Abstract
The amino acids in the placenta have multiple functions; however, the therapeutic effects of proline remain poorly for relief postmenopausal symptoms. The aim of present study was to evaluate the effects of proline in the treatment of menopause using in vitro and in vivo models. We assessed the therapeutic effects and regulatory mechanisms of proline by using MCF-7 estrogen-dependent cells, MG63 osteoblast cells, and ovariectomized mice model. An in vivo study was carried out in eight-week-old sham and ovariectomized group. The ovariectomized mouse was further subdivided into two groups administered orally with 17β-estradiol or proline (10 mg/kg/day) for eight weeks. Proline significantly increased cell proliferation and Ki-67 levels in MCF-7 cells and enhanced cell proliferation, alkaline phosphatase activity, extracellular signal-regulated kinase phosphorylation, and glutamyl-prolyl-tRNA synthetase activation in MG63 cells. The estrogen receptor-β and estrogen-response elements luciferase activity were significantly increased by proline in MCF-7 and MG63 cells. In ovariectomized mice, oral administration of proline (10 mg/kg/day) for eight weeks significantly reduced body and vaginal weights. Proline also significantly increased serum estradiol and alkaline phosphatase levels, whereas serum luteinizing hormone was decreased by proline. In addition, detailed microcomputed tomography analysis showed that the proline notably enhanced bone mineral density, trabecular bone volume, and trabecular number in ovariectomized mice. Those findings implied that proline can be a promising candidate for the treatment of menopause.
Keywords: Proline, menopause, estrogen receptor, estradiol, MCF-7 cells, MG-63 cells
Introduction
Menopause was characterized as the permanent cessation of woman’s reproductive ability and is induced by significantly hormonal changes such as estrogen deficiency in women.1 Although women experience it typically during their late 40s or early 50s, nowadays, menopause can happen at any age.2 Most of the menopausal women suffer from abnormal vaginal bleeding, bone loss, headache, weight gain, hot flashes, vaginal and urinary symptoms, agitation, forgetfulness, and mood changes.3 These symptoms negatively impact on overall quality of life. Furthermore, it caused osteoporosis and heart disease.4
Hormone replacement therapy (HRT) has been mostly used to modulate menopausal symptoms. However, alternative therapies such as botanical and dietary supplements are still being sought as a substitute for pharmacological HRT because of the high risk of adverse side-effects such as thromboembolic events, breast cancer, vaginal bleeding, and heart attack during and after menopause.5
The placenta is a vital organ with the role of allowing protein, vitamins, minerals, and nutrient uptake, and antibody provisions to the developing fetus in a mother’s womb.6,7 The placenta and its extract have been used as folk remedies for menopausal symptoms in Asian countries, because placental extract contains a wide range of bioactive components molecules including hormone, cytokines, chemokines, amino acids, steroids, fatty acids, nucleic acids, and growth factors.8 Several studies have been reported that placenta has immune modulation, wound healing, antiaging, postpartum depression, and cellular regeneration properties.9–11 Recently, Han et al.12 reported that amino acids are active ingredients of the porcine placenta. The amino acids in the placenta are so called phytohormones and have multiple functions, such as antioxidant, fuel provision, energy homeostasis, immune responses, recovery, development and growth.13 Arginine, lysine, and carnitine are very effective on menopausal symptoms such as hot flushes, osteoporosis, and weight loss.14,15 However, proline which is a major amino acid found in the placenta remains poorly defined as potential therapeutic agents for relief postmenopausal symptoms. Here, we report the effects of proline on estrogen-dependent cell line, osteoblast cell line, and the ovariectomized (OVX) menopausal mice model.
Materials and methods
Materials
Proline, dimethyl sulfoxide (DMSO), and 17β-estradiol were purchased from Sigma chemical Co. (St. Louis, MO, USA). Antibodies of antiextracellular signal-regulated kinase (ERK) and antiphosphorylated ERK (pERK), glutamyl-prolyl-tRNA synthetase (EPRS), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dulbeco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA). Proline was dissolved in distilled water and filtered with 0.22 µm syringe filter. 17β-Estradiol was dissolved in DMSO, filtered with 0.22 µm syringe filter, and prepared at a dose of 100 nM.
Cell culture
Michigan Cancer Foundation (MCF)-7 human breast cancer cell line and MG63 osteoblast-like cell line were purchased from Korean Cell Line Bank (KCLB®, Seoul, Republic of Korea) and were cultured in culture dishes in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37℃ under 5% CO2 and 95% humidity.
Bromodeoxyuridine (BrdU) assay
The growth rates of both MG63 and MCF-7 cells were measured using a colorimetric immunoassay based on the measurement of bromodeoxyuridine (BrdU) incorporated by DNA synthesis (Roche Diagnostics GmbH, Mannheim, Germany).
MTT assay
Cell viability was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 100 µl of cells suspension (1 × 104 cells/well) was cultured in 96-well plates after pretreatment with proline. The MTT solution (5 mg/ml) was added, and the cells were incubated at 37℃ for 4 h. After washing the supernatant out, the insoluble formazan product was dissolved in DMSO. Then, the optical density was measured using an ELISA reader at 540 nm.
Quantitative real-time PCR analysis
Total RNA was isolated from MCF-7 cells or MG63 cells according to the manufacturer’s protocol using an easy-BLUE™ RNA extraction kit (iNtRON Biotech, Republic of Korea). The cDNA was synthesized using the commercial cDNA synthesis kit (Bioneer Corporation, Daejeon, Republic of Korea). Quantitative real-time polymerized chain reaction (PCR) was performed using a SYBR Green master mix and the detection of mRNA was analyzed using an ABI StepOne real-time PCR system (Applied Biosystems, Foster City, CA, USA). Data were normalized to house-keeping gene GAPDH. Primers were as follows: GAPDH (5′ TCG ACA GTC AGC CGC ATC TTC TTT 3′; 5′ACC AAA TCC GTT GAC TCC GAC CTT 3′), estrogen receptor (ER)-β (5′ TTC CCA GCA ATG TCA CTA ACT T3′; 5′TTG AGG TTC CGC ATA CAG A 3′); Ki-67 (5′ ATA AAC ACC CCA ACA CAC ACA A 3′; 5′GCC ACT TCT TCA TCC AGT TAC 3′). Typical profile times used were the initial step, 95℃ for 10 min followed by a second step at 95℃ for 15s and 60℃ for 30s for 40 cycles with a melting curve analysis. The level of target mRNA was normalized to the level of the GAPDH and compared with the control. Data were analyzed using the ΔΔCT method.
Alkaline phosphatase (ALP) assay
The MG63 cells were plated at a density of 3 × 105 cells/well in 24-well plates. Proline (10 µg/ml) was added and cultured for 24 h. Activity of alkaline phosphatase (ALP) was evaluated according to the manufacturer’s instructions (Abcam, Cambridge, UK).
Western blot analysis
Samples were extracted from cultured MG63 cells. For whole cell extracts, the collected cells were then lysed in an ice-cold RIPA buffer containing 1% protease inhibitor cocktail (Sigma). The sample was centrifuged at 12000 g for 10 min at 4℃ and collected. The protein concentration was measured by the modified Bradford assay (Bio-Rad, Hercules, USA), and then the proteins were separated through 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes. Next, the membrane was blocked with 5% skim milk in PBS-tween-20 for 2 h at room temperature and probed with primary antibodies against ERK, pERK, EPRS, and GAPDH (dilution 1:1000; SantaCruz, Cambridge, USA); overnight at 4℃ followed by the incubation with the corresponding horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. The signal was detected using an enhanced chemiluminescence reagent (Amersham Co., Newark, NJ, USA) following the manufacturer’s instructions.
Transient transfections and estrogen-response element (ERE) luciferase reporter assay
The reporter gene estrogen-response element (ERE)-TATA-Luc was constructed using the enhanced luciferase reporter gene. For the transfection, we seeded MCF-7 and MG63 cells (1 × 107) in a 100 mm culture dish. We then used Lipofectamine™ 2000 purchased from Invitrogen (Carlsbad, CA, USA) to transiently transfect pERE-TATA-Luc and pSV40-Luc reporter gene constructs into each MCF-7 and MG63 cells. To measure the luciferase activity, we used a luminometer 1420 luminescence counter purchased from Perkin Elmer (Waltham, MA, USA) in accordance with the manufacturer’s protocol. The relative luciferase activity was defined as the ratio of firefly luciferase activity to renilla luciferase activity.
Experimental animals
Female Balb/c mice (seven-week-old) were purchased from Dae-Han Experimental animal center (Eumsung, Chungbuk, Republic of Korea). Animals were kept for a week with free access to water and chow and then maintained at a temperature of 22 ± 1℃ and a relative humidity of 55 ± 10% under a 12:12 light/dark cycle throughout the study. All protocols for animals were approved by the institutional animal care committee of Kyung Hee University (KHUASP (SE)-14-024). For the study, mice were OVX. Mice were randomized into the following groups: non-treated group, OVX group, OVX + 100 nM 17β-estradiol group, and OVX + proline (10 mg/kg/day). Proline or 17β-estradiol was administered orally for eight weeks. The body weight was measured weekly from the beginning to the end of the administration. At the end of experiment, blood samples were collected. The dissected bones were fixed with 10% formaldehyde.
Analysis of serum
The levels of serum ALP, 17β-estradiol (Invitrogen, Carlsbad, CA, USA), and luteinizing hormone (LH, Abcam, Cambridge, UK) were analyzed according to the manufacturer’s instructions.
Microcomputed tomography (µCT)
The 2D/3D information on bone geometry was provided by microcomputed tomography (µCT) according to previous study.16
Statistical analysis
All data were checked for normality using the Shapiro-Wilk test. In vitro data are shown as mean ± standard error mean (SEM) from at least three independent experiments performed in duplicates or triplicates. A power analysis in serum estradiol levels was used to estimate the required sample size for in vivo study. We set G power to calculate the sample size needed for a two independent sample t-tests. Sample size (type I error 0.05; power 99.89%) was based on results from a pilot study, and in vivo data are represented as the mean ± SEM. A value of P < 0.05 was regarded to be statistically significant.
Results
Proline increased cell proliferation and Ki-67 mRNA expression in MCF-7 cells
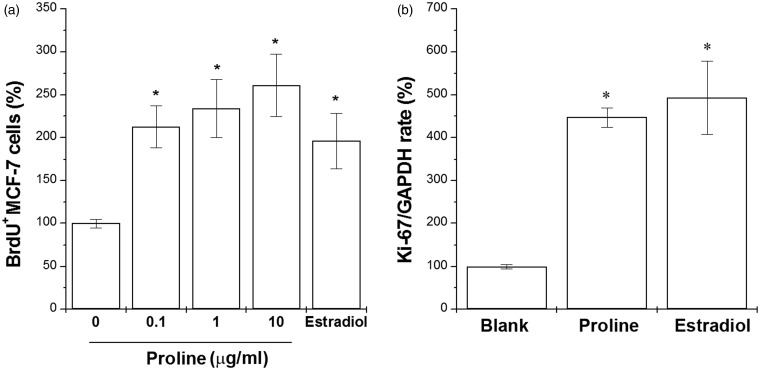
We first studied the effect of proline on proliferation of MCF-7 cells, an estrogen-dependent cell line. Treatment of 17β-estradiol significantly increased the cell proliferation compared with the untreated cells. Treatment of proline (0.1, 1, 10 µg/ml) also significantly increased the cell proliferation in a dose-dependent manner (Figure 1(a), P < 0.05). To assess the mechanism of increased growth of MCF-7 cells, Ki-67 mRNA expression (a marker for cell proliferation) was evaluated. The high proliferation rate of MCF-7 cells is associated with the high nuclear Ki-67. Proline or 17β-estradiol also significantly increased the mRNA expression of Ki-67 (Figure 1(b), P < 0.05).
Figure 1.
Proline increased cell proliferation and Ki-67 mRNA expression in MCF-7 cells. (a) MCF-7 cells were treated with proline (0.1, 1, and 10 µg/ml) for 48 h. Proliferation was measured with a BrdU incorporation assay. (b) MCF-7 cells were treated with proline (10 µg/ml) or 17β-estradiol (100 nM) for 24 h. The Ki-67 mRNA expression was analyzed with the quantitative real-time PCR analysis. *P < 0.05; significantly different from untreated cells. Blank, untreated cells
Proline increased cell proliferation, ALP production, and ERK and EPRS activation in MG63 cells
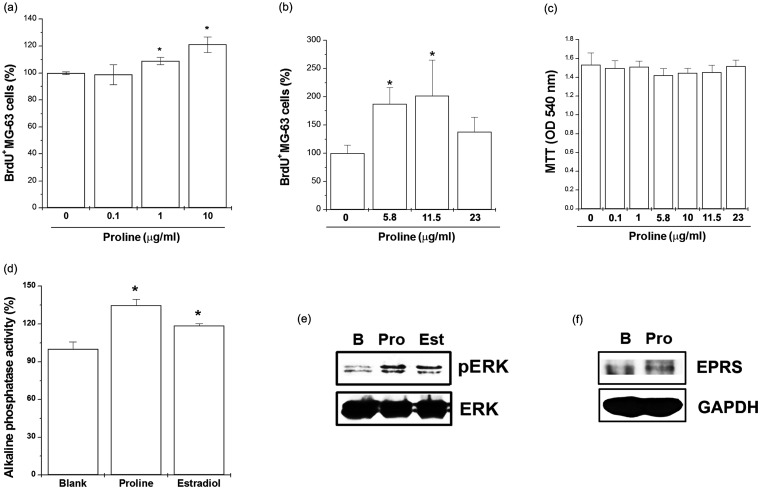
We investigated the proliferative effect of proline on osteoblast cell line, MG63. For this purpose, MG63 cells were incubated with various doses of proline (0.1, 1, and 10 µg/ml) or 17β-estradiol for 48 h and then BrdU assay was performed. Treatment of 17β-estradiol significantly increased the cell proliferation compared with the untreated cells. Proline induced a significant dose-dependent increase in cell proliferation compared with the untreated cells (Figure 2(a)). Proline at higher levels (11.5 µg/ml) also increased the cell proliferation compared with the untreated cells (Figure 2(b), P < 0.05) but decreased the cell proliferation at 23 µg/ml. A maximal effect of proline was seen at 11.5 µg/ml (Figure 2(b)). Cytotoxicity was not observed at doses of 0.1, 1, 5.8, 10, 11.5, and 23 µg/ml of proline (Figure 2(c)). Next, we evaluated the cell-associated ALP activity, which is a marker of bone differentiation. As shown in Figure 2(d), 17β-estradiol significantly increased the ALP activity. In cultures treated with proline for 24 h, ALP activity was significantly increased (Figure 2(d), P < 0.05). Estrogen rapidly phosphorylates the mitogen-activated protein kinases, ERK. We showed that proline or 17β-estradiol increased phosphorylation of ERK in MG63 cells (Figure 2(e), P < 0.05). Proline regulates cell proliferation by stimulating the activity of EPRS.17 We also showed that proline increased activation of EPRS in MG63 cells (Figure 2(f)).
Figure 2.
Proline increased cell proliferation, ALP production, ERK phosphorylation, and EPRS activation in MG63 cells. (a) MG63 cells were treated with proline (0.1, 1, and 10 µg/ml) for 48 h. Proliferation was measured with a BrdU incorporation assay. (b) MG63 cells were treated with proline (5.8, 11.5, and 23 µg/ml) for 48 h. Proliferation was measured with a BrdU incorporation assay. (c) MG63 cells were treated with proline (0.1, 1, 5.8, 10, 11.5, and 23 µg/ml) for 24 h. Cell viability was evaluated by a MTT assay. (d) MG63 cells were treated with proline (10 µg/ml) or 17β-estradiol (100 nM) for 24 h. The ALP activity was measured using an ALP assay kit. (e) MG63 cells were treated with proline (10 µg/ml) or 17β-estradiol (100 nM) for 5 min. The levels of ERK were assessed by Western blotting. (f) MG63 cells were treated with proline (10 µg/ml) for 2 h. The levels of EPRS were assessed by Western blotting. *P < 0.05; significantly different from untreated cells. B, untreated cells; Pro, Proline; Est, 17β-estradiol
Proline increased ER-β mRNA expression and ERE luciferase activity in MCF-7 and MG63 cells
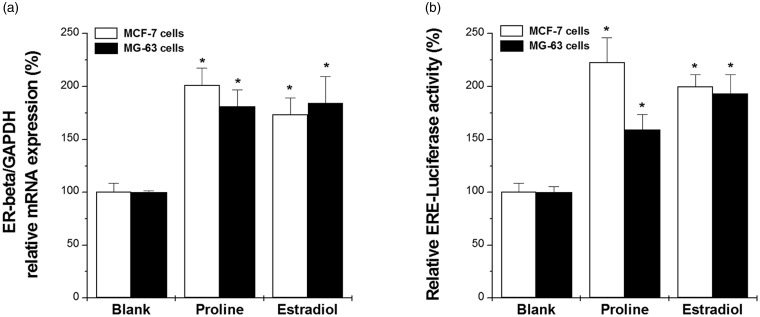
The effect of proline on the expression of ER-β mRNA was monitored in MCF-7 and MG63 cells. After treatment of MCF-7 or MG63 cells with proline (10 µg/ml) or 17β estradiol, mRNA levels were measured by real-time PCR. ER-β mRNA levels were up-regulated by proline or estradiol treatment compared to the untreated cells. (Figure 3(a), P < 0.05). Proline also significantly increased the ERE luciferase activity in MG63 and MCF-7 cells (Figure 3(b), P < 0.05).
Figure 3.
Proline increased ER-β mRNA expression and estrogenic activity in MG63 and MCF-7 cells. (a) MG63 and MCF-7 cells were treated with proline (10 µg/ml) or 17β-estradiol (100 nM) for 10 h. The ER-β mRNA expression was analyzed with the quantitative real-time PCR analysis. (b) MG63 and MCF-7 cells were treated with proline (10 µg/ml) or 17β-estradiol (100 nM) for 48 h. The ERE-luc activity was measured with a luciferase assay. *P < 0.05; significantly different from untreated cells. Blank, untreated cells
Effect of proline on body, vaginal, and uterus weights
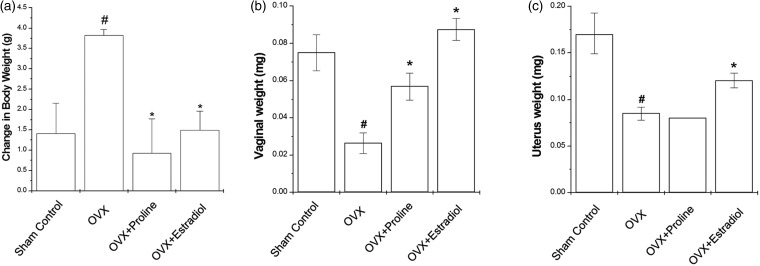
Estrogen is important modulator of body weight. We determined the estrogenic activity of proline using OVX mice as a menopausal model system. Figure 4(a) represents the time-course changes in body weight for eight weeks. OVX mice showed higher body weight than sham controls (Figure 4(a), P < 0.05). Administration of proline or 17β-estradiol to OVX mice significantly decreased the body weight gain compared with the OVX mice (Figure 4(a), P < 0.05). After eight weeks of treatment, the changes of vaginal and uterus weight were observed (Figure 4(b)). Vaginal and uterus weights after OVX were decreased compared with the sham controls. However, 17β-estradiol treatment maintained vaginal and uterus weight comparable to that of the sham controls (Figure 4(b) and (c), P < 0.05). Proline also significantly increased vaginal weight but not uterine weight (Figure 4(b) and (c)).
Figure 4.
Effect of proline on body, vaginal, and uterus weights in the OVX mice. Proline (10 mg/kg) or 17β-estradiol (100 nM) was orally administered daily for eight weeks. (a) Body weight was measured once a week for eight weeks. The weights of (b) vagina and (c) uterus were measured at the end of the eight weeks treatment period. #P < 0.05; significantly different from the sham control mice. *P < 0.05; significantly different from the OVX mice
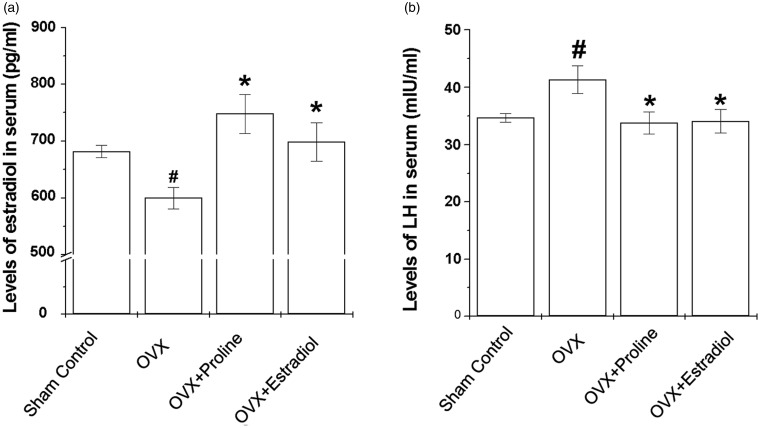
Effect of proline on levels of estradiol and LH in the serum
In the OVX mice, the level of estradiol was statistically decreased in comparison with the sham controls (Figure 5(a), P < 0.05). Administration of proline or 17β-estradiol tended to increase the serum estradiol level (Figure 5(a), P < 0.05). OVX mice resulted in a marked increasing serum LH levels (Figure 5(b), P < 0.05). However, treatment of OVX mice with proline (10 mg/ml) or 17β-estradiol resulted in significant suppression of serum LH levels (Figure 5(b), P < 0.05).
Figure 5.
Effects of proline on production of estradiol and LH in serum in the OVX mice. Proline (10 mg/kg) or 17β-estradiol was orally administered daily for eight weeks. Serum levels of (a) 17β-estradiol and (b) LH were measured at the end of the treatment period as described in the Materials and methods section. #P < 0.05; significantly different from the sham control mice. *P < 0.05; significantly different from OVX mice
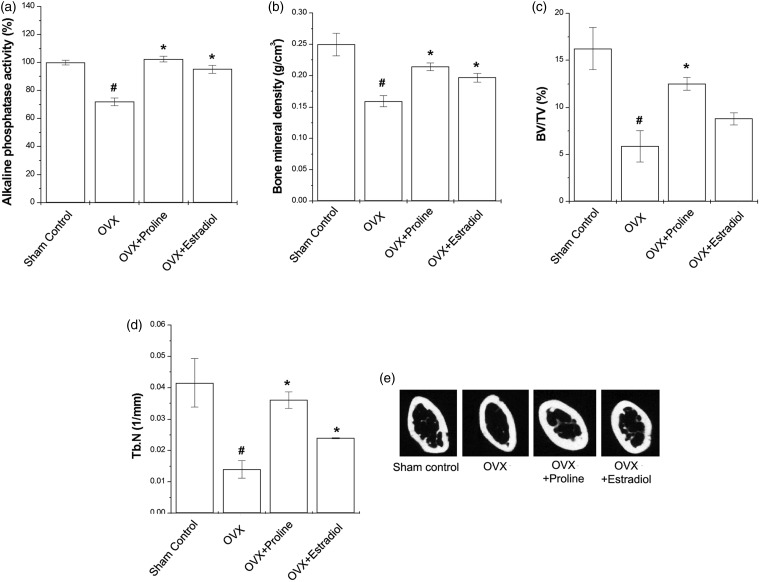
Effect of proline on bone parameters
The OVX mice had significantly lower serum ALP levels than the sham control group. Proline or 17β-estradiol-treated mice significantly increased the serum ALP levels compared with the OVX mice (Figure 6(a), P < 0.05). The OVX mice had remarkably reduced bone mineral density (BMD), trabecular bone volume (BV/TV), and trabecular number (Tb. N) (Figure 6(b) to (d), P < 0.05). Compared with the OVX mice, proline or 17β-estradiol groups showed significant increase of these parameters (Figure 6(b) to (d), P < 0.05).
Figure 6.
Effect of proline on bone parameters in the OVX mice. Proline (10 mg/kg) or 17β-estradiol (100 nM) was orally administered daily for eight weeks. (a) ALP activity in the serum was measured. (b) BMD was determined using 2D µCT. Quantified parameters of proximal tibia were determined using 3D µCT (0.9 mm–1.8 mm regions from growth plate). (c) BV/TV, trabecular bone volume (d) Tb.N, trabecular number (e) these images are representative cross-sectional µCT scanning of the tibia. #P < 0.05; significantly different from the sham control mice. *P < 0.05; significantly different from the OVX mice
Discussions and conclusions
This is the first report verifying the estrogenic efficacy of proline in the treatment of menopause in vitro and OVX animal models. Oral administration with proline for eight weeks could significantly relieved the menopausal symptoms induced by estrogen decline, suggesting that proline is another supplementation option in treating menopausal symptoms.
Proline is required for the multiple biochemical and physiological actions in cells.18 It was recently reported that porcine placenta containing proline have therapeutic value in treating osteoporosis in OVX rats.12 Furthermore, Vadlamudi et al.19 reported that a novel human protein containing proline acts as a co-activator of the ER pathways. Proline is also associated with higher BMD value at the lumbar spine.20 In the present study, proline shows the estrogenic agonistic effects on MG-63 osteoblasts, MCF-7 cells, and OVX mice.
To evaluate the estrogenic effects of proline, we used an estrogen-dependent cell line derived from human breast tumor, MCF-7 cells. This cell line is well-known as ER positive and is widely used as experimental models for menopausal symptoms.21 Our results showed that proline significantly increased the cell proliferation and Ki-67 mRNA expression in MCF-7 cells, suggesting that proline acts as a functional estrogenic ligand in MCF-7 cells.
Osteoporosis is the most common disease in women after menopause and caused by an imbalance between activity of osteoblasts and osteoclasts. BMD contributing bone fragility is reduced through decreasing proliferation and mineralization of osteoblast.22 Thus, we evaluated the effect of proline on the proliferation and differentiation of MG63 cells, which is generally used for the treatment of postmenopausal osteoporosis.23 As shown in this study, proline is capable of promoting proliferation of osteoblasts. ALP, a classical biomarker of early stage of osteoblast differentiation, is required for the initiation of bone mineralization.24 In our results, we revealed that proline significantly increased the ALP activity in MG63 cells, showing beneficial effects on cell differentiation. The ERK signaling pathway is required for proliferation, differentiation, and survival of osteoblast cells.25 Furthermore, Chu et al.17 reported that proline plays an important role in cell proliferation by binding EPRS. In this report, we also demonstrate that proline enhanced the phosphorylation levels of ERK and activation of EPRS. The results of the present study clearly confirm our speculation that proline induces the cell proliferation via the activation of the ERK and EPRS pathway.
ER signaling is a key factor to promote the bone growth and bone remodeling.26 There are two subtypes of ERs, usually referred to ER-α and ER-β, which are expressed during osteoblast differentiation by the link with estrogen. Several studies reported that both ER-α and ER-β is co-linked to osteoporosis and BMD.27 Therefore, ERs may be a possible molecular target for treating menopausal symptoms. In the present study, we showed that proline up-regulated the expression of ER-β mRNA in MG63 and MCF-7 cells. Also, proline increased the ERE luciferase activity in both cells. These results indicated that estrogenic activity of proline in MG63 and MCF-7 cells is mediated via the ER signaling pathway.
OVX is a widely used standard surgical procedure for the study of menopausal symptoms and postmenopausal osteoporosis, because OVX animals are characterized by reduced ovarian function and cancellous bone mass and strength.27 Estrogen, a reproductive hormone, is important in the maintenance body weight and estrogen deficiency following menopause is a serious cause of obesity in females.28 Thus, it is important to inhibit body weight gain to maintain a healthy life in postmenopausal women. Kolta et al.29 reported that treating OVX mice with estradiol prevented the development of obesity. In our study, OVX mice showed a significant increase in body weight, which was also observed in other studies in comparison with the sham controls.30,31 However, proline showed a significant decrease in body weight. Furthermore, proline also restored the vaginal weight decreased by OVX. OVX mice produced a marked reduction in serum estrogen concentrations, because estrogen is synthesized in the testis, adrenal gland, or ovary.3,28,32 The present study showed that proline increased serum estrogen levels. This result suggests that proline may stimulate the biosynthesis of estrogen. The serum level of LH is a marker of estrogenic effect.33 Proline seems to be effective in suppressing the production of LH. Another characteristic of menopausal symptoms is bone loss. ALP is a serum biomarker for bone formation in vivo.34 As shown in our data, ALP levels were decreased in OVX mice. However, ALP levels were elevated in proline treated group compared with the OVX group, indicating that proline is capable of promoting osteoblast differentiation. Estrogen deficiency also plays an important role in bone loss of postmenopausal women.34 The current investigation compared the long-term efficacy of 17β-estradiol and proline in enhancing bone mass in OVX mice. OVX mice are characterized by an osteopenia in the proximal tibia.35 Administration of proline significantly restored the levels of BMD, BV/TV, and Tb.N. Therefore, proline appears to be very effective for inhibiting bone resorption and improving bone formation.
Estrogen has been widely used for relief of menopausal symptoms. However, it causes serious side-effects such as breast cancer and lupus erythematosus.36,37 Amino acids such as glycine and glutamine are key factors for regulating menopause.38 Furthermore, food supplements such as dietary soy isoflavones decreases menopause-induced osteoporotic bone loss.39 In the present study, proline showed osteogenic and estrogenic activities in vitro and in vivo experimental models, suggesting proline as an alternative instead of estrogen therapy during menopause. However, further study is also necessary to evaluate the side-effect of nutritional therapy including proline.
In conclusion, this is the first report to provide the evidence to support the use of proline as a potent agent for menopausal diseases. Therefore, we suggest that proline has beneficial effects in alleviation and treatment of menopausal symptoms.
Acknowledgements
This research was supported by the Ministry of Trade, Industry and Energy(MOTIE) through the Special Education program for Industrial Convergence (2015).
Authors’ contributions
This study was carried out in collaboration between all authors. H-JJ and H-MK designed the research scheme. S-YN and M-SY contributed equally in this article. H-JJ, S-YN and M-SY carried out the laboratory experiments and analyzed the data. S-YN wrote the manuscript and discussed issues with the coauthors.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Buckler H. The menopause transition: endocrine changes and clinical symptoms. J Br Menopause Soc 2005; 11: 61–5. [DOI] [PubMed] [Google Scholar]
- 2.Lindh-Åstrand L, Hoffmann M, Järvstråt L, Fredriksson M, Hammar M, Spetz Holm AC. Hormone therapy might be underutilized in women with early menopause. Hum Reprod 2015; 30: 848–52. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HD. Menopause. Lancet 2008; 371: 760–70. [DOI] [PubMed] [Google Scholar]
- 4.Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, Lumsden MA, Mack WJ, Shapiro S, Baber RJ. Prevention of diseases after menopause. Climacteric 2014; 17: 540–56. [DOI] [PubMed] [Google Scholar]
- 5.Panay N, Hamoda H, Arya R, Savvas M. British Menopause Society and Women’s Health Concern. British Menopause Society and Women’s Health Concern. The 2013 British Menopause Society & Women’s Health Concern recommendations on hormone replacement therapy. Menopause Int 2013; 19: 59–68. [DOI] [PubMed] [Google Scholar]
- 6.Ober WB. Notes on placentophagy. Bull N Y Acad Med 1979; 55: 591–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Young SM, Benyshek DC. In search of human placentophagy: a cross-cultural survey of human placenta consumption, disposal practices, and cultural beliefs. Ecol Food Nutr 2010; 49: 467–84. [DOI] [PubMed] [Google Scholar]
- 8.Biswas TK, Auddy B, Bhattacharya NP, Bhattacharya S, Mukherjee B. Wound healing activity of human placental extracts in rats. Acta Pharmacol Sin 2001; 22: 1113–6. [PubMed] [Google Scholar]
- 9.Datta P, Bhattacharyya D. Spectroscopic and chromatographic evidences of NADPH in human placental extract used as wound healer. J Pharm Biomed Anal 2004; 34: 1091–8. [DOI] [PubMed] [Google Scholar]
- 10.Hong HT, Kim HJ, Lee TK, Kim DW, Kim HM, Choo YK, Park YG, Lee YC, Kim CH. Inhibitory effect of a Korean traditional medicine, Honghwain-Jahage (water extracts of Carthamus tinctorius L. Seed and Hominis placenta) on interleukin-1-mediated bone resorption. J Ethnopharmacol 2002; 79: 143–8. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Lee JW, Kim YL, Lee MH. The effect of placental extract on the expression of tyrosinase, TRP-1 and TRP-2 in SK30 melanoma cells. Korean J Dermatol 2003; 41: 1612–8. [Google Scholar]
- 12.Han NR, Kim KY, Kim MJ, Kim MH, Kim HM, Jeong HJ. Porcine placenta mitigates protein-energy malnutrition-induced fatigue. Nutrition 2013; 29: 1381–7. [DOI] [PubMed] [Google Scholar]
- 13.Cruzat VF, Krause M, Newsholme P. Amino acid supplementation and impact on immune function in the context of exercise. J Int Soc Sports Nutr 2014; 11: 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torricelli P, Fini M, Giavaresi G, Giardino R, Gnudi S, Nicolini A. q-arginine and L-lysine stimulation on cultured human osteoblasts. Biomed Pharmacother 2002; 56: 492–7. [DOI] [PubMed] [Google Scholar]
- 15.Odo S, Tanabe K, Yamauchi M. A pilot clinical trial on L-carnitine supplementation in combination with motivation training: effects on weight management in healthy volunteers. Food Nutr Sci 2013; 4: 222–31. [Google Scholar]
- 16.Kim MH, Jeong H, Park M, Moon PH. Effect of KH-BaRoKer-SeongJangTang based on traditional medicine theory on longitudinal bone growth. TANG 2014; 4: e14–e14. [Google Scholar]
- 17.Chu TL, Guan Q, Nguan CY, Du C. Halofuginone suppresses T cell proliferation by blocking proline uptake and inducing cell apoptosis. Int Immunopharmacol 2013; 16: 414–23. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 2011; 40: 1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, Kumar R. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem 2001; 276: 38272–9. [DOI] [PubMed] [Google Scholar]
- 20.Jennings A, MacGregor A, Spector T, Cassidy A. Amino acid intakes are associated with bone mineral density and prevalence of low bone mass in women: evidence from discordant monozygotic twins. J Bone Miner Res. Epub ahead of print 3 September 2015. Doi: 10.1002/jbmr.2703. [DOI] [PMC free article] [PubMed]
- 21.Shim MK, Lee YJ. Estrogen receptor is activated by Korean red ginseng in vitro but not in vivo. J Ginseng Res 2012; 36: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamdy RC. Fracture risk assessment in postmenopausal women. Rev Endocr Metab Disord 2010; 11: 229–36. [DOI] [PubMed] [Google Scholar]
- 23.Ji ZN, Zhao WY, Liao GR, Choi RC, Lo CK, Dong TT, Tsim KW. In vitro estrogenic activity of formononetin by two bioassay systems. Gynecol Endocrinol 2006; 22: 578–84. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Peng J, Wu S, Jin Y, Xia F, Wang C, Liu K, Sun H, Liu M. Dioscin promotes osteoblastic proliferation and differentiation via Lrp5 and ER pathway in mouse and human osteoblast-like cell lines. J Biomed Sci 2014; 21: 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol 2008; 215: 442–51. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Huang YL, Liao JF, Chiou WF. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur J Pharmacol 2012; 676: 26–33. [DOI] [PubMed] [Google Scholar]
- 27.Cho P, Schneider GB, Krizan K, Keller JC. Examination of the bone-implant interface in experimentally induced osteoporotic bone. Implant Dent 2004; 13: 79–87. [DOI] [PubMed] [Google Scholar]
- 28.Dubuc PU. Effects of estrogen on food intake, body weight, and temperature of male and female obese mice. Proc Soc Exp Biol Med 1985; 180: 468–73. [DOI] [PubMed] [Google Scholar]
- 29.Kolta S, De Vernejoul MC, Meneton P, Fechtenbaum J, Roux C. Bone mineral measurements in mice: comparison of two devices. J Clin Densitom 2003; 6: 251–8. [DOI] [PubMed] [Google Scholar]
- 30.Mori-Okamoto J, Otawara-Hamamoto Y, Yamato H, Yoshimura H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J Ethnopharmacol 2004; 92: 93–101. [DOI] [PubMed] [Google Scholar]
- 31.Zaid SS, Sulaiman SA, Sirajudeen KN, Othman NH. The effects of Tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats – animal model for menopause. BMC Complement Altern Med 2010; 10: 82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev 2008; 57: 470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimoldi G, Christoffel J, Seidlova-Wuttke D, Jarry H, Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect 2007; 115(Suppl 1): 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, Lu L, Xiao DQ. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res 2014; 34: 467–77. [DOI] [PubMed] [Google Scholar]
- 35.Riggs BL, Khosla S, Melton LJ. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 1998; 13: 763–73. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, Flanders WD, Berkelman RL. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 1991; 266: 1362–1362. [PubMed] [Google Scholar]
- 37.Sánchez-Guerrero J, Liang MH, Karlson EW, Hunter DJ, Colditz GA. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann Intern Med 1995; 122: 430–3. [DOI] [PubMed] [Google Scholar]
- 38.Auro K, Joensuu A, Fischer K, Kettunen J, Salo P, Mattsson H, Niironen M, Kaprio J, Eriksson JG, Lehtmäki T, Raitakario O, Jula A, Tiitinen A, Jauhiainen M, Soininen P, Kangas AJ, Kähönen M, Havulinna AS, Ala-Korpela M, Salomaa V, Metspalu A, Perola M. A metabolic view on menopause and ageing. Nat Commun 2014; 5: 4708–4708. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Lee SK, Chun OK. Soy isoflavones and osteoporotic bone loss: a review with an emphasis on modulation of bone remodeling. J Med Food. Epub ahead of print 19 January 2015. DOI: 10.1089/jmf.2015.0045. [DOI] [PMC free article] [PubMed]