Abstract
Previous studies have revealed multiple functional roles of long non-coding RNA taurine upregulated gene 1 in different types of malignant tumors, except for human glioma. Here, it was designed to study the potential function of taurine upregulated gene 1 in glioma pathogenesis focusing on its regulation on cell apoptosis. The expression of taurine upregulated gene 1 in glioma tissues was detected by quantitative RT-PCR and compared with that in adjacent normal tissues. Further correlation analysis was conducted to show the relationship between taurine upregulated gene 1 expression and different clinicopathologic parameters. Functional studies were performed to investigate the influence of taurine upregulated gene 1 on apoptosis and cell proliferation by using Annexin V/PI staining and cell counting kit-8 assays, respectively. And, caspase activation and Bcl-2 expression were analyzed to explore taurine upregulated gene 1-induced mechanism. taurine upregulated gene 1 expression was significantly inhibited in glioma and showed significant correlation with WHO Grade, tumor size and overall survival. Further experiments revealed that the dysregulation of taurine upregulated gene 1 affected the apoptosis and cell proliferation of glioma cells. Moreover, taurine upregulated gene 1 could induce the activation of caspase-3 and-9, with inhibited expression of Bcl-2, implying the mechanism in taurine upregulated gene 1-induced apoptosis. taurine upregulated gene 1 promoted cell apoptosis of glioma cells by activating caspase-3 and -9-mediated intrinsic pathways and inhibiting Bcl-2-mediated anti-apoptotic pathways, acting as a tumor suppressor in human glioma. This study provided new insights for the function of taurine upregulated gene 1 in cancer biology, and suggested a potent application of taurine upregulated gene 1 overexpression for glioma therapy.
Keywords: Taurine upregulated gene 1, human glioma, cell apoptosis, caspase, Bcl-2
Introduction
Human glioma is one common malignancy in the central nervous system of brain, accounting for up to 10%,1 and of which glioblastoma (GBM) is the most aggressive subtype with less than 5% of five-year survival rates.2 Currently, some advances have been achieved in multimodal treatments, including surgical extirpation, local irradiation and conventional chemotherapy; however, the fatality rate in patients within two years of diagnosis is still high according to recent reports.3,4 Mounting efforts have been made to explore the complex gene interaction and molecular modulation network involved in the development of glioma for reliable diagnostic markers and effective therapeutic targets.5,6 However, the mechanism is still far from clearly understood, and the identification of genes or proteins that show potential effect on glioma development is still imperative.
Long non-coding RNAs (LncRNAs), a new class of non-coding RNAs with more than 200 bases have been reported to function in various biologcal process due to their regulation on gene expression.7 Emerging evidence suggests that LncRNAs show close correlation with diverse malignancies, and act as oncogenes or tumor suppressors in different types.8 LncRNA taurine upregulated gene 1 (TUG1), with a length of 7.1 kb, was initially detected in a genomic screening study for upregulated genes in retinal cells under taurine treatment.9 More studies show that p53, one common tumor suppressor, can induce the expression of TUG1, while TUG1 can bind to the PRC2 complex, acting as a repressor for specific genes involved in cell-cycle regulation.10 And, TUG1 downregulation has been detected in the non-small cell lung carcinoma tissues, showing positive association with advanced tumor development and poor overall survival.11 These studies imply a role of TUG1 as one potential tumor suppressor by inhibiting cell proliferation and promoting apoptosis. Conversely, however, significantly higher TUG1 expression is reported in osteosarcoma, indicating another potential role of TUG1 as one oncogene,12 while similar function of TUG1 as cell growth promoter has been also reported in esophageal squamous cell carcinoma,13 as well as mouse pancreatic β cells.14
Although one latest research has reported the downregulation of TUG1 in doxorubicin-induced necrosis of human glioma cells,15 the functional role of TUG1 in glioma pathogenesis is still unrevealed. In this study, TUG1 expression was detected and analyzed in glioma tissues with different clinicopathologic parameters. Further in vitro studies were performed to identify the functional role of TUG1 as one potential oncogene or tumor suppressor, involving the affected apoptosis and cell proliferation of human glioma cell lines.
Materials and methods
Tissue collection
A total of 120 paired primary glioma tissues and adjacent normal ones were obtained from patients who had undergone surgical treatment at the Affiliated Xuzhou Hospital of College of Medicine of Dongnan University between 2013 and 2014, with written informed consent. And, all patients were free from chemotherapy or radiotherapy before the surgery. All procedures were approved by the Ethics Committee of the Affiliated Xuzhou Hospital.
Cell culture
U251 and SHG-44, two human glioma cell lines, were purchased from Chinese Academy of Science Cell Bank (Shanghai, China). These cells were incubated in the DMEM (Invitrogen, Shanghai, China), supplemented with 10% FBS (Invitrogen), 2 mM glutamine, and 100 U/ml penicillin, at 37℃ with 5% CO2.
Plasmids, small interference RNA, and cell transfection
The full length of human TUG1 cDNA was synthesized and sub cloned into a pCDNA3.1 (Invitrogen) vector, resulting in TUG1-pCDNA for its overexpression. For small interfering RNAs (siRNAs) analysis, three TUG1 siRNAs and negative control siRNA (si-NC) were provided by Invitrogen, and the siRNA sequences targeting the sequence of TUG1 transcript were as follows: siTUG1-1# 5′-GGGAUAUAGCCAGAGAACAAUUCUA-3′; siTUG1-2# 5′-GCUUGGCUUCUAUUCUGAAUCCUUU-3′; siTUG1-3# 5′-CAGCUGUUACCAUUCAACUUCUUAA-3′. Cells were pre-incubated to 40–60% confluence on a six-well plate and then transfected by incubation with plasmids or siRNAs with Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocols. At indicated time point post the transfection, cells were harvested for further analysis.
Quantitative real-time PCR
TRIzol reagent (Invitrogen, Grand Island, NY, USA) was used to isolate total RNA from tissues or cultured cells, which was then transcribed into cDNA by the PrimeScript RT Reagent Kit (Takara, Dalian, China) with provided random primers, according to the manufacturers’ protocol. Quantitative PCR was performed on ABI 7500 real-time PCR system (Applied Biosystems; Foster, CA, USA) by using the SYBR PrimeScript RT-PCR kit (Takara). The gene expression was quantified by calculating the ΔCT value, and results were normalized to the expression of GAPDH. The sequences of primers used here were as follows: TUG1-F 5′-TAGCAGTTCCCCAATCCTTG-3′, TUG1-R 5′-CACAAATTCCCATCATTCCC-3′; GAPDH-F 5′-GTGTCTGAGCGATGTGGCT-3′, GAPDH-R 5′-GGATTTGGTCGTATTGGGC-3′.
Cell apoptosis analysis
Annexin V-FITC apoptosis detection kit (BD Biosciences; San Jose, CA, USA) was used to analyze cell apoptosis, following the manufacturer’s protocols. In brief, cells were collected after the dissociation with EDTA-free trypsin, and then washed with cold phosphate-buffered saline (PBS). Then, cells were resuspended in the binding buffer with the addition of Annexin V-FITC and PI for an incubation of 15 min in the darkness. Finally, flow cytometry analysis was performed immediately on the BD FACSCalibur (BD Biosciences).
Cell proliferation assay
Glioma cells from different groups were seeded at 3 × 104 cells/well and grown in 150 µL cultured medium. Cell viability was assayed by using cell counting kit (CCK)-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s protocols. Briefly, CCK8 reagents were added into corresponding wells at indicated time points and incubated at 37℃ for 2 h. The absorbance at 450 nm was recorded with a microwell plate reader (HynergyTM HT, BIO-TEK).
Caspases analysis
Since caspases activation is an important indicator for apoptotic process, the activities of caspase- 3, and -9 were analyzed to explore the pathways during TUG1-induced apoptosis, by using corresponding caspase Colorimetric Assay Kit (KeyGen, China) following the manufacturer’s protocols. Briefly, cultured cells at 72 h post-transfection were lysed at 4℃ for 30 min, and protein concentration in the supernatant was determined. Samples with equal protein concentration were incubated with provided reaction buffer and respective substrate at 37℃ in the darkness for 4 h. The absorbance was measured at 405 nm, and caspase activity was expressed as the ratio of OD405 nm of transfected cells in that of parental cells.
Statistical analysis
All data were expressed as the mean ± SD or means ± SEM. Student’s t-test or one-way ANOVA analysis was performed to calculate the statistical significance of the means. The unpaired t-test was used to compare the TUG1 expression in groups showing different clinical parameters (WHO grading or tumor size). Survival rate was analyzed with the Kaplan–Meier method, and log-rank tests were used to test the difference between stratified survival groups. P value of less than 0.05 was considered as significant difference.
Results
TUG1 downregulation was observed in human glioma tissues and correlated with advanced development and poor prognosis
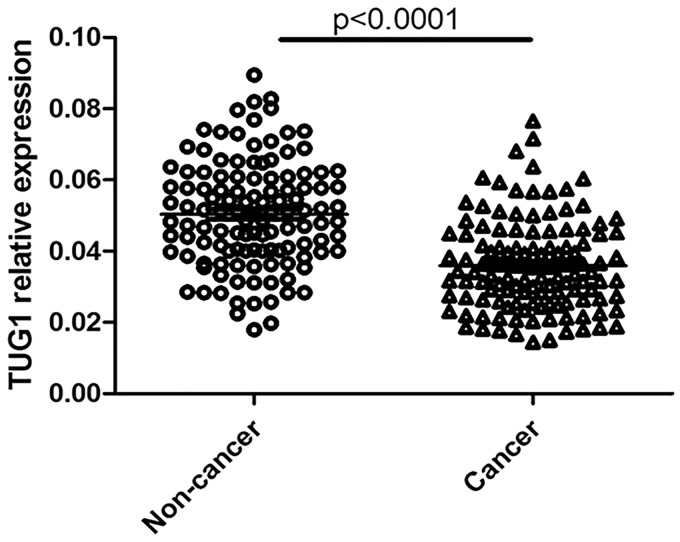
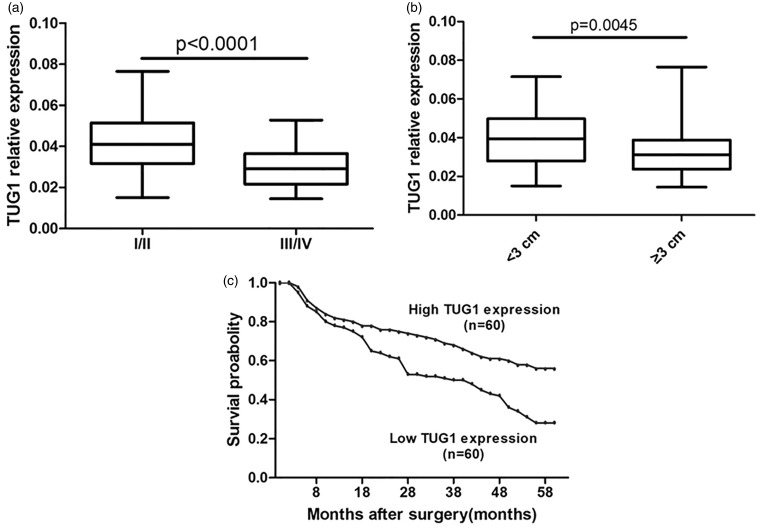
The expression levels of TUG1 were determined using qRT-PCR in glioma tissues and adjacent normal tissues from 120 glioma patients. The clinical features of these patients are provided in Table 1. In cancerous tissues, the average expression level of TUG1 was 0.036 after normalized to GAPDH, and lower than the value of 0.051 in normal specimens (Figure 1). Further correlation analysis showed that TUG1 downregulation was closely related to advanced pathological stage and larger tumor size (Figure 2(a) and (b)).
Table 1.
Characteristic of individuals
| Variable | Patients |
|---|---|
| Number of cases | 120 |
| Sex | |
| Male/female | 79/41 |
| Age at diagnosis (mean) | 49.5 |
| Clinical pathology features | |
| Extent of resection | |
| Subtotal | 49 |
| Total | 71 |
| Radiographic pattern | |
| Solitary lesion | 75 |
| Invasive and multifocal lesions | 45 |
| WHO grading | |
| I–II | 57 |
| III–IV | 63 |
| Tumor size | |
| ≥3 cm | 60 |
| <3 cm | 60 |
| KPS score | |
| ≥80 | 79 |
| <80 | 41 |
WHO: World Health Organization; KPS: Karnofsky performance score.
Figure 1.
Downregulated TUG1 expression in human glioma tissues.
TUG1 expression was analyzed in 120 pairs of human glioma tissues by real-time PCR. The levels of TUG1 in glioma tissues (Cancer) were significantly lower than those in non-tumorous tissues (non-cancer) (P < 0.0001)
Figure 2.
Analysis for TUG1 expression in the clinical parameters of human glioma. The levels of TUG1 were compared in human glioma tissues according to the WHO grading (I/II vs. III/IV; (a) or tumor size (>3 cm vs. <3 cm; (b) The values are reported means ± SEM. TUG1 expression was significantly lower in patients with a higher pathological stage (P < 0.001) and big tumor size (P = 0.0045). (c) Patients with low levels of TUG1 expression showed reduced survival probability by Kaplan–Meier method, compared with those with high levels of TUG1 expression
The association of TUG1 expression and glioma patient prognosis was also detected following Kaplan–Meier survival analysis and log-rank tests by using patient postoperative survival. On the basis of the mean ratio of relative TUG1 expression in different tumor tissues, total 120 glioma patients were classified into two groups: high TUG1 expression group (n = 60, TUG1 expression ratio ≥ 0.036) and low TUG1 expression group (n = 60, TUG1 expression ratio ≤ 0.036). According to the results of overall survival, significantly poorer prognosis was indicated in patients with lower TUG1 expression than those with higher TUG1 expression (P < 0.001, log-rank tests) (Figure 2(c)). Taken together, TUG1 downregulation might play important roles in human glioma.
TUG1 overexpression contributed to the cell apoptosis of human glioma cells
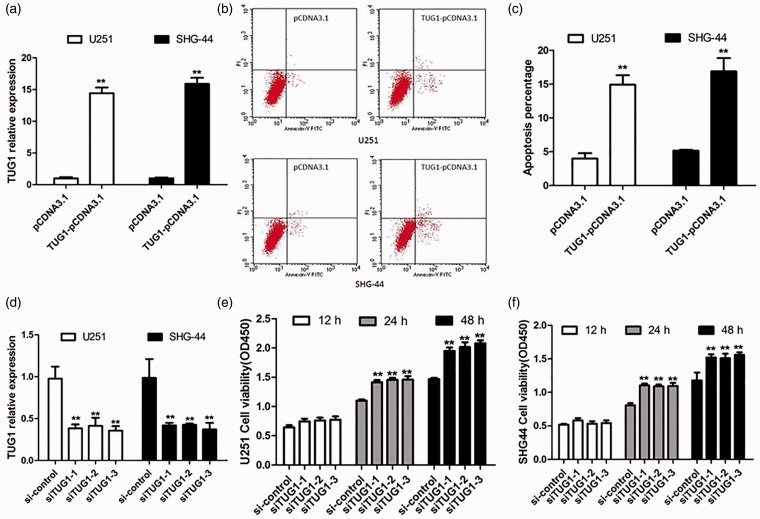
Since the significant decrease of TUG1 expression in glioma tissues showed positive correlation with poor prognosis, the possible biological significance of TUG1 in tumor genesis was investigated through gain- and loss-of-function studies in vitro. Enforced TUG1 overexpression was performed using the TUG1-pCDNA transfection in U251 and SHG-44 cells, with expression level change confirmed by qRT-PCR analysis at 24 h post-transfection. Around 15-fold increase of TUG1 expression was achieved in TUG1 overexpressing cells (Figure 3(a)). Subsequently, Annexin V/PI staining analysis was performed to determine TUG1-induced apoptosis at 48 h post-transfection. And, the percentage of apoptotic cells significantly increased in TUG1 overexpressing cells when compared to respective controls (Figure 3(b) and (c)). These results indicated that TUG1 can induce glioma cell apoptosis in vitro.
Figure 3.
TUG1 affected cell viability of human glioma cells. Induced or inhibited TUG1 expression was conducted in U251 and SHG-44 cells through TUG1 overexpression plasmid (TUG1-pCDNA3.1) or siRNAs (siTUG1) transfection, respectively. Dysregulated TUG1 expression was confirmed by real-time PCR (a) and (d). TUG1-induced cell apoptosis were detected by flow cytometric analysis. The Annexin V-FITC positive cells in the top (PI positive) and bottom (PI negative) right quadrants were induced by TUG1 overexpression (b), and the number of apoptotic cells was also shown here (c). Besides, increased cell viability was detected in TUG1 inhibiting cells after transfection for 12, 24, or 48 h through CCK8 analysis (e) and (f). **P < 0.01. (A color version of this figure is available in the online journal.)
TUG1 inhibition improved the cell proliferation of human glioma cells
To further examine whether TUG1 inhibition could promote glioma cell proliferation, TUG1 knockdown with siRNA transfection was performed in U251 and SHG-44 cells. The expression levels of TUG1 strongly decreased in cells with si-TUG1s transfection compared with si-NC-transfected cells (Figure 3(d)). After transfection for 48 h, the same number of U251 and SHG-44 cells from each group (si-TUG1-1, si-TUG1-2, si-TUG1-3, and si-NC group) was inoculated and undergone CCK8 analysis at indicated time points. The results revealed that inhibition of TUG1 promoted cell proliferation of both U251 and SHG-44 cells (Figure 3(e) and (f)). These data showed that downregulated TUG1 can promote glioma cell proliferation.
TUG1-induced caspases activation, with inhibited BCL-2 expression
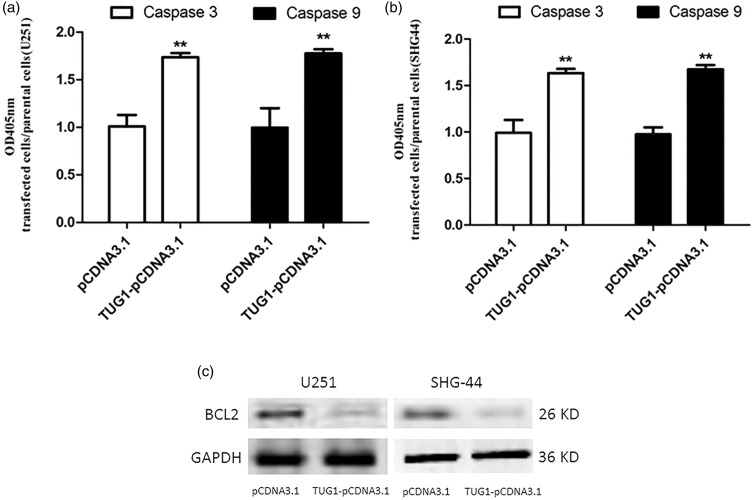
In order to further investigate the mechanism underlying TUG1 regulation on glioma cell apoptosis, caspases pathways, involving caspase-3 and -9 activation, were tested in TUG1 overexpressing cells. As shown in Figure 4(a) and (b), significant activation of both caspase-3 and -9, key executors of cell apoptosis, was observed in U251 and SHG-44 cells 72 h after transfection with TUG1-pcDNA, as compared to respective controls. These results suggested that the upregulated expression of TUG1 promoted the apoptosis of glioma cells via the activation of caspase-3 and -9 pathway. Moreover, to examine involvement of Bcl-2, a key anti-apoptotic protein, in TUG1-induced apoptosis, changes in its protein levels was also examined in TUG1 overexpressing cells with western blotting analysis. Significant downregulation of Bcl-2 was achieved under TUG1 overexpression 48 h after transfection (Figure 4(c)). Taken together, it was concluded that the mechanism of TUG1 induced glioma cell apoptosis involves caspase activation and Bcl-2 downregulation, showing anti-tumor like potential.
Figure 4.
TUG1 promoted the caspases activation with inhibited Bcl-2 expression in human glioma cells. The activation of caspases, including caspase-3 and caspase-9, was detected using colorimetric method in TUG1 overexpressing cells after TUG1-pCDNA3.1 transfection, with normal parental cells as control (a) and (b). And, Bcl-2 expression was analyzed at the same time through western blot analysis. (c) Results showed significantly activated caspases, as well as decrease of Bcl-2 expression, by TUG1 overexpression. **P < 0.01
Discussion
Glioma is the most common malignant brain tumor with remarkable characters like vascular proliferation, aggressive invasion, and poor prognosis.16 It is difficult to be cured for most glioma patients due to the resistance to conventional therapies, and the median survival time remains approximately 14 months.17 The dysregulation of TUG1 expression has been reported to influence tumor cell proliferation, apoptosis and invasion in urothelial carcinoma of the bladder, osteosarcoma, non-small cell lung carcinoma and esophageal squamous cell carcinoma,11–13,18 indicating its potential role as diagnostic marker or therapeutic target. But the study of TUG1 in glioma is very less, let alone its mechanism in glioma tumorigenesis. Of note, a latest research has reported the downregulation of TUG1 in doxorubicin-induced necrosis of human glioma cell lines.15 Thus, the purpose of this study was to identify whether TUG1 expression was disordered in glioma tissues, or involved in glioma incidence and proliferation.
Firstly, the expression profiles of TUG1 were detected in human glioma tissues and compared normal tissues through qRT-PCR analysis, and results showed decreasing tendency of TUG1 expression in cancer tissues. It was also found that decrease of TUG1 expression showed significant correlation with Grade, tumor size, and KPS scores of glioma, indicating the important role of TUG1 expression in glioma tumorigenesis. Therefore, TUG1 was speculated to negatively regulate glioma cell proliferation, showing anti-tumor like effects. To further explore the functional role of TUG1 in glioma cells, ectopic expression of TUG1 was executed in both U251 and SHG-44 glioma cells, as well as the introduction of three TUG1 siRNAs. The results showed that TUG1 overexpression could promote cell apoptosis, with significant increased apoptotic cells, while downregulated TUG1 expression resulted in increased proliferation of U251 and SHG-44 cells.
Generally, apoptosis can be stimulated by extrinsic pathway, intrinsic or mitochondrial pathway and granzyme B pathway, while the three different pathways will converge to a same execution phase that needs the caspases-3 and/or -7 activation.19,20 Through cascade reactions and/or death receptors function, cytochrome c is released from mitochondria, leading to cell death. Thus, caspase activation was also investigated here to get insights for TUG1-induced apoptosis. And, obvious activation of both caspase-3 and -9 was observed in TUG1 overexpressing glioma cells, suggesting that TUG1 promoted glioma cell apoptosis via intrinsic pathways mediated by caspase-3 and -9.21 Besides, the expression level of Bcl-2, one anti-apoptotic Bcl-2 family member, was also detected by western blotting, and data showed significant decrease of Bcl-2 level in cells with TUG1 overexpression. Therefore, it was speculated that TUG1 promoted glioma cell apoptosis via the caspase-3 and -9-mediated intrinsic pathway, involving inhibited anti-apoptotic pathways that mediated by Bcl-2.22 The precise mechanisms will require further study and elucidation.
In conclusion, TUG1 expression was significantly repressed in human glioma tissues, while this inhibition showed significant correlation with WHO Grade, tumor size, and overall survival, implying the important role of TUG1 in glioma tumorigenesis. Further gain- and loss-of-function studies revealed that the dysregulation of TUG1 affected apoptosis and cell proliferation in glioma cells. Moreover, mechanism studies for TUG1 induced apoptosis revealed the involvement of caspase 3- and -9-mediated intrinsic pathway, as well as Bcl-2-mediated anti-apoptotic pathways. This study provided new insight for TUG1 function in carcinogenesis, and suggested a potent therapeutic tool in preventing glioma by TUG1 overexpression. Further research could be performed to explore the precise mechanism between TUG1 and cell proliferation, as well as invasion, in glioma development.
Authors’ contribution
LJ was responsible for the main conceive of the study and the draft of the manuscript, and carried out most molecular genetic studies. ZM carried out the immunoassays and participated in the sequence alignment. AG helped to design the study and performed the statistical analysis. MQ helped to revise the manuscript and participated in its design. All authors have read and approved the final manuscript.
Declaration of Conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
There was no specific funding for this work.
References
- 1.Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol 2013; 2013: 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 2013; 15(suppl 2): ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol 2010; 9: 717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O'Neill B, Faul C. A clinical review of treatment outcomes in glioblastoma multiforme – the validation in a non-trial population of the results of a randomised phase III clinical trial: has a more radical approach improved survival? Br J Radiol 2012; 85: e729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta K, Salunke P. Molecular markers of glioma: an update on recent progress and perspectives. J Cancer Res Clin Oncol 2012; 138: 1971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochberg FH, Atai NA, Gonda D, Hughes MS, Mawejje B, Balaj L, Carter RS. Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn 2014; 14: 439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136: 629–41. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol 2013; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young T, Matsuda T, Cepko C. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol 2005; 15: 501–12. [DOI] [PubMed] [Google Scholar]
- 10.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 2009; 106: 11667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS, De W, Shu YQ, Wang ZX. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis 2014; 5: e1243–e1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Geng P-L, Yin P, Wang X-L, Jia J-P, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev 2013; 14: 2311–5. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, Yin R, Xu L. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumor Biol 2014; 36: 1643–51. [DOI] [PubMed] [Google Scholar]
- 14.Yin DD, Zhang EB, You LH, Wang N, Wang LT, Jin FY, Zhu YN, Cao LH, Yuan QX, De W, Tang W. Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic beta cells. Cell Physiol Biochem 2015; 35: 1892–904. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Sun S, Yu W, Jiang J, Zhuo F, Qiu G, Xu S, Jiang X. Altered expression of long non-coding RNAs during genotoxic stress-induced cell death in human glioma cells. J Neurooncol 2015; 122: 283–92. [DOI] [PubMed] [Google Scholar]
- 16.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008; 359: 492–507. [DOI] [PubMed] [Google Scholar]
- 17.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 2010; 60: 166–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol 2013; 107: 555–9. [DOI] [PubMed] [Google Scholar]
- 19.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ. Apoptosis and cancer: mutations within caspase genes. J Med Genet 2009; 46: 497–510. [DOI] [PubMed] [Google Scholar]
- 20.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 2011; 351: 41–58. [DOI] [PubMed] [Google Scholar]
- 21.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 2013; 14: 32–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15: 49–63. [DOI] [PubMed] [Google Scholar]