Abstract
Previously, hepatic ischemia followed by reperfusion (hepatic I/R) has been found to cause cognitive impairment. Hydrogen sulfide (H2S) attenuates hepatectomy induced cognitive deficits and also protects against cognitive dysfunction induced by neurodegenerative diseases. In this study, we aim to determine whether sodium hydrosulfide (NaHS), a H2S donor, could alleviate hepatic I/R-induced cognitive impairment and the underlying mechanisms. Rats were injected intraperitoneally with NaHS (5 mg/kg/d) for 11 days. A segmental hepatic I/R model was established on the fourth day. Cognitive function, proinflammatory cytokines levels, and hippocampal ionized calcium-binding adaptor molecule 1 (Iba1) expression was analyzed. We found hepatic I/R increased proinflammatory cytokines levels in serum and hippocampus, up-regulated Iba1 expression, leading to cognitive impairment in rats. However, treatment with NaHS alleviated hepatic I/R induced these neuroinflammatory changes and effectively improved cognitive function. Thus, NaHS appears to protect against cognitive impairment in rats undergoing hepatic I/R by attenuating neuroinflammation in the hippocampus.
Keywords: Hydrogen sulfide, hepatic ischemia and reperfusion, cognition, cytokines, microglia
Introduction
Hepatic vascular clamping and release is a surgical procedure that is frequently performed during hepatic resection and liver transplantation. During this process, microcirculation dysfunction, different cells and mediators, as well as complex signaling mechanisms induce hepatic ischemia and reperfusion (hepatic I/R) damage.1 Meanwhile, hepatic I/R may evoke systemic inflammatory response that leads to damages to remote organs.2,3 A systemic inflammatory response can also lead to entry of peripheral proinflammatory cytokines into the brain to affect neurocognitive function.4–6 In combination with microglia activation (also referred to as, neuroinflammation), cognitive deficits may develop.7,8
Hydrogen sulfide (H2S) was known as gaseous signaling molecule that was generated in mammalian cells.9 In a recent study, it was demonstrated that H2S mediates multiple functions under various physiological and pathological conditions.10 In addition, several studies have revealed that H2S can modulate inflammatory processes;11–13 it can reduce lipopolysaccharide-induced overexpression of proinflammatory cytokines by inhibiting NF-κB pathways and finally attenuates cognitive impairment.14 Furthermore, in the brain, H2S has been shown to suppress hippocampal neuroinflammation and to alleviate memory deficits.15 The ability of H2S to enhance N-methyl-D-aspartate (NMDA) receptors activity and potentiate long-term potentiation (LTP) induction also suggests that H2S contributes to the process of learning and memory.16,17
Previous experiment has demonstrated that H2S could alleviate hepatic I/R injury.18 Recent study showed that H2S attenuates hippocampal neuronal damages in rats subjected to brain ischemia.19 However, the neuroprotective role of H2S after hepatic I/R remains unknown. Therefore, we designed this experiment to determine whether H2S could attenuate hepatic I/R-induced cognitive impairment and the potential role of neuroinflammation.
Materials and methods
Animals
Male Sprague-Dawley rats (220–250 g) were provided by the Laboratory Animal Center of North Sichuan Medical College. The rats were maintained in a controlled environment that included suitable temperature and humidity, as well as 12 h light and dark cycle and were fed with regular standard rat chow and water. All rats underwent the training trials (four trials per day) for five continuous days, and those rats exhibiting abnormal behavior were eliminated. The experiments were approved by the Committee of Animal Care at North Sichuan Medical College and complied with the guidelines of the National Institutes of Health for use of experimental animals.
Experimental design and hepatic I/R model
In the first experiment, ninety-six rats were assigned to four groups (n = 24): vehicle-treated group (NS group), sodium hydrosulfide group (NaHS group), hepatic I/R group (HIR group), and hepatic I/R + sodium hydrosulfide group (HIR + NaHS group). Rats in the NaHS group and the HIR + NaHS group were intraperitoneally injected with NaHS (5 mg/kg, Sigma, USA). The NaHS dosage was determined from our pilot study and previous report.14 The rats in the NS and the HIR group received normal saline. Both sets of injections were administered once a day for 11 days. Rats in the HIR and the HIR + NaHS group underwent 70% hepatic I/R according to a previously described protocol on the fourth day;20 the rats were subjected to midline laparotomy after they were anesthetized with pentobarbital (50 mg/kg, intraperitoneally, i.p.), and then a microaneurysm clamp was used to occlude the portal vein and hepatic artery to induce ischemia of the hepatic left lateral and median lobes, then liver was reperfused by removing the clamp after 60 min. Rats in the NS and the NaHS group underwent the same laparotomy procedure but not clamped the vessels. Bupivacaine (0.25%) was applied to each incision and the abdomen was closed by sterile suture. After recovery from anesthesia, the rats were individually housed and then were sacrificed on the first, third, fifth, and seventh day after the operation (n = 6, per day/group). A behavior test was performed prior to sacrifice. Blood samples and hippocampus and liver tissues were collected for biochemical assay, measurement of proinflammatory cytokines, and histopathologic examination.
In the second experiment, forty-eight rats were assigned to four groups (NS, NaHS, HIR, and HIR + NaHS, n = 12). The treatment of NaHS or saline, as well as application of the hepatic I/R model, was performed as described in the first experiment. The rats were killed on the first and third day after the operation (n = 6, per day/group) for ionized calcium-binding adaptor molecule 1 (Iba1) analysis.
Biochemical analysis
Serum AST and ALT levels were measured by using an autoanalyzer (Hitachi, Tokyo, Japan).
Histopathologic examination
After fixed with formalin and embedded in paraffin, liver samples were cut into 5 µm thick sections and then stained with hematoxylin–eosin. Liver pathological injury was assessed by using a light microscope.
MWM test
MWM tests were used to evaluate the cognitive abilities.21 The maze included an open circular pool with a diameter of 120 cm and a height of 50 cm that was filled up to 25 cm with cylinder water at a temperature of 21–23℃. An opaque appearance to the water was achieved with the addition of white nontoxic paint. Several distal cues were added around the pool. The tank was divided into four equal quadrants; in the center of one quadrant, a circular platform (10 cm in diameter) was located 2 cm below the water. The swimming behaviors of animals were analyzed by a computer tracking system. The animals received a five-day consecutive training trials (four trials per day). In each trial, facing the tank wall, the rats were put into the water from one of the four start points and allowed to navigate until they touched the platform. The time needed to reach the platform was limited to 90 s. Animals would have 20 s to have a rest after arriving at the platform. If a rat could not find the platform within 90 s, it would be directed to the platform where it could rest for 20 s. Swim distance, swim speed, and escape latency were measured. A probe trial was performed on the first, third, fifth, and seventh day after operation, and the percentage of time spent in the target quadrant, the number of crossings over the platform, and swim speed were recorded.
Measurement of serum TNF-α, IL-1β, and IL-6
Serum TNF-α, IL-1β, and IL-6 levels were determined by using ELISA kits (Nanjing Jiancheng Corp., China) according to the manufacturer’s instructions.
Western blot analysis
Hippocampus was homogenized in M-PER lysis buffer (Pierce) containing a freshly added protease inhibitor cocktail (Roche). The homogenates were centrifuged (12,000 rpm, 15 min, 4℃) and the supernatant was collected. A total of 80 µg of total proteins was obtained from the supernatant for each per lane and were resolved with 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The protein was transferred to polyvinylidene difluoride membranes (Millipore). These membranes were blocked with 5% (W/V) skim milk for 2 h and incubated with rabbit anti-TNF-α (1:1000; Abcam), IL-1β (1:700; Abcam), IL-6 (1:500; Abcam), or β-actin (1:1000; Abcam) overnight at 4℃. Blots were washed for 3 × 5 min in Tris buffered saline with 0.5% Tween (TBST) and incubated with goat anti-rabbit peroxidase-conjugated secondary antibody (1:100,000) (Jackson, ImmunoResearch) for 1 h. Bound antibodies were visualized with Immobilon substrate (ECL; Millipore) and band density was analyzed with Kodak Digital Science and ID Image Analysis Software (Kodak, Rochester, NY).
Immunofluorescence
In the second experiment, the brains were immediately resected and fixed in 4% paraformaldehyde at 4℃ and paraffin embedded. Coronal sections (4 µm) were exposed to a goat anti-Iba1 polyclonal antibody (1:500, Abcam) overnight at 4℃. After the sections were incubated with PBS, they were exposed to a fluorescein isothiocyanate-conjugated anti-goat secondary antibody (1:400; Santa Cruz Biotechnology) at room temperature (RT). After 1 h, the sections were incubated with PBS and were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, USA). Positively stained cells were observed with an Olympus optical microscope (BX51; Olympus, Japan) and the mean number was recorded for each section.
Statistical analysis
All data were expressed as mean ± SEM, and the data were analyzed by using one-way ANOVA followed by least significant difference multiple comparison test between groups. Swim distance, swim speed, and escape latency of the MWM training phase were analyzed by using repeated measures ANOVA followed by post hoc Bonferroni multiple comparison test between groups. P < 0.05 was considered significant.
Results
NaHS attenuated hepatic I/R injury
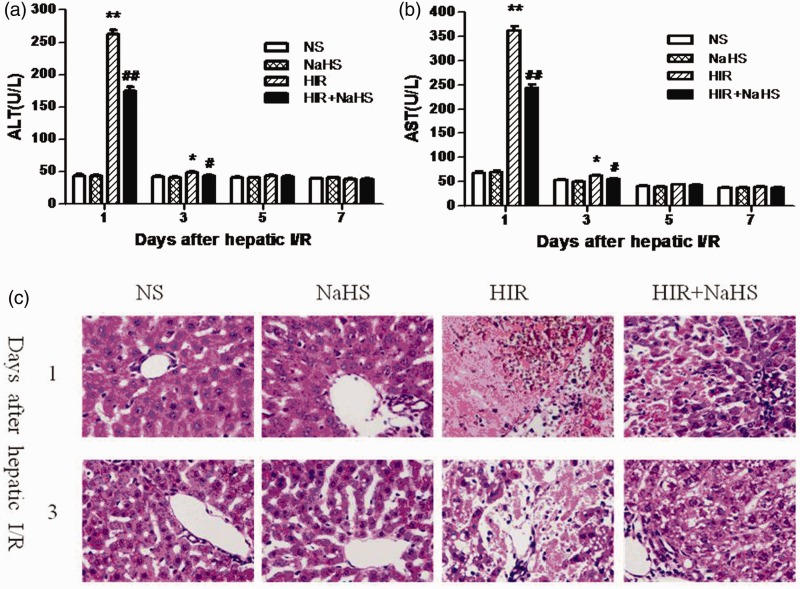
Liver injury was surveyed by using biochemical analysis and histopathologic examination. Rats undergoing hepatic I/R exhibited significant higher ALT and AST levels when compared to the rats in the NS and the NaHS group on the first and third day after the operation (Figure 1(a) and (b)). Contrastingly, treatment with NaHS significantly reduced ALT and AST levels (Figure 1(a) and (b)). On the other hand, the HIR group showed marked deformation of the hepatic lobule, hepatocyte necrosis, and inflammatory cell infiltration on the first and third day after the operation, but NaHS treatment significantly attenuated these pathological injuries (Figure 1(c)).
Figure 1.
NaHS attenuated hepatic I/R injury. (a) ALT level, (b) AST level, (c) representative sections of median lobe liver stained with hematoxylin–eosin were taken on the first and third day after hepatic I/R (400 × magnification). The data are expressed as the mean ± SEM. *P < 0.05 HIR versus NS, **P < 0.01 HIR versus NS, #P < 0.05 HIR + NaHS versus HIR, ##P < 0.01 HIR + NaHS versus HIR. (A color version of this figure is available in the online journal.)
NaHS ameliorated cognitive impairment after hepatic I/R
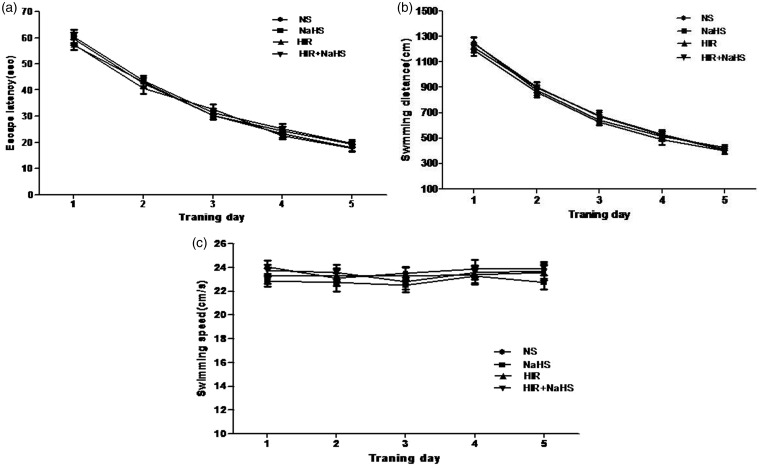
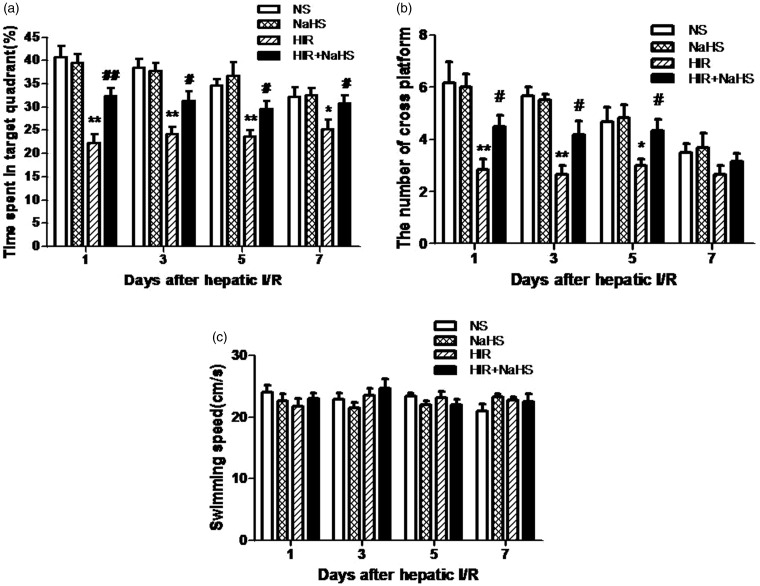
During the water maze training, all animals learned the spatial task and exhibited progressively reduced escape latency and swimming distance over time (Figure 2(a) and (b)), but swimming speed remained constant (Figure 2(c)). No significant differences in these swim data among groups were found during the five training days. In the probe trail, animals in the HIR group showed a marked decrease in percentage of time spent in the target quadrant and the number of crossings over the platform (Figure 3(a) and (b)), which indicates that cognitive function was impaired after hepatic I/R. However, NaHS treatment significantly alleviated such cognitive impairment (Figure 3(a) and (b)). The findings suggested that NaHS markedly ameliorated cognitive deficits after hepatic I/R.
Figure 2.
Performance in the Morris water maze for five continuous days. (a) Escape latency, (b) swimming distance, (c) swimming speed. The data are expressed as mean ± SEM
Figure 3.
NaHS ameliorated cognitive impairment after hepatic I/R. (a) Percentage of time spent in the target quadrant, (b) number of crossings over the platform, (c) swimming speed during the probe phase. The data are expressed as mean ± SEM. *P < 0.05 HIR versus NS, **P < 0.01 HIR versus NS, #P < 0.05 HIR + NaHS versus HIR, ##P < 0.01 HIR + NaHS versus HIR
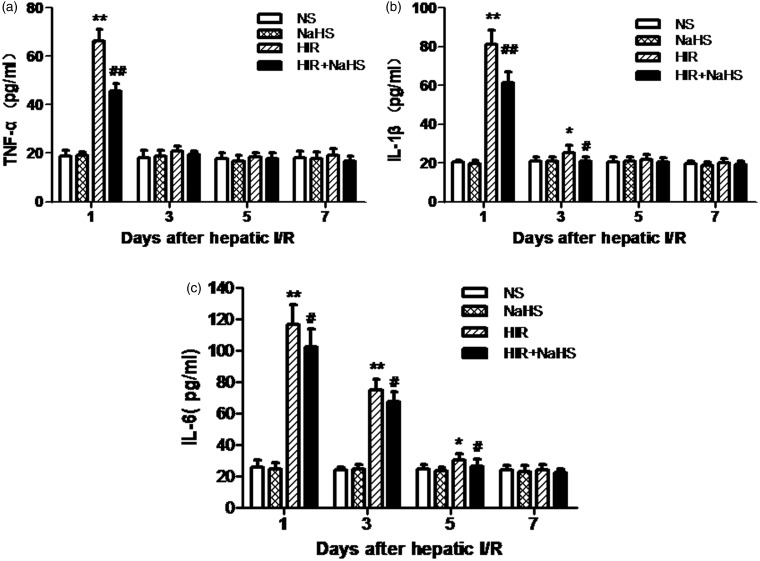
NaHS decreased the production of serum proinflammatory cytokines
Compared with the NS group and the NaHS group, the HIR group displayed significantly elevated TNF-α, IL-1β, and IL-6 on the first day after operation (Figure 4(a) to (c)) and increased IL-1β and IL-6 on the third and fifth day after operation (Figure 4(b) and (c)). In contrast, treatment with NaHS significantly decreased serum TNF-α, IL-1β, and IL-6 levels at the same time point (Figure 4(a) to (c)).
Figure 4.
NaHS decreased the production of serum proinflammatory cytokines. (a) TNF-α level, (b) IL-1β level, (c) IL-6 level. The data are expressed as mean ± SEM. *P < 0.05. HIR versus NS, **P < 0.01 HIR versus NS, #P < 0.05 HIR + NaHS versus HIR, ##P < 0.01 HIR + NaHS versus HIR
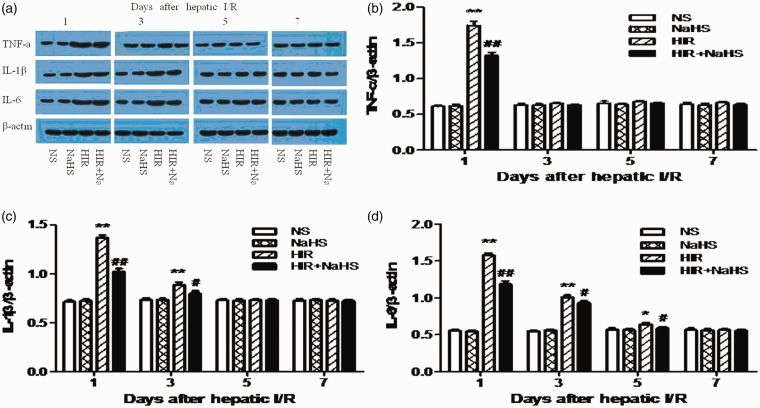
NaHS lowered hippocampal proinflammatory cytokines expression
Compared with the NS group and the NaHS group, the HIR group exhibited higher hippocampal TNF-α, IL-1β, and IL-6 expression (Figure 5(a) to (d)). However, after treatment with NaHS, the HIR + NaHS group displayed significantly reduced protein levels of proinflammatory cytokines in hippocampus when compared with the HIR group (Figure 5(a) to (d)).
Figure 5.
NaHS lowered hippocampal proinflammatory cytokines expression. (a) Western blot of TNF-α, IL-1β, and IL-6 protein levels in the hippocampus. (b) TNF-α level, (c) IL-1β level, (d) IL-6 level. The data are expressed as mean ± SEM. *P < 0.05 HIR versus NS, **P < 0.01 HIR versus NS, #P < 0.05 HIR + NaHS versus HIR, ##P < 0.01 HIR + NaHS versus HIR. (A color version of this figure is available in the online journal.)
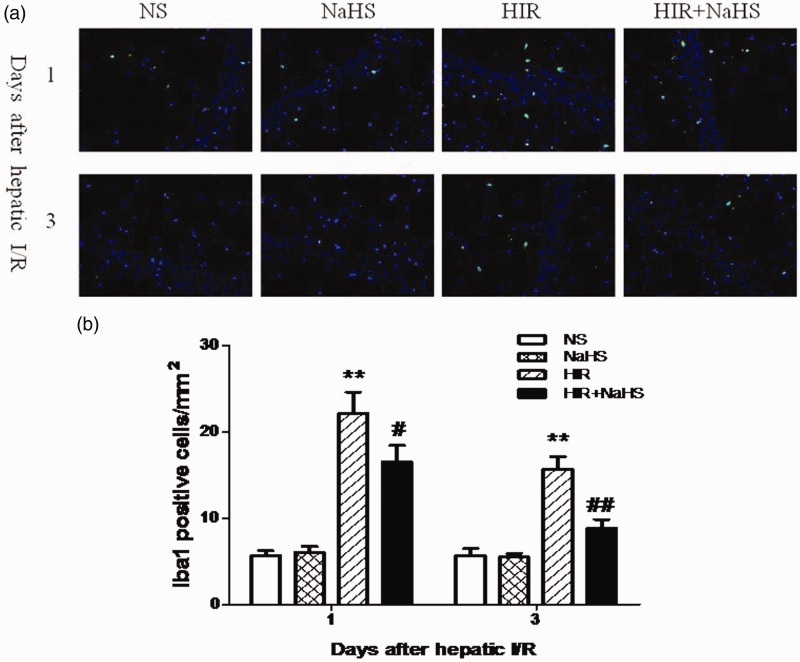
NaHS decreased the expression of Iba1 in the hippocampus
The HIR group exhibited increased hippocampal Iba1 expression when compared with the NS and the NaHS group (Figure 6(a) and (b)). But the expression of Iba1 was markedly decreased after administration of NaHS. These data indicated NaHS effectively ameliorated the activation of microglia.
Figure 6.
NaHS decreased hippocampal Iba1 expression. (a) The distribution and number of hippocampal Iba1-positive cells (magnification × 400). (b) Quantitative analysis of hippocampal Iba1-positive cells. The data are presented as mean ± SEM. **P < 0.01 HIR versus NS, #P < 0.05 HIR + NaHS versus HIR, ##P < 0.01 HIR + NaHS versus HIR. (A color version of this figure is available in the online journal.)
Discussion
In this study, we found that hepatic I/R induced the high-level expression of proinflammatory cytokine and the activation of microglia in the hippocampus, thereby leading to impaired cognitive function in rats. Treatment with NaHS (a H2S donor) effectively alleviated these neuroinflammatory changes and cognitive impairment. The present work demonstrates that neuroinflammation is involved in cognitive deficits after hepatic I/R, and NaHS may represent an effective agent for the treatment of this cognitive dysfunction.
Liver diseases may induce some alterations of the brain functions, and patients with liver diseases frequently present different degrees of cognitive deficits.22 To date, the exact mechanisms responsible for such cognitive impairment have not been sufficiently clarified. Previous studies have indicated that neuroinflammation and hyperammonemia contribute to these cognitive deficits.23,24 Herein, we used 70% hepatic I/R model, which is implicated in clinical scenario, including liver surgery, and which is different from cirrhosis and liver failure, to explore the mechanisms of the relations between hepatic I/R and cognitive impairment. We found that hepatic I/R evokes a peripheral inflammatory response, demonstrated by the increase of serum proinflammatory cytokines. Peripheral proinflammatory cytokines could enter the brain and induce the activation of microglia;25,26 activated microglia might further release the same proinflammatory cytokines. Furthermore, proinflammatory cytokines could suppress LTP and induce cognitive dysfunction.6,27,28 In this study, the cognitive dysfunction induced by hepatic I/R was associated with neuroinflammation. Based on these results and those of a previous study,24 neuroinflammation is involved in the mechanisms of cognitive impairment not only in liver diseases, such as cirrhosis and liver failure, but also in liver surgery, including hepatic resection.
H2S has been reported to exhibit protective effects against inflammation caused by lipopolysaccharide in various microglia.13 Another study revealed that H2S releasing compounds attenuated activated glia-induced neuroinflammation,29 but the specific mechanisms remain to be determined. Possible mechanisms included activated KATP channels, increased cAMP and glutathione levels.16,29 In the present study, it was found that hepatic I/R induces marked neuroinflammation, which was ameliorated after NaHS treatment. This is in line with previous report suggesting that NaHS could attenuate neuroinflammation in the hippocampus.15 On the other hand, our data suggested that hepatic I/R could induce cognitive decline in rats and treatment with NaHS efficiently ameliorated such cognitive deficits. In sum, these results suggest NaHS alleviates hepatic I/R-induced cognitive impairment via inhibiting neuroinflammation, and NaHS has a significant anti-inflammatory effect in the central nervous system.
This experiment indicated that hepatic I/R caused the injury of liver itself, which could be attenuated with the treatment of NaHS. Previous studies demonstrated that NaHS attenuated hepatic I/R injury through inhibiting inflammatory response.18 In this study, the HIR + NaHS group exhibited a marked decrease in serum proinflammatory cytokines levels, speculating that peripheral proinflammatory cytokines entering into the brain was also reduced. Accordingly, it produced lower levels of inflammatory responses in the hippocampus. In addition, another study has demonstrated that NaHS could attenuate neuroinflammation and neuronal injury in brain.15 Taken together, we speculated that NaHS protected against hepatic I/R-induced cognitive deficits that are associated with reduced peripheral proinflammatory cytokines entering the brain and decreased local brain inflammation response and neuronal damages.
The increases of proinflammatory cytokines and Iba1 expression in the hippocampus did not last for seven days, but the impairment of cognitive function lasted for seven days, which suggests that neuroinflammation induced by hepatic I/R may not directly lead to the late cognitive deficits, and other mechanisms may participate in the late cognitive impairment. For example, it has been suggested that activated microglia could release reactive oxygen species and cause memory dysfunction.30 Additionally, apoptosis-induced hippocampal neuronal loss may induce cognitive decline.31 The roles of other factors, such as neurotoxic mediators and apoptosis, associated with the late impairment of cognitive function after hepatic I/R are required to clarify in further study.
In conclusion, we found that NaHS alleviates hepatic I/R-induced cognitive impairment in rats by attenuating neuroinflammation, which suggests that NaHS may be an alternative and valuable treatment for cognitive dysfunction after liver surgery.
Acknowledgements
This research was funded by the Department of Anaesthesiology, North Sichuan Medical College.
Authors’ contributions
FT and JL contributed equally to this work. FT, JW, and WC are responsible for the idea and design of this study. FT, JW, and QL performed the experiments. JW performed all immunohistochemical measurements. FT performed all behavioral assessments. JL participated in data analysis. FT and WC interpreted the results of the experiments. FT and JW drafted the manuscript. All authors have approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg 2012; 2012: 176723–176723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin HI, Chou SJ, Wang D, Feng NH, Feng E, Chen CF. Reperfusion liver injury induces down-regulation of eNOS and up-regulation of iNOS in lung tissues. Transpl Proc 2006; 38: 2203–6. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Maher JM, Chen C, Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol 2007; 71: 817–25. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 1993; 47: 169–76. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 1991; 259: 988–96. [PubMed] [Google Scholar]
- 6.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 2007; 106: 436–43. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc 2002; 50: 2041–56. [DOI] [PubMed] [Google Scholar]
- 8.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007; 32: 1106–15. [DOI] [PubMed] [Google Scholar]
- 9.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002; 16: 1792–8. [DOI] [PubMed] [Google Scholar]
- 10.Łowicka E, Bełtowski J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacologists. Pharmacol Rep 2007; 59: 4–24. [PubMed] [Google Scholar]
- 11.Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol 2006; 290: L1193–201. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Bhatia M, Moore PK. Hydrogen sulphide—a novel mediator of inflammation? Curr Opin Pharmacol 2006; 6: 125–9. [DOI] [PubMed] [Google Scholar]
- 13.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 2007; 100: 1121–8. [DOI] [PubMed] [Google Scholar]
- 14.Gong QH, Wang Q, Pan LL, Liu XH, Huang H, Zhu YZ. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a proinflammatory pathway in rats. Pharmacol Biochem Behav 2010; 96: 52–8. [DOI] [PubMed] [Google Scholar]
- 15.Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-myeloid rat model of Alzheimer’s disease. J Neuroinflammation 2012; 9: 202–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 2000; 267: 129–33. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996; 16: 1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl 2009; 15: 1306–14. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Wang Y, Xie Y, Yang Z, Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem Res 2011; 36: 1840–9. [DOI] [PubMed] [Google Scholar]
- 20.Kuncewitch M, Yang WL, Molmenti E, Nicastro J, Coppa GF, Wang P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock 2013; 39: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monfort P, Cauli O, Montoliu C, Rodrigo R, Llansola M, Piedrafita B, El Mlili N, Boix J, Agustí A, Felipo V. Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochem Int 2009; 55: 106–12. [DOI] [PubMed] [Google Scholar]
- 23.Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol 2002; 67: 259–79. [DOI] [PubMed] [Google Scholar]
- 24.Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol 2015; 5: S21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 1993; 47: 169–76. [DOI] [PubMed] [Google Scholar]
- 26.Gaykema RP, Goehler LE, Tilders FJ, Bol JG, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation 1998; 5: 234–40. [DOI] [PubMed] [Google Scholar]
- 27.Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience 2004; 124: 319–26. [DOI] [PubMed] [Google Scholar]
- 28.Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol 2005; 90: 663–70. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Sparatore A, Del Soldato P, McGeer E, McGeer PL. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia 2010; 58: 103–13. [DOI] [PubMed] [Google Scholar]
- 30.Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010; 7: 354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 2002; 946: 221–31. [DOI] [PubMed] [Google Scholar]