Abstract
Anabolic bone accruement through osteogenic differentiation is important for the maintenance of physiological bone mass and often disrupted in various inflammatory diseases. Epigallocatechin-3-gallate, as an antioxidant and anti-inflammatory agent, has been suggested for potential therapeutic use in this context, possibly by the inhibition of bone resorption as well as the enhancement of bone formation through directly activating osteoblast differentiation. However, the reported effects of epigallocatechin-3-gallate modulating osteoblast differentiation are mixed, and the underlying molecular mechanism is still elusive. Moreover, there is limited information regarding the effects of epigallocatechin-3-gallate on osteogenic potential of mesenchymal stem cell in inflammation. Here, we examined the in vitro osteogenic differentiation of human mesenchymal stem cells. We found that the cell viability and osteoblast differentiation of human bone marrow-derived mesenchymal stem cells are significantly inhibited by inflammatory cytokine TNFα treatment. Epigallocatechin-3-gallate is able to enhance the cell viability and osteoblast differentiation of mesenchymal stem cells and is capable of reversing the TNFα-induced inhibition. Notably, only low doses of epigallocatechin-3-gallate have such benefits, which potentially act through the inhibition of NF-κB signaling that is stimulated by TNFα. These data altogether clarify the controversy on epigallocatechin-3-gallate promoting osteoblast differentiation and further provide molecular basis for the putative clinical use of epigallocatechin-3-gallate in stem cell-based bone regeneration for inflammatory bone loss diseases, such as rheumatoid arthritis and prosthetic osteolysis.
Keywords: EGCG, TNFα, osteoblast differentiation, mesenchymal stem cell, inflammatory bone disease, NF-κB
Introduction
Patients with chronic inflammatory bone diseases, including arthritis, osteomyelitis, periodontal bone loss, and wear debris-induced osteolysis, not only suffered from the acceleration of bone resorption (osteoclast activation) but also devastated by a dramatic loss of bone formation activity (osteoblast differentiation).1–5 Such a dilemma remains a challenge for treating numerous inflammatory diseases that are frequently associated with severe bone loss.3,6,7 In complementary to the current treatment strategies involving the blockade of bone resorption,8–10 it is often needed for the regeneration of collagen architecture through bone neo-formation.3,6 The feasibility of cell-based approaches,11 particular using bone marrow-derived mesenchymal stem cell (BM-MSC), thus presents an exciting option for such purposes to repair bone lesions by promoting the differentiation of autologous bone cell (including chondrocyte, osteoblast, and osteocyte). Preliminary successes of MSC therapy on animal models of inflammatory bone diseases12–16 further prompted basic research to investigate the potential mechanisms on the refinement of bone tissue repair by MSCs.
As a critical mediator of pro-inflammation, TNFα stimulates strong systemic effects as well as local responses in bone disease.17–19 Both arms of bone remodeling can be affected by TNFα, resulting synergistic bone loss. The activation of TNFα on bone resorption has been extensively studied in each step of osteoclast activation,20–23 including expansion of osteoclast precursor, amplification of RANKL/RANK-mediated osteoclast differentiation, and facilitation of osteoclast specialized function. The less clear is the effect of inflammation on bone formation. Consistent with a negative impact on bone formation, TNFα can induce cell death of osteogenic cells and block osteoblast differentiation in vitro.24–26 However, its molecular target is unknown. Important candidates include bone maturation marker (e.g. Runx2, alkaline phosphatase (ALP)) and Wnt signaling,27,28 both of which are repressed by TNFα through a variety of pathways (e.g. oxidative stress and/or its major downstream activator NF-κB29). In addition, its effect on MSC differentiation is unclear, and whether it could also inhibit bone regeneration from BM-MSC is an open question.
Epigallocatechin-3-gallate (EGCG), or catechins in green tea polyphenols, is best known as an antioxidant and anti-inflammatory agent.30 It, therefore, has been proposed to use in many clinic scenarios such as inhibiting cancer progression, preventing metabolic disorders, and modulating immune diseases. It is known to block a wide array of inflammatory stimuli through inhibiting intracellular NF-κB activation.31 Importantly, it has been reported that the association occurred between higher EGCG consumption and a decreased risk of fractures with improved bone health.32–34 The inhibitory effects of EGCG on osteoclastogenesis are relatively well established, while its action on osteoblast differentiation is still controversial, with both increasing and suppressing bone formation/osteoblast differentiation reported in the literature.35–38 In addition, there is even less information about the effect of EGCG on osteogenic differentiation of MSCs.35,39 Given the strong evidence of anti-inflammatory effect of EGCG and inhibition of bone formation by inflammatory cytokine such as TNFα, we designed and carried out the experiments to examine if EGCG would promote osteogenic differentiation in human MSCs isolated from bone marrow (BM-hMSCs) and to identify potential interplays between EGCG and TNFα in this setting. In the present study, our data support that EGCG has a protective effect on the osteogenic response inhibited by TNFα in MSCs, which is potentially mediated through an important downstream player of TNFα, namely NF-κB signaling.
Methods and materials
Isolation and culture of BM-hMSC
Bone marrow cells were placed in heparinized tubes prefilled with a-MEM and then washed twice with HBSS followed by Ficoll-Paque centrifugation (1800 × g for 30 min at room temperature). Buffy coat was then carefully collected from the Ficoll-HBSS interface and washed again by HBSS. Viable cells were counted with a hemocytometer using Trypan blue and plated at a cell density of 50–100 cells/cm2 in 175 cm2 flasks or 150 mm dishes. After 24 h to remove, the adherent cells were cultured in CCM media at 37℃ with 5% humidified CO2. Vehicle controls, TNFα (1, 5, 10, and 20 ng/mL), or EGCG (5, 10, 20, and 40 μM) were added in the media throughout the primary cultures until harvest for assays. For the blockade of NF-κB pathway, IκB-AA1 (super-repressor of NF-κB, cloned into a commercially available Rc/CMV expression vector) was transfected. The protocol was approved by the Committee on the Ethics of The Second Affiliated Hospital of Nantong University.
Osteogenic differentiation of BM-hMSC
BM-hMSCs were cultured and grown to reach complete confluence (24–72 h) and then induced with MSC osteogenic differentiation medium containing 10 mmol/L β-glycerol phosphate, 10−8 mol/L dexamethasone, and 50 mg/L ascorbic acid 2-phosphate.39 Differentiation medium was changed every third day. Vehicle controls, TNFα (1, 5, 10, and 20 ng/mL), or EGCG (5, 10, 20, and 40 μM) were added in the differentiation media throughout the differentiation processes until harvest for assays.
Characterization of BM-hMSC by flow cytometry analysis
Adherent MSCs were trypsinized and resuspend in PBS containing 5% fetal bovine serum. After washing, cells were counted and aliquoted into tubes for different staining by antibodies recognize various surface proteins, including CD29, CD45, CD105, and CD90. Isotype control antibody-stained cells were used to optimize photomultiplier tube and compensation in the analysis using BD-FACScan (Becton Dickinson, San Jose, CA). Flow cytometry data were analyzed with CellQuest software.
ALP activity assay
Cultured cells after eight days in osteogenic differentiation media were fixed in 4% paraformaldehyde for 15 min at room temperature and then washed twice with PBS followed by staining in freshly prepared 0.1% naphthol AS-MX phosphate, 56 mM 2-amino-2-methyl-1-3-propanediol, and 0.1% fast red violet LB salt. Quantitative analysis was determined by colorimetric assay of enzyme activity using the ALP kit (Sigma, St. Louis, MO, USA). Briefly, total protein lysates were extracted and then mixed with the freshly prepared colorimetric substrate para-nitrophenyl phosphate at 37℃ for 30 min. The enzymatic reaction was stopped by adding NaOH (0.2 M). The optical density of the yellow product para-nitrophenol was determined by a spectrometer plate reader (Molecular Devices, CA, USA) at a wavelength of 405 nm. The ALP activity was normalized by protein amount and expressed as relative fold compared with vehicle control-treated samples.
Mineralization assays
The mineralization nodules formed 16 days after osteogenic induction of mesenchymal stem cells in dishes were determined by alizarin red-sulfate staining.39 The washed cells were treated by 40 mM AR-S (pH 4.2) with gentle rocking for 30 min on room temperature. After three times of water rinse followed by a PBS washing step, the positively stained mineralization were examined by light microscopy.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from cell cultures using the RNeasy kit (Qiagen, Duesseldorf, Germany) and was reverse transcribed to complementary cDNAs using Superscript II according to manufacturer’s instructions (Invitrogen, NY, USA). Primers specific for Runx2 and Ostrix were used. Duplicated polymerase chain reaction (PCR) reactions were carried out in ABI 7300 real-time PCR machine (Life Technologies, USA), using n = 3 for each sample. SYBR Green dye-based detection method was used by using the SYBR Green PCR Master Mix assay (TaKaRa Biotechnology). A series of duplicate dilutions of cDNA from control samples were used to optimize the standard curve and validate the melting curves for each primer set.
Western blotting
For the protein analysis, cells were lysed in hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.2 mM PMSF) to separate cytoplasmic fractions from nuclear extracts. Both fractions were then measured and the same amounts of proteins were subjected to SDS-PAGE, followed by electric transfer into PVDF membrane. The antibodies used in western blot included anti-p65, anti-IκBα, and anti-phosphorylated IκBα (Cell Signaling). Anti-GAPDH and anti-HDAC1 (Cell Signaling) were used as loading controls for the cytoplasm and nuclear factions, respectively.
Statistics
All data in graphs are expressed as the means ± SEM as indicated. The two-way ANOVA was used for Figure 1 and one-way ANOVA was used in Figure 2. The comparisons were determined by Bonferroni post test with *P < 0.05 and #P < 0.01 (vs. the controls) considered as significant.
Figure 1.
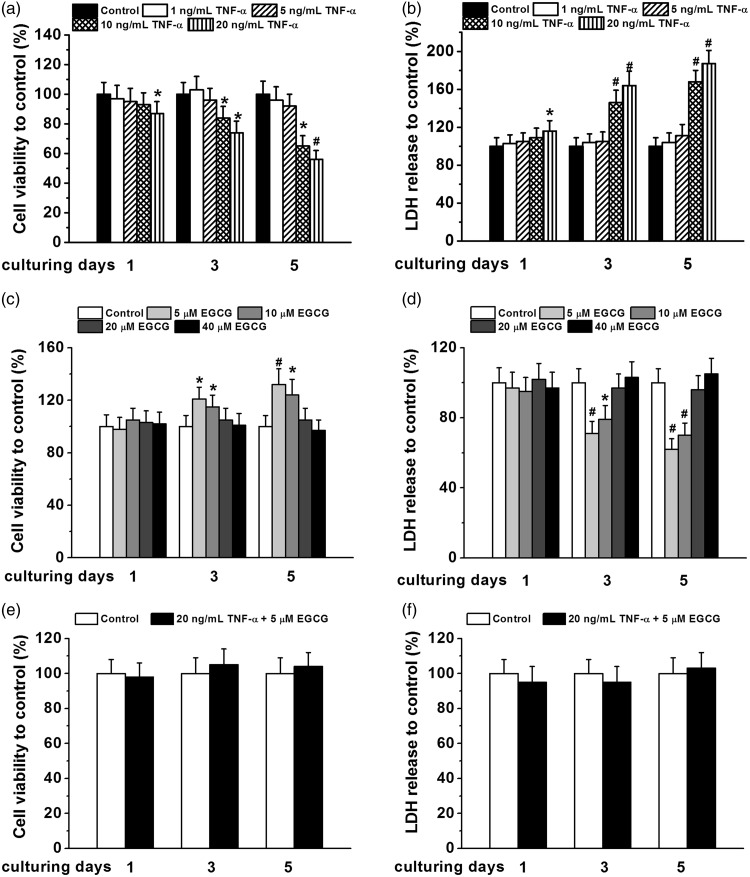
Cell viability of human BM-MSCs under different concentrations of TNF-α (1, 5, 10, and 20 ng/mL) and EGCG (5, 10, 20, and 40 μM) treatment. Cell viability was measured by MTT assay and cell death was indicated by LDH release assay. Cell viability (a) and LDH release (b) of BM-MSCs normalized to that of control under TNF-α treatment for one, three, and five days, respectively. Cell viability (c) and LDH release (d) of BM-MSCs normalized to that of control under EGCG treatment for one, three, and five days, respectively. Cell viability (e) and LDH release (f) of BM-MSCs normalized to control under co-treatment of 20 ng/mL TNF-α and 5 μM EGCG. All the experiments were independently repeated in triplicate. Data were presented by mean ± SEM. *P < 0.05 and #P < 0.01 vs. control
Figure 2.
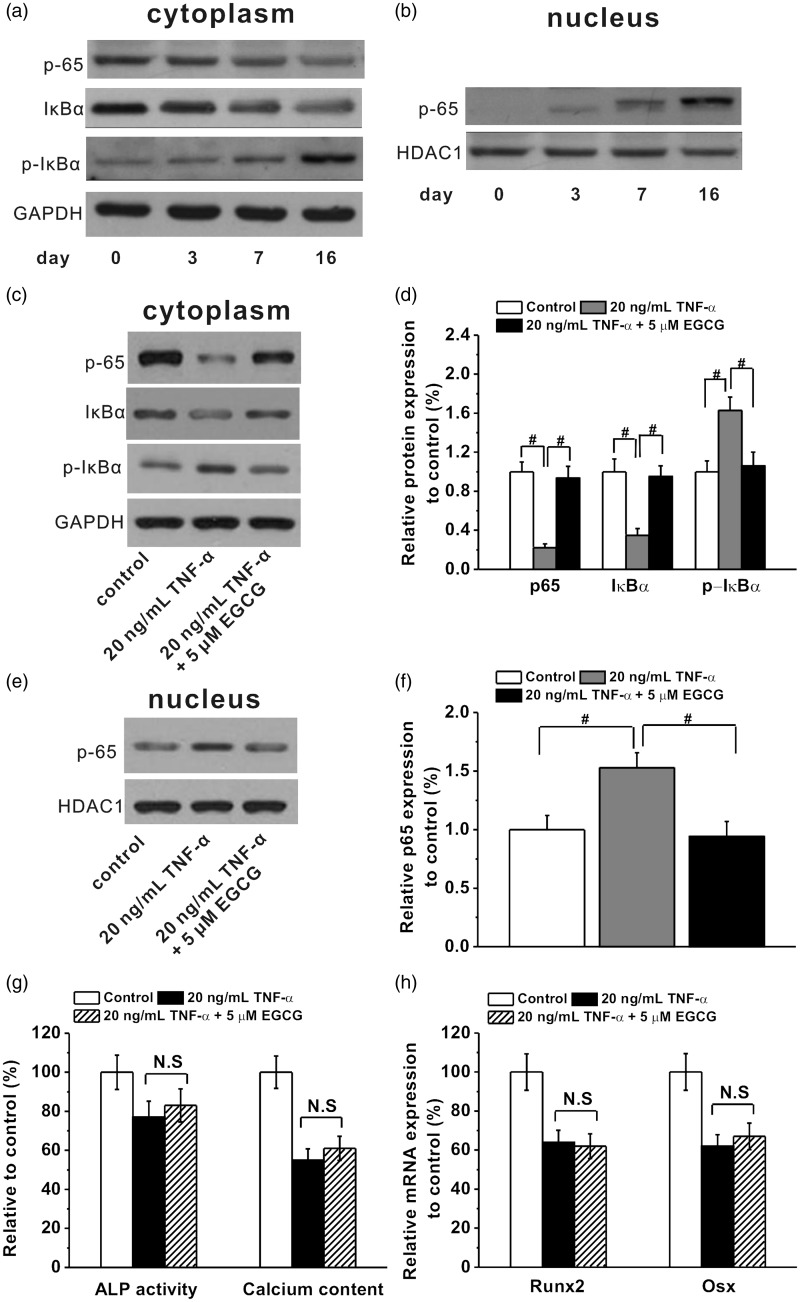
NF-кB signaling pathway was involved in the protective effect of EGCG on the TNF-α-induced inhibition of osteogenic differentiation of human BM-MSCs. The changes of p65, IкBα, and p-IкBα protein bands in the cytoplasm (a) and p65 protein band in the nucleus (b) during the osteogenic differentiation at indicated time (day 0, 3, 7, and 16) in the cultures of human BM-MSC under 20 ng/mL TNF-α treatment. EGCG (5 μM) was capable of inhibiting NF-кB signaling pathway activated by 20 ng/mL TNF-α. (c and d) The p65, IкBα, and p-IкBα protein expressions in the cytoplasm in the cultures at day 16 during osteogenic differentiation in the experimental groups. (e and f) The p65 expression in the nucleus at day 16 during osteogenic differentiation in the experimental groups. (g and h) EGCG failed to rescue TNF-α-induced inhibition of osteogenic differentiation in BM-hMSCs when NF-κB was blocked by its super-repressor IκB-AA1, as indicated by no significant differences of the ALP activity, calcium content, Runx2, and Osx mRNA expression between non-EGCG and EGCG treated groups. All the experiments were independently repeated in triplicate. Data were presented by mean ± SEM. #P < 0.01 vs. control
Results
Isolation and characterization of human bone marrow-derived MSCs
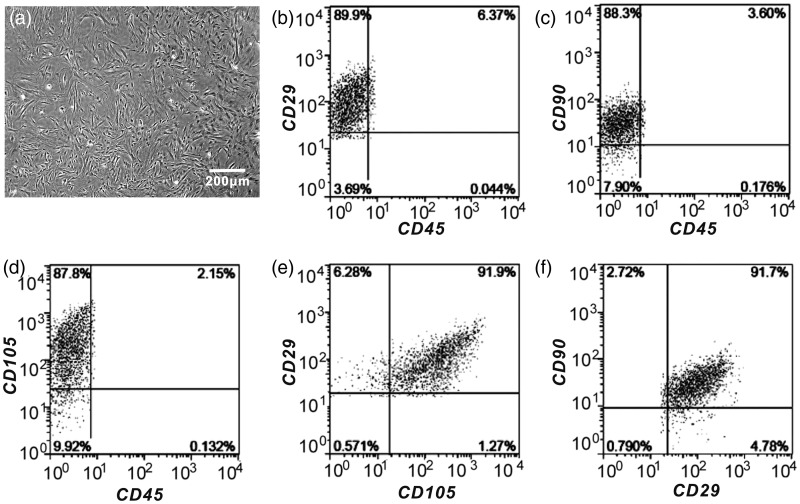
We used a conventional isolation protocol to obtain the mesenchymal stem cell derived from human bone marrow (Figure 3(a)). The identity of the progenitor cells was also confirmed by the analysis of well-known surface markers, including positive for CD29, CD105, CD90, and negative for CD45 (Figure 3(b) to (f)).
Figure 3.
Phenotypic characterization of human BM-MSCs. (a) Representative image of human BM-MSCs after seeding for five days. (b to f) Flow cytometry identified BM-MSCs positive marker CD29, CD90, CD105, and negative marker CD45. (A color version of this figure is available in the online journal)
The effects of TNFα and EGCG on cell viability of BM-hMSCs
In order to determine the effects of TNFα on MSCs, we tested a series of concentrations of TNFα (1, 5, 10, and 20 ng/mL) based on published reports and our own preliminary experiments. As shown by Figure 1(a) and (b), increasing concentrations of TNFα (particularly at 10 and 20 ng/mL) can significantly reduce cell viability and increase cell death in MSCs. These effects were found as early as one day at the highest concentration of TNFα (20 ng/mL) treatment and accumulated up to five days (more than 40% decrease in cell viability and around 80% increase in cell death). Similarly, we examined a series of doses of EGCG (5, 10, 20, and 40 μM) on BM-hMSCs by the same assays. Interestingly, we found an inverse dose response of EGCG, with only lower doses (5 and 10 μM) increasing the cell viability and decreasing cell death of BM-hMSCs (Figure 1(c) and (d)). To further test the cross-talk between these two agents, we chose 20 ng/mL TNFα (the most significant damage in cell survival) and 5 μM EGCG (the most effective increase in cell viability). We found that at 5 μM EGCG concentration, the inhibitory effect of TNFα in BM-hMSCs was completely corrected in both cell viability (Figure 1(e)) and cell death assays (Figure 1(f)) during five-day treatment.
The effects of TNFα and EGCG on osteogenic differentiation of BM-hMSCs
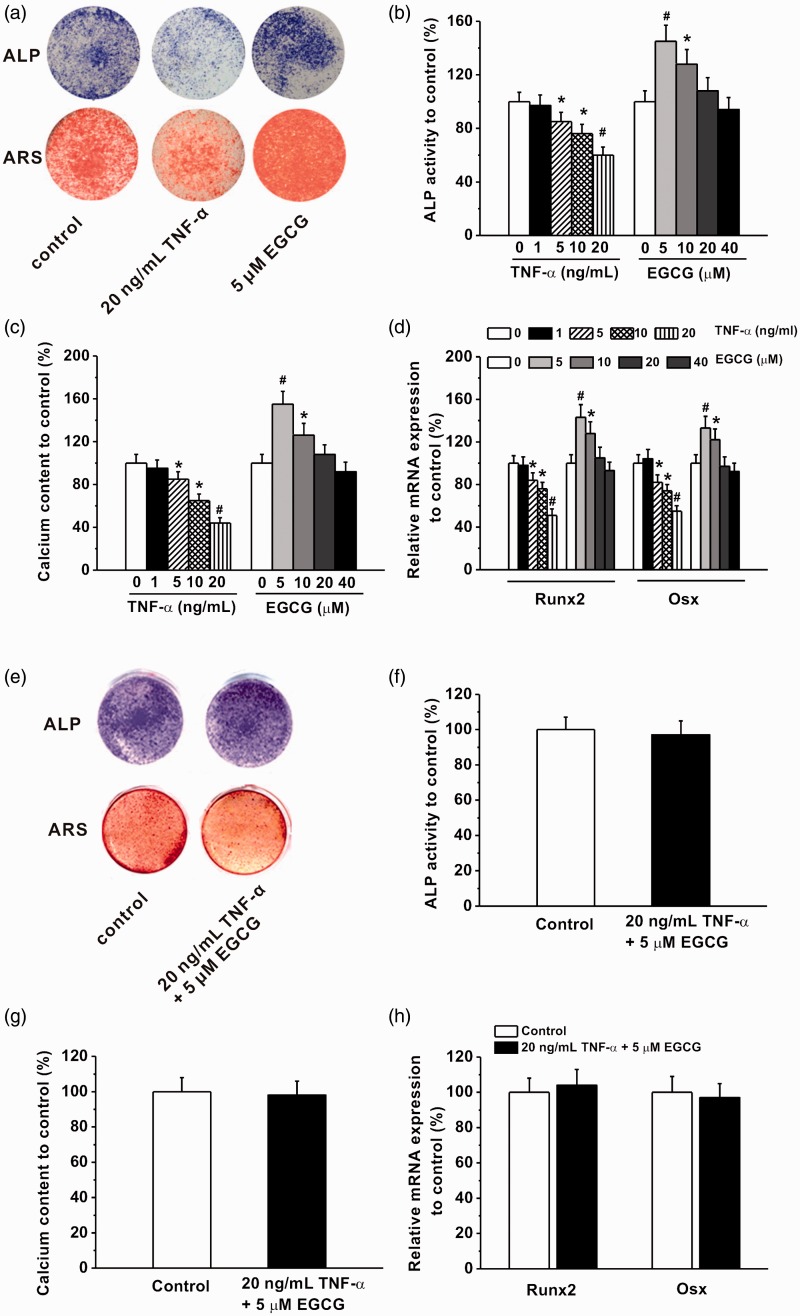
Next, we sought to investigate the effects of TNFα and EGCG on osteogenic differentiation of BM-hMSCs. Similarly as the previous findings, both reagents showed dose-dependent effects on the osteoblast differentiation processes (Figure 4). TNFα (as low as 5 ng/mL) inhibited the ALP activity, mineralization ability, calcium content (Figure 4(a) to (c)), and gene expressions of bone formation markers (Runx2 and Osterix in Figure 4(d)) with the maximal effects at the highest dose 20 ng/mL. On the contrary, EGCG promoted osteogenic differentiation (Figure 4(a) to (d)) only at lower doses (5 and 10 μM), with no effects at higher doses (20 and 40 μM). Finally, in the presence of a low-dose EGCG (5 μM), TNFα could no longer block the BM-hMSC osteogenic differentiation, shown by ALP activity, mineralization nodule formation, and Runx2 and Osterix expressions (Figure 4(d) to (h)).
Figure 4.
Effects of different concentrations of TNF-α (1, 5, 10, and 20 ng/mL) and EGCG (5, 10, 20, and 40 μM) treatment on osteogenic differentiation of human BM-MSCs. (a) Representative photos for ALP staining in the cultures at day 8 and Alizarin red staining at day 16. Relative ALP activity (b) and relative calcium content (c) in the experimental groups. (d) Relative mRNA expression of Runx2 and Osx in the experimental groups, quantified by RT-PCR analysis. (e) Representative photos for ALP staining in the cultures at day 8 and Alizarin red staining at day 16 after co-treatment of 20 ng/mL TNF-α and 5 μM EGCG. Relative ALP activity (f), relative calcium content (g) and relative expression of Runx2 and Osx mRNAs (h) in the cultures after co-treatment of 20 ng/mL TNF-α and 5 μM EGCG. All the experiments were independently repeated in triplicate. Data were presented by mean ± SEM. *P < 0.05 and #P < 0.01 vs. control. (A color version of this figure is available in the online journal.)
TNFα stimulates NF-κB signaling in BM-hMSCs that is inhibited by EGCG
To better ascertain the importance of intracellular signaling originated from the classic inflammation mediator, we examined NF-κB pathway in BM-hMSCs in response to TNFα treatment. During the cultures of human BM-MSC under 20 ng/mL TNF-α treatment from day 3 to day 16, we found an increased phosphorylation-associated degradation of IκBα protein in the cells (Figure 2(a)) accompanied by a sustained nuclear translocation of p65 (Figure 2(b)) from the cytoplasm (Figure 2(a)). These data demonstrated that TNFα activates classic NF-κB signaling in bone marrow-derived MSCs. Importantly, EGCG blocked such activation by TNFα (again at a low dose of 5 μM). Treating cells with EGCG, we found that the levels of IκBα protein remained in the cytoplasm even in the presence of TNFα (20 ng/mL on day 16, Figure 2(c) and (d)), possibly due to an attenuation of IκBα phosphorylation (Figure 2(c) and (d)). As a result, p65 protein was still quenched in the cytoplasm (compared with TNFα treatment only, Figure 2(c) and (d)) after EGCG treatment, losing the ability to shuttle into the nucleus for transcription activation (Figure 2(e) and (f)). Besides, when NF-κB was blocked by NF-κB super-repressor IκB-AA1 in BM-hMSCs, EGCG treatment was not able to rescue the TNFα-induced inhibition of osteogenic differentiation any more, as there were no significant differences in ALP activity, calcium content, Runx2, and Osx mRNA expressions between EGCG-treated group and EGCG-non-treated group (Figure 2(g) and (h)). This furthermore indicates the involvement of NF-κB signaling pathway in the protective effects of EGCG against TNFα-induced impairments of osteogenesis.
Discussion
Based on prior evidences that inflammation can perturb osteoblast differentiation, we aimed to investigate the possible functions played by EGCG, a known anti-inflammatory agent, in protecting bone formation inhibited by a pro-inflammatory cytokine TNFα. In particular, we focused on BM-hMSCs and found out that TNFα and EGCG have counter effects on the osteogenic differentiation process in MSCs, which might be mediated through a classic NF-κB signaling pathway.
As others have suggested,26,27 we found an inhibitory effect of TNFα on the osteogenic potential of BM-MSCs, which is consistent with the notion that in the inflammatory milieu, not only bone resorption is activated but bone formation activity is also attenuated.7 Using highly pure progenitors of bone marrow cells (CD29+, CD90+, CD105+, and CD45− populations) in primary cultures, our data have shown that TNFα is able to suppress ALP activity/mineralization and repress Runx2 and Osterix gene expressions in the differentiation of MSCs towards the osteoblastic lineages (Figure 4). TNFα can induce primary mesenchymal stem cell death (Figure 1), which could not be solely responsible for its inhibition on the bone differentiation since a low dose of TNFα (which didn’t stimulate MSC death at 5 ng/mL) still significantly suppressed osteogenic differentiation (Figure 4, e.g. up to 20% decrease of Runx2 and Osterix gene expression). On the other hand, TNFα was first proposed as a crucial player in promoting postnatal bone repair, including intramembranous bone formation40 and fracture repair,41 and later found to enhance expression of osteogenic factors like bone morphogenetic protein 2 (BMP-2) in mesenchymal cells.42 This discrepancy may be resulted from the differences in heterogeneous progenitor populations in MSC pools and the loss-of-function studies using TNFα knockout mice.40,41 In fact, TNFα-dependent osteoclast activation may be required to initiate bone remodeling that integrates bone resorption/formation cycles.7,21 Thus, the integrity of TNFα signaling might be indispensable for bone repair process, while the direct action of TNFα on osteoblast differentiation and/or maturation could be detrimental, as suggested by our data and many others.26,27
The recent explosion of technique advances in isolation and expansion of adult stem/progenitor cells from human bone marrow11,12,15 has contributed significantly in developing cell-based therapies for inflammatory bone diseases. In order to restore the three-dimensional collagen structure and facilitate the integration of the newly synthesized matrix with the resident tissues, MSCs that can differentiate into all bone cell types have become an excellent choice for bone regeneration approaches. In addition, the ease of genetically manipulated bone marrow-derived stem cells makes them attractive vehicles for gene therapy, if necessary.11 Given the drastically negative effects of TNFα on the in vitro bone differentiation from bone marrow-hMSCs reported here, we hypothesize that a counter strategy is necessary in restraining the actions of TNFα and other pro-inflammatory cytokines.15
Based on this rationale and previous findings, EGCG could be a good candidate. First, as a dietary supplement, EGCG or green tea extracts have been known for their effective benefits on bone health. A number of human studies have reported a positive correlation between tea drinking and bone mineral density in osteoporosis patients or postmenopausal women and reduced risk of hip fractures in aged people.32–34 Second, EGCG has anti-inflammatory activity and antioxidative stress action.32,39,43 Both of which most likely can mitigate the adverse impacts of inflammation damages on bone regeneration. For instance, a recent study revealed that EGCG is able to suppress the lipopolysaccharide-induced inflammatory bone resorption in mice.44 Also, our present study provides an in vitro analysis to support this acclaim in human mesenchymal stem cells. Finally, direct pre-clinical tests using green tea polyphenols have approved its ameliorating effects on the animal models of rheumatoid arthritis,45 cadmium toxicity-associated bone disorder,46 and estrogen deficiency-induced bone loss.47 Altogether, our results are consistent with the hypothesis that EGCG and related green tea extracts could be useful in the treatment of inflammatory disease, or as supplementary approaches for other therapies such as using hormones (glucocorticoids, PTH), nutrition (vitamin D), or Wnt signaling antagonist (DKK1).3,7
Compared with the well-characterized benefit of EGCG on bone metabolism, the underlying mechanism is unclear, and particularly confusing in terms of the direct action of EGCG on osteoblast differentiation. EGCG was first shown to enhance bone formation activity in a osteosarcoma Saos-2 cell line,36 increase osteogenesis in a murine bone marrow mesenchymal stem cell line D1,35 and upregulate Runx2-mediated differentiation of bone cells.48 However, conflicting data exist. In an osteoblastic cell line MC3T3-L1, EGCG appeared to suppress the ALP activity and osteoblastic differentiation,37 which was compatible with another prior report in which an ectopic bone formation model induced by BMP was shown to be repressed by EGCG.49 Our data support the positive effects of EGCG on osteoblastic differentiation in general and surprisingly have identified an inverse dose-dependent action on MSC cell survival and osteogenic differentiation (Figures 1, 2, and 4). The finding that the increasing doses of EGCG diminished its otherwise beneficial effects on the bone formation suggested that cells may respond differently to the various levels of EGCG, even in the opposite directions, which may explain the contradictory results obtained previously. These interesting findings are also consistent with a recent study on alveolar bone cells in which EGCG at a low concentration increases, whereas at higher concentrations decreases osteogenic differentiation.38
The molecular basis of EGCG protecting TNFα-induced bone formation repression can be complicated, and our data pointed to an important role played by NF-κB pathway. Since its discovery, NF-κB signaling has been extensively investigated and known as one of the best characterized intracellular pathway in inflammation.29 We have found a dramatic stimulation of classic NF-κB signaling during long-term MSCs culturing in the presence of TNFα (Figure 2), which included enhanced IκBα phosphorylation accompanied by decreased IκBα protein (presumably due to proteasome degradation of IκBα dependent on phosphorylation29). Consequently, the nuclear translocation of p65 was released from the lack of IκBα sequestration (Figure 2), supposedly inducing transcription activation/repression of its target genes. Interestingly, a low dose of EGCG (5 μM) completely abolished the NF-κB signaling activated by TNFα, which is consistent with previous studies on osteoclast cells, or other cell types.35,45 These data suggest that EGCG reverses the inhibitory effects of TNFα, at the least in part, through preventing the activation of NF-κB signaling in BM-hMSCs during osteogenic differentiation. In addition, other mechanisms could involve, such as a matrix metalloproteinase (MT1-MMP) in mesenchymal stromal cells.50 Importantly, oxidative stress can induce inflammatory responses through NF-κB-dependent or -independent pathways, which is also a widely recognized target of EGCG due to its ability to capture and quench reactive oxygen species.30–32,39 The potential antioxidant functions of EGCG in suppressing the inhibition effects on mesenchymal stem cells by TNFα require further investigation in the future.
Conclusion
In conclusion, our studies have shown that EGCG can prevent the inhibition by TNFα on the survival and osteogenic differentiation of human BM-MSCs. These effects are only found at low concentrations of EGCG, potentially through suppressing NF-κB signaling stimulation by TNFα. Therefore, besides the efforts in reducing bone resorption in the inflammation-associated bone diseases, strategies such as using green tea extracts to promote bone formation while preventing the damaging of bone repair by inflammatory insult may directly benefit these bone diseases.
Acknowledgements
The study was supported by grants from Nantong provincial key personnel fund for medical science, China Postdoctoral Science Foundation (2013M541707) and Nantong science and technology project (HS2012047). We thank Dr M-M Zhou for the valuable suggestions on this manuscript.
Author contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript; WL, JBF, DWX, and JZ conducted the experiments; WL and ZMC wrote the manuscript; WL and JBF contributed equally to this work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteoporos Rep 2014; 12: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Benedetti F, Rucci N, Del Fattore A, Peruzzi B, Paro R, Longo M, Vivarelli M, Muratori F, Berni S, Ballanti P, Ferrari S, Teti A. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum 2006; 54: 3551–63. [DOI] [PubMed] [Google Scholar]
- 3.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 2012; 11: 234–50. [DOI] [PubMed] [Google Scholar]
- 4.Sela J, Applebaum J, Uretzky G. Osteogenesis induced by bone matrix is inhibited by inflammation. Biomater Med Devices Artif Organs 1986; 14: 227–37. [DOI] [PubMed] [Google Scholar]
- 5.Thakker MM, Zhang J, Sires BS. Chronic inflammation from polycarbonate motility peg inhibits osteogenesis in a human hydroxyapatite orbital implant. Ophthal Plast Reconstr Surg 2005; 21: 399–401. [DOI] [PubMed] [Google Scholar]
- 6.Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med 2007; 13: 133–4. [DOI] [PubMed] [Google Scholar]
- 7.Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev 2010; 233: 301–12. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004; 292: 490–5. [DOI] [PubMed] [Google Scholar]
- 9.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med 2004; 10: 617–24. [DOI] [PubMed] [Google Scholar]
- 10.Romas E, Sims NA, Hards DK, Lindsay M, Quinn JW, Ryan PF, Dunstan CR, Martin TJ, Gillespie MT. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol 2002; 161: 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8: 726–36. [DOI] [PubMed] [Google Scholar]
- 12.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 2007; 56: 1175–86. [DOI] [PubMed] [Google Scholar]
- 13.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009; 60: 813–23. [DOI] [PubMed] [Google Scholar]
- 14.Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 2006; 39: 678–83. [DOI] [PubMed] [Google Scholar]
- 15.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther 2008; 10: 223–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009; 60: 1006–19. [DOI] [PubMed] [Google Scholar]
- 17.Edwards CK, 3rd, Bendele AM, Reznikov LI, Fantuzzi G, Chlipala ES, Li L, Moldawer LL, Mountz JD, Li YY, Dinarello CA. Soluble human p55 and p75 tumor necrosis factor receptors reverse spontaneous arthritis in transgenic mice expressing transmembrane tumor necrosis factor alpha. Arthritis Rheum 2006; 54: 2872–85. [DOI] [PubMed] [Google Scholar]
- 18.Aloe L, Probert L, Kollias G, Bracci-Laudiero L, Spillantini MG, Levi-Montalcini R. The synovium of transgenic arthritic mice expressing human tumor necrosis factor contains a high level of nerve growth factor. Growth Factors 1993; 9: 149–55. [DOI] [PubMed] [Google Scholar]
- 19.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest 2002; 110: 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000; 106: 1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 2000; 275: 4858–64. [DOI] [PubMed] [Google Scholar]
- 22.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 2003; 111: 821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 2005; 115: 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998; 13: 793–802. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita M, Otsuka F, Mukai T, Otani H, Inagaki K, Miyoshi T, Goto J, Yamamura M, Makino H. Simvastatin antagonizes tumor necrosis factor-alpha inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J Endocrinol 2008; 196: 601–13. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000; 141: 3956–64. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem 2002; 277: 2695–701. [DOI] [PubMed] [Google Scholar]
- 28.Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, Fazzalari NL, Evdokiou A, Atkins GJ. Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res 2009; 24: 1434–49. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–34. [DOI] [PubMed] [Google Scholar]
- 30.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 2003; 43: 89–143. [DOI] [PubMed] [Google Scholar]
- 31.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 2001; 480–481: 243–68. [DOI] [PubMed] [Google Scholar]
- 32.Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res 2009; 29: 437–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devine A, Hodgson JM, Dick IM, Prince RL. Tea drinking is associated with benefits on bone density in older women. Am J Clin Nutr 2007; 86: 1243–7. [DOI] [PubMed] [Google Scholar]
- 34.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Lopes Vaz A, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Cano PR, Rapado A, Ribot C. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res 1995; 10: 1802–15. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int 2005; 16: 2039–45. [DOI] [PubMed] [Google Scholar]
- 36.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem 2007; 18: 341–7. [DOI] [PubMed] [Google Scholar]
- 37.Kamon M, Zhao R, Sakamoto K. Green tea polyphenol (-)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol Int 2010; 34: 109–16. [DOI] [PubMed] [Google Scholar]
- 38.Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, Moon SJ, Kim JH, Choi HJ. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch Oral Biol 2014; 59: 539–49. [DOI] [PubMed] [Google Scholar]
- 39.Yagi H, Tan J, Tuan RS. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J Cell Biochem 2013; 114: 1163–73. [DOI] [PubMed] [Google Scholar]
- 40.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs 2001; 169: 285–94. [DOI] [PubMed] [Google Scholar]
- 41.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 2003; 18: 1584–92. [DOI] [PubMed] [Google Scholar]
- 42.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 2009; 45: 367–76. [DOI] [PubMed] [Google Scholar]
- 43.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol 1999; 69: 148–53. [PubMed] [Google Scholar]
- 44.Tominari T, Matsumoto C, Watanabe K, Hirata M, Grundler FM, Miyaura C, Inada M. Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS Open Bio 2015; 5: 522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, Sakai Y, Kurosaka M, Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum 2008; 58: 2012–8. [DOI] [PubMed] [Google Scholar]
- 46.Choi JH, Rhee IK, Park KY, Kim JK, Rhee SJ. Action of green tea catechin on bone metabolic disorder in chronic cadmium-poisoned rats. Life Sci 2003; 73: 1479–89. [DOI] [PubMed] [Google Scholar]
- 47.Shen CL, Wang P, Guerrieri J, Yeh JK, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int 2008; 19: 979–90. [DOI] [PubMed] [Google Scholar]
- 48.Jin P, Wu H, Xu G, Zheng L, Zhao J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res 2014; 356: 381–90. [DOI] [PubMed] [Google Scholar]
- 49.Takita H, Kikuchi M, Sato Y, Kuboki Y. Inhibition of BMP-induced ectopic bone formation by an antiangiogenic agent (epigallocatechin 3-gallate). Connect Tissue Res 2002; 43: 520–3. [DOI] [PubMed] [Google Scholar]
- 50.Zgheib A, Lamy S, Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem 2013; 288: 13378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]