Abstract
Conventional chemo-immunotherapy fails to cure the majority of mantle cell lymphoma patients and causes substantial toxicity. Resistant mantle cell lymphoma cells commonly overexpress and are dependent on the anti-apoptotic protein, Mcl-1, for survival. In this study, we use potent lipidoid nanoparticles to deliver siRNA to silence Mcl-1 expression. Studies were conducted using two different mantle cell lymphoma cell lines, a normal (JeKo-1) and an aggressive (MAVER-1) line, to assess the ability of lipidoid nanoparticles to be used broadly in the treatment of mantle cell lymphoma. Mcl-1 mRNA silencing and protein knockdown was observed as early as one day after treatment and the lipidoid nanoparticles achieved sustained silencing of Mcl-1 mRNA for at least four days in both JeKo-1 and MAVER-1 cells. Eighty percent silencing was achieved at three days post-transfection in JeKo-1 cells while 50% silencing was achieved in MAVER-1 cells, which are more resistant to transfection. Interestingly, silencing of Mcl-1 induced apoptosis in nearly 30% of both JeKo-1 and MAVER-1 cells three days post-transfection. Additionally, Mcl-1 silencing and the resultant apoptosis in mantle cell lymphoma cells were dose dependent. These data suggest that lipidoid nanoparticles siRNA therapy targeting Mcl-1 has potential as a new treatment modality for mantle cell lymphoma and many other cancers that overexpress Mcl-1. The combination of anti-Mcl-1 lipidoid nanoparticles with other forms of targeted therapy offers hope for reducing or replacing cytotoxic chemotherapy as standard treatment for mantle cell lymphoma.
Keywords: Mcl-1, siRNA, nanomedicine, nanoparticles, mantle cell lymphoma
Introduction
Non-Hodgkin lymphoma (NHL) is the seventh most common cancer in the United States, with nearly 70,000 people diagnosed each year.1 NHL is a particularly heterogeneous cancer, with over 60 known subtypes that differ in genetic landscape, cell surface receptor expression, and aggressiveness. Of the 12 most commonly diagnosed subtypes of NHL, mantle cell lymphoma is the most formidable. Considered incurable, it has an extremely poor prognosis, with a five-year survival rate of 55.8%.1
Mantle cell lymphoma cells typically have several genetic mutations compared to healthy, naïve B-cells. The genetic hallmark of mantle cell lymphoma is a t(11;14)(q13;q32) translocation, which places the cyclin D1 gene downstream of IgH enhancer elements. This errant positioning results in overexpression of cyclin D1, a cell cycle regulator that facilitates the G1/S phase transition. Additionally, mantle cell lymphoma tumors overexpress several other survival promoting genes, including Mcl-1.2–4 Mcl-1 is an anti-apoptotic protein that is part of the Bcl-2 family,5 and its overexpression is associated with many types of cancer, including multiple myeloma, acute myeloid leukemia, breast cancer, and lung cancers.6–8 Although novel treatments have been developed that inhibit Bcl-2,9–11 their use in mantle cell lymphoma has been stymied by high levels of Mcl-1.4,10 Although several Mcl-1 inhibitors have recently achieved encouraging results in vitro and in vivo, no selective and direct Mcl-1 inhibitor has been approved by the Food and Drug Administration to the authors’ knowledge.12,13
This study investigates the therapeutic potential of Mcl-1 gene downregulation using RNA interference (RNAi). It is anticipated that RNAi therapy would be used in conjunction with the standard treatment for mantle cell lymphoma, such as R-HyperCVAD followed by autologous stem cell transplantation for younger patients or R-CHOP for older patients, who generally cannot tolerate the intensity of R-HyperCVAD.14 R-HyperCVAD pairs immunotherapy (rituximab) with chemotherapy (cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with methotrexate, and cytarabine).15 R-CHOP, on the other hand, combines rituximab with cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone.16 Unfortunately, patients that undergo these therapeutic regimens inevitably relapse, and the cumulative cardiotoxicity of chemotherapy, particularly in older patients, prevents its continued aggressive use. The introduction of a mechanistically distinct anti-cancer therapy, such as RNA interference, has the potential to lower the overall doses of chemotherapy needed to achieve remission. Should patients relapse, cardiotoxicity would no longer prevent the use of additional chemotherapy.
Several groups have attempted to knockdown Mcl-1 in mantle cell lymphoma cells with short interfering RNA (siRNA) via electroporation or lipofectamine-mediated delivery.4,17–20 Although the results associated with Mcl-1 knockdown were encouraging, the delivery methods used were unfortunately not viable for use in animals or in the clinic. Through this study, we have, therefore, sought to use a more robust delivery system, lipidoid nanoparticles (LNPs), to deliver siRNA targeting Mcl-1 into mantle cell lymphoma cells for therapeutic effect. These LNPs have been previously shown to be potent and viable for in vivo delivery to numerous cell and organ targets, including hepatocytes and immune cells.21 In this article, we harness their potency for the treatment of difficult-to-transfect human mantle cell lymphoma cells through the silencing of the anti-apoptotic protein, Mcl-1.
Method and materials
Materials
JeKo-1 and MAVER-1 human mantle cell lymphoma cells were obtained from ATCC (Manassas, VA). Tridecyl acrylate was purchased from Pfaltz & Bauer (Waterbury, CT). 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (C14 PEG2000) were ordered from Avanti Polar Lipids (Alabaster, AL). 3,3′-Diamino-N-methyldipropylamine and cholesterol were purchased from Sigma–Aldrich (St. Louis, MO). Roswell Park Memorial Institute (RPMI) Medium 1640, phosphate buffered saline (PBS), fetal bovine serum (FBS), penicillin/streptomycin, Quant-iT™ Ribogreen Reagent, TaqMan Gene Expression Assays, TaqMan Gene Expression Master Mix, and High Capacity cDNA Reverse Transcription Kits were purchased from Life Technologies (Grand Island, NY). Myeloid cell leukemia-1 (Mcl-1) and alpha-tubulin rabbit monoclonal antibodies were purchased from Abcam (Cambridge, MA).
306O13 lipidoid is synthesized via Michael addition chemistry
The lipidoid 306O13 was synthesized as described previously.21 Briefly, tridecyl acrylate and 3,3′-diamino-N-methyldipropylamine at a 3:1 ratio were stirred in a glass scintillation vial at 90℃ for two days. Lipidoids were purified using flash chromatography (Isco Teledyne, Lincoln, NE) and inspected using electrospray ionization mass spectrometry.
Nanoparticle formation and characterization
Nanoparticles were formed as described previously.22 Briefly, a lipid solution containing lipidoid, DSPC, cholesterol, and C14 PEG2000 in a ratio of 50:10:38.5:1.5 in ethanol, respectively, was added to equal volume of siRNA in citrate buffer. This solution was further diluted 1:2 in PBS. The lipid:siRNA ratio was 5:1. Nanoparticles were briefly vortexed after each addition. Nanoparticle size was determined using a Malvern Zetasizer Nano ZSP (Westborough, MA). siRNA entrapment was determined using Quant-iT™Ribogreen RNA Reagent.
Cell culture
Jeko-1 cells were cultured in RPMI 1640 medium supplemented with 20% FBS, 20 U/ml penicillin, and 20 U/ml streptomycin with 5% CO2 and 37℃ in a Heracell 240i CO2 incubator (Thermo Fisher Scientific, Grand Island, NY). MAVER-1 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 20 U/ml penicillin, and 20 U/ml streptomycin.
Cell transfection with siRNA LNPs
In each experiment, cells were seeded at a density of 250,000 cells per ml. For 24-well plates, 400 µl of media/cells were added to each well. For 12-well plates, 800 µl of media/cells were added to each well. The LNP solution was added to the cells such that it made up 10% of the final volume. Treatments were completed in triplicate. Mcl-1 siRNA was purchased from Sigma–Aldrich (St. Louis, MO) (siRNA ID: SASI_Hs01_00162658).
Gene expression
RNA was extracted using an RNeasy Mini (250) kit (Qiagen, Valencia, CA) and QIAshredder columns (Qiagen, Valencia, CA). Reverse transcriptase PCR was completed using High Capacity cDNA Reverse Transcription Kits with RNase Inhibitor following the manufacturer’s protocol. qPCR was performed using an Applied Biosystems (Grand Island, NY) ViiA 7 Real-Time PCR System, Taqman Gene Expression Mastermix, and TaqMan gene expression assays. The TaqMan Gene Expression Assays were Hs01050896_m1 and Hs02758991_g1 for Mcl-1 and GAPDH genes, respectively. All experiments were run in comparative Ct mode with temperatures at 50℃ for two min, 95℃ for 10 min, followed by 40 cycles of 95℃ for 15 s and 60℃ for 1 min. Two technical replicates were completed for each cDNA sample. The mRNA expression of Mcl-1 was normalized with GAPDH mRNA expression. The Mcl-1 mRNA expression is presented as relative to the untreated control group.
Mcl-1 protein expression determined via Western blotting
For protein harvesting, cells were centrifuged for five min at 2000 rpm and washed with ice-cold PBS. PBS was removed and ice-cold lysis buffer was added to each tube. The lysis buffer is composed of 50 nM Tris, 150 mM NaCl, 1% Triton X-100. A mammalian protease inhibitor cocktail (Amresco, Solon, OH) was added to the buffer in a 1:100 cocktail:lysis buffer ratio. Cells were shaken for 30 min on a thermal shaker at 400 rpm. Every 10 min, the cells were vortexed for 10 s at 3000 rpm. Then the cells were centrifuged at 14,000 rpm for 10 min at 4℃. The supernatant was removed and put in a fresh tube. Total protein concentration was quantified using a Pierce Bicinchoninic acid assay kit (Thermo Fisher Scientific, Rockford, IL) and analyzed using a NanoDrop 2000 (Thermo Scientific, Grand Island, NY) by following the manufacturer’s protocol. Equal amounts of protein were added to each well in a mini 4%–15% SDS-PAGE (TGX Mini-Protean) gel (Bio-Rad Laboratories, Hercules, CA). The gel was run in a Mini-PROTEAN 3 tank (Bio-Rad Laboratories, Hercules, CA) in 1× Tris/Glycine/SDS running buffer (Bio-Rad Laboratories, Hercules, CA) for 30 min at 200 V. The gel was then blotted onto a polyvinylidene difluoride (PDVF) membrane using an iBlot system (Life Technologies, Grand Island, NY). Immunodetection of proteins on the gel was performed using a WesternBreeze Chemiluminescent kit (Thermo Fisher Scientific, Grand Island, NY). Chemiluminesence was detected using an ImageQuant LAS 4000 system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), and band densities were measured using ImageJ.
Results
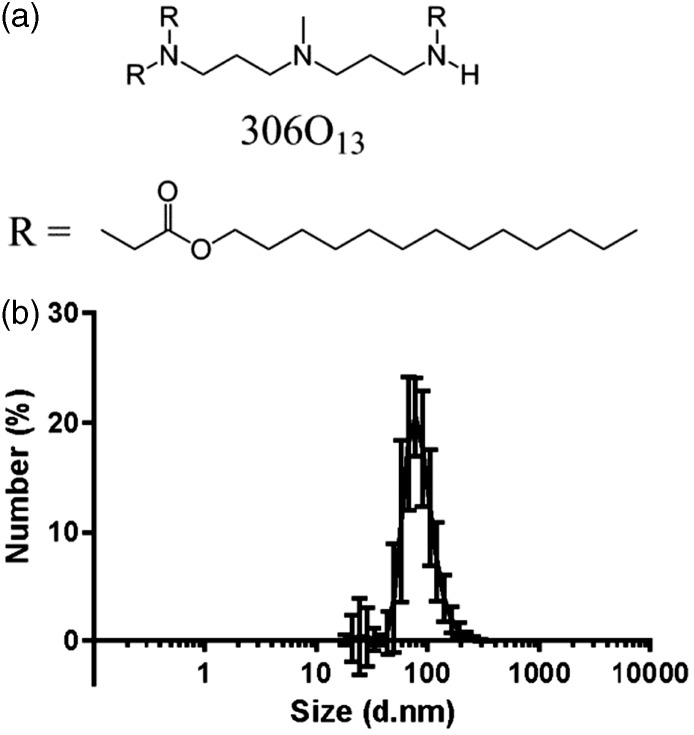
In this study, we investigated the ability of LNPs to silence the anti-apoptotic protein, Mcl-1, for therapeutic effect in the treatment of mantle cell lymphoma. The lipidoid used for these studies, 306O13, is a potent siRNA delivery material with proven efficacy, both in vitro and in vivo, in a variety of cell types, including epithelial cells, hepatocytes, and immune cells (Figure 1(a)).21,22 Prior to siRNA transfection, the lipidoid 306O13 was formulated into nanoparticles along with cholesterol (for stability), DSPC, C14 PEG2000 (for reduced phagocytosis in vivo), and siRNA. Although the use of a polyethylene glycol (PEG) compound was not necessary for in vitro experiments, we included it in the nanoparticle formulation to facilitate translation to eventual in vivo studies. Resultant nanoparticles were relatively monodisperse, with a number average diameter of 90 nm as measured by dynamic light scattering (Figure 1(b)).
Figure 1.
Lipidoid nanoparticle formulation and characterization. (a) The lipidoid 306O13 is the active component of the delivery vehicles used in this study. It is formulated into nanoparticles along with cholesterol, DSPC, C14 PEG2000, and siRNA. (b) Dynamic light scattering indicated that the number average size of 306O13 LNPs was 87.7 ± 12.2 nm with a PDI of 0.116 ± 0.036 (n = 76)
LNPs effectively silence Mcl-1 in human mantle cell lymphoma cells
Mcl-1, which is an anti-apoptotic Bcl-2 family protein, was chosen as a potential therapeutic gene target because higher Mcl-1 levels have been commonly observed in mantle cell lymphoma cells compared to normal mantle zone lymphocytes.2,3 Among other anti-apoptotic properties, Mcl-1 protein sequesters the pro-apoptotic protein BAK away from the mitochondrial membrane, preventing cells from undergoing apoptosis.5,23 Human cancers that overexpress Mcl-1 have been shown to overly depend on Mcl-1 for cell survival compared to control cells.8
Despite its potential as a therapeutic gene target, there are no direct Mcl-1 inhibitors approved for use or currently in clinical trials, to the authors’ knowledge. Therefore, we sought to specifically silence Mcl-1 expression using LNPs. Unfortunately, B cells are notoriously difficult to transfect, with a limited number of studies reporting siRNA delivery efficacy.24–26 The lipidoid 306O13, however, is potent enough to facilitate transfection in mantle cell lymphoma cells.
In a first set of experiments, JeKo-1 cells, a standard mantle cell lymphoma cell line, were transfected with LNPs at an siMcl-1 dose of 100 nM. Three days post-transfection, Mcl-1 mRNA expression was reduced by 80% (Figure 2(a)). Silencing was quite durable, with greater than 60% silencing being maintained over a period of one week. Over the same time period, control LNPs containing control siRNA did not induce gene knockdown, suggesting that the LNP delivery vehicle was not causing cytotoxicity or otherwise affecting Mcl-1 levels. Western blotting confirmed corresponding Mcl-1 protein knockdown 24 hours post-transfection in cells treated with 100 nM siMcl-1 (Figure 2(b)). Again, no Mcl-1 downregulation was observed for control LNPs.
Figure 2.
Lipid nanoparticles induce Mcl-1 silencing in human mantle cell lymphoma cells. Knockdown was achieved at the (a, c) mRNA and (b, d) protein level for two genetically heterogeneous mantle cell lymphoma cell lines: (a, b) JeKo-1 and (c, d) MAVER-1. For Western blotting experiments, siRNA doses were 100 nM and protein was isolated from cells 24 h post-transfection. Alpha-tubulin was used as a loading control. Error bars correspond to s.d. (n = 3)
One challenge associated with genetic therapies for cancer is the heterogeneity of gene expression across different patients with the same diagnosis. To assess whether or not siMcl-1 LNP therapy might be broadly applicable to mantle cell lymphomas with disparate genetic background, we assessed gene silencing in MAVER-1 cells in addition to the aforementioned JeKo-1 cells. The MAVER-1 cell line contains many of the genetic features of aggressive, poor prognosis mantle cell lymphoma and is the only known mantle cell lymphoma line that contains a MYC rearrangement.27 Indeed, we show in this work that it is more difficult to treat. A single LNP dose containing 100 nM siMcl-1 silenced 50% of Mcl-1 mRNA expression relative to untreated cells (Figure 2(c)). Again, Western blotting suggests robust Mcl-1 silencing at the protein level for MAVER-1 cells (Figure 2(d)).
Mcl-1 silencing induces apoptosis in lymphoma cells
In parallel experiments, we analyzed the fraction of cells undergoing apoptosis in response to Mcl-1 gene silencing. Apoptosis was determined using an Annexin V/PI assay, with quantification by flow cytometry. For JeKo-1 cells receiving a 100 nM siMcl-1 LNP treatment, apoptosis rates increased from about 5%–15% the first two days following treatment. Three days post-treatment, however, the percentage of apoptotic cells further increased to nearly 30% (Figure 3(a)). This was in contrast to MAVER-1 cells, which underwent constant increased levels of apoptosis (∼30%) at each time point following treatment (Figure 3(b)). For both JeKo-1 and MAVER-1 cells, cells treated with control LNPs did not experience higher levels of apoptosis than untreated cells (Figure 3). Again, this suggests that the delivery vehicle does not contribute to the cell killing effect.
Figure 3.
Silencing Mcl-1 causes apoptosis in human mantle cell lymphoma cells. (a) JeKo-1 and (b) MAVER-1 cells were transfected with a 100 nM dose of either siMcl-1 or control siRNA. Every 24 h, the fraction of cells undergoing apoptosis was analyzed with an Annexin V/PI apoptosis assay using flow cytometry. Error bars represent s.d. (n = 3). (A color version of this figure is available in the online journal.)
Mcl-1 silencing and the ensuing apoptosis were dose dependent
Dose response experiments in both JeKo-1 and MAVER-1 cell lines were completed to determine what doses of siRNA are needed for the maximum fraction of cells to undergo apoptosis. For JeKo-1 cells, maximal apoptosis was observed at 200 nM doses of siMcl-1 LNPs (Figure 4(a)). The dose response appears to be more evident in the apoptosis measurement compared to the gene expression (Figure 4(b)). For MAVER-1 cells, a 200 nM dose was also responsible for maximally observed apoptosis (Figure 4(c)). Of note, although 100 and 200 nM doses of siMcl-1 LNPs did not result in statistically different levels of mRNA silencing (p = 0.15) (Figure 4(d)), they did cause statistically significant differences in apoptosis levels (p = 0.01).
Figure 4.
Mcl-1 silencing and resultant apoptosis were siRNA dose dependent. Lipid nanoparticles containing siRNA doses between 1 and 200 nM were delivered to (a, b) JeKo-1 and (c, d) MAVER-1 cells. (a, c) The percentage of cells undergoing apoptosis was determined by flow cytometry 72 h post-transfection and compared to (b, d) Mcl-1 gene silencing as determined by qPCR. Error bars represent s.d. (n = 3).*p < 0.05 and **p < 0.01. (A color version of this figure is available in the online journal.)
Discussion
In the past 15 years, NHL outcomes have improved dramatically with the introduction of the R-CHOP chemotherapy regimen.1,28 Despite these advances, mantle cell lymphoma is still considered incurable using currently approved regimens. This stubbornness of mantle cell lymphoma necessitates the development of alternative therapies that are mechanistically distinct from chemotherapy and immunotherapy. Although RNAi therapeutics could meet this need, the delivery of siRNA to B-cells has historically been particularly challenging. To the authors’ knowledge, only three studies have delivered siRNA for successful gene silencing in B-cell NHL without the use of lipofectamine or electroporation.24–26
Here, we use potent LNPs to deliver siRNA to two genetically heterogeneous lines of mantle cell lymphoma and demonstrate that Mcl-1 protein knockdown induces apoptosis. Both JeKo-1 and MAVER-1 mantle cell lymphoma cells were examined to preliminarily assess the broader utility of Mcl-1 directed therapy. That is, siRNA drugs will have the widest impact if they can be used for most patients, regardless of genetic differences. While JeKo-1 cells represent a standard manifestation of mantle cell lymphoma, MAVER-1 cells are considered to be the most challenging from a treatment perspective. MAVER-1 cells are double hit lymphoma cells that contain the MYC/8q24 translocation as well as the classic t(11;14)(q13;q32) translocation.27 This double hit lymphoma is an aggressive variant of mantle cell lymphoma with a very poor prognosis.27,29 JeKo-1 cells do not contain this MYC rearrangement, though they do express MYC.27,30
As anticipated, the Mcl-1 gene silencing that we achieved in MAVER-1 cells was significantly lower than that obtained in JeKo-1 cells (40% vs. 80%, respectively, three days post-transfection, Figure 2). The dose response study conducted in Figure 4 shows even a wider discrepancy: 25% vs. 80% Mcl-1 silencing for a 200 nM dose. This disparity could be a result of either (1) lower transfection rates or (2) higher initial Mcl-1 mRNA expression in MAVER-1 cells compared to JeKo-1 cells. Our qPCR data for both cell lines, however, suggests that Mcl-1 levels are nearly equivalent in each cell line. Therefore, we conclude that MAVER-1 cells are more difficult to transfect. Interestingly, the very different levels of Mcl-1 knockdown in the two cell lines resulted in nearly identical apoptosis rates of 25%–30% three days post-transfection (Figure 4(a) and (c)). Furthermore, MAVER-1 cells saw higher levels of apoptosis within one day of siRNA delivery, compared to the three days required for JeKo-1 cells (Figure 3), while both cell lines experienced robust protein knockdown 24 h of post-treatment (Figure 2(b) and (d)). Together, these data suggest that MAVER-1 cells rely more heavily on Mcl-1 for apoptosis avoidance compared to JeKo-1 cells. To our knowledge, this is the first demonstration of siRNA-mediated gene silencing in MAVER-1 cells, irrespective of the delivery method.
This gene modulatory therapy has the potential to be combined with chemotherapy, immunotherapy, or small molecule drug inhibitors to enhance the treatment of NHLs, including mantle cell lymphoma. Furthermore, to prevent resistance to chemotherapeutics or small molecule drug inhibitors, siRNA and the inhibitors could potentially be co-delivered using LNPs. Nanoparticle delivery of chemotherapeutics, nucleic acids, small molecule drug inhibitors, and other cytotoxic material has been used to improve efficacy, biodistribution, and tolerability compared to free drug.31 Adding targeting ligands to the nanoparticle can potentially improve cancer cell localization and efficacy compared to non-actively targeting nanoparticles.32–35 Several groups have co-delivered nucleic acids and other drugs in nanoparticles leading to an improvement in the treatment.36–38 Although LNPs have not yet been used to co-deliver nucleic acids and small molecule drug inhibitors, LNPs have been shown to effectively deliver a variety of cargo, including protein, DNA, mRNA, and siRNA. As such, we believe LNPs are a promising vehicle option for co-delivery.39–43 Hydrophilic drugs can be stored in the hydrophilic core and hydrophobic drugs within the hydrophobic lipid bilayer of these particles. Alternatively, co-delivery of siRNAs with different targets could potentially negate the need for co-delivery of siRNA and inhibitors or chemotherapeutics.
In this study, we have shown that LNPs are sufficiently potent to silence Mcl-1 at the mRNA and protein levels in two distinct lines of mantle cell lymphoma, leading to an increase in the fraction of the cells undergoing apoptosis. In particular, cells that have developed resistance to chemotherapy, glucocorticoids, or inhibitors often have higher levels of Mcl-1 compared to normal cells.4,10,44–48 By reducing Mcl-1 levels, it is expected that improvements in efficacy and reductions in relapse rates may be realized for mantle cell lymphoma patients.
Acknowledgements
Funding was provided by the Department of Chemical Engineering at Carnegie Mellon University.
Author contributions
CMK, JL, and KAW participated in the design of the studies. CMK and KAW participated in interpretation of the studies and analysis of the data. CMK and JH conducted the experiments. CMK and KAW wrote the manuscript. All authors reviewed the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute, http://seer.cancer.gov/csr/1975_2011/ (2014, accessed 18 April 2014).
- 2.Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol 1995; 146: 1309–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury JD, Medeiros LJ, Rassidakis GZ, McDonnell TJ, Abruzzo LV, Lai R. Expression of Mcl-1 in mantle cell lymphoma is associated with high-grade morphology, a high proliferative state, and p53 overexpression. J Pathol 2003; 199: 90–7. [DOI] [PubMed] [Google Scholar]
- 4.Touzeau C, Dousset C, Bodet L, Gomez-Bougie P, Bonnaud S, Moreau A, Moreau P, Pellat-Deceunynk C, Amiot M, Gouill SL. ABT-737 induces apoptosis in mantle cell lymphoma cells with a Bcl-2high/Mcl-1low profile and synergizes with other antineoplastic agents. Clin Cancer Res 2011; 17: 5973–81. [DOI] [PubMed] [Google Scholar]
- 5.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett 2010; 584: 2981–9. [DOI] [PubMed] [Google Scholar]
- 6.Wuillème-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, Harousseau J-L, Amiot M, Bataille R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005; 19: 1248–52. [DOI] [PubMed] [Google Scholar]
- 7.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, Alexander WS, Lowe SW, Robb L, Strasser A. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 2012; 26: 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho Y-J, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao M-S, DeMichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park C-M, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–8. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary GS, Al-harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, Almasan A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 2015; 6: e1593–e1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Harbi S, Hill BT, Mazumder S, Singh K, DeVecchio J, Choudhary G, Rybicki LA, Kalaycio M, Maciejewski JP, Houghton JA, Almasan A. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood 2011; 118: 3579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJC, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 2015; 6: e1590–e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abulwerdi F, Liao C, Liu M, Azmi AS, Aboukameel A, Mady ASA, Gulappa T, Cierpicki T, Owens S, Zhang T, Sun D, Stuckey JA, Mohammad RM, Nikolovska-Coleska Z. A novel small-molecule inhibitor of Mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther 2014; 13: 565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood 2015; 125: 48–55. [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Romaguera J, Kantarjian H, Palmer JL, Pugh WC, Korbling M, Hagemeister F, Samuels B, Rodriguez A, Giralt S, Younes A, Przepiorka D, Claxton D, Cabanillas F, Champlin R. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol 1998; 16: 3803–9. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B, Thieblemont C, Neste EVD, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Fermé C, Tilly H. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010; 116: 2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogner C, Dechow T, Ringshausen I, Wagner M, Oelsner M, Lutzny G, Licht T, Peschel C, Pastan I, Kreitman RJ, Decker T. Immunotoxin BL22 induces apoptosis in mantle cell lymphoma (MCL) cells dependent on Bcl-2 expression. Br J Haematol 2010; 148: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller A, Zang C, Chumduri C, Dörken B, Daniel PT, Scholz CW. Concurrent inhibition of PI3K and mTORC1/mTORC2 overcomes resistance to rapamycin induced apoptosis by down-regulation of Mcl-1 in mantle cell lymphoma. Int J Cancer 2013; 133: 1813–24. [DOI] [PubMed] [Google Scholar]
- 19.Hussain S-RA, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, Lucas DM, Byrd JC. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res 2007; 13: 2144–50. [DOI] [PubMed] [Google Scholar]
- 20.Shirsath NP, Manohar SM, Joshi KS. P276-00, a cyclin-dependent kinase inhibitor, modulates cell cycle and induces apoptosis in vitro and in vivo in mantle cell lymphoma cell lines. Mol Cancer 2012; 11: 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt V, Chen D, Barros S, Klebanov B, Novobrantseva T, Langer R, Anderson DG. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun 2014; 5: 4277–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball RL, Knapp CM, Whitehead KA. Lipidoid nanoparticles for siRNA delivery to the intestinal epithelium: in vitro investigations in a Caco-2 model. PLoS ONE 2015; 10: e0133154–e0133154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci CMLS 2009; 66: 1326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palanca-Wessels MC, Convertine AJ, Cutler-Strom R, Booth GC, Lee F, Berguig GY, Stayton PS, Press OW. Anti-CD22 antibody targeting of pH-responsive micelles enhances small interfering RNA delivery and gene silencing in lymphoma cells. Mol Ther 2011; 19: 1529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis SM, Taylor CA, Tang T, Liu Z, Zheng Q, Dondero R, Thompson JE. SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide. Mol Ther 2014; 22: 1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Tiemann K, Chomchan P, Alluin J, Swiderski P, Burnett J, Zhang X, Forman S, Chen R, Rossi J. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res 2013; 41: 4266–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamo A, Ott G, Katzenberger T, Adam P, Parolini C, Scarpa A, Lestani M, Menestrina F, Chilosi M. Establishment of the MAVER-1 cell line, a model for leukemic and aggressive mantle cell lymphoma. Haematologica 2006; 91: 40–7. [PubMed] [Google Scholar]
- 28.Sehn LH. A decade of R-CHOP. Blood 2010; 116: 2000–1. [DOI] [PubMed] [Google Scholar]
- 29.Aukema SM, Siebert R, Schuuring E, Imhoff GW van, Kluin-Nelemans HC, Boerma E-J, Kluin PM. Double-hit B-cell lymphomas. Blood 2011; 117: 2319–31. [DOI] [PubMed] [Google Scholar]
- 30.Jeon HJ, Kim CW, Yoshino T, Akagi T. Establishment and characterization of a mantle cell lymphoma cell line. Br J Haematol 1998; 102: 1323–6. [DOI] [PubMed] [Google Scholar]
- 31.Knapp CM, Whitehead KA. In pursuit of a moving target: nanotherapeutics for the treatment of non-Hodgkin B-cell lymphoma. Expert Opin Drug Deliv 2014; 11: 1923–37. [DOI] [PubMed] [Google Scholar]
- 32.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Contr Release 2012; 161: 175–87. [DOI] [PubMed] [Google Scholar]
- 33.Loomis K, Smith B, Feng Y, Garg H, Yavlovich A, Campbell-Massa R, Dimitrov DS, Blumenthal R, Xiao X, Puri A. Specific targeting to B Cells by lipid-based nanoparticles conjugated with a novel CD22-ScFv. Exp Mol Pathol 2010; 88: 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuscano JM, Martin SM, Ma Y, Zamboni W, O’Donnell RT. Efficacy, biodistribution, and pharmacokinetics of CD22-targeted pegylated liposomal doxorubicin in a B-cell non-hodgkin’s lymphoma xenograft mouse model. Clin Cancer Res 2010; 16: 2760–8. [DOI] [PubMed] [Google Scholar]
- 35.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010; 115: 4778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Co-delivery of Doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 2009; 5: 2673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Yuan Y, Cheng D, Chen J, Wang L, Shuai X. Co-delivery of doxorubicin and siRNA with reduction and pH dually sensitive nanocarrier for synergistic cancer therapy. Small Weinh Bergstr Ger 2014; 10: 2678–87. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013; 34: 2547–64. [DOI] [PubMed] [Google Scholar]
- 39.Sun S, Wang M, Knupp SA, Soto-Feliciano Y, Hu X, Kaplan DL, Langer R, Anderson DG, Xu Q. Combinatorial library of lipidoids for in vitro DNA delivery. Bioconjug Chem 2012; 23: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun S, Wang M, Alberti KA, Choy A, Xu Q. DOPE facilitates quaternized lipidoids (QLDs) for in vitro DNA delivery. Nanomed Nanotechnol Biol Med 2013; 9: 849–54. [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Sun S, Alberti KA, Xu Q. A combinatorial library of unsaturated lipidoids for efficient intracellular gene delivery. ACS Synth Biol 2012; 1: 403–7. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Alberti K, Sun S, Arellano CL, Xu Q. Combinatorially designed lipid-like nanoparticles for intracellular delivery of cytotoxic protein for cancer therapy. Angew Chem Int Ed 2014; 53: 2893–8. [DOI] [PubMed] [Google Scholar]
- 43.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett 2013; 13: 1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michels J, Obrist F, Vitale I, Lissa D, Garcia P, Behnam-Motlagh P, Kohno K, Wu GS, Brenner C, Castedo M, Kroemer G. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem Pharmacol 2014; 92: 55–61. [DOI] [PubMed] [Google Scholar]
- 45.Song L, Coppola D, Livingston S, Cress WD, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther 2005; 4: 267–76. [DOI] [PubMed] [Google Scholar]
- 46.Van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DCS. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006; 10: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham L, Zhang L, Tao W, Zhao D, Zhang H, Xie J, Wang J, Nomie K, Wang M. Developing novel therapeutic strategies to overcome ibrutinib resistance in mantle cell lymphoma. Orlando, FL, https://ash.confex.com/ash/2015/webprogram/Paper85213.html (2015, accessed 9 March 2016).
- 48.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006; 10: 331–42. [DOI] [PubMed] [Google Scholar]