Abstract
The aim of this work was to assess the influence of nutritional intervention on inflammatory status and wellness in people with multiple sclerosis. To this end, in a seven-month pilot study we investigated the effects of a calorie-restricted, semi-vegetarian diet and administration of vitamin D and other dietary supplements (fish oil, lipoic acid, omega-3 polyunsaturated fatty acids, resveratrol and multivitamin complex) in 33 patients with relapsing-remitting multiple sclerosis and 10 patients with primary-progressive multiple sclerosis. At 0/3/6 months, patients had neurological examination, filled questionnaires and underwent anthropometric measurements and biochemical analyses. Serum fatty acids and vitamin D levels were measured as markers of dietary compliance and nutritional efficacy of treatment, whereas serum gelatinase levels were analyzed as markers of inflammatory status. All patients had insufficient levels of vitamin D at baseline, but their values did not ameliorate following a weekly administration of 5000 IU, and rather decreased over time. Conversely, omega-3 polyunsaturated fatty acids increased already after three months, even under dietary restriction only. Co-treatment with interferon-beta in relapsing-remitting multiple sclerosis was irrelevant to vitamin D levels. After six months nutritional treatment, no significant changes in neurological signs were observed in any group. However, serum levels of the activated isoforms of gelatinase matrix metalloproteinase-9 decreased by 59% in primary-progressive multiple sclerosis and by 51% in relapsing-remitting multiple sclerosis patients under nutritional intervention, including dietary supplements. This study indicates that a healthy nutritional intervention is well accepted by people with multiple sclerosis and may ameliorate their physical and inflammatory status.
Keywords: Multiple sclerosis, diet, vitamin D, interferon-β, gelatinases, inflammation, quality of life, complementary and alternative medicine
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of the central nervous system (CNS), leading to disabilities in young adults, largely women.1,2 MS is a T-cell-mediated autoimmune disease occurring in genetically susceptible individuals, and characterized by focal breakdown of the myelin sheath and neuronal and axonal damage.3,4 Although multiple experimental models of MS have been developed,5 they can facilitate the understanding of only particular aspects of MS pathogenesis and study of the disease in MS patients remain an essential issue for its treatment.
There are two main forms of the human disease: (i) the relapsing-remitting MS (RRMS) (about 85% of clinical cases), and [ii] the primary-progressive MS (PPMS) (about 15% of the clinical cases).6,7 RRMS is characterized by the presence of relapses, associated with systemic inflammation and lesions in the brain, followed by partial or complete remissions. RRMS usually evolves in secondary-progressive MS (SPMS). Conversely, the pathogenesis of PPMS is characterized by progressive neurological damages, rather than relapses and remissions.7,8
Disease-modifying therapies (DMT) are focused in the prevention of relapses by suppression of the immune system and are currently available only for RRMS.9–11 They, can only slow disease progression and prevent some disability symptoms. This partial failure may be due to the fact that MS is a complex and multifactorial disease. In fact, in addition to the genetic and immunological components, various environmental and/or metabolic factors or conditions may have a role in its development: i) viral infections,12–14 ii) heavy metal poisoning,15,16 iii) smoking,14,17 iv) childhood obesity,18 v) incorrect life style, mostly including wrong dietary habits,19,20 or vi) low vitamin D status.21
The most significant difference between the different causative agents listed above is that, unlike the others, dietary habits and lifestyle (v) and vitamin D levels (vi) can be modified to provide beneficial effects on the course of the disease. In fact, physical exercise, the assumption of “healthy” dietary factors, and restoration of optimal concentrations of vitamin D, have an anti-inflammatory action and may ameliorate the course of the disease.
However, although mounting evidence indicates that nutrition can play an important role in MS, MS therapy is not currently combined with any dietary and/or lifestyle recommendation. This may be due in part to the lack of information from clinical studies.
To date, only few clinical trials have been performed. They were focused on the administration of dietary supplements22 or on the assignment diets low in saturated fat, either without supplements23 or combined with omega-3 fatty acids supplements.24,25 Other clinical trials were based only on the administration of single dietary supplements such as vitamin D (several, mostly ongoing studies), fish oil (n-3 polyunsaturated fatty acids, PUFA),26,27 or lipoic acid.28 No studies evaluated the effect of multiple dietary supplements in association to a fixed dietary regimen in MS patients, as in the present investigation.
In this framework, the main objective of our pilot study was to evaluate in a preliminary clinical trial, the effects of a low-energy diet, mainly based on the principles of the Mediterranean diet, combined with the administration of vitamin D3, and other dietary supplements (fish oil, lipoic acid, resveratrol, multivitamin complex), in MS patients with RRMS, under interferon-beta (IFN-β) treatment, or with PPMS.
Both the low-energy, mainly vegetarian diet and the dietary supplements were intended to down-regulate systemic inflammation and, in addition, restore or maintain a healthy symbiotic gut microbiota. This may be achieved by different mechanisms involving anti-inflammatory nuclear receptors, transcriptional factors and/or enzymes.19,20 In particular, the rationale for the administration of fish oil containing the n-3 PUFA, eicosapentanoic acid (EPA) and docosaexaenoic acid (DHA), was to rebalance towards higher levels the low ratio n-3/n-6 PUFA, which is often associated with the hyper-caloric diets rich in animal fats.29 This allows the switch from a pro-inflammatory condition (due also to the synthesis of pro-inflammatory eicosanoids from the n-6 arachidonic acid [ARA]) towards a reduced inflammation, in a similar manner as statins30,31 and IFN-β32,33 do. Fish oil and n-3 PUFA inhibit the pro-inflammatory transcription factor nuclear transcription factor-kB (NF-kB) and lower the level of the gelatinase B, also known as matrix metalloproteinase-9 (MMP-9) (another important mediator of inflammation).20,25,26,34
In addition, n-3 PUFA have neuroprotective properties and may be very useful in the complementary treatment in MS patients. DHA is present at high concentration in the brain and its levels decreases dramatically in MS patients.
On the basis of its anti-inflammatory and neuroprotective action, fish oil supplementation was found to be highly effective in reducing the levels of cytokines and nitric oxide in patients with RRMS under treatment with IFN-β,27 and in improving motor performances in healthy rat pups.35 Finally, both EPA and DHA can form new anti-inflammatory bioactive molecules, called resolvins, protectins, and maresins, which are able to reduce cellular inflammation and inflammatory pain.36–38
With regard to the other supplements, alfa-lipoic acid has immune-modulatory and anti-inflammatory properties, stabilizes the integrity of the blood–brain barrier (BBB) and stimulates the production of cAMP and the activity of protein kinase A.39 Resveratrol, a non-flavonoid polyphenol, acts as a non-steroidal anti-inflammatory molecule, has neuroprotective effects, and reverses experimental allergic encephalomyelitis (EAE).40,41
With regard to vitamin D3, it is now known that, besides regulating bone metabolism, vitamin D3 is involved in cell signaling and many metabolic processes, and this might explain why low levels of vitamin D3 are associated with chronic inflammatory diseases.42 Actually, vitamin D3 insufficiency or deficiency has been observed in patients with MS, type 1 diabetes mellitus, inflammatory bowel disease (IBD) and systemic lupus erythematosus (SLE). On these grounds, the therapeutic effects of vitamin D3 supplementation on MS severity and progression are becoming more and more attractive and interesting. Altogether, vitamin D and all the additional dietary supplements given in this study are able to up-regulate anti-inflammatory activity, down-regulate inflammation and protect the nervous system.
The efficacy of the nutritional treatment in this study was evaluated by several targets: i) neurological status, including the Disability and Fatigue Severity Scale; ii) compliance of MS patients to follow dietary restrictions and their appreciation of quality of life and evaluation of the Hamilton scale of depression; iii) inflammatory status as determined by the zymographic analysis of serum gelatinases, biochemical analysis and anthropometric measures; iv) wellness of PPMS patients; v) efficacy of RDA doses of vitamin D in restoring or sustaining sufficient levels of vitamin D; vi) compatibility of the chosen dietary regimen with IFN treatment in RRMS patients.
Methods
Study design, settings and patients
The study was conducted for seven months at the Department of Neurology—Center for Multiple Sclerosis—Hospital “Madonna Delle Grazie” 75100 Matera, Region Basilicata, in Southern Italy (approval n. 132 of the Ethics Committee of “San Carlo” Hospital, Potenza, Basilicata, Italy). Patient selection and study were performed at Matera Hospital.
Inclusion criteria
Subjects diagnosed with RRMS o PPMS, according to the revised McDonald Criteria (2005), regardless of gender, duration of disease, age of onset or disability level.
Subjects diagnosed with RRMS treated with DMDs (IFNβ-1a or IFNβ-1b) and subjects diagnosed with PPMS without treatment.
Signed informed consent. Eligible patients were informed about the study protocol and outcomes, and then enrolled in the study after signing of an informed consent form.
Exclusion criteria
Participation in other clinical trials.
Any visual or physical impairment that could preclude the subject’s compliance.
Serious or acute other diseases.
Primary end points
To investigate the effects of a nutritional intervention on the wellness of RRMS and PPMS patients, and to test their compliance with dietary prescriptions.
Secondary end points
Mean number of relapses at T3 and T6.
Rate of subjects with any adverse reaction to treatment and/or dietary prescription.
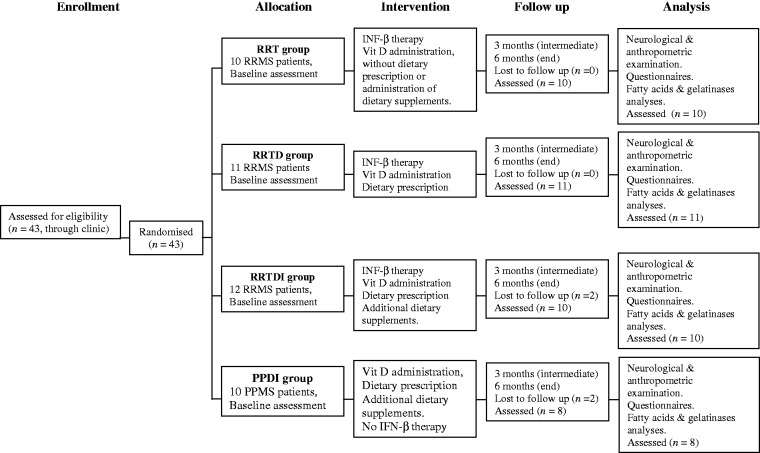
The study profile is described in Figure 1. Forty-three patients were enrolled in the study (33F/10M, aged between 22 and 52 years; mean age 38 ± 8.45 years, [years of the disease from diagnosis: 9.0 ± 5.3]).
Figure 1.
Study profile. RR: relapsing remitting; PP: primary progressive; T: therapy; D: diet; I: dietary supplements. Diets were primarily based on high intake of plant-based food, comprised of whole grains, legumes, vegetables, fruit, soy, but also yogurt and fishery products, and low intake of meat, saturated animal fat, fried food and trans fatty acids, sweetened drinks and alcohol. Vitamin D3 (cholecalciferol), 714 IU/day (5000 IU, once the week, for five months). Additional dietary supplements: 1) fish oil pearls containing n-3 polyunsaturated fatty acids: 5 g EPA + DHA, 60 mg vit. E and 300 mg lipoic acid/day (for six months); 2) resveratrol 150 mg/day (for three months). 3) Multivitamin complex, 300 mg/day (for four months)
Patients were treated independently from this study. The 33 patients with RRMS were treated with IFNβ-1b (Betaferon) or IFNβ-1a (Rebif or Avonex).43,44 Of the 10 patients with PPMS, only one was under treatment with Copaxone.
Enrolled patients were randomly assigned to one of four treatment groups namely RRT, RRTD, RRTDI, and PPDI, where T is for therapy, D is for diet, and I is for dietary supplements. All patients received a weekly administration of 5000 IU of cholecalciferol (vitamin D3), corresponding to a total of 914 IU/day (including the 200 IU administered daily with a multivitamin complex).
The differences among treatments in the four groups are reported below:
RRT group included RRMS patients under therapy with IFN-β and administration of vitamin D3 (cholecalciferol), but without dietary prescription or administration of dietary supplements.
RRTD group included RRMS patients that, in addition to IFN-β therapy and vitamin D administration, had a dietary prescription.
RRTDI group included RRMS patients under IFN-β therapy, vitamin D, and dietary restriction as in the group RRTD, but in addition had a daily consumption of dietary supplements.
PPDI group included patients with PPMS. All PPMS patients were under dietary control and received cholecalciferol and dietary supplements. One patient was under treatment with Copaxone.
At baseline and every three months the MS patients reached the Center for Multiple Sclerosis in the Hospital. In these occasions height, weight, waist and hip circumferences were measured and a blood sample (10 mL) was collected. Tubes were immediately centrifuged at 3000 g per 15 min at 4℃ and stored at −20℃ until analyses. During these occasions patients were also interviewed about their diets and physical activities and they were requested to drop the results of biochemical analyses that were performed during the previous week at a private laboratory.
Recommended diet and life style
A calorie-restricted diet of 1700–800 kcal was assigned to each subject. Diet, made of 50% carbohydrates, 30% fat (mainly from olive oil, fish and vegetables), and 20% proteins, was divided in five meals in the day, with typical glycemic loads of ca. 19, 12, 62, 12, 34, respectively. Diet was based on: 1) low intake or exclusion of salt, sugar, animal fat, red meat (allowed only once-twice the week); fried food (every two weeks); processed condiments; cakes and pies (once the week); long shelf snacks; spirits and sugar-sweetened beverages; 2) preferential assumption of raw and cooked vegetables, legumes, fresh fruit and nuts; fish, seafood and shellfish; bread and pasta (possibly whole grain) (half portions, ca. 60–70 g), fruit juice with no added sugar, fibers, soy, skimmed milk and yogurt, extra virgin olive oil, coffee, tea, still water. In general, the diet was based mainly on the principles of Mediterranean diet but with some important modifications: it was semi-vegetarian, with a preference for fish instead of meat, and lower gluten content, than the usual Mediterranean diet. A mild physical activity (walking, cycling or even dancing), for 20–30 min, twice the day was also suggested to patients.
Dietary supplements, daily dosage, and duration of administration
Dibase Abiogen Pharma, vitamin D3 (cholecalciferol), 714 IU/day (5000 IU, once the week, for five months) + 200 IU with a multivitamin complex (Vit M O.T.I.) (daily administration for four months).
SeaLife O.T.I., fish oil pearls containing omega-3 (n-3) PUFA. Daily intake: 5 g EPA + DHA, 60 mg vit. E and 300 mg lipoic acid (for six months);
Resvital O.T.I., Resveratrol. daily intake: 150 mg, for three months;
Vit M O.T.I., multivitamin complex (including oligoelements), per day, for four months: vitamins: C (120 mg), PP (36 mg), E (20 mg), B6 (4 mg), B2 (3.2 mg), B1 (2.8 mg), B12 (2 mcg), A (1.6 mg), D3 (5 mcg); biotin (0.3 mg), pantothenic acid (12 mg), folic acid (0.4 mg); oligoelements: zinc (30 mg), iron (28 mg), copper (3 mg), manganese (3 mg), selenium (120 mcg), fluorine (3 mg), iodine (0.3 mg).
Neurological examination and questionnaires
The patients had a neurological examination including Kurtzke Expanded Disability Status Scale (EDSS) and Fatigue Severity Scale (FSS) at t0/t3/t6 months.
They also completed the Quality of life measured by The Short Form (SF-36) questionnaire and the Hamilton Rating Scale for Depression (Ham-D) questionnaire at t0, t3, and t6.
Biochemical analyses
Serum fibrinogen, cholesterol, transaminases, triglycerides, creatinine, and calcidiol [25(OH)D3] analyses were performed in private laboratories following gold-standard methods of analysis for each parameter.
Fatty acids determination
Serum concentration of n-3 (EPA, DHA) and n-6 PUFA (ARA) was measured following the method described by Marangoni et al.,45 with some modifications. Briefly, five hundred microliters of plasma were transferred to teflon screw-capped glass vials, with 1 mL of 3 N MeOH/HCl and maintained in a dry bath at 90℃ for 1 h. Then 1 mL of a saturated solution of KCl was sequentially added to each sample, and the fatty acid methyl esters (FAME) were finally extracted using 1 mL of n-exane twice. The samples were injected into an Agilent Technologies 6850 Series II gas chromatography (Santa Clara, CA) equipped with split/splitless injection port, programmed temperature vaporizer (PTV), flame ionization detector (FID), and a 100-m fused-silica capillary column (Supelco, St. Louis, MO) coated with cyanopropyl methyl silicone (0.25 µm film thickness). Helium was used as the carrier gas and the flow rate was 1.8 mL min−1 at a split ratio of 1:30. The PTV temperature was 60℃ × 0.1 min., up to 260℃ with a rate of 500℃/min. The FID temperature was 260℃. The oven temperature was set at 140℃ for 5 min, ramped to 175℃ at 4℃/min, hold 20 min and finally ramped to 240℃ and hold 1 min for gas chromatography separation. The analyses were done in duplicates for each sample.
Zymographic analysis of serum gelatinases
Serum gelatinolytic activity was assessed by 1D and 2D gelatin gel zymography according to the established procedures.46 1D zymography (1-DZ) was carried out as follows. Aliquots of sera containing 25 µg of proteins were supplemented with 15 µL of the non-reducing electrophoresis loading buffer (4% SDS, 12% glycerol, 0.01% bromophenol blue, 50 mmol/L Tris-HCl at pH 6.8) and analyzed in 8% polyacrylamide gel copolymerized with 0.1% (w/v) gelatin. Stacking gel contained 4% polyacrylamide. Electrophoresis was carried out on a Mini-PROTEAN Tetra Cell system (Bio-Rad) at 4℃ for 90 min at 150 V. Precision Plus, Protein Standards Dual Color (Bio-Rad) were used as molecular weight markers. After electrophoresis, the gel was washed (2 × 40 min) in 2.5% (w/v) Triton X-100, 20 mmol/L Tris-HCl, pH 7.5 (washing buffer) in order to remove SDS, then incubated for 14 h at 37℃ in developing buffer: 1% (w/v) Triton X-100, 20 mmol/L Tris-HCl pH 7.5, 10 mmol/L CaCl2. For the development of enzyme activity, the gels were stained with Coomassie Brilliant Blue R-250, destained in methanol/acetic acid/H2O and scanned using an ImageMaster DTS (Pharmacia Biotech) scanner.
Statistical analyses
To test for mean differences in each of the variables (waist circumference, cholesterol, creatinine, fibrinogen, etc.) among experimental groups, nested model ANOVAs with “group” (PPDI, RRT, RRTD, and RRTDI) as main effect and “time” (0, 3, and 6 months) nested within “group” were used.
Tukey post hoc tests for multiple comparisons of means were also performed to detect significant differences among the treatments of significant factors in the ANOVAs.
All the analyses in the present study were performed with R.3.0.0 software (R Core Team, 2013).
Results
Patients
Of 43 patients, 49 (29F/10M) followed the prescriptions to completion. Two patients of the RRTDI group, and two patients of the PPDI group, withdrew from the study, because they did not like fish or fish oil and were not satisfied with the nutritional treatment (Figure 1);
Enrolled (43) To completion (39)
10 RRT (8F/2M) 10 (8F/2M)
11 RRTD (10F/1M) 11 (10F/1M)
12 RRTDI (10F/2M) 10 (8F/2M)
10 PPDI (5F/5M) 08 (3F/5M)
All patients, including the control group of RRMS, received vitamin D3. One out of the two RRMS groups under dietary control and the PPMS group received dietary supplements in addition to vitamin D3. These dietary supplements given in addition to vitamin D3 (2 out of four groups) were: i) fish oil containing the omega-3 (n-3) long-chain PUFA EPA and DHA, alfa-lipoic acid and vitamin E; ii) resveratrol and iii) the multivitamin complex.
Vitamin D status
Vitamin D3 status was assessed by measurement of serum concentration of calcidiol, 25 hydroxyvitamin D3, and [25(OH)D3].
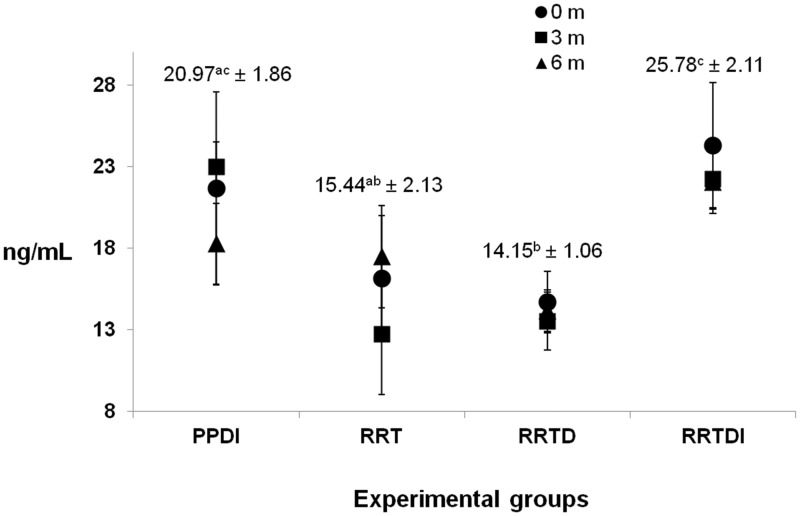
As reported in Table 1, insufficient or sub-optimal levels of calcidiol [25(OH)D3] were found in all groups at baseline (16.14 ± 3.23 ng/mL [RRT]; 14.66 ± 1.92 ng/mL [RRTD]; 26.30 ± 5.46 ng/mL [RRTDI] and 21.66 ± 5.92 ng/mL [PPDI]). The reference scale used for 25-hydroxyvitamin D3 (25(OH)D3) was: deficiency <25 nmol/L (<10 ng/mL), insufficiency (25–50 nmol/L or 10–20 ng/mL), and sub-optimal status (50–75 nmol/L, 20–30 ng/mL). Figure 2 shows the plasma concentrations of vitamin D at baseline, after three months and six months, respectively. No significant effect of cholecalciferol administration for three or five months was recorded. Mean values of 25(OH)D3 were significantly higher in RRTDI group, when compared with the corresponding RRT and RRTD, and in PPDI group when compared with RRTD.
Table 1.
Mean values of all subjects grouped in the four treatment groups at baseline
| RRT |
RRTD |
RRTDI |
PPDI |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SEM | Range | Mean ± SEM | Range | Mean ± SEM | Range | Mean ± SEM | Range |
| Vit D (ng/mL) | 16.14ab ± 3.23 | 5–31 | 14.66b ± 1.92 | 5–25 | 26.30c ± 5.46 | 22–32 | 21.66ac ± 5.92 | 7–36 |
| ARA (%) | 8.60a ± 0.78 | 5–12 | 9.20a ± 0.53 | 5–11 | 9.56a ± 0.62 | 7–12 | 9.98a ± 1.01 | 4–14 |
| EPA (%) | 0.60a ± 0.09 | 0.3–0.8 | 0.54a ± 0.05 | 0.3–0.8 | 0.81b ± 0.09 | 0.3–1.3 | 0.46a ± 0.04 | 0.4–0.7 |
| DHA (%) | 1.58a ± 0.19 | 1.3–2.7 | 2.13ab ± 0.37 | 0.8–4.4 | 2.76b ± 0.32 | 1.8–5.1 | 1.19a ± 0.23 | 0.6–2.1 |
| Cholesterol (mg/dL) | 184.71a ± 9.50 | 164–236 | 185.80a ± 9.52 | 166–256 | 190.56a ± 10.76 | 150–234 | 227.75b ± 17.65 | 189–259 |
| HDL (mg/dL) | 42.71a ± 3.16 | 36–59 | 61.33b ± 6.02 | 40–89 | 64.00b ± 3.31 | 46–83 | 58.40b ± 4.27 | 45–69 |
| LDL (mg/dL) | 118.28ab ± 12.72 | 101–158 | 106.00a ± 7.12 | 77–125 | 122.67ab ± 15.43 | 73–185 | 130.50b ± 9.40 | 114–156 |
| TGL (mg/dL) | 114.43a ± 29.03 | 52–279 | 109.14a ± 29.97 | 65–282 | 108.38a ± 16.71 | 43–153 | 110.50a ± 12.93 | 81–144 |
| Fibrinogen (mg/dL) | 279.50a ± 18.28 | 199–334 | 312.90ab ± 28.04 | 147–496 | 319.00b ± 14.58 | 277–364 | 269.00a ± 18.01 | 148–417 |
| Creatinine (mg/dL) | 0.73a ± 0.06 | 0.5–0.8 | 0.62a ± 0.05 | 0.4–0.9 | 0.69a ± 0.04 | 0.5–0.9 | 0.95b ± 0.05 | 0.8–1.1 |
| GOT (U/L) | 21.17a ± 3.19 | 13–33 | 16.88a ± 1.46 | 11–23 | 17.89a ± 2.47 | 12–34 | 18.25a ± 1.60 | 16–23 |
| GPT (U/L) | 26.17a ± 5.60 | 15–51 | 17.25b ± 2.26 | 6–30 | 19.67b ± 4.48 | 8–50 | 16.25b ± 1.70 | 13–21 |
| Waist circum. (cm) | 83.78a ± 2.68 | 72–101 | 89.64a ± 3.55 | 67–108 | 89.60a ± 2.55 | 79–106 | 96.25b ± 2.48 | 75–108 |
| Hip circum. (cm) | 97.22a ± 2.39 | 88–114 | 102.66b ± 1.24 | 89–117 | 102.00ab ± 1.01 | 90–106 | 105.71b ± 2.99 | 96–122 |
| Weight (kg) | 61.55a ± 3.59 | 52–80 | 67.55ab ± 4.57 | 45–87 | 65.40ab ± 3.16 | 51–80 | 73.81b ± 4.28 | 60–90 |
| EDSS | 1.80a ± 0.26 | 1–4 | 1.91a ± 0.24 | 1–4 | 2.80a ± 0.69 | 1–6 | 4.19b ± 0.67 | 1–6 |
| FSS | 23.40a ± 4.73 | 9–58 | 30.09ab ± 3.44 | 11–50 | 31.30ab ± 4.85 | 9–63 | 36.50b ± 5.64 | 9–59 |
| SF-36 | 103.00a ± 2.04 | 95–113 | 100.18a ± 3.38 | 72–118 | 97.95a ± 3.10 | 75–116 | 101.40a ± 2.45 | 88–112 |
| HAM-D | 5.80a ± 1.62 | 0–22 | 9.00b ± 1.76 | 3–17 | 10.50b ± 2.58 | 0–28 | 10.50b ± 2.65 | 0–22 |
EDSS: Expanded Disability Status Scale; FSS: Fatigue Severity Scale; SF-36:Short Form (36) Health Survey; HAM-D: Hamilton Depression Rating Scale.
Values are reported as mean ± SEM. Values with different letters superscript in the same row are significantly different (P < 0.05, Tukey’s test) as analyzed by one-way analysis of variance (ANOVA).
Figure 2.
Serum concentration of 25 hydroxyvitamin D3 in the different groups of patients in the course of time. Numbers correspond to the mean value (±SEM) of t0, t3, and t6 times for each group. Values with different letters superscript are significantly different (P < 0.05; Tukey’s test) as analyzed by one-way analysis of variance (ANOVA)
Taken together, data showed no significant differences in the same group of patients during the treatment period. Diet or diet combined with dietary supplements had no significant influence on diet on vitamin status of patients. No seasonal fluctuation in 25(OH)D3 was observed, although its levels had in all groups a slight increase in the spring.
Serum n-3 PUFA
Table 2 shows serum concentrations of PUFA. Data showed that the magnitude of concentrations was in the order ARA >> DHA > EPA. In general, the concentration of n-3 PUFA (EPA and DHA) increased in all groups under dietary regimen, except in the RRT group. In particular, the mean value of EPA was significantly higher after three months of treatment whereas no differences were found between t3 and t6. For DHA, in RRTD and RRTDI groups, the increase was observed after six months, while in PPDI, the increase was significant from the third month of treatment.
Table 2.
Polyunsaturated fatty acids (PUFAs) in patient groups in the course of time
| RRT | RRTD | RRTDI | PPDI | ||
|---|---|---|---|---|---|
| PUFA | (%) | (%) | (%) | (%) | |
| ARA | t0 | 8.60a ± 0.78 | 9.20a ± 0.53 | 9.56a ± 0.62 | 9.98a ± 1.01 |
| t3 | 8.03a ± 0.90 | 8.07a ± 0.68 | 6.75b ± 0.41 P < 0.05 | 8.11a ± 0.76 | |
| t6 | 9.44a ± 0.72 | 9.33a ± 0.73 | 7.61b ± 0.63 P < 0.05 | 8.18a ± 0.58 | |
| EPA | t0 | 0.68a ± 0.19 | 0.54a ± 0.05 | 0.61a ± 0.09 | 0.46a ± 0.04 |
| t3 | 1.05a ± 0.18 | 1.06b ± 0.11 P < 0.05 | 1.37b ± 0.37 P < 0.05 | 1.32b ± 0.12 P < 0.01 | |
| t6 | 0.98a ± 0.13 | 1.26b ± 0.19 P < 0.01 | 1.15b ± 0.19 P < 0.05 | 1.75b ± 0.34 P < 0.01 | |
| DHA | t0 | 1.58a ± 0.19 | 2.13a ± 0.37 | 2.66a ± 0.32 | 1.19a ± 0.23 |
| t3 | 1.47a ± 0.21 | 2.31a ± 0.21 | 2.75a ± 0.14 | 3.89b ± 0.36 P < 0.001 | |
| t6 | 2.01a ± 0.38 | 2.96b ± 0.24 P < 0.05 | 3.33b ± 0.29 P < 0.05 | 3.75b ± 0.44 P < 0.001 | |
Values are reported as mean ± SEM. For each fatty acids the mean values with different letters superscript in the same column are significantly different (Tukey’s test) as analyzed by one-way analysis of variance (ANOVA).
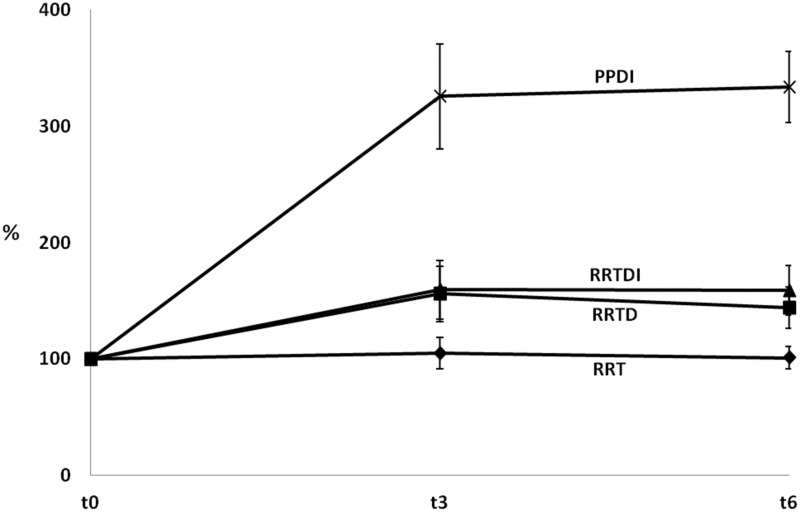
Looking at the ratio EPA + DHA/ARA (Figure 3), data indicated that it did not change significantly over time in the RRT group. Conversely in the other three groups, this ratio increased already after three months. In the PPDI group, there were statistically significant higher levels of EPA and DHA between t3 and t0, as well as between t6 and t0 (Tukey t test P < 0.001). In the other groups (RRTD and RRTDI), a trend to an increased ratio EPA + DHA/ARA was observed.
Figure 3.
Percentages of the (EPA + DHA)/ARA ratios in the different groups of patients in the course of time. The ratio was first calculated for each individual patient (within each group), and then averaged for each group at various times
Neurological examination and questionnaires
The results obtained with the neurological examinations of the patients at times t0/t3/t6 are shown in Table 3 for the EDSS and FSS. Both EDDS and FFS values did not change in any group in the course of time.
Table 3.
Neurological examination and questionnaires of MS patients
| Index | RRT | RRTD | RRTDI | PPDI | |
|---|---|---|---|---|---|
| EDSS | t0 | 1.80a ± 0.26 | 1.91a ± 0.24 | 2.80a ± 0.69 | 4.19a ± 0.37 |
| t3 | 1.80a ± 0.26 | 1.91a ± 0.24 | 2.80a ± 0.69 | 4.19a ± 0.37 | |
| t6 | 1.80a ± 0.26 | 1.91a ± 0.24 | 2.80a ± 0.69 | 4.19a ± 0.37 | |
| Mean | 1.80a ± 0.04 | 1.91a ± 0.13 | 2.80b ± 0.38 P < 0.05 | 4.19c ± 0.37 P < 0.01 | |
| FSS | t0 | 23.40a ± 4.73 | 30.09a ± 13.44 | 31.30a ± 4.85 | 36.50a ± 5.64 |
| t3 | 22.20a ± 3.70 | 26.73a ± 3.52 | 28.50a ± 5.47 | 36.13a ± 5.07 | |
| t6 | 25.90a ± 5.32 | 29.91a ± 3.73 | 27.00a ± 6.00 | 33.38a ± 5.35 | |
| Mean | 23.83a ± 2.60 | 28.91b ± 2.01 P < 0.05 | 28.93ab ± 3.06 P < 0.05 | 35.33b ± 2.67 P < 0.05 | |
| SF-36 | t0 | 103.00a ± 2.04 | 100.18a ± 3.38 | 97.75a ± 3.10 | 101.40a ± 2.45 |
| t3 | 102.10a ± 1.26 | 99.09a ± 3.98 | 100.00a ± 2.94 | 96.80a ± 2.65 | |
| t6 | 105.10a ± 2.00 | 98.73a ± 2.92 | 102.55a ± 2.06 | 98.89a ± 2.02 | |
| Mean | 103.41a ± 1.26 | 99.33a ± 1.93 | 100.22a ± 1.72 | 99.32a ± 1.49 | |
| HAM-D | t0 | 5.80a ± 1.62 | 9.00a ± 1.76 | 10.00a ± 2.58 | 10.50a ± 2.56 |
| t3 | 5.40a ± 1.49 | 7.64a ± 1.51 | 8.90a ± 2.98 | 9.75a ± 2.88 | |
| t6 | 4.80a ± 2.06 | 5.91a ± 1.38 | 7.80a ± 2.68 | 10.13a ± 2.43 | |
| Mean | 5.33a ± 0.97 | 7.52ab ± 0.90 P < 0.05 | 8.90b ± 1.54 P < 0.05 | 10.13b ± 1.47 P < 0.05 | |
EDSS: Expanded Disability Status Scale; FSS: Fatigue Severity Scale; HAM-D: Hamilton Depression Rating Scale; SF-36: Short Form (36) Health Survey.
Values are reported as mean ± SEM. For each groups the values with different letters superscript in the same column are significantly different (Tukey’s test), whereas mean values with different letters subscript in the same row indicate significant differences within groups (Tukey’s test) as analyzed by one-way analysis of variance (ANOVA).
The results obtained with the SF-36 and Ham-D questionnaires at t0, t3, and t6 are also shown in Table 3. In both cases no significant differences were observed.
Zymographic assessment of serum gelatinases as a measure of inflammatory status
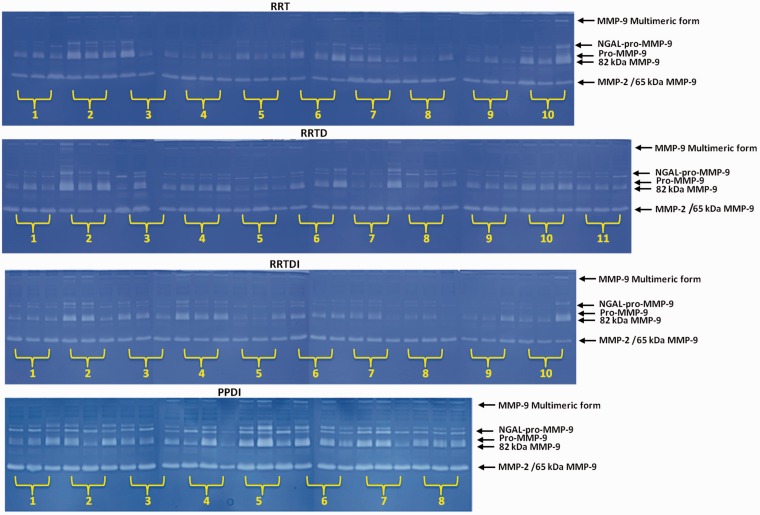
The inflammatory status of patients at baseline and during the study was assessed by zymographic analyses of serum gelatinases MMP-9 and MMP-2. These MMPs were chosen as markers of the inflammatory status of the patients, and their levels were evaluated by 1-DZ and 2-DZ. This type of assay allows the fine tune of the inflammatory status and the influence of therapy under dietary control and dietary supplements administration also in the short period of the clinical trial. Among the different gelatinase isoforms indicated in Figure 4, those more indicative for an active inflammatory status are the activated forms of MMP-9, i.e. the 82 kDa and the 65 kDa MMP-9. This latter can be distinguished from MMP-2 by separation through concanavalin A47 or by 2-DZ,46 not shown here. However, in routine assay, 1-DZ is sufficient to provide useful data to assess the presence of activated MMP-9 isoforms, long before that clinical signs or RMI evidences appear.
Figure 4.
Monodimensional zymography (1-DZ) on 8% polyacrylamide containing 0.1% gelatine. Gels of sera (25 µg of proteins) from patients with RRMS or PPMS. Groups: (T: therapy; D: diet; I: dietary supplements). (A color version of this figure is available in the online journal.)
1-DZs of serum gelatinases are shown in Figure 4 for each patient of the four groups. Densitometric analyses of the band intensities indicate if there is a decrease or increase in serum gelatinases activity and therefore can give information on the effectiveness of the treatment. On the basis of 2-DZ evidences, not shown here, changes in MMP band intensities were here ascribed to the activated forms of MMP-9 and not to MMP-2.
As shown in Figure 4 for group RRT, after six months treatment with IFN, a clear reduction of the activated forms of MMP-9 was observed in 5 out of 10 patients (patients 1–3, 5, 7). MMP-9 increased only in patient 10, whereas remained practically unchanged in the other four patients. Going into more detail, in patients 1, 2, 3, and 5, the 82 kDa MMP-9 decreased by 50%, 20%, 80%, and 15%, respectively), whereas in patient 7 both the 82 kDa MMP-9 and the 65 kDa MMP-9 were found to decrease (both by 30%). In patient 10 a 40% increase of the 82 kDa MMP-9 was observed. After six months the mean decrease of the activated 82 kDa MMP-9 level in the RRT group was 39%.
In the RRTD group, the 82 kDa MMP-9 level varied in five patients. In patients 2 and 9, band intensity decreased (by 15% and 20%) whereas in patients 3, 7, and 8, the 82 kDa MMP-9 increased (by 30%, 80% and 20%, respectively). The 65 kDa MMP-9 band intensity decreased only in patient 10 (by 10%). In average, the decrease of the 82 kDa MMP-9 level in the RRTD group was 17.5%.
In the RRTDI group, only changes of the 82 kDa MMP-9 intensities were observed. The intensity of the 82 kDa MMP-9 band decreased in five patients (1–3, 7, 9), by 10%, 40%, 20%, 90%, and 95%, respectively and increased in four patients (4, 5, 9, 10) (by 10%, 40%, 30% and 70%, respectively), and remained unchanged only in patient 6. At t6, the mean decrease of the 82 kDa MMP-9 level in the RRTDI group was 51%.
In the PPDI group, a decrease of the MMP-9 level was observed in patients 4–7. In patients 4, 5, and 7 the 82 kDa MMP-9 was found to decrease (by 50%, 40% and 85%, respectively), whereas in patient 6 both the 82 kDa MMP-9 and the 65 kDa MMP-9 were found to decrease (by 60% and 20%, respectively). An increase of the 82 kDa MMP-9 was observed in patients 1 (by 40%). Altogether, after six months the decrease of the 82 kDa MMP-9 level in the PPDI group was 59% in average.
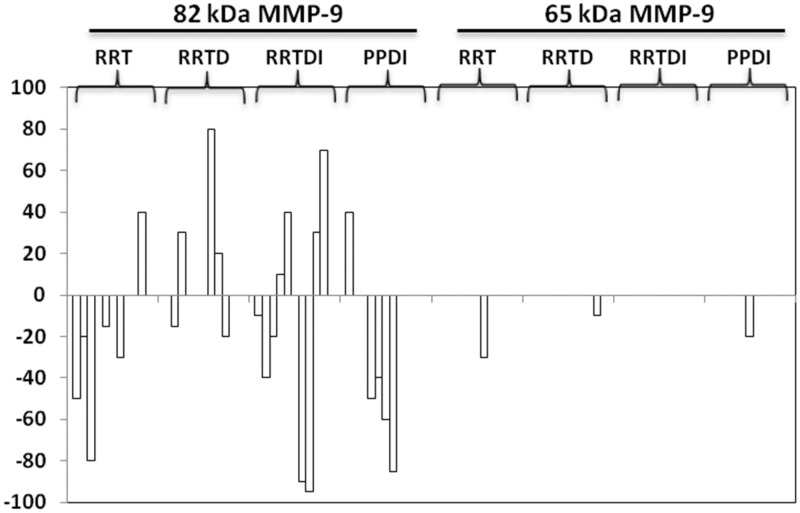
In Figure 5 are shown the differences of densitometric values of the bands between t6 and t0. Taken together, the 82 kDa MMP-9 was the only one MMP-9 isoform that increased its activity and this occurred only in nine out of 39 patients (23%). Increase of the 65 kDa MMP-9 was never observed. Conversely, the decrease of the 82 kDa was observed in 16 patients (41%), and the decrease of the 65 kDa was observed in three patients (8%).
Figure 5.
Differences of densitometric values of the 65 and 82 kDa MMP-9 bands between t6 and t0
Anthropometric measures
Anthropometric data collected over the study are summarized in Table 4. No significant differences were found over time. For waist circumference, waist/hip and weight, the mean values were higher in PPDI group compared to the RRT group (P < 0.01; P < 0.05 and P < 0.001, respectively), while the mean hip circumference was lower for patients of the RRT group compared to the RRTD (P < 0.05) and PPTD groups (P < 0.05).
Table 4.
Anthropometric measures in patient groups in the course of time
| Parameter | RRT | RRTD | RRTDI | PPDI | |
|---|---|---|---|---|---|
| Waist circumference | t0 | 83.78a ± 2.68 | 89.64a ± 3.55 | 89.60a ± 2.55 | 96.25a ± 2.48 |
| t3 | 83.11a ± 2.03 | 82.09a ± 3.96 | 84.30a ± 2.82 | 90.38a ± 2.08 | |
| t6 | 81.56a ± 2.31 | 88.09a ± 3.53 | 86.20a ± 3.13 | 92.00a ± 2.07 | |
| Mean | 82.81a ± 1.32 | 86.61a ± 2.11 | 86.70a ± 1.79 | 92.88b ± 2.09 p < 0.01 | |
| Hip circumference | t0 | 97.22a ± 2.39 | 102.66a ± 1.24 | 102.00a ± 1.01 | 105.71a ± 2.99 |
| t3 | 95.00a ± 2.50 | 102.91a ± 2.6 | 100.09a ± 1.95 | 102.43a ± 2.85 | |
| t6 | 94.44a ± 2.17 | 101.55a ± 2.94 | 100.09a ± 2.19 | 99.29a ± 3.55 | |
| Mean | 95.96a ± 1.34 | 102.37b ± 1.55 p < 0.05 | 100.73ab ± 1.14 | 102.48b ± 1.71 p < 0.05 | |
| Waist/Hip | t0 | 0.86a ± 0.02 | 0.87a ± 0.02 | 0.89a ± 0.02 | 0.95a ± 0.05 |
| t3 | 0.88a ± 0.02 | 0.81a ± 0.03 | 0.84a ± 0.02 | 0.90a ± 0.05 | |
| t6 | 0.87a ± 0.02 | 0.87a ± 0.03 | 0.86a ± 0.03 | 0.94a ± 0.04 | |
| Mean | 0.87a ± 0.01 | 0.85a ± 0.02 | 0.86a ± 0.01 | 0.93b ± 0.02 p < 0.05 | |
| Weight | t0 | 61.55a ± 3.59 | 67.55a ± 4.57 | 65.40a ± 3.16 | 73.81a ± 4.28 |
| t3 | 59.28a ± 3.20 | 67.32a ± 4.45 | 63.72a ± 2.72 | 71.16a ± 4.04 | |
| t6 | 61.63a ± 3.83 | 67.14a ± 4.56 | 64.05a ± 2.56 | 71.38a ± 3.99 | |
| Mean | 60.82a ± 0.24 | 67.33a ± 0.04 | 64.39a ± 0.31 | 77.12b ± 0.30 p < 0.001 | |
| BMI | t0 | 23.40a ± 1.19 | 25.21a ± 1.51 | 24.15a ± 0.89 | 24.92a ± 1.13 |
| t3 | 23.32a ± 1.20 | 24.84a ± 1.40 | 23.57a ± 0.81 | 24.07a ± 1.22 | |
| t6 | 23.40a ± 1.22 | 25.02a ± 1.45 | 23.70a ± 0.76 | 24.65a ± 1.18 | |
| Mean | 23.37a ± 0.69 | 25.03a ± 0.83 | 23.81a ± 0.47 | 24.55a ± 0.68 | |
Values are reported as mean ± SEM. For each groups the values with different letters superscript in the same column are significantly different (Tukey’s test), whereas mean values with different letters subscript in the same row indicates significant differences within groups (Tukey’s test) as analyzed by one-way analysis of variance (ANOVA).
Biochemical analyses
No significant variations of cholesterol, HDL, LDL, TGL, fibrinogen, GOT, GPT, and creatinine serum concentration over time were found (Table 5).
Table 5.
Biochemical analyses of sera from the different groups of patients in the course of time
| Parameter | RRT | RRTD | RRTDI | PPDI | |
|---|---|---|---|---|---|
| Cholesterol | t0 | 184.71a ± 7.96 | 185.80a ± 9.04 | 190.56a ± 10.21 | 227.75a ± 12.48 |
| t3 | 183.00a ± 8.05 | 186.70a ± 6.73 | 189.78a ± 8.27 | 225.75a ± 23.10 | |
| t6 | 179.29a ± 6.65 | 187.20a ± 6.38 | 190.00a ± 10.04 | 220.50a ± 19.69 | |
| Mean | 182.33a ± 4.17 | 186.57a ± 4.17 | 188.88a ± 5.30 | 224.67b ± 8.14 p < 0.01 | |
| HDL | t0 | 42.71a ± 2.65 | 61.38a ± 5.17 | 64.00a ± 3.24 | 58.40a ± 3.40 |
| t3 | 41.00a ± 2.07 | 66.88a ± 6.99 | 59.56a ± 2.67 | 53.92a ± 4.27 | |
| t6 | 44.43a ± 2.84 | 61.75a ± 5.73 | 64.44a ± 5.44 | 57.08a ± 4.94 | |
| Mean | 42.71a ± 1.30 | 63.33b ± 3.35 P < 0.001 | 62.67b ± 2.38 P < 0.001 | 56.47c ± 2.31 P < 0.05 | |
| LDL | t0 | 121.50a ± 8.04 | 108.00a ± 7.26 | 122.67a ± 11.95 | 130.50a ± 6.65 |
| t3 | 121.50a ± 8.55 | 107.67a ± 8.81 | 117.57a ± 14.17 | 127.00a ± 13.96 | |
| t6 | 121.90a ± 7.61 | 121.50a ± 8.25 | 107.00a ± 12.12 | 129.75a ± 4.99 | |
| Mean | 122.00a ± 4.26 | 112.18a ± 6.63 | 115.78a ± 9.04 | 129.08a ± 4.91 | |
| TGL | t0 | 114.43a ± 24.33 | 109.14a ± 23.95 | 100.38a ± 15.01 | 110.50a ± 9.14 |
| t3 | 106.71a ± 14.57 | 113.57a ± 12.87 | 90.38a ± 14.08 | 92.50a ± 8.83 | |
| t6 | 112.86a ± 19.20 | 104.86a ± 14.78 | 109.00a ± 19.42 | 85.83a ± 3.10 | |
| Mean | 111.33a ± 10.85 | 109.19a ± 9.81 | 99.92a ± 9.12 | 94.79a ± 6.26 | |
| TGL/HDL | t0 | 2.88a ± 0.72 | 1.76a ± 0.36 | 1.56a ± 0.26 | 2.08a ± 0.29 |
| t3 | 2.68a ± 0.40 | 1.85a ± 0.26 | 1.50a ± 0.26 | 1.92a ± 0.25 | |
| t6 | 2.73a ± 0.56 | 1.72a ± 0.25 | 1.76a ± 0.32 | 1.32b ± 0.05 P < 0.05 | |
| Mean | 2.76a ± 0.32 P < 0.05 | 1.78b ± 0.16 | 1.59b ± 0.16 | 1.77b ± 0.11 | |
| Fibrinogen | t0 | 279.50a ± 14.16 | 312.90a ± 28.38 | 319.00a ± 10.33 | 269.00a ± 48.27 |
| t3 | 324.00a ± 23.81 | 340.70a ± 24.56 | 287.60a ± 13.01 | 274.67a ± 26.96 | |
| t6 | 322.50a ± 17.63 | 302.60a ± 21.49 | 264.00a ± 14.25 | 237.00a ± 17.22 | |
| Mean | 308.67a ± 10.99 | 318.73a ± 13.57 | 290.20ab ± 7.98 | 259.22b ± 17.06 P < 0.05 | |
| GOT | t0 | 21.17a ± 2.46 | 16.88a ± 1.21 | 17.89a ± 2.35 | 18.25a ± 1.13 |
| t3 | 21.17a ± 2.19 | 19.75a ± 3.18 | 20.89a ± 2.11 | 21.75a ± 0.93 | |
| t6 | 25.00a ± 3.78 | 17.25a ± 1.80 | 19.89a ± 1.93 | 22.50a ± 2.54 | |
| Mean | 22.44a ± 1.61 | 17.96a ± 1.25 | 19.56a ± 1.21 | 20.83a ± 0.97 | |
| GPT | t0 | 26.17a ± 4.34 | 17.25a ± 2.13 | 19.67a ± 4.39 | 16.25a ± 1.19 |
| t3 | 24.83a ± 2.73 | 22.00a ± 4.43 | 25.00a ± 4.39 | 22.00a ± 1.90 | |
| t6 | 22.33a ± 4.81 | 20.38a ± 2.84 | 24.33a ± 4.78 | 21.00a ± 2.90 | |
| Mean | 24.44a ± 1.61 | 19.91a ± 1.88 | 23.00a ± 2.55 | 19.75a ± 1.24 | |
| Creatinine | t0 | 0.73a ± 0.04 | 0.62a ± 0.04 | 0.69a ± 0.04 | 0.95a ± 0.03 |
| t3 | 0.74a ± 0.05 | 0.65a ± 0.04 | 0.72a ± 0.04 | 0.99a ± 0.09 | |
| t6 | 0.85a ± 0.13 | 0.64a ± 0.05 | 0.66a ± 0.09 | 0.93a ± 0.01 | |
| Mean | 0.77a ± 0.05 P < 0.05 | 0.63b ± 0.02 P < 0.001 | 0.69b ± 0.01 P < 0.001 | 0.96c ± 0.11 P < 0.001 | |
Values are reported as mean ± SEM. For each groups the values with different letters superscript in the same column are significantly different (Tukey’s test), whereas mean values with different letters subscript in the same row indicates significant differences within groups (Tukey’s test) as analyzed by one-way analysis of variance (ANOVA).
The mean cholesterol and creatinine concentrations were higher in patients of the PPDI group compared to the other three groups. For cholesterol, P < 0.01, in all cases. For creatinine, P < 0.05, for RRT, and P < 0.001 in all the other cases.
For HDL, the mean value was lower for patients of the RRT group compared to the other three groups (P < 0.05 for PPDI and P < 0.001 in all the other cases).
In the case of fibrinogen, a lower mean value was found for patients of the RRT group compared to RRT and RRTD groups (P < 0.05).
Higher values of waist and hip circumferences and higher levels of creatinine and cholesterol were observed in the PPDI group when compared with the other groups of RRMS. In the same group, a significant decrease of the triglycerides/HDL ratio was observed after six months (Table 5). In RRT group significant lower levels of HDL were determined.
Discussion
Usefulness of a complementary dietary intervention in PPMS patients and in RRMS patients under treatment with IFN-β
The main aim of this study was to investigate the effects of a complementary dietary intervention on the wellness of RRMS and PPMS patients, and to test the compliance of the patients with dietary guidelines. Dietary intervention may be particularly important for PPMS patients, as no therapy is still available for them, but it may be relevant as add-on treatment in RRMS patients receiving IFN-β as well.
It can be actually expected that a nutritional intervention based on a calorie-restricted, semi-vegetarian diet, and administration of vitamin D3, with or without the other “healthy” dietary supplements chosen for this study, may contribute to counteract chronic inflammation, which is a common characteristic of both PPMS and RRMS. The anti-inflammatory effect is achieved by the activation of nuclear receptors and enzymes that up-regulate oxidative metabolism, down-regulate the synthesis of pro-inflammatory molecules, and restore or maintain a healthy symbiotic gut microbiota.20
While low level of vitamin D3 has been recognized as a potential risk factor for MS, the effects on MS patients of specific diets and dietary supplements, alone or in combination, are still largely unknown. Some studies, focused on the association of vitamin D with IFN-β, suggested that together they can synergistically counteract the disease.48 A similar synergic effect could be expected from the semi-vegetarian diet and the dietary supplements in this study. If so, one of the benefits that may derive from a nutritional intervention in RRMS patients could be to reduce the IFN-β dosage and hence to obtain the decrease of antibody formation vs. IFN-β. With regard to PPMS, the efficacy of the dietary intervention in lowering the level of chronic inflammation or to prevent its worsening, would mean to slow-down the disease progression.
On these grounds, the added value in associating vitamin D treatment with dietary guidelines is given by the fact that “healthy”, i.e. anti-inflammatory, dietary factors and life style act both at systemic and gut level. It is indeed well known that endotoxins released from a dysbiotic gut microbiota may contribute to the development of a systemic inflammatory status that in turn may favor MS relapses. Therefore, modification of a dysbiotic gut microbiota by a diet, to balance its composition towards eubiosis, may be a possible strategy to help managing of MS progression.
Reasons that may explain the persistence of insufficient values of vitamin D3
It is now commonly accepted that vitamin D3 is also an immune-modulatory and anti-inflammatory agent, and it is ascertained that patients with MS and other chronic inflammatory diseases have a low level of vitamin D3.21,49 So, low vitamin D3 level may represent a common con-cause of chronic inflammation.
Insufficient levels of active vitamin D may be due to insufficient exposure to sunlight, in consequence of living at low latitudes, as in the geographical distribution of MS. However, this cannot be a relevant aspect in sunny countries as Southern Italy and the area of Matera in particular, having plenty sunshine (9–15 h). To explain this discrepancy, it can be assumed that the insufficient levels of active vitamin D3, observed in all MS patients in this study, may be also due to an altered metabolism or function of this vitamin.
In our study administration of RDA doses of vitamin D3 were insufficient to achieve the target serum level ≥75 nmol/L (30 ng/mL) of calcidiol (25OHD3). Values higher than 75 nmol/L are considered to be adequate, because this is the minimal value at which vitamin D3 is significantly bound to its receptor VDR, immunological properties become evident and the parathormone is reaching a plateau.50–52 RDA equivalent doses of vitamin D were chosen for the treatment of MS patients, because they are the most frequent doses of administration in common praxis. So, the question arises why in the present study cholecalciferol supplementation failed to increase vitamin D3 levels. Can this failure be due to metabolic or functional defects?
Low vitamin D levels are increasingly associated with unfavorable metabolic phenotypes, including type 2 diabetes and cardiovascular diseases, which are commonly linked with overweight and obesity. Has already reported, MS patients with low content of vitamin D have significantly higher body mass index (BMI), which could account for the involvement of an altered metabolism, and high BMI before age 20 is associated with 2x increased risk of MS,53 and with tendency to gut dysbiosis.
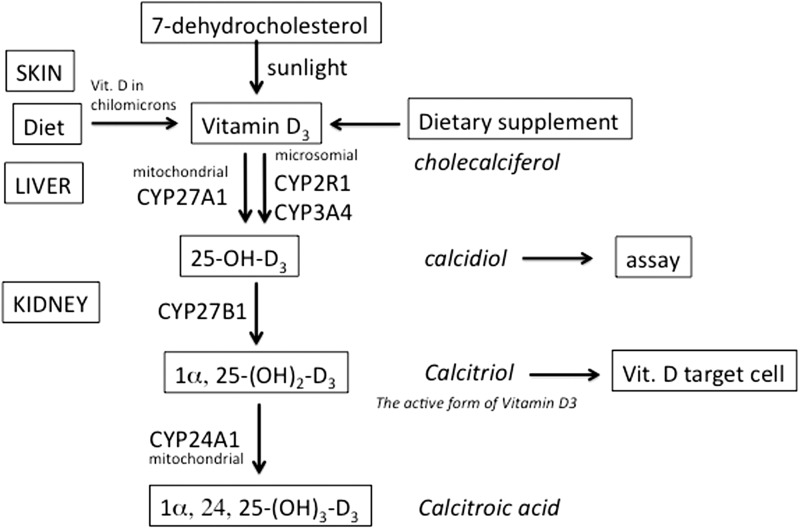
As shown in Figure 6, the metabolic pathway leading to active vitamin D3 starts in the skin with the UV light-mediated formation of vitamin D3 (cholecalciferol). In the liver, vitamin D3 is hydroxylated in position 25 by various cytochrome P450 isoforms (CYP). The most important 25-hydroxylases are the mitochondrial CYP21A1 and the microsomial CYP2R1. Their product, the 25(OH)D3 (calcidiol), is modified in the kidney in the active form of vitamin D3: 1α-25(OH)2D3 (calcitriol). This latter is inactivated by a 24(OH) hydroxylase (CYP24A1), which is induced by its substrate.
Figure 6.
Schematic representation of the metabolic pathway leading to the activation of vitamin D3 to calcitriol
This means that the down-regulation of the CYP2R154,55 and/or CYP27B1—the enzymes responsible of the synthesis of 25(OH)D3—and/or the up-regulation of gene expression of the CYP24A156—the enzyme responsible for the inactivation of 1α-25(OH)2D3 (calcitriol)—may lead to misbalance of vitamin D3. In other words, the levels of active vitamin D3 depend on the relative rates of its synthesis via CYP27B1 and its modification via CYP24A1.57 High CYP24A1 expression (that can be induced, for example, by xenobiotics) might lead to low levels of vitamin D3 and cause or worsen chronic inflammatory diseases and cancer. If vitamin D3 levels remain low, in spite of cholecalciferol administration, the expression of CYP24A1 mRNA should be examined, and determination of CYP27B1 and CYP24A1 activities and their inhibition should be tested.58,59
It is important to note that vitamin D has a direct effect on the brain, as circulating 25(OH)D3 can pass through the BBB cells and can be converted in neuronal and glial cells directly to 1α,25(OH)2D3, which then binds to its receptor, VDR.60
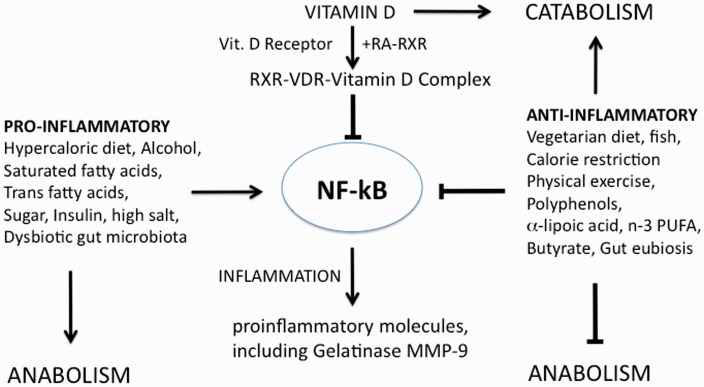
The receptor VDR is the tool by which vitamin D3 exerts its most important actions. The VDR-D complex controls the expression of several genes involved in processes relevant to inflammation and chronic diseases. In particular, as schematically shown in Figure 7, the VDR-D complex binds to the retinoic acid–retinoid X receptor (RA–RXR) complex, in competition with other ligand-activated nuclear receptors such as peroxisome proliferator-activated receptors (PPARs) or liver X receptors (LXRs). The heterodimeric complex formed by RA–RXR with VDR-D binds to the pro-inflammatory transcription factor NF-kB and down-regulate the synthesis of pro-inflammatory molecules, such as the gelatinase MMP-9. The dietary supplements given in this study (indicated on the right in Figure 7) also inhibit inflammation, whereas a “wrong” hyper-caloric high animal-fat diet up-regulates the inflammatory processes and favors a dysbiotic gut microbiota. As at least 75 nmol/L of hydroxycholecalciferol are needed to achieve full activation of the vitamin D receptor,50 it may be concluded that the vitamin D levels detected in the MS patients in our study are insufficient for the formation of the VDR-D complex and VDR activity.
Figure 7.
Schematic description of how nutritional intervention can influence the inflammatory status. VDR: vitamin D receptor; RXR: retinoid X-receptor; RA: 9 cis-retinoic acid; NF-kB: nuclear transcription factor-kB; MMP: metalloproteinase; n-3 PUFA: omega-3 polyunsaturated fatty acids
Another aspect to be taken into consideration, when evaluating the effectiveness of vitamin D supplementation in the course of MS, is to consider the eventual polymorphisms affecting the VDR, which has been recently associated with obesity, inflammation, and alterations of gut permeability.61 In fact, genetic factors might be as important as the effect of season, as it has been shown that besides the vitamin D receptor polymorphism, 25(OH)D3 levels are significantly related also to single nucleotide polymorphisms (SNPs) in the vitamin D binding protein (DBP), RXR, and CYP2R1 genes.55,62 Finally, the significance of the increased circulating levels of DBP observed in MS patients should be investigated, in regard to vitamin D bioavailability and neuroinflammation.63
Moreover, the finding that that VDR-D activate the Sirtuin SIRT-1, an anti-inflammatory, and anti-aging enzyme64,65 suggests that vitamin D can influence also cell metabolism and therefore can indirectly up-regulate oxidative metabolism and down-regulate biosynthetic pathways including those related to the synthesis of pro-inflammatory molecules (Figure 7).
Accordingly, vitamin D was reported to inhibit IL-17 and improve other markers of inflammation and MRI findings in IFN-β-treated patients, although no benefits in terms of clinical efficacy were observed.66 Furthermore vitamin D can inhibit IFN-γ production and is also effective in regulating gastrointestinal homeostasis by inducing regulatory T cells and innate immune responses, as well as favoring epithelial cell integrity, and a healthy composition of the gut microbiota.67 Furthermore, vitamin D appears to be a potent antioxidant, as it inhibits the formation of free radicals by nitric oxide synthase and gamma-glutamyl transpeptidase.60 And finally, vitamin D appears to have an important role in brain development by regulating the level of neurotrophic factors.60
On these grounds, vitamin D may represent a very promising dietary molecule for the treatment of chronic inflammatory diseases as MS.21,68–70 However, when studying vitamin D in experimental models, it should be considered that there might be differences with humans. Actually, in humans, unlike in mice, obesity is associated with poor vitamin D status.71
Duration of dietary treatment and usefulness of monitoring the inflammatory status by zymography
In this study no effect of vitamin D supplementation on vitamin D nutritional status of patients was found. This was in disagreement with previous studies showing that six months treatments with cholecalciferol slightly above RDA dosage were sufficient to increase 25(OH)D3 levels.55,72,73 The baseline levels of vitamin D3 in our patients, lower than in other studies, might explain the persistence of low values of vitamin D3 despite the administration of RDA doses of the vitamin. On the contrary, fish oil supplementation was sufficient to increase the ratio EPA + DHA/ARA (n-3/n-6 PUFA) to a nearly satisfactory level already after three months (Figure 3), suggesting that a dietary regimen alone, as in the RRTD group, may be sufficient to increase the EPA + DHA/ARA ratio, thus potentially ameliorating inflammatory status of patients.
In our study, the anti-inflammatory efficacy of combined therapy—vitamin D, diet, and dietary supplements—was effectively monitored by the zymographic analysis of the gelatinase MMP-9. Our results show that 1-DZ can represent very well the individual response to treatment and that in general there was an improvement in the inflammatory status. In fact, the levels of activated gelatinase MMP-9 increased only in a minority of patients, and the involved gelatinase was only the 82 kDa MMP-9. The 65 kDa MMP-9 level never increased. Among the two gelatinases, the 82 kDa isoform is the first to increase in case of MS worsening.46 This is understandable, as the 82 kDa MMP-9 can still be inhibited by its natural inhibitor tissue inhibitor of metalloproteinase-1 (TIMP-1), whereas the 65 kDa MMP-9 is TIMP-1 resistant and is the predominant active MMP-9 isoform in the course of MRI activity in MS.74
The disease worsening observed in eight out of the 29 patients with dietary prescription, might be a matter of both the short treatment duration and inter-individual differences of gut dysbiotic microbiota to change in response to dietary intervention.
Conclusions
In conclusion, the data obtained in this study provided important indications for the nutritional management of MS patients and future studies aimed to associate treatment with dietary guidelines.
In our study, it was shown that:
All MS patients had insufficient or sub-optimal levels of vitamin D3. It can be suggested that this is a common condition in MS patients;
Vitamin D3 nutritional status of patients was not ameliorated, neither by administration of vitamin D3 at ca. 1.5 fold the RDA dose nor by combined administration of IFN-β, with or without dietary management or dietary supplements;
A dietary regimen, mainly based on principles of Mediterranean diet, with or without administration of dietary supplements, determined an increase of the rate n-3/n-6 PUFA serum concentration thus supporting the general trend towards an amelioration of inflammatory status. This was established in 12 out of 29 patients, on the basis of reduced active MMP-9 levels in the RRTD, RRTDI and PPMS groups), and of anthropometric parameters as well. PPMS patients were more responsive to the nutritional intervention with fish oil and lipoic acid.
From a practical point of view, data are supporting the idea that the assessment of the nutritional status of vitamin D3 should be made on a routine basis in MS patients and dosage of vitamin D3 should be much higher than that used in this study to counteract the sub-optimal vitamin D3 status. In this respect, it is also evident the need to standardize 25(OH)D3 measurements towards a specific method.75
In regard to the assessment of serum gelatinases (by 1-DZ or another method) to evaluate patients inflammatory status, it appears to be a valuable tool to follow the individual response to the therapy. In fact, the disease is complex in nature and unique in the individual course, and no patient responds to therapy in the same way.10 Furthermore, there are no truly reliable biomarkers that allow for everyone to evaluate the effectiveness of treatment and it is therefore important to discover novel markers of the disease.76
Further research in larger randomized controlled trials is necessary. However, it is important to note that in all the studies done so far no adverse effects for a nutritional treatment in MS or interferences with therapy were observed. In most cases it has been reported rather an improvement of well-being, if not a clinical improvement.
Recent small size trials based on different nutritional plans to treat MS confirm this position. The McDougall diet had some favorable general health marker change but no significant change in fatigue or MRI,77 whereas a modified Paleo diet study on SPMS showed significant results in reduction of fatigue severity.78,79 Dietary treatments with omega-3 fatty acids80 or a nutraceutical mixture of omega-3 and omega-6 fatty acids were found to improve patient wellness.81 In particular, administration of a nutraceutical formula of omega-3 and omega-6 fatty acids including gamma-tocopherol (but without dietary prescriptions) significantly reduced the annualized relapse rate vs. placebo.81 No adverse effects but a high rate of dropouts (49%) were reported. In our study, the rate of dropouts was just 9%, but only in the groups receiving dietary supplements. We may conclude that patient compliance and acceptability is higher towards dietary prescription than towards supplements, and choice of dietary supplements may be a critical step in nutritional interventions.
Acknowledgments
The authors are grateful to Prof Vincenzo Fogliano, Wageningen, Nederland, for his advices in the measurement of lipid levels.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Italian Foundation for Multiple Sclerosis (FISM) for the Projects ‘‘Healthy and Functional Foods for MS patients’’ [Grant 2007/R/15], ‘‘The Molecular Basis for Nutritional Intervention in Multiple Sclerosis’’ [Grant 2010/R/35], and “Nutritional facts in multiple sclerosis: why they are important and how they should be managed” [Grant 2014/S/2] to PR.
Author contributions
PR conceived and designed the study (with MGC, RR, ME and ADS) and obtained the funding. PR and RR collected and analyzed the data, wrote the article and prepared the figures. ML and RR performed, analyzed and interpreted the zymographic data. VT performed the statistical analyses. IM and PV performed, analyzed and interpreted the lipid analyses. ME designed the dietary regimen. AG and ADS were involved in the selection and dosage of the dietary supplements. EDM and MGC were responsible for the clinical part of the study: ethics approval, diagnosis of the disease, enrollment, information about study protocol, therapy, anthropometric measures, neurological examinations including Kurtzke Expanded Disability Status Scale (EDSS) and Fatigue Severity Scale (FSS), the Quality of life measured by The Short Form (SF-36) questionnaire and the Ham-D (Hamilton Rating Scale for Depression) questionnaire at t0, t3, and t6 months.
Declaration of conflicting interests
Two of the co-authors (ADS, AG) are employees of a company that manufactures the products used in this study.
References
- 1.Constantinescu CS, Gran B. Multiple sclerosis: autoimmune associations in multiple sclerosis. Nat Rev Neurol 2010; 6: 591–2. [DOI] [PubMed] [Google Scholar]
- 2.Miller E. Multiple sclerosis. Adv Exp Med Biol 2012; 724: 222–38. Review. [DOI] [PubMed] [Google Scholar]
- 3.Lassmann H. Multiple sclerosis: Lessons from molecular neuropathology. Exp Neurol 2014; 262: 2–7. [DOI] [PubMed] [Google Scholar]
- 4.Naegele M, Martin R. The good and the bad of neuroinflammation in multiple sclerosis. Handb Clin Neurol 2014; 122: 59–87. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J Autoimmun 2014; 54: 33–50. [DOI] [PubMed] [Google Scholar]
- 6.Jacques FH, Lublin FD. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2015; 84: 963–963. [DOI] [PubMed] [Google Scholar]
- 7.Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol 2014; 27: 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AJ, Polman CH, Miller DH, McDonald WI, Brochet B, Filippi M, Miller DH, Polman CH, Stevenson VL, McDonald WI. Primary progressive multiple sclerosis. Brain 1997; 120: 1085–96. [DOI] [PubMed] [Google Scholar]
- 9.Filippini G, Del Giovane C, Vacchi L, D’Amico R, Di Pietrantonj C, Beecher D, Salanti G. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Db Syst Rev 2013; 6 CD008933. Review. [DOI] [PubMed] [Google Scholar]
- 10.Loleit V, Biberacher V, Hemmer B. Current and future therapies targeting the immune system in multiple sclerosis. Curr Pharm Biotechnol 2014; 15: 276–96. [DOI] [PubMed] [Google Scholar]
- 11.Bridel C, Lalive PH. Update on multiple sclerosis treatments. Swiss Med Wkly 2014; 144: 14012–14012. [DOI] [PubMed] [Google Scholar]
- 12.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012; 8: 602–12. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesan A, Johnson RT. Infections and multiple sclerosis. Handb Clin Neurol 2014; 122: 151–71. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015; 14: 263–73. [DOI] [PubMed] [Google Scholar]
- 15.Zanella SG, Roberti di Sarsina P. Personalization of multiple sclerosis treatments: using the chelation therapy approach. Explore 2013; 9: 244–8. [DOI] [PubMed] [Google Scholar]
- 16.Latronico T, Branà MT, Merra E, Fasano A, Di Bari G, Casalino E, Liuzzi GM. Impact of manganese neurotoxicity on MMP-9 production and superoxide dismutase activity in rat primary astrocytes. Effect of resveratrol and therapeutical implications for the treatment of CNS diseases. Toxicol Sci 2013; 135: 218–28. [DOI] [PubMed] [Google Scholar]
- 17.Jafari N, Hintzen QR. The association between cigarette smoking and multiple sclerosis. J Neurol Sci 2011; 311: 78–85. Review. [DOI] [PubMed] [Google Scholar]
- 18.Munger KL. Childhood obesity is a risk factor for multiple sclerosis. Mult Scler 2013; 19: 1800–1800. [DOI] [PubMed] [Google Scholar]
- 19.Riccio P. The molecular basis of nutritional intervention in multiple sclerosis: a narrative review. Complement Ther Med 2011; 19: 228–37. [DOI] [PubMed] [Google Scholar]
- 20.Riccio P, Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro 2015; 7 1759091414568185. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalbán X, Edan G, Barkhof F, Pleimes D, Radü EW, Sandbrink R, Kappos L, Pohl C. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014; 71: 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farinotti M, Vacchi L, Simi S, Di Pietrantonj C, Brait L, Filippini G. Dietary interventions for multiple sclerosis. Cochrane Db Syst Rev 2012; 12 CD004192. Review. [DOI] [PubMed] [Google Scholar]
- 23.Swank RL, Goodwin JV. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003; 19: 161–2. [DOI] [PubMed] [Google Scholar]
- 24.Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scan 2000; 102: 143–9. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, Venkatraman J, Meksawan K, Deinehert S, Pendergast D, Awad AB, Ramanathan M, Munschauer F, Rudick R. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids 2005; 73: 397–404. [DOI] [PubMed] [Google Scholar]
- 26.Shinto L1, Marracci G, Baldauf-Wagner S, Strehlow A, Yadav V, Stuber L, Bourdette D. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot Essent Fatty Acids 2009; 80: 131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Ramirez V1, Macias-Islas MA, Ortiz GG, Pacheco-Moises F, Torres-Sanchez ED, Sorto-Gomez TE, Cruz-Ramos JA, Orozco-Aviña G, Celis de la Rosa AJ. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid Med Cell Longev 2013; 2013: ID709493–ID709493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav V1, Marracci GH, Munar MY, Cherala G, Stuber LE, Alvarez L, Shinto L, Koop DR, Bourdette DN. Pharmacokinetic study of lipoic acid in multiple sclerosis: comparing mice and human pharmacokinetic parameters. Mult Scler 2010; 16: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Progr Lipid Res 2008; 47: 147–55. [DOI] [PubMed] [Google Scholar]
- 30.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006; 83: 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 31.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem 2007; 101: 577–99. [DOI] [PubMed] [Google Scholar]
- 32.Liuzzi GM, Latronico T, Fasano A, Carlone G, Riccio P. Interferon-beta inhibits the expression of metalloproteinases in rat glial cell cultures: implications for multiple sclerosis pathogenesis and treatment. Mult Scler 2004; 10: 290–7. [DOI] [PubMed] [Google Scholar]
- 33.Liuzzi GM, Latronico T, Rossano R, Viggiani S, Fasano A, Riccio P. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells-implications for complementary multiple sclerosis treatment. Neurochem Res 2007; 32: 2184–93. [DOI] [PubMed] [Google Scholar]
- 34.Mehta LR, Dworkin RH, Schwid SR. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat Clin Pract Neurol 2009; 5: 82–92. [DOI] [PubMed] [Google Scholar]
- 35.Coluccia A, Borracci P, Renna G, Giustino A, Latronico T, Riccio P, Carratù MR. Developmental omega-3 supplementation improves motor skills in juvenile-adult rats”. Int J Dev Neurosci 2009; 27: 599–605. [DOI] [PubMed] [Google Scholar]
- 36.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 201;16:592–7. [DOI] [PMC free article] [PubMed]
- 37.Hong S, Lu Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Front Immunol 2013; 4: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 2015; 1851: 397–413. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinthone S, Yadav V, Schillace RV, Bourdette DN, Carr DW. Lipoic acid attenuates inflammation via cAMP and protein kinase A signaling. PLoS One 2010; 5: e13058–e13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 2007; 6: 168–73. [DOI] [PubMed] [Google Scholar]
- 41.Cheng G, Zhang X, Gao D, Jiang X, Dong W. Resveratrol inhibits MMP-9 expression by up-regulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci Lett 2009; 451: 105–8. [DOI] [PubMed] [Google Scholar]
- 42.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys 2012; 523: 103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marziniak M, Meuth S. Current perspectives on interferon Beta-1b for the treatment of multiple sclerosis. Adv Ther 2014; 31: 915–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasper LH, Reder AT. Immunomodulatory activity of interferon-beta. Ann Clin Trans Neurol 2014; 1: 622–31. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marangoni F, Colombo C, Galli C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: applicability to nutritional and epidemiological studies. Anal Biochem 2004; 326: 267–72. [DOI] [PubMed] [Google Scholar]
- 46.Rossano R, Larocca M, Riviello L, Coniglio MG, Vandooren J, Liuzzi GM, et al. Heterogeneity of serum gelatinases MMP-2 and MMP-9 isoforms and charge variants. J Cell Mol Med 2014; 18: 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellini T, Trentini A, Manfrinato MC, Tamborino C, Volta CA, Di Foggia V, Fainardi E, Dallocchio F, Castellazzi M. Matrix metalloproteinase-9 activity detected in body fluids is the result of two different enzyme forms. J Biochem 2012; 151: 493–9. [DOI] [PubMed] [Google Scholar]
- 48.Stewart N, Simpson S, Jr, van der Mei I, Ponsonby AL, Blizzard L, Dwyer T, Pittas F, Eyles D, Ko P, Taylor BV. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012; 79: 254–60. [DOI] [PubMed] [Google Scholar]
- 49.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res 2014; 7: 69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Handel AE, Sandve GK, Disanto G, Berlanga-Taylor AJ, Gallone G, Hanwell H, Drabløs F, Giovannoni G, Ebers GC, Ramagopalan SV. Vitamin D receptor ChIP-seq in primary CD4+ cells: relationship to serum 25-hydroxyvitamin D levels and autoimmune disease. BMC Med 2013; 11: 163–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–81. Review. [DOI] [PubMed] [Google Scholar]
- 52.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med 1998; 338: 777–83. [DOI] [PubMed] [Google Scholar]
- 53.Hedström AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler 2012; 18: 1334–6. [DOI] [PubMed] [Google Scholar]
- 54.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 2004; 101: 7711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Didriksen A, Grimnes G, Hutchinson MS, Kjærgaard M, Svartberg J, Joakimsen RM, Jorde R. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol 2013; 169: 559–67. [DOI] [PubMed] [Google Scholar]
- 56.Ramasamy A, Trabzuni D, Forabosco P, Smith C, Walker R, Dillman A, Sveinbjornsdottir S; North American Brain Expression Consortium (NABEC), UK Brain Expression Consortium (UKBEC), Hardy J, Weale ME, Ryten M. Genetic evidence for a pathogenic role for the vitamin D3 metabolizing enzyme CYP24A1 in multiple sclerosis. Mult Scler Relat Disord 2014;3:211–9. [DOI] [PMC free article] [PubMed]
- 57.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta 2011; 1814: 186–99. Review. [DOI] [PubMed] [Google Scholar]
- 58.Chiellini G, Rapposelli S, Zhu J, Massarelli I, Saraceno M, Bianucci AM, Plum LA, Clagett-Dame M, DeLuca HF. Synthesis and biological activities of vitamin D-like inhibitors of CYP24 hydroxylase. Steroids 2012; 77: 212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kósa JP, Horváth P, Wölfling J, Kovacs D, Balla B, Matyus P, Horváth E, Speer G, Takács I, Nagy Z, Horváth H, Lakatos P. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J Gastroenterol 2013; 19: 2621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holick MF. Vitamin D and brain health: the need for vitamin D supplementation and sensible sun exposure. J Int Med 2015; 277: 90–3. [DOI] [PubMed] [Google Scholar]
- 61.Al-Daghri NM, Guerini FR, Al-Attas OS, Alokail MS, Alkharfy KM, Draz HM, Agliardi C, Costa AS, Saulle I, Mohammed AK, Biasin M, Clerici M. Vitamin D receptor gene polymorphisms are associated with obesity and inflammosome activity. PLoS One 2014; 9: e102141–e102141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grzegorzewska AE, Jodłowska E, Mostowska A, Sowińska A, Jagodziński PP. Single nucleotide polymorphisms of vitamin D binding protein, vitamin D receptor and retinoid X receptor alpha genes and response to hepatitis B vaccination in renal replacement therapy patients. Expert Rev Vaccines 2014; 13: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 63.Rinaldi AO, Sanseverino I, Purificato C, Cortese A, Mechelli R, Francisci S, Salvetti M, Millefiorini E, Gessani S, Gauzzi MC. Increased circulating levels of vitamin D binding protein in MS patients. Toxins 2015; 7: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, White JH. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol 2010; 30: 4890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polidoro L, Properzi G, Marampon F, Gravina GL, Festuccia C, Di Cesare E, Scarsella L, Ciccarelli C, Zani BM, Ferri C. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res 2013; 6: 221–31. [DOI] [PubMed] [Google Scholar]
- 66.Golan D, Halhal B, Glass-Marmor L, Staun-Ram E, Rozenberg O, Lavi I, Dishon S, Barak M, Ish-Shalom S, Miller A. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: a randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol 2013; 13: 60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med 2014; 239: 1524–30. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 2008; 194: 7–17. [DOI] [PubMed] [Google Scholar]
- 69.Pierrot-Deseilligny C. Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 2009; 256: 1468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor BV. Sunshine and multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84: 1066–1066. [DOI] [PubMed] [Google Scholar]
- 71.Bouillon R, Carmeliet G, Lieben L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol 2014; 10: 79–87. Review. [DOI] [PubMed] [Google Scholar]
- 72.Dörr J, Döring A, Paul F. Can we prevent or treat multiple sclerosis by individualized vitamin D supply? EPMA J 2013; 4: 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shab-Bidar S, Bours S, Geusens PP, Kessels AG, van den Bergh JP. Serum 25(OH)D response to vitamin D3 supplementation: a meta-regression analysis. Nutrition 2014; 30: 975–85. Review. [DOI] [PubMed] [Google Scholar]
- 74.Trentini A, Manfrinato MC, Castellazzi M, Tamborino C, Roversi G, Volta CA, Baldi E3, Tola MR4, Granieri E1, Dallocchio F1, Bellini T1, Fainardi E5. Emilia-Romagna network for Multiple Sclerosis (ERMES) study group. TIMP-1 resistant matrix metalloproteinase-9 is the predominant serum active isoform associated with MRI activity in patients with multiple sclerosis. Mult Scler J 2015; 21: 1121–30. [DOI] [PubMed] [Google Scholar]
- 75.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, Fabian D, Hauptlorenz S, Halwachs-Baumann G. 25-Hydroxy-vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab 2014; 60: 1541–50. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez O1, Martin R, Rovira A, Llufriu S, Vidal-Jordana A, Fernandez-Sanchez VE, Alvarez-Cermeno JC, Izquierdo G, Arroyo-Gonzalez R, Rodriguez-Antiguedad A, Casanova-Estruch B, Montalban X. Biomarkers in multiple sclerosis: an update for 2014. Rev Neurol 2014; 58: 553–70. [PubMed] [Google Scholar]
- 77.Yadav V MG, Kim E, Spain R, Cameron M, Overs S, McDougall J, Bourdette D. Effects of a low fat plant based diet in multiple sclerosis (MS): results of a 1 year long randomized controlled study (P6.152). Neurology 2014; 82(10 suppl): 6.152–6.152. [Google Scholar]
- 78.Bisht B, Darling WG, Shivapour ET, Lutgendorf SK, Snetselaar LG, Chenard CA, Wahls TL. Multimodal intervention improves fatigue and quality of life in subjects with progressive multiple sclerosis: a pilot study. Degen Neurol Neuromuscular Dis 2015; 5: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bisht B, Darling WG, Grossmann RE, Shivapour ET, Lutgendorf SK, Snetselaar LG, Hall MJ, Zimmerman MB, Wahls TL. A multimodal intervention for patients with secondary progressive multiple sclerosis: feasibility and effect on fatigue. J Altern Complem Med 2014; 20: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torkildsen O, Wergeland S, Bakke S, Beiske AG, Bjerve KS, Hovdal H, Midgard R, Lilleås F, Pedersen T, Bjørnarå B, Dalene F, Kleveland G, Schepel J, Olsen IC, Myhr KM. Omega-3 Fatty Acid Treatment in Multiple Sclerosis (OFAMS study): a randomised, double-blind, placebo-controlled trial. Archiv Neurol 2012; 69: 1044–51. [DOI] [PubMed] [Google Scholar]
- 81.Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013; 3: e002170–e002170. [DOI] [PMC free article] [PubMed] [Google Scholar]