatory response.84 At the same time, ECFCs became incorporated into retinal vasculature, preventing neovascularization in a mouse model of ischemic retinopathy.84 Thus, ECFCs appear to be the true EPCs, making their clinical assessment as a homogenous cell type an important issue.
There are some problems with regard to EPCs use for cell replacement. First, it should be noted that intravenously injected EPCs tend to accumulate in liver and spleen.89 Also, similar to MSCs, a diabetic host environment is a hostile one for EPCs and hinders their migration and adhesion,90 thus decreasing EPCs reparative potential. Noteworthy, the eye has an advantage, compared to other organs, associated with the opportunity to directly and accurately deliver therapeutic cells to the area of ischemia. This helped to start a clinical trial on the safety of intravitreally injected BM-derived CD34+ cells in hereditary retinal dystrophy.91
Diabetic retinopathy, EPC dysfunction and cell therapy
Retinal vasodegeneration is a hallmark of an earlier DR stage, that is, NPDR. In the diabetic environment that is characterized by elevated levels of reactive oxygen species (ROS), vascular progenitors switch to producing pathologic cytokines such as tumor necrosis factor (TNF)-α, IL-8, and to increased expression of pathologic inducible nitric oxide synthase (iNOS) instead of eNOS.21 As a result, diabetic EPCs have reduced bioavailable NO due to either decreased eNOS activity or increased generation of ROS via upregulated NADPH oxidase.92 NO-mediated signaling events are also important for the mobilization of EPCs from the bone marrow and in their homing to ischemic regions.93,94 As shown by the Grant’s group, the function of diabetic EPCs can be partially restored by increasing eNOS expression, either by using NO donors, or by reducing NADPH oxidase-dependent ROS production.95,96 Besides increased ROS and reduced bioavailable NO, several other molecular alterations have been found in dysfunctional diabetic EPCs, including decreased cathepsin L activity97 and elevated expression of thrombospondin-1.98
Regarding the late stage proliferative disease, or PDR, recent studies suggest that the high numbers of bone marrow-derived EPCs constitute a major factor in the development of such serious complications as pathologic neovascularization of ischemic tissues. Although increased numbers of circulating CD34+CD45− endothelial colony-forming cells (ECFCs) were found in PDR patients compared with controls,99 these cells were defective in their ability to migrate toward SDF-1, incorporate into and form vascular tubes. These data suggest that even though the ECFCs from PDR patients are mobilized into the circulation, they are unable to properly migrate and repair damaged vascular endothelium.99
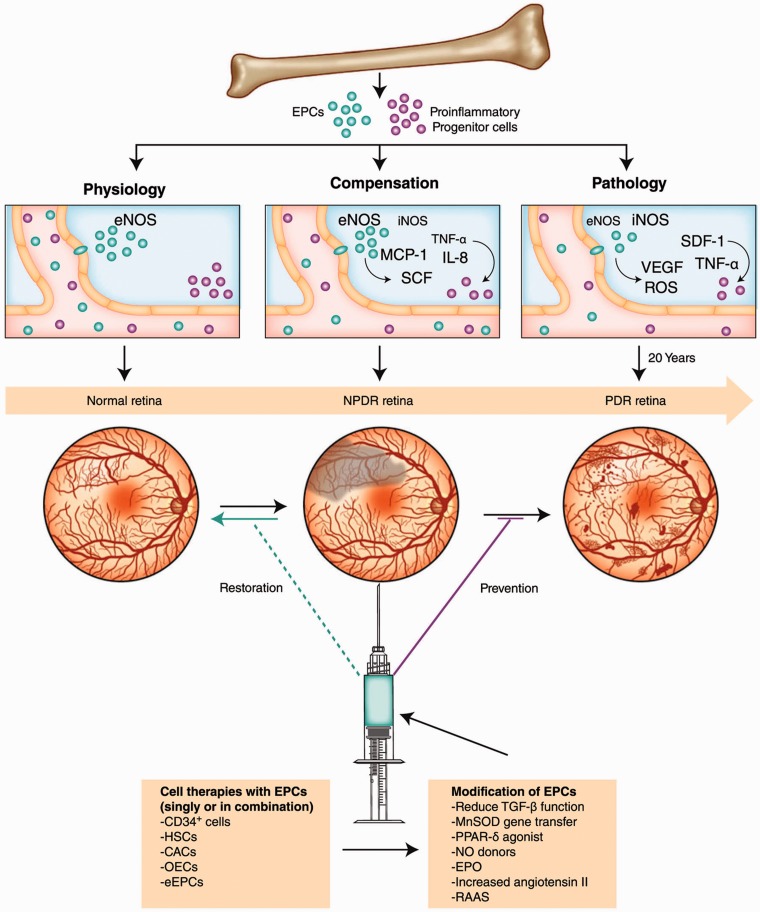
Despite these caveats, cell-based therapy may still represent an effective alternative strategy for the current approaches to the treatment of end-stage DR and other ischemic retinopathies. Cell therapies are designed to target early and intermediate stages of vasodegeneration to promote vascular repair, reverse ischemia, reduce hypoxic or inflammatory signaling, and prevent progression to the late and sight-threatening DR stages.21 This strategy may prove to be successful if autologous progenitors could be modified to function properly. In recent years, a number of approaches have been developed to reverse EPC defects in diabetic patients, including improvement of EPC mobilization and homing with G-CSF100 and SDF-1,101,102 or use of an NO donor to alleviate SDF-1-mediated migration defects.95 Further, some evidence suggests that diabetic EPC dysfunction can be improved or corrected by treatment with peroxisome proliferator-activated receptor (PPAR)-δ and -γ agonists GW501516,103 rosiglitazone,104 or atorvastatin.105 Additionally, it was found that the levels of transforming growth factor (TGF)-β1 were significantly increased both in the EPCs (Figure 2) and in the serum of type 2 diabetic patients.106 TGF-β1 inhibition in CD34+ cells increased cell survival, NO release, and in vivo vascular reparative ability (Figure 2), suggesting that this approach could be used for improving the vasoreparative potential of dysfunctional diabetic CD34+ cells for autologous therapy.106
Figure 2.
Diabetic dysfunction in the BM mobilization of stem/progenitor cells and paracrine regulation of ischemic vascular repair. In normal conditions, factors released by ischemic/injured tissue cause mobilization of BM cells. In diabetes, there is reduced mobilization of BM cells into circulation. Cell therapy in diabetic retinopathy would ideally restore perfusion to areas of the retina that have undergone vasodegeneration associated with NPDR and would prevent the development of advanced disease, PDR
BM: bone marrow; CACs: circulating angiogenic cells; eEPCs: early endothelial progenitor cells; eNOS: endothelial nitric oxide synthase; EPCs: endothelial progenitor cells; EPO: erythropoietin; HSCs: hematopoietic stem cells; IL: interleukin; iNOS: inducible nitric oxide synthase; MCP-1: monocyte chemoattractant protein-1; MnSOD: manganese superoxide dismutase; NO: nitric oxide; NPDR: nonproliferative diabetic retinopathy; OECs: outgrowth endothelial cells; PDR: proliferative diabetic retinopathy; PPAR-δ: peroxisome proliferator-activated receptor-δ; RAAS: renin-angiotensin-aldosterone system; ROS: reactive oxygen species; SCF: stem cell factor; SDF-1: stromal cell–derived factor-1; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α; VEGF: vascular endothelial growth factor. Reproduced with permission from Shaw et al.21 (A color version of this figure is available in the online journal.)
Another potential approach for fighting pathological neovascularization at the late proliferative stage of DR may be based on inhibiting protein kinase CK2 that is involved in retinal angiogenesis.107,108 CK2 inhibitors prevented recruitment of EPCs (Sca-1+/c-kit+ BM-derived HSC) to areas of retinal neovascularization in mouse oxygen-induced retinopathy (OIR) model.109
ASC and iPSC
Adipose stem cells (ASCs) are another class of progenitor cells that share characteristics of both MSCs and EPCs. They can be relatively easily harvested by liposuction, isolated from stromal-vascular fraction of fat, and expanded in vitro to promote angiogenesis.110 CD34+ cells isolated from the adipose tissue prevent endothelial apoptosis and stabilize vasculature,111 and are believed to originate from resident pericytes.112
Intravitreally injected ASCs incorporate into retinal vasculature, acquire pericyte position, and prevent retinal endothelial apoptosis and capillary dropout by about 50% and 80%, as was shown in OIR mouse model and Akimba diabetic mice, respectively.113 Interestingly, similar to native retinal pericytes, the pericyte phenotype of ASCs can be enhanced by TGF-β1 treatment making such ASCs more suitable for cell therapy.113 Moreover, ASCs intravitreal inoculation into type I diabetic athymic nude rat led to improvement of electroretinogram, thus also providing neuroprotection.114
Pluripotent stem cells (PSC) represented until recent discovery of induced PSC (iPSC) mostly by embryonic stem cells (ESC) are able to differentiate into any cell type of all three main lineages. ESC-derived retinal progenitor cells showed their ability to integrate and differentiate into functional photoreceptors, as evidenced by a significant improvement of vision in mouse models of retinal degeneration,115,116 although it is unclear whether neuroretinal replacement is likely to be successful in diabetes. Much promise for treatment of retinal pigment epithelium (RPE) dystrophies, such as age-related macular degeneration, is associated with ESC-derived RPE117 that are currently being tested in clinical trials.118 In diabetes, tight junctions of RPE are compromised leading to breakdown of RPE barrier at late stage hypoxia,119,120 which suggests that RPE replacement might be also important for managing DR.
Latest advancements in reprogramming adult somatic cells into iPSCs may allow developing a promising strategy for DR treatment. Recently, vascular progenitors have been generated from iPSC derived from CD34+ cord blood cells.121 Those iPSCs were stimulated to become CD31+/CD146+ vascular progenitors by treatment with high levels of VEGF.122 Interestingly, engraftment of vascular progenitors generated from iPSCs may occur in different positions in the capillary depending on the way of their delivery. CD31+/CD146+ vascular progenitors injected into the vitreous of NOD/SCID mice that had endured ischemic injury to retina resulting in acellular capillaries, migrated to the abluminal pericyte location of the acellular capillaries. When the cells were administered intravenously, they were incorporated into a lumenal position, assuming their role as endothelial cells.123 The use of iPSCs made from cord blood CD34+ cells presents a feasible approach to regenerate acellular capillaries. As during reprogramming, iPSC may shed some of their epigenetic changes that are the basis of diabetic metabolic memory, their use for generation of relatively normal autologous vascular progenitors may constitute a viable strategy for an auxiliary DR treatment.
Corneal stem cells changes in diabetes
If one considers diabetic eye disease at large, stem cells could contribute to future treatments not only for retinal vasculature, but also for diabetic corneal alterations known as diabetic keratopathy. The severity of these alterations, e.g. of neuropathy, correlates with the severity of retinopathy.124 We have recently documented a significant decrease in the expression of a number of putative stem cell markers in the corneolimbal epithelial stem cell compartment.125 As corneal epithelium is renewed by limbal stem cells, this might explain clinically observed delays in diabetic wound healing, for example, after epithelial debridement for vitrectomy126 or refractive surgery.127 Adenoviral gene therapy with overexpression of c-met proto-oncogene and/or silencing of matrix metalloproteinase-10 and cathepsin F normalized epithelial wound healing and stem cell marker expression in human organ-cultured diabetic corneas.125,128 Importantly, gene therapy of the limbal stem cell niche only produced the same normalization of stem cell marker expression and wound healing.129 In the future, gene therapy or replacement of ailing stem cells with cultured normal cells including those made from iPSC130 could become viable options for alleviating diabetic corneal disease.
In summary, stem cells may offer new ways of retarding progression or alleviating symptoms of DR. Currently, their use is considered for earlier stages of DR, before the onset of PDR. Some of these cells secreting special growth factors could serve for neuroprotection in the diabetic retina. Endothelial progenitor cells could be used for preventing and/or repairing capillary closure and reduce pericyte dropout. New strategies to normalize functions of diabetic progenitors offer ways to use them for autologous therapy. The emerging ESC and iPSC technologies may also help generate bankable and renewable sources of stem cells capable upon proper differentiation to enhance cellular regeneration in the diabetic retina.
Acknowledgements
This work was supported by the grants from NIH (R01 EY013431, R01 EY023429).
This paper was presented in part at the 11th International Symposium on ocular pharmacology and therapeutics (ISOPT), June 2014, Reykjavik, Iceland, and at the 12th ISOPT, July 2015, Berlin, Germany.
Authors’ contributions
All authors participated in the writing, review, and editing of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sharma N, Gardiner T, Archer D. A morphologic and autoradiographic study of cell death and regeneration in the retinal microvasculature of normal and diabetic rats. Am J Ophthalmol 1985; 100: 51–60. [DOI] [PubMed] [Google Scholar]
- 2.Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim C. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diab Obes Metab 2008; 10: 53–63. [DOI] [PubMed] [Google Scholar]
- 3.Tolentino MJ, Husain D, Theodosiadis P, Gragoudas ES, Connolly E, Kahn J, Cleland J, Adamis AP, Cuthbertson A, Miller JW. Angiography of fluorestricted anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch Ophthalmol 2000; 118: 78–84. [DOI] [PubMed] [Google Scholar]
- 4.Tolentino MJ, McLeod DS, Taomoto M, Otsuji T, Adamis AP, Lutty GA. Pathological features of vascular endothelial growth factor-induced retinopathy in non-human primates. Am J Ophthalmol 2002; 133: 373–85. [DOI] [PubMed] [Google Scholar]
- 5.Stitt A, O’Neill C, O’Doherty M, Archer D, Gardiner T, Medina R. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res 2011; 30: 149–66. [DOI] [PubMed] [Google Scholar]
- 6.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. JDRF Diabetic Retinopathy Center Group. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006; 55: 2401–11. [DOI] [PubMed] [Google Scholar]
- 7.Gardner T, Antonetti D, Barber A, LaNoue K, Levison S. Diabetic retinopathy: More than meets the eye. Surv Ophthalmol 2002; 47(Suppl 2): S253–62. [DOI] [PubMed] [Google Scholar]
- 8.Barber A. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog Neuro-Psychopharmacol Biol Psychiatry 2003; 27: 283–90. [DOI] [PubMed] [Google Scholar]
- 9.Tzekov R, Arden G. The electroretinogram in diabetic retinopathy. Surv Ophthalmol 1999; 44: 53–60. [DOI] [PubMed] [Google Scholar]
- 10.Semenza G. HIF-1 and human disease: one highly involved factor. Genes Dev 2000; 14: 1983–91. [PubMed] [Google Scholar]
- 11.Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol 1992; 110: 267–76. [DOI] [PubMed] [Google Scholar]
- 12.Fong D, Aiello L, Gardner T, King G, Blankenship G, Cavallerano J, Ferris FL, 3rd, Klein R. Retinopathy in diabetes. Diab Care 2004; 27: S84–7. [DOI] [PubMed] [Google Scholar]
- 13.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977–86. [DOI] [PubMed] [Google Scholar]
- 14.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–65. [PubMed] [Google Scholar]
- 15.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000; 342: 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–53. [PubMed] [Google Scholar]
- 17.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985; 103: 1796–806. [PubMed] [Google Scholar]
- 18.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98: 766–85. [PubMed] [Google Scholar]
- 19.Aiello L. Perspectives on diabetic retinopathy. Am J Ophthalmol 2003; 136: 122–35. [DOI] [PubMed] [Google Scholar]
- 20.Ljubimov AV, Shaw LC, Li Calzi S, Kielczewski JV, Caballero S, Boulton ME, Grant MB. The vascular stem cell. In: Dartt DA, Besharse JC, Dana R. (eds). Encyclopedia of the eye, San Diego, CA: Elsevier, 2010, pp. 289–99. [Google Scholar]
- 21.Shaw LC, Neu MB, Grant MB. Cell-based therapies for diabetic retinopathy. Curr Diab Rep 2011; 11: 265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima H, Kim J, Chan L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol Metab 2014; 25: 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megaw R, Dhillon B. Stem cell therapies in the management of diabetic retinopathy. Curr Diab Rep 2014; 14: 498–506. [DOI] [PubMed] [Google Scholar]
- 24.Porada C, Zanjani E, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther 2006; 1: 365–9. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007; 25: 2739–49. [DOI] [PubMed] [Google Scholar]
- 26.Prockop D. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71–4. [DOI] [PubMed] [Google Scholar]
- 27.Caplan A, Dennis J. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076–84. [DOI] [PubMed] [Google Scholar]
- 28.Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 2007; 25: 602–11. [DOI] [PubMed] [Google Scholar]
- 29.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res 2007; 85: 234–41. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci 2010; 51: 3742–8. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Li X, Yuan J. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol 2009; 247: 503–14. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Zhang Y, Liu X, Ghazaryan E, Li Y, Xie J, Su G. Recent advances of stem cell therapy for retinitis pigmentosa. Int J Mol Sci 2014; 15: 14456–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong L, Wu Q, Song B, Lu B, Zhang Y. Differentiation of rat mesenchymal stem cells transplanted into the subretinal space of sodium iodate-injected rats. Clin Exp Ophthalmol 2008; 36: 666–71. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang QS, Grompe M, Adamus G, Appukuttan B, Lund R. Noninvasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One 2010; 5: e9200–e9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan P, Xu H, Zeng Y, Wang Y, Yin ZQ. Human bone marrow stromal cells can differentiate to a retinal pigment epithelial phenotype when co-cultured with pig retinal pigment epithelium using a transwell system. Cell Physiol Biochem 2013; 31: 601–13. [DOI] [PubMed] [Google Scholar]
- 36.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120–6. [DOI] [PubMed] [Google Scholar]
- 37.Nauta A, Fibbe W. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499–506. [DOI] [PubMed] [Google Scholar]
- 38.Abdi R, Fiorina P, Adra C, Atkinson M, Sayegh M. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 2008; 57: 1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal S, Pittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–22. [DOI] [PubMed] [Google Scholar]
- 40.Volarevic V, Arsenijevic N, Lukic M, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells 2011; 29: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic M. Interleukin-1 receptor antagonist (IL-1Rα) and IL-1Rα producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010; 43: 255–63. [DOI] [PubMed] [Google Scholar]
- 42.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009; 183: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Xia Y, Wang Z, Cao X, Da Z, Guo G, Qian J, Liu X, Fan Y, Sun L, Sang A, Gu Z. Suppression of the PI3K-Akt pathway is involved in the decreased adhesion and migration of bone marrow-derived mesenchymal stem cells from non-obese diabetic mice. Cell Biol Int 2011; 35: 961–6. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Gu Z, Shen B, Xu G, Zhou T, Jiang J, Xing J, Liu S, Li M, Tan W, Feng G, Sang A, Li L. Roles of Wnt/β- catenin signaling in retinal neuron-like differentiation of bone marrow mesenchymal stem cells from nonobese diabetic mice. J Mol Neurosci 2013; 49: 250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmon AB, Pérez VI, Bokov A, Jernigan A, Kim G, Zhao H, Levine RL, Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J 2009; 23: 3601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res 2009; 34: 2030–9. [DOI] [PubMed] [Google Scholar]
- 47.Kim EY, Lee KB, Yu J, Lee JH, Kim KJ, Han KW, Park KS, Lee DS, Kim MK. Neuronal cell differentiation of mesenchymal stem cells originating from canine amniotic fluid. Hum Cell 2014; 27: 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frausin S, Viventi S, Verga Falzacappa L, Quattromani MJ, Leanza G, Tommasini A, Valencic E. Wharton’s jelly derived mesenchymal stromal cells: Biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta Histochem 2015; 117: 329–38. [DOI] [PubMed] [Google Scholar]
- 49.Yang JD, Cheng H, Wang JC, Feng XM, Li YN, Xiao HX. The isolation and cultivation of bone marrow stem cells and evaluation of differences for neural-like cells differentiation under the induction with neurotrophic factors. Cytotechnology 2014; 66: 1007–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE 2008; 1: e1451–e1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu LF, Wang NN, Liu YS, Wei X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A 2009; 15: 2865–73. [DOI] [PubMed] [Google Scholar]
- 52.Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: Perspectives and future endeavors. Int J Biochem Cell Biol 2014; 46: 90–102. [DOI] [PubMed] [Google Scholar]
- 53.Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev 2010; 19: 423–38. [DOI] [PubMed] [Google Scholar]
- 54.Zhang YN, Lie PC, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy 2009; 11: 548–58. [DOI] [PubMed] [Google Scholar]
- 55.Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev 2011; 7: 342–63. [DOI] [PubMed] [Google Scholar]
- 56.Jadalannagari S, Aljitawi OS. Ectodermal differentiation of Wharton’s jelly mesenchymal stem cells for tissue engineering and regenerative medicine applications. Tissue Eng Part B Rev 2015; 21: 314–22. [DOI] [PubMed] [Google Scholar]
- 57.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7. [DOI] [PubMed] [Google Scholar]
- 58.Jiang S, Walker L, Afentoulis M, Anderson DA, Jauron-Mills L, Corless CL, Fleming WH. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci U S A 2004; 101: 16891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walshe TE, D’Amore PA. The role of hypoxia in vascular injury and repair. Annu Rev Pathol 2008; 3: 615–43. [DOI] [PubMed] [Google Scholar]
- 60.Fadini GP. A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia 2014; 57: 4–15. [DOI] [PubMed] [Google Scholar]
- 61.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2012; 2: a006692–a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urbich C, Dimmeler S. Endothelial progenitor cells characterization and role in vascular biology. Circ Res 2004; 95: 343–53. [DOI] [PubMed] [Google Scholar]
- 63.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011; 29: 1650–5. [DOI] [PubMed] [Google Scholar]
- 64.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600. [DOI] [PubMed] [Google Scholar]
- 65.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004; 53: 195–9. [DOI] [PubMed] [Google Scholar]
- 66.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781–6. [DOI] [PubMed] [Google Scholar]
- 67.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia 2008; 51: 1296–305. [DOI] [PubMed] [Google Scholar]
- 68.Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, Weaver JU, Shaw JA, Home PD. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009; 52: 1464–73. [DOI] [PubMed] [Google Scholar]
- 69.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes the role of reparatory mechanisms. Diab Care 2011; 34: S285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, Avogaro A. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diab Care 2010; 33: 1097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferraro F, Lymperi S, Méndez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, Scadden DT. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 2011; 3: 104ra101–104ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fadini GP, Avogaro A. Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: a meta-regression analysis. Int J Cardiol 2013; 168: 892–7. [DOI] [PubMed] [Google Scholar]
- 73.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diab Care 2013; 36: 943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dominguez JM, 2nd, Yorek MA, Grant MB. Combination therapies prevent the neuropathic, proinflammatory characteristics of bone marrow in streptozotocin-induced diabetic rats. Diabetes 2015; 64: 643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia–reperfusion injury in rats. Diabetologia 2006; 49: 3075–84. [DOI] [PubMed] [Google Scholar]
- 76.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J Clin Invest 2007; 117: 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006; 124: 407–21. [DOI] [PubMed] [Google Scholar]
- 78.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Hematopoietic stem cell release is regulated by circadian oscillations. Nature 2008; 452: 442–7. [DOI] [PubMed] [Google Scholar]
- 79.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009; 206: 2897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peichev M, Naiyer A, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000; 95: 952–8. [PubMed] [Google Scholar]
- 82.Yoder M, Ingram D. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: Is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta 2009; 1796: 50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoder M, Mead L, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007; 109: 1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010; 3: 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He T, Lu T, D’Uscio L, Lam C, Lee H, Katusic Z. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res 2008; 103: 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rehman J, Li J, Orschell C, March K. Peripheral blood ‘endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003; 107: 1164–9. [DOI] [PubMed] [Google Scholar]
- 87.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005; 112: 1618–27. [DOI] [PubMed] [Google Scholar]
- 88.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006; 355: 1199–209. [DOI] [PubMed] [Google Scholar]
- 89.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003; 107: 2134–9. [DOI] [PubMed] [Google Scholar]
- 90.Li M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res 2006; 98: 697–704. [DOI] [PubMed] [Google Scholar]
- 91.Siqueira R, Messias A, Voltarelli J, Scott I, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A phase I trial. Retina 2011; 31: 1207–14. [DOI] [PubMed] [Google Scholar]
- 92.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002; 105: 1656–62. [DOI] [PubMed] [Google Scholar]
- 93.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension 2005; 45: 321–5. [DOI] [PubMed] [Google Scholar]
- 94.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res 2006; 98: 1405–13. [DOI] [PubMed] [Google Scholar]
- 95.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 2006; 55: 102–9. [PubMed] [Google Scholar]
- 96.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res 2010; 106: 854–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urbich C, Dernbach E, Rossig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol 2008; 45: 429–36. [DOI] [PubMed] [Google Scholar]
- 98.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetesinduced delay in reendothelialization following arterial injury. Circ Res 2006; 98: 697–704. [DOI] [PubMed] [Google Scholar]
- 99.Tan K, Lessieur E, Cutler A, Nerone P, Vasanji A, Asosingh K, Erzurum S, Anand-Apte B. Impaired function of circulating CD34(+) CD45(−) cells in patients with proliferative diabetic retinopathy. Exp Eye Res 2010; 91: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho HJ, Kim HS, Lee MM, Kim DH, Yang HJ, Hur J, Hwang KK, Oh S, Choi YJ, Chae IH, Oh BH, Choi YS, Walsh K, Park YB. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation 2003; 108: 2918–25. [DOI] [PubMed] [Google Scholar]
- 101.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 2005; 115: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hwang JH, Kim SW, Park SE, Yun HJ, Lee Y, Kim S, Jo DY. Overexpression of stromal cell-derived factor-1 enhances endothelium-supported transmigration, maintenance, and proliferation of hematopoietic progenitor cells. Stem Cells Dev 2006; 15: 260–8. [DOI] [PubMed] [Google Scholar]
- 103.Cheang WS, Fang X, Tian XY. Pleiotropic effects of peroxisome proliferator-activated receptor γ and δ in vascular diseases. Circ J 2013; 77: 2664–71. [DOI] [PubMed] [Google Scholar]
- 104.Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: Restoration by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Circulation 2007; 116: 163–73. [DOI] [PubMed] [Google Scholar]
- 105.Mohler ER, 3rd, Shi Y, Moore J, Bantly A, Hamamdzic D, Yoder M, Rader DJ, Putt M, Zhang L, Parmacek M, Wilensky RL. Diabetes reduces bone marrow and circulating porcine endothelial progenitor cells, an effect ameliorated by atorvastatin and independent of cholesterol. Cytometry A 2009; 75: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhatwadekar AD, Guerin EP, Jarajapu YP, Caballero S, Sheridan C, Kent D, Kennedy L, Lansang MC, Ruscetti FW, Pepine CJ, Higgins PJ, Bartelmez SH, Grant MB. Transient inhibition of transforming growth factor-β1 in human diabetic CD34+ cells enhances vascular reparative functions. Diabetes 2010; 59: 2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ljubimov AV, Caballero S, Aoki AM, Pinna LA, Grant MB, Castellon R. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci 2004; 45: 4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kramerov AA, Saghizadeh M, Pan H, Kabosova A, Montenarh M, Ahmed K, Penn JS, Chan CK, Hinton DR, Grant MB, Ljubimov AV. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am J Pathol 2006; 168: 1722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kramerov AA, Saghizadeh M, Caballero S, Shaw LC, Li Calzi S, Bretner M, Montenarh M, Pinna LA, Grant MB, Ljubimov AV. Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites. Mol Cell Biochem 2008; 316: 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao R. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005; 332: 370–9. [DOI] [PubMed] [Google Scholar]
- 111.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109: 1292–8. [DOI] [PubMed] [Google Scholar]
- 112.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 2008; 102: 77–85. [DOI] [PubMed] [Google Scholar]
- 113.Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, Katz AJ, Herman IM, Peirce SM, Yates PA. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS ONE 2013; 8: e65691–e65691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS ONE 2014; 9: e84671–e84671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJ, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JW, Sowden JC, Ali RR. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 2013; 31: 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Rep 2014; 2: 853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog Retin Eye Res 2015; pii: S1350–9462(15)00046-4. [DOI] [PubMed] [Google Scholar]
- 118.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012; 379: 713–20. [DOI] [PubMed] [Google Scholar]
- 119.Xu H, Le Y-Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 2011; 52: 2160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier – implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol 2010; 2010: 190724–190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park TS, Huo JS, Peters A, Talbot CC, Jr, Verma K, Zimmerlin L, Kaplan IM, Zambidis ET. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate nonintegrated iPSC reprogramming of human myeloid progenitors. PLoS ONE 2012; 7: e42838–e42838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytometry A 2013; 83: 114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, Rufaihah AJ, Talbot C, Aguilar J, Grebe R, Merges C, Reijo-Pera R, Feldman RA, Rassool F, Cooke J, Lutty G, Zambidis ET. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation 2014; 129: 359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 2000; 41: 2915–21. [PubMed] [Google Scholar]
- 125.Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis 2011; 17: 2177–90. [PMC free article] [PubMed] [Google Scholar]
- 126.Chen WL, Lin CT, Ko PS, Yeh PT, Kuan YH, Hu FR, Yang CM. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology 2009; 116: 1038–47. [DOI] [PubMed] [Google Scholar]
- 127.Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM. Laser in-situ keratomileusis in patients with diabetes mellitus: A review of the literature. Clin Ophthalmol 2012; 6: 1665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saghizadeh M, Epifantseva I, Hemmati DM, Ghiam CA, Brunken WJ, Ljubimov AV. Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing. Invest Ophthalmol Vis Sci 2013; 54: 8172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saghizadeh M, Dib CM, Brunken WJ, Ljubimov AV. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp Eye Res 2014; 129: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sareen D, Saghizadeh M, Ornelas L, Winkler MA, Narwani K, Sahabian A, Funari VA, Tang J, Spurka L, Punj V, Maguen E, Rabinowitz YS, Svendsen CN, Ljubimov AV. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl Med 2014; 3: 1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]