Abstract
Surface fouling and undesired adhesion are nearly ubiquitous problems in the medical field, complicating everything from surgeries to routine daily care of patients. Recently, the concept of immobilized liquid (IL) interfaces has been gaining attention as a highly versatile new approach to antifouling, with a wide variety of promising applications in medicine. Here, we review the general concepts behind IL layers and discuss the fabrication strategies on medically relevant materials developed so far. We also summarize the most important findings to date on applications of potential interest to the medical community, including the use of these surfaces as anti-thrombogenic and anti-bacterial materials, anti-adhesive textiles, high-performance coatings for optics, and as unique platforms for diagnostics. Although the full potential and pitfalls of IL layers in medicine are just beginning to be explored, we believe that this approach to anti-adhesive surfaces will prove broadly useful for medical applications in the future.
Keywords: Anti-adhesion, anti-biofouling, anti-thrombogenic, liquid-infused, immobilized liquid layer, SLIPS
Introduction
Adhesion to medical devices is the initiator of a number of detrimental, often fatal, clinical events such as thrombosis and biofouling. Such adhesion causes further problems ranging from necessitating the administration of systemic anticoagulants, which increase risk of bleeding and drug-related death,1 to requiring increasingly powerful antibiotics to combat the high number of healthcare-related infections caused by biofilm formation on medical devices.2 Despite all the attempts made to design surfaces that prevent biological adhesion or mitigate biological processes, current techniques are generally only able to provide solutions that are transient at best. Chemical approaches include hydrophilic coatings that prevent non-specific protein adhesion (e.g. PEG)3 or minimize van der Waals forces (e.g. Teflon),4 and materials that present or release biocidal compounds (e.g. antibiotics, silver nanoparticles).5 These surfaces have shown some success but are vulnerable to degradation, depletion, or desorption of surface molecules over time, and any defects in the surface chemistry can become nucleation sites for adhesion.6,7 Physical tactics often use micro- or nanoscale surface topography to reduce the contact area for organisms and thus their adhesion strength. However, these structures can be damaged, resulting in adhesion sites. Moreover, many film-forming organisms such as bacteria produce a growth-facilitating conditioning layer that can mask underlying surface chemistry.6
Recently, the concept of immobilized liquid (IL) layers has been proposed as a new approach to the problem of unwanted adhesion.8 This approach, which leverages the inherent dynamic and self-healing nature of liquids to prevent contaminants from adhering to a substrate, has shown promise toward a variety of industrial applications including anti-icing and marine biofouling.9–11 Increasing realization of the versatility of IL layers also suggests enormous potential in medical applications. In this mini-review, we give an overview of IL layers with the perspective of their eventual application in the medical field. We discuss the overall concept behind this technology, emerging strategies for applying IL layers to a wide variety of materials, and applications of direct relevance to the medical community that have been explored thus far. We also identify the most critical directions in need of development to adapt this new approach in materials science to the clinic, where it can begin to improve patient outcomes and reduce the burden of healthcare costs associated with medical device failure.
Concept
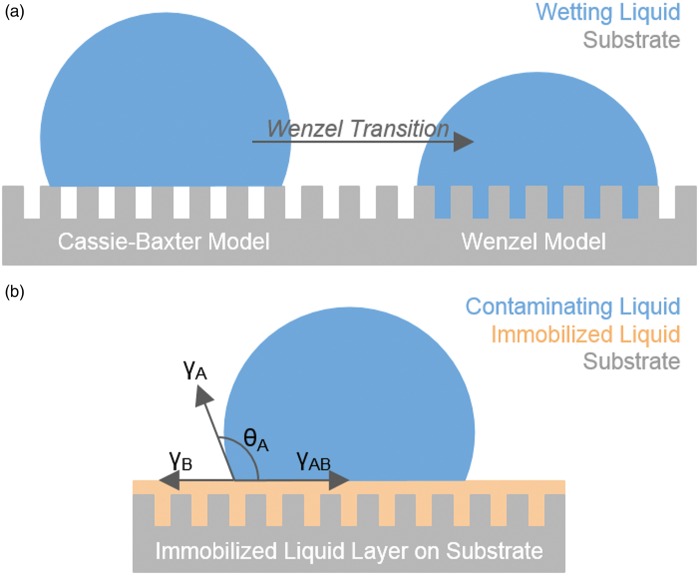
One common method of producing superhydrophobic, liquid-repellent surfaces makes use of hierarchical surface structure to repel liquids via the Cassie–Baxter model (Figure 1(a)). Inspired by the leaves of the Lotus plant,12 these methods are known to perform well against high-surface-tension liquids such as water under ambient temperature and pressure, but often fail when exposed to complex fluids,13 high pressure,14 humidity,15 or extreme temperatures.13 This is due to the fact that the air pocket supporting the droplet is inherently compressible, allowing it to be overcome by addition of pressure to the air/droplet interface or by low-surface-tension liquids. Attempts to expand the ability of these surfaces to repel such liquids, including hexane and oils, using re-entrant geometries or fluorinated porous structures have had some success,16,17 however, the question of performance against complex fluids or under high pressure still remains. In addition, the dependence of Lotus-inspired approaches on physical surface characteristics to suspend liquid droplets makes these surfaces susceptible to physical damage, reducing the overall longevity of the design.14
Figure 1.
(a) Schematic of Cassie–Baxter (left) and Wenzel (right) models for wetting. (b) Schematic of a contaminating droplet on an immobilized liquid layer over a structured substrate. (A color version of this figure is available in the online journal.)
Recently, a new approach to fouling-resistant surfaces was proposed: drawing inspiration from the carnivorous Nepenthes pitcher plant, which uses a highly wettable microstructure to force insects to aquaplane into its pitcher-like stomach,18 Wong et al.8 were the first to introduce IL layers as a broad solution to this problem with their Slippery Liquid-Infused Porous Surfaces (SLIPS).
The concept of ILs as omniphobic surfaces is based on providing and maintaining a superficially dynamic yet stable and IL layer (Figure 1(b)). The stability of the liquid on the solid can be achieved by designing the system to meet the thermodynamic requirement of
where γSB is the interfacial tension of substrate (S) and infusing liquid (B), γA the surface tension of the contaminating liquid (A), γSA the interfacial tension of substrate and infused liquid, and γB surface tension of the infusing liquid. In other words, it must be more energetically favorable for the infusing liquid to wet the surface than the contaminating liquid. This is accomplished through (1) physical entrapment of the infusing liquid through capillary forces and (2) increasing chemical affinity of the solid substrate for the infusing liquid by functionalization with appropriate chemical modifiers. Condition (1) is met by creating roughness at the micro-, nano-, or molecular scales, with the latter being defined as having a molecular structure into which the liquid molecules can penetrate.
Providing the conditions of chemical affinity and physical entrapment are met, the result is an extremely stable, homogeneous, and highly repellent interface. Flow tests have shown that the liquid over-layer is not stripped at physiological shear rates,19 and certain IL layers have demonstrated the ability to repel water and liquid hydrocarbons at pressures as high as 676 atm.8 Additionally, samples with up to 33% mechanical surface damage have been shown to remain slippery,20 as the liquid-based interface inherently exhibits self-healing characteristics.
Fabrication
For use in medical applications, IL layers should be fabricated (1) using biocompatible materials, (2) on a wide range of scales, and (3) requiring minimal effort and time. They should also be (4) sterilizable and (5) stable in their intended use. To address these issues, multiple fabrication strategies have recently emerged which demonstrate the flexibility inherent in this system.
Liquids
Although a number of different liquids have been used to create IL surfaces, so far only a few have been shown to be biocompatible or have been previously used in other clinical applications. Of these, the medical-grade perfluorocarbons perfluorodecalin and perfluoroperhydrophenanethrene have been used clinically as a blood substitute21 and vitreonretinal tamponade,22 respectively. A number of edible oils have also been used as liquid layers, including almond,23 coconut, olive, and canola.24 A larger number of studies on ILs have used silicone oil as the infusing liquid, which as a medical-grade product has been used for ocular tamponades25 and off-label for cosmetic reconstructions.26 Perfluoropolyethers and perfluorotripentylamines have also been extensively used as ILs due to their repellency of both water- and oil-based compounds,8,9,11,27–33 however, these have not yet been thoroughly evaluated for toxicity.
Plastics
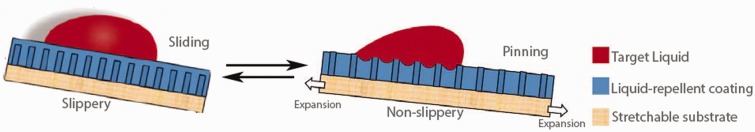
Plastics have most frequently been used to-date as substrates for IL interfaces due to their wide variety of chemical and physical properties and the unique ways in which they can be manipulated. Leslie et al.27 created a ‘tethered-liquid perfluorocarbon’ (TLP) IL system based on surface fluorosilanization and applied it to a wide range of medically relevant plastics including polyvinyl chloride (e.g. perfusion tubing), polyurethane (e.g. catheters), polycarbonate (e.g. barbed connectors), and polysulfone (e.g. dialysis membranes), among others. Wong et al. used expanded polytetrafluoroethylene (ePTFE) to make their SLIPS with perfluoropolyether liquids.8 ePTFE is well-known in the medical community as a material used for vascular grafts,34 hernia grafts,35 thyroplasty,36 augmentation of bone growth around implants,37 and even cosmetic surgery,38 and when coated with IL layers has been shown to be highly resistant against blood8 and bacteria.11 Membranes of ePTFE have further been used to make dynamic slippery/sticky materials through a simple stretch-and-release process (Figure 2).39 Such an approach may prove useful in applications requiring a switchable surface, such as one in which immediate bacterial resistance is needed, but eventual tissue integration is desired.
Figure 2.
Dynamic control of surface slipperiness is achieved by the stretching of a liquid-infused substrate, causing droplet pinning.39 (A color version of this figure is available in the online journal.)
Infused polymers
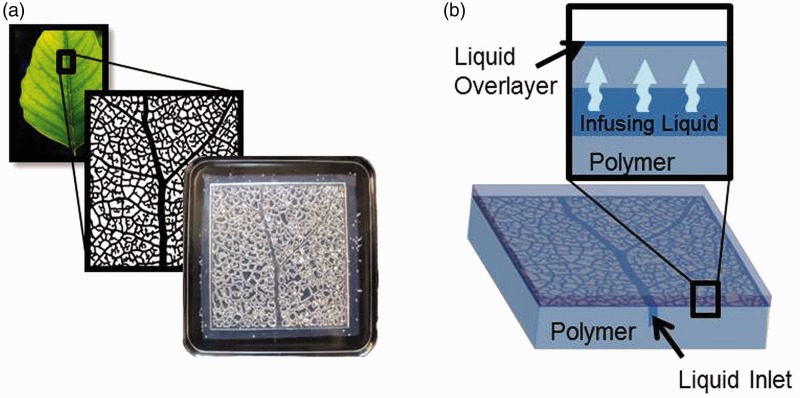
Recently, reports of creating ILs by infusing liquid into the molecular structure of a polymer have become more prevalent. Liu et al.40 used an ultra-thin (∼110 nm) organogel layer coated on copper, iron, silicone, and aluminum surfaces and infused with silicone oil to produce slippery self-cleaning surfaces. Urata et al.41 designed infused polydimethylsiloxane (PDMS) which could spontaneously release its liquid upon environmental changes such as a temperature decrease to −15℃ or humidity increase to 60% relative humidity. These polymers were prepared as a premixed liquid-polymer system which could make it an easily applicable overcoat for other materials. Other researchers have used widely available PDMS mixtures42 and commercially available pre-made silicone rubbers43 to create IL layers simply by soaking them in silicone oil for a matter of hours. Furthermore, these materials can be made self-replenishing through the incorporation of an embedded vascular system (Figure 3(a)), which can be continuously refilled with silicone oil from an outside source (Figure 3(b)). The infusing liquid filling the vascular network can diffuse through the polymer and replenish the surface liquid layer when it becomes depleted.42 The wide use of PDMS as catheters and tubing in current medical applications, and to a lesser extent silicone oils,26,27 may make the infused polymer approach to IL interfaces both cost-effective and scalable.
Figure 3.
(a) Bio-inspired vascular systems can be embedded in a polymer prior to infusion to provide self-replenishment properties. (b) Mechanism of self-replenishment of the slippery over-layer: the infusing liquid diffuses up through the polymer and replenishes the surface liquid layer when it becomes depleted. (A color version of this figure is available in the online journal.)
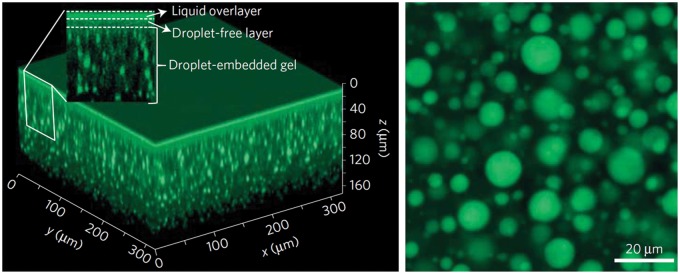
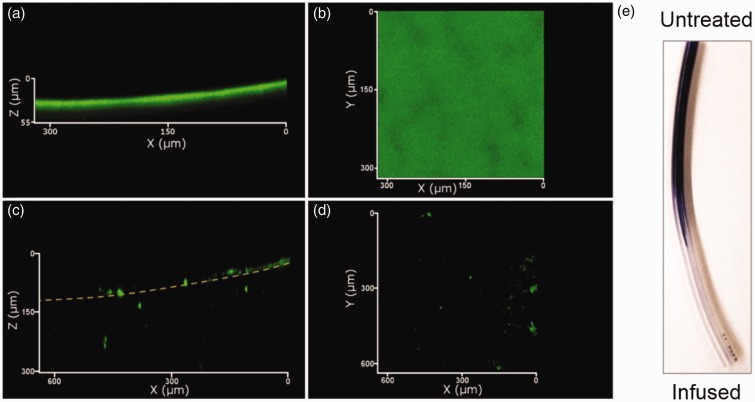
Alternative methods have been explored to create infused polymers, including the creation of fluoro-organogels which can be infused with perfluorinated lubricants.28 Supramolecular polymer approaches have been used to create a fibrous polymer for infusion that can be healed upon microwave treatment44 or which can automatically self-replenish to a 200 nm-thick liquid over-layer from a reservoir of embedded droplets of liquid (Figure 4).45 While these are powerful demonstrations of the ability of polymer chemistry to create infused materials to exhibit novel properties, further work must be done to either validate the safety of the component chemicals to biological systems or engineer them from currently standard medical materials before they can be used in a medical setting.
Figure 4.
Confocal fluorescence images of a supramolecular organogel embedded with silicone oil droplets (green). Visualized in 3D (left) and in horizontal cross-section (right).45 (A color version of this figure is available in the online journal.)
Metals
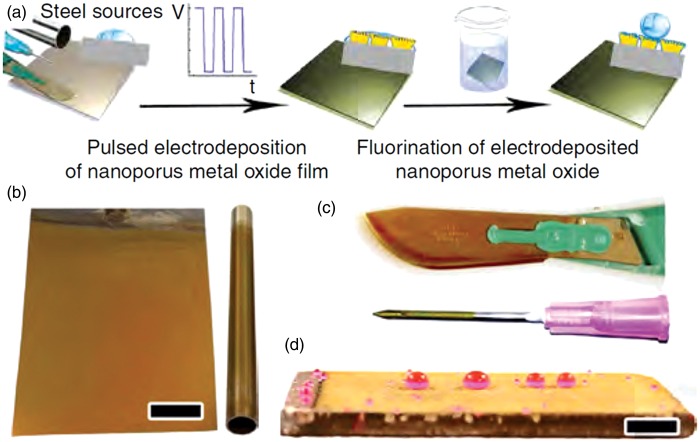
Metal substrates are also of interest for immobilizing liquids due to their wide use in applications from scalpel blades to implants. Tesler et al.29 created IL layers on steel through electrodeposition of nanoporous tungsten oxide, followed by surface fluorination and lubrication to create a perfluoropolyether liquid over-layer (Figure 5(a)). The result (Figure 5 (b) to (d)) was a highly durable system able to withstand impingement of solid particles, UV exposure, and temperatures from –196℃ to 300℃. Studies applying IL layers to titanium,27 aluminum,9 and low-alloy steel46 have also been reported. However, these materials have not yet been thoroughly tested in medical applications and more work is necessary prior to widespread use.
Figure 5.
Electrodeposited tungsten oxide films on various steel grades and surface geometries. (a) Preparation procedure on rough steel films deposited on (b) stainless-steel sheet and pipe, (c) surgical-grade steel scalpel blade and syringe needle, (d) naval construction steel plate. Scale bar, 1 cm.29 (A color version of this figure is available in the online journal.)
Arbitrarily shaped substrates
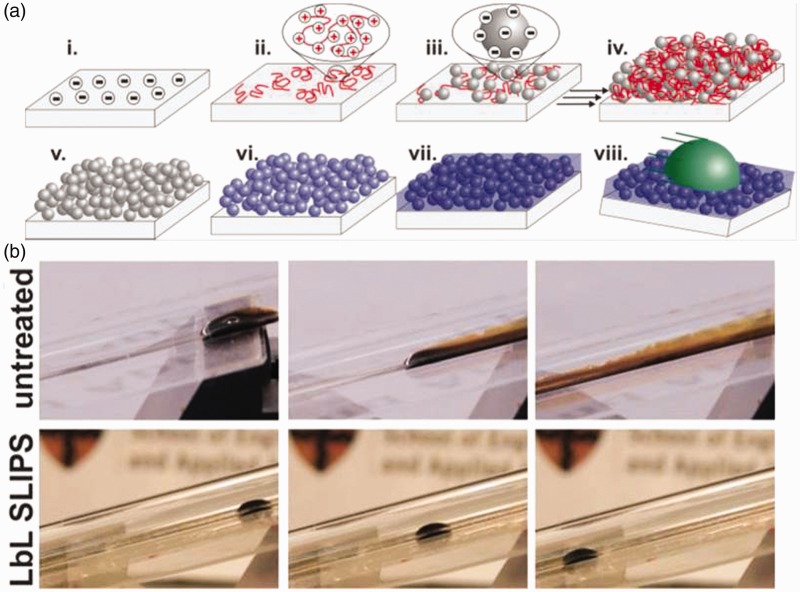
Layer-by-layer deposition47 (LbL) has been demonstrated to be a versatile and robust way to create porous surfaces on objects of arbitrary shape for IL layers. Huang et al.48 used LbL deposition of two water-soluble weak polyelectrolytes to create substrates for immobilizing liquids on curved glass surfaces. Sunny et al.30 used 20 nm silica nanoparticles in their LbL deposition (Figure 6) and further demonstrated the mechanical robustness of the resulting layer in a series of tape tests. Other researchers have recently taken the LbL approach in new directions by demonstrating how they can be used to make selectively non-slippery materials by coating only certain areas of the substrate23 or by making them out of entirely biocompatible and biodegradable materials such as alginate, polyvinylphyrrolidone, and almond oil.24
Figure 6.
Layer-by-layer fabrication of nanoscale, liquid-coated infused silica. (a) (i) Substrate is negatively charged. (ii) Positively charged polyelectrolyte and (iii) negatively charged silica nanoparticles are deposited, creating a thin film (iv) that is (v) calcined and (vi) functionalized with fluorinated silanes, then (vii) coated with a fluorinated lubricant to make it non-adhesive (viii). (b) An LbL coating on the inside of a glass tube repels crude oil compared to an untreated glass tube.30 (A color version of this figure is available in the online journal.)
Applications
To date, most applications of IL layers have been industrial.8,49 However, strides have been made in applying this technology to preventing adhesion of microorganisms, specifically in marine settings.10,42 As the versatility of this technology becomes increasingly apparent, pursuits in medical applications are beginning to progress as well.
Anti-thrombogenic surfaces
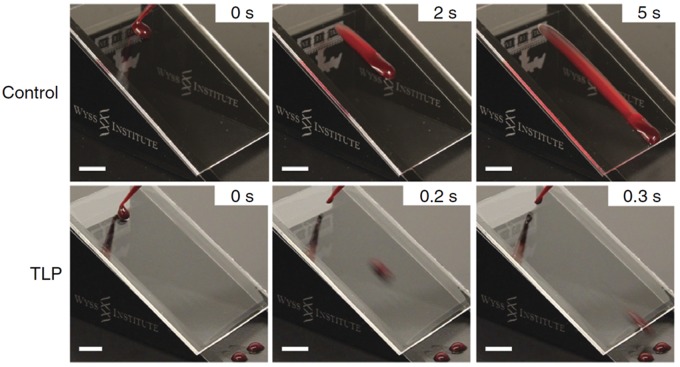
Medical device thrombosis and its consequences remain a ubiquitous problem.50–53 IL layers have been shown in several systems to prevent adhesion of proteins and blood both in vitro and in vivo, which is a promising step toward decreasing the need for systemic anticoagulants. Fluorocarbon-infused wrinkled surfaces showed a 96% reduction in bovine fibrinogen deposition, a significant reduction (P < .001) in platelet adhesion and reduction in whole blood clotting time by up to 95% (correlated to anti-thrombogenicity) compared to controls.54 The previously mentioned TLP coating has shown great promise in both in vitro and in vivo studies. In addition to macroscopically repelling droplets of blood on tilted surfaces (Figure 7), TLP-coated substrates showed reduced adhesion and polymerization of fibrin compared to controls, as well as suppression of both adhesion and activation of platelets in in vitro thrombogenicity tests. These results were replicated on ethylene oxide-sterilized TLP coatings on medical-grade cardiopulmonary perfusion tubing that were exposed to physiological flow rates. In vivo tests using TLP-coated medical-grade cannulae and the same perfusion tubing in a porcine femoral arteriovenous shunt model showed sustained patency for at least 8 h without the use of anticoagulants, as compared to controls, 80% of which fully occluded in that timeframe.27 The readiness with which IL layers can be applied to medical tubing and devices lends itself to commercial translation, an essential step toward making this technology accessible to patients.
Figure 7.
Tethered liquid perfluorocarbon (TLP) coating on acrylic surface prevents blood adhesion in sliding angle tests (30° tilt), compared to non-coated control. Scale bars, 1 cm.24 (A color version of this figure is available in the online journal.)
Bacterial anti-adhesive surfaces
In response to the prevalence of nosocomial infections and the rise in multi-drug resistant (MDR) pathogens,55 IL layers present the possibility to prevent bacterial attachment and biofilm formation without the need for antibiotics.27 Epstein et al.11 demonstrated that IL layers on ePTFE reduced biofilm coverage of Staphylococcus aureus by 97.2% and Escherichia coli by 96% after 48 h, as well as Pseudomonas aeruginosa by 99.6% after seven days of growth under gentle flow (10 mL/min). Researchers working on IL layers fabricated using other methods have found similar results with these species both under static27,54 and flow conditions27,43 (Figure 8). However, Li et al.32 found that not all bacterial strains are equally repelled by lubricant-infused surfaces. In high-nutrient medium, a MDR P. aeruginosa strain isolated from wastewater exhibited up to 12% surface coverage over seven days of incubation, compared to less than 1% coverage for other less virulent strains tested. More work is needed to uncover the reasons for such discrepancies, as well as the mechanics behind bacterial interactions with IL interfaces.
Figure 8.
Confocal microscopy of a ∼40um thick P. aeruginosa biofilm (green) on untreated (a,b) and infused (c,d) silicone tubing. (e) Photograph of crystal violet-stained biofilm on a variably infused tube.40 (A color version of this figure is available in the online journal.)
Optics
Another promising direction for IL layers is that of optics, where complications related to staining, coloration, and opacity caused by fouling agents can impair visibility. Zhang et al.56 developed a method for easily producing large-area (up to 100 cm2) transparent liquid surfaces on glass using breath figure patterns or water droplets sprayed onto surfaces followed by evaporation. Sunny et al.30 demonstrated that the transparency of their LbL-textured surfaces became even higher than that of bare glass once the IL layer was added, and that it could easily repel protein solutions. Such work opens the door for high-performance coatings of optical devices such as cameras and endoscopes.
Diagnostics
Beyond fouling of medical devices, controlling the adhesion of materials may also be applied to the development of novel diagnostic devices. Boreyko et al.57 have demonstrated how a phospholipid bilayer can be suspended between two droplets on an IL layer, allowing single-channel gating events across the bilayer to be measured. This is possible due to the fact that droplets on IL surfaces will coalesce orders of magnitude more slowly than on other surfaces due to the presence of an oil layer between them. This concept may prove useful in creating functional droplet networks that can be used for applications such as detecting airborne or exhaled compounds. Other researchers have created patterned IL layers for controlling droplet mobility,23 have manipulated droplets using ILs impregnated with magnetic nanoparticles,58 and have transferred magnetically doped droplets between IL layers using magnets.59 Still others have manipulated droplets using IL layers fabricated on fluorinated paper,60 which could aid in the development of low-cost, paper-based diagnostics.61
Textiles
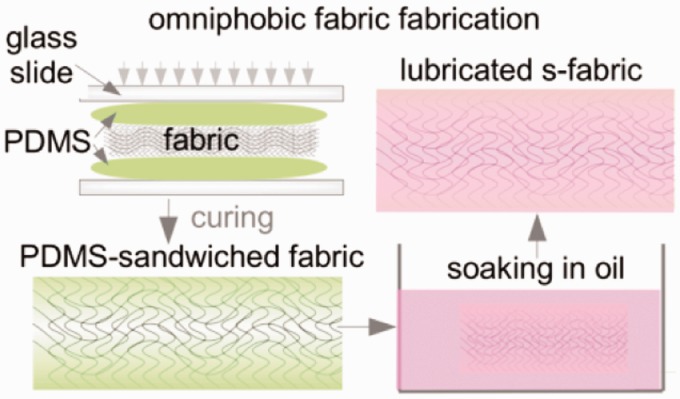
The development of fabrics which will absorb fluids but not stick to wounds or facilitate biofilm growth is highly desired for bandages, dressings, surgical meshes, and sutures. Both cotton and polyester fabrics have been used as IL substrates, created either by depositing a roughening layer of silica microbeads, followed by surface fluorination and lubrication,31 or by embedding the fabrics in a thin layer of oil-infused PDMS (Figure 9).62 Both approaches displayed increased liquid repellency as demonstrated by decreased droplet sliding angles. The embedded fabrics further showed retention of these properties in the face of repeated pressurized contact with absorbent cellulose fiber wipes. Testing regarding these infused fabrics’ absorption of fluids remains to be done.
Figure 9.
Fabrication of a fabric-supported lubricant-swollen polymeric film resulting in PDMS-sandwiched (s-) fabric with an IL layer.62 PDMS: polydimethylsiloxane (A color version of this figure is available in the online journal.)
Scaling and corrosion
The accumulation of inorganic solids on surfaces within the body can also cause significant problems. Afflictions such as the calcification of joints and gout are caused by the deposition of calcium pyrophosphate63 and monosodium urate crystal,64 respectively, and can cause significant pain and discomfort. Investigations into the ability of IL layers to mitigate mineral fouling have shown an 18-fold decrease in CaSO4 deposition on fluorinated IL surfaces compared to untreated stainless-steel33 and a 10-fold decrease in CaSO4 scaling on silicone oil-impregnated surfaces.65 While these studies were focused on addressing scaling problems in industry, this concept may prove useful for medical implants including joint replacements.
Discussion
IL layers have demonstrated promise in a number of medical applications, including those aimed towards anti-thrombosis, anti-biofouling, diagnostics, textiles, and anti-corrosion. Despite this, a great deal of work remains to be done in order to realize the full potential of this technology. Perhaps most importantly, in order for IL layers to become a commercialized technology in the clinic, the substrate and liquid combinations must eventually be validated by regulatory agencies for their intended uses. This may present a challenge for two reasons. First, the thermodynamic requirement for the solid surface and liquid to chemically match may prove difficult depending on the application, such as one in which only the use of a particular solid substrate is acceptable. Second, the liquid layer must be water-immiscible to remain stable against water-based foreign liquids. Such liquids, including the previously mentioned silicone oil and fluorocarbons, while not inherently toxic, may not be appropriate for certain applications due their tendency to form emulsions in the body.66 Furthermore, depending on the liquid, complications may arise in applications where an immiscible liquid can migrate within the body.26 However, given the versatility of fabrication methods and materials available for these layers, as well as the great potential benefits of the application of this technology, efforts to overcome issues such as these or find alternatives are likely to prove successful.
As with any emerging area of technology, the hurdles associated with design requirements, testing, and safety must be overcome through rigorous experimentation and documentation. For example, some safety issues raised concerning clinically used perfluorocarbons were found to be a consequence of the emulsifying agent and potentially emulsion size rather than the perfluorocarbons themselves.21 Nevertheless, safety profiles must be generated for the liquids that can be immobilized, potentially for each application. Additionally, more studies need to be done on the longevity of IL layers, with respect to their stability under flow and ability to survive sterilization processes. Recent testing of perfluorocarbon liquid stability under physiological flow rates in water found that the IL layer remained stable as long as it was not exposed to an air/water interface.19 This type of interface has been suggested to allow for the energetically favorable wrapping of the air bubble with the lubricant. If this air bubble is in motion, the lubricant may be carried away with the bubble, reducing the volume of lubricant remaining on the surface, and therefore the omniphobicity of the surface.19 As discussed previously, one method to maintain surface functionality could be through the incorporation of a vascular system within the material, allowing for continual replenishment of the surface layer through diffusion.42 This would additionally allow for the entire system to be refilled with liquid through a single point. Nevertheless, it will be crucial to obtain further information for more complex fluids and physiological conditions for this work to eventually enable the practical application of IL layers in the medical field.
We expect to see a rise in the investigation and use of IL layers in the medical field in a variety of applications. First and foremost, the ability of this technology to reduce or prevent bacterial adhesion may prove highly useful for reducing infection of grafts and synthetic implants. Second, the ability of these materials to be made optically transparent may be helpful in endoscopy, where their dual slipperiness and transparency can offer physicians and surgeons a heightened advantage in visualization and patient treatment. Finally, designing IL layers to be controllably slippery could aid advancement in fields such as tissue engineering, medical diagnostics, and any areas where the controlled presentation or release of proteins, cells, or biomolecules are desirable. With proper experimentation and development, IL layers have enormous potential to improve current therapies, create new directions in the medical field, and ultimately improve patient care.
Acknowledgements
CH acknowledges funding from a Wyss Technology Development Fellowship. All authors thank Dr. Ida Pavlichenko, Adrian Arias Palomo, and Edythe Johnson for helpful discussions and technical editing.
Author Contributions
IS and CH designed the manuscript. All authors wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths reported in United States vital statistics, 1999-2006. Ann Pharmacother 2012; 46(2): 169–75. [DOI] [PubMed] [Google Scholar]
- 2.Guggenbichler JP, Assadian O, Boeswald M, Kramer A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials - catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip 2011; 6(1): Doc18–Doc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KD, Kim YS, Han DK, Kim YH, Lee EH, Suh H, Choi KS. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998; 19(7–9): 851–9. [DOI] [PubMed] [Google Scholar]
- 4.Ebnesajjad S. Fluoroplastics, volume 1: non-melt processible fluoroplastics. Waltham, MA, USA: William Andrew (Elsevier), 2000, 370 p.
- 5.Mahltig B, Fiedler D, Boettcher H. Antimicrobial Sol-Gel Coatings. J Sol-Gel Sci Technol 2004; 32(1–3): 219–22. [Google Scholar]
- 6.Banerjee I, Pangule RC, Kane RS. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater 2011; 23(6): 690–718. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander RS, Vlamakis H, Kim P, Khan M, Kolter R, Aizenberg J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci U S A 2013; 110(14): 5624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong T-S, Kang SH, Tang SKY, Smythe EJ, Hatton BD, Grinthal A, Aizenberg J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011; 477(7365): 443–7. [DOI] [PubMed] [Google Scholar]
- 9.Kim P, Wong T-S, Alvarenga J, Kreder MJ, Adorno-Martinez WE, Aizenberg J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012; 6(8): 6569–77. [DOI] [PubMed] [Google Scholar]
- 10.Xiao L, Li J, Mieszkin S, Di Fino A, Clare AS, Callow ME, Callow JA, Grunze M, Rosenhahn A, Levkin P. Slippery liquid-infused porous surfaces showing marine antibiofouling properties. ACS Appl Mater Interfaces 2013; 5(20): 10074–80. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AK, Wong T-S, Belisle Ra, Boggs EM, Aizenberg J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc Natl Acad Sci U S A 2012; 109(33): 13182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997; 202: 1–8. [Google Scholar]
- 13.Liu Y, Chen X, Xin JH. Can superhydrophobic surfaces repel hot water? J Mater Chem 2009; 19(31): 5602–5602. [Google Scholar]
- 14.Bocquet L, Lauga E. A smooth future? Nat Mater 2011; 10(5): 334–7. [DOI] [PubMed] [Google Scholar]
- 15.Varanasi KK, Deng T, Smith JD, Hsu M, Bhate N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl Phys Lett 2010; 97(23): 234102–234102. [Google Scholar]
- 16.Deng X, Mammen L, Butt H-J, Vollmer D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012; 335(6064): 67–70. [DOI] [PubMed] [Google Scholar]
- 17.Tuteja A, Choi W, Ma M, Mabry JM, Mazzella SA, Rutledge GC, McKinley GH, Cohen RE. Designing superoleophobic surfaces. Science 2007; 318(5865): 1618–22. [DOI] [PubMed] [Google Scholar]
- 18.Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc Natl Acad Sci U S A 2004; 101(39): 14138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell C, Vu TL, Johnson CP, Hou X, Ahanotu O, Alvarenga J, Leslie DC, Uzun O, Waterhouse A, Kim P, Super M, Aizenberg M, Ingber DE, Aizenberg J. Stability of surface-immobilized lubricant interfaces under flow. Chem Mater 2015; 27(5): 1792–800. [Google Scholar]
- 20.Vogel N, Belisle RA, Hatton B, Wong T-S, Aizenberg J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat Commun 2013; 4: 2167–2167. [DOI] [PubMed] [Google Scholar]
- 21.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs 2010; 34(8): 622–34. [DOI] [PubMed] [Google Scholar]
- 22.Kertes PJ, Wafapoor H, Peyman GA, Calixto N, Thompson H. The management of giant retinal tears using perfluoroperhydrophenanthrene. Ophthalmology 1997; 104(7): 1159–65. [DOI] [PubMed] [Google Scholar]
- 23.Manna U, Lynn DM. Fabrication of liquid-infused surfaces using reactive polymer multilayers: principles for manipulating the behaviors and mobilities of aqueous fluids on slippery liquid interfaces. Adv Mater 2015; 27(19): 3007–12. [DOI] [PubMed] [Google Scholar]
- 24.Manabe K, Kyung K-H, Shiratori S. Biocompatible slippery fluid-infused films composed of chitosan and alginate via layer-by-layer self-assembly and their antithrombogenicity. ACS Appl Mater Interfaces 2015; 7(8): 4763–71. [DOI] [PubMed] [Google Scholar]
- 25.Spandau U, Pavlidis M. 27-Gauge Vitrectomy. Cham, Switzerland: Springer International Publishing, 2015.
- 26.Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg 2006; 118: 77S–84S. [DOI] [PubMed] [Google Scholar]
- 27.Leslie DC, Waterhouse A, Berthet JB, Valentin TM, Watters AL, Jain A, Kim P, Hatton BD, Nedder A, Donovan K, Super EH, Howell C, Johnson CP, Vu TL, Bolgen DE, Rifai S, Hansen AR, Aizenberg M, Super M, Aizenberg J, Ingber DE. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat Biotechnol 2014; 32(11): 1134–40. [DOI] [PubMed] [Google Scholar]
- 28.Yao X, Dunn SS, Kim P, Duffy M, Alvarenga J, Aizenberg J. Fluorogel elastomers with tunable transparency, elasticity, shape-memory, and antifouling properties. Angew Chem Int Ed Engl 2014; 53(17): 4418–22. [DOI] [PubMed] [Google Scholar]
- 29.Tesler AB, Kim P, Kolle S, Howell C, Ahanotu O, Aizenberg J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat Commun 2015; 6: 8649–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunny S, Vogel N, Howell C, Vu TL, Aizenberg J. Lubricant-infused nanoparticulate coatings assembled by layer-by-layer deposition. Adv Funct Mater 2014; 24(42): 6658–67. [Google Scholar]
- 31.Shillingford C, MacCallum N, Wong T-S, Kim P, Aizenberg J. Fabrics coated with lubricated nanostructures display robust omniphobicity. Nanotechnology 2014; 25: 014019–014019. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Kleintschek T, Rieder A, Cheng Y, Baumbach T, Obst U, Schwartz T, Levkin PA. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl Mater Interfaces 2013; 5(14): 6704–11. [DOI] [PubMed] [Google Scholar]
- 33.Charpentier TVJ, Neville A, Baudin S, Smith MJ, Euvrard M, Bell A, Wang C, Barker R. Liquid infused porous surfaces for mineral fouling mitigation. J Colloid Interface Sci 2015; 444: 81–6. [DOI] [PubMed] [Google Scholar]
- 34.Chlupác J, Filová E, Bacáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res Acad Sci Bohemoslov 2009; 58(Suppl 2): S119–39. [DOI] [PubMed] [Google Scholar]
- 35.Bauer JJ, Salky BA, Gelernt IM, Kreel I. Repair of large abdominal wall defects with expanded polytetrafluoroethylene (PTFE). Ann Surg 1987; 206(6): 765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stasney CR, Beaver ME, Rodriguez M. Minifenestration type I thyroplasty using an expanded polytetrafluoroethylene implant. J Voice 2001; 15(1): 151–7. [DOI] [PubMed] [Google Scholar]
- 37.Becker W, Dahlin C, Becker BE, Lekholm U, van Steenberghe D, Higuchi K, Kultje C. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: a prospective multicenter study. Int J Oral Maxillofac Implants 1994; 9(1): 31–40. [PubMed] [Google Scholar]
- 38.Hanke CW. A new ePTFE soft tissue implant for natural-looking augmentation of lips and wrinkles. Dermatol Surg 2002; 28(10): 901–8. [DOI] [PubMed] [Google Scholar]
- 39.Yao X, Hu Y, Grinthal A, Wong T-S, Mahadevan L, Aizenberg J. Adaptive fluid-infused porous films with tunable transparency and wettability. Nat Mater 2013; 12(6): 529–34. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Zhang P, Liu M, Wang S, Jiang L. Organogel-based thin films for self-cleaning on various surfaces. Adv Mater 2013; 25(32): 4477–81. [DOI] [PubMed] [Google Scholar]
- 41.Urata C, Dunderdale GJ, England MW, Hozumi A. Self-lubricating organogels (SLUGs) with exceptional syneresis-induced anti-sticking properties against viscous emulsions and ices. J Mater Chem A 2015; 3: 12626–30. [Google Scholar]
- 42.Howell C, Vu TL, Lin JJ, Kolle S, Juthani N, Watson E, Weaver JC, Alvarenga J, Aizenberg J. Self-replenishing vascularized fouling-release surfaces. ACS Appl Mater Interfaces 2014; 6(15): 13299–307. [DOI] [PubMed] [Google Scholar]
- 43.MacCallum N, Howell C, Kim P, Sun D, Friedlander R, Ranisau J, Ahanotu O, Lin JJ, Vena A, Hatton B, Wong T, Aizenberg J. Liquid-infused silicone as a biofouling-free medical material. ACS Biomater Sci Eng 2015; 1: 43–51. [DOI] [PubMed] [Google Scholar]
- 44.Wei Q, Schlaich C, Prévost S, Schulz A, Böttcher C, Gradzielski M, Qi Z, Haag R, Schalley CA. Supramolecular polymers as surface coatings: rapid fabrication of healable superhydrophobic and slippery surfaces. Adv Mater 2014; 26(43): 7358–64. [DOI] [PubMed] [Google Scholar]
- 45.Cui J, Daniel D, Grinthal A, Lin K, Aizenberg J. Dynamic polymer systems with self-regulated secretion for the control of surface properties and material healing. Nat Mater 2015; 14: 790–5. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Qiu R, Song H, Wang P, Shi Z, Wang Y. Slippery liquid-infused porous surface based on perfluorinated lubricant/iron tetradecanoate: preparation and corrosion protection application. Appl Surf Sci 2015; 328: 491–500. [Google Scholar]
- 47.Tang Z, Wang Y, Podsiadlo P, Kotov NA. Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering. Adv Mater 2006; 18(24): 3203–24. [Google Scholar]
- 48.Huang X, Chrisman JD, Zacharia NS. Omniphobic slippery coatings based on lubricant-infused porous polyelectrolyte multilayers. ACS Macro Lett 2013; 2(9): 826–9. [DOI] [PubMed] [Google Scholar]
- 49.Wilson PW, Lu W, Xu H, Kim P, Kreder MJ, Alvarenga J, Aizenberg J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys Chem Chem Phys 2013; 15(2): 581–5. [DOI] [PubMed] [Google Scholar]
- 50.Segesser LK, Weiss BM, Pasic M, Garcia E, Turina MI. Risk and benefit of low systemic heparinization during open heart operations. Ann Thorac Surg 1994; 58(2): 391–8. [DOI] [PubMed] [Google Scholar]
- 51.Conn G, Kidane AG, Punshon G, Kannan RY, Hamilton G, Seifalian AM. Is there an alternative to systemic anticoagulation, as related to interventional biomedical devices? Expert Rev Med Devices 2006; 3(2): 245–61. [DOI] [PubMed] [Google Scholar]
- 52.Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: still the best option. Semin Dial 2011; 23(5): 510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bannan S, Danby A, Cowan D, Ashraf S, Martin PG. Low heparinization with heparin-bonded bypass circuits: is it a safe strategy? Ann Thorac Surg 1997; 63(3): 663–8. [DOI] [PubMed] [Google Scholar]
- 54.Yuan S, Luan S, Yan S, Shi H, Yin J. Facile fabrication of lubricant-infused wrinkling surface for preventing thrombus formation and infection. ACS Appl Mater Interfaces 2015; 7: 19466–73. [DOI] [PubMed] [Google Scholar]
- 55.Schachter B. Slimy business — the biotechnology of biofilms new insight into the biology of biofilms is spawning novel commercial strategies for treating the. Nat Biotechnol 2003; 21: 361–5. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, Chen H, Zhang L, Ran T, Zhang D. Transparent self-cleaning lubricant-infused surfaces made with large-area breath figure patterns. Appl Surf Sci 2015; 355: 1083–90. [Google Scholar]
- 57.Boreyko JB, Polizos G, Datskos PG, Sarles SA, Collier CP. Air-stable droplet interface bilayers on oil-infused surfaces. Proc Natl Acad Sci 2014; 111(21): 7588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalil KS, Mahmoudi SR, Abu-Dheir N, Varanasi KK. Active surfaces: ferrofluid-impregnated surfaces for active manipulation of droplets. Appl Phys Lett 2014; 105: 18–22. [Google Scholar]
- 59.Chen L, Geissler A, Bonaccurso E, Zhang K. Transparent slippery surfaces made with sustainable porous cellulose lauroyl ester films. ACS Appl Mater Interfaces 2014; 6(9): 6969–76. [DOI] [PubMed] [Google Scholar]
- 60.Glavan AC, Martinez RV, Subramaniam AB, Yoon HJ, Nunes RMD, Lange H, Thuo MM, Whitesides GM. Omniphobic “RF Paper” produced by silanization of paper with fluoroalkyltrichlorosilanes. Adv Funct Mater 2014; 24(1): 60–70. [Google Scholar]
- 61.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 2010; 82(1): 3–10. [DOI] [PubMed] [Google Scholar]
- 62.Damle VG, Tummala A, Chandrashekar S, Kido C, Roopesh A, Sun X, Doudrick K, Chinn J, Lee JR, Burgin TP, Rykaczewski K. “Insensitive” to touch: fabric-supported lubricant-swollen polymeric films for omniphobic personal protective gear. ACS Appl Mater Interfaces 2015; 7(7): 4224–32. [DOI] [PubMed] [Google Scholar]
- 63.Guggenbuhl P, Chalès G. From chondrocalcinosis to rheumatism with calcium pyrophosphate dehydrate. Rev Prat 2015; 65(5): 677, 679–80, 682. [PubMed] [Google Scholar]
- 64.Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology 2005; 44(9): 1090–6. [DOI] [PubMed] [Google Scholar]
- 65.Subramanyam SB, Azimi G, Varanasi KK. Designing lubricant-impregnated textured surfaces to resist scale formation. Adv Mater Interfaces 2014; 1: 1300068–74. [Google Scholar]
- 66.Chan YK, Cheung N, Chan WSC, Wong D. Quantifying silicone oil emulsification in patients: are we only seeing the tip of the iceberg? Graefe’s Arch Clin Exp Ophthalmol 2014; 253(10): 1671–5. [DOI] [PubMed] [Google Scholar]