Abstract
Tumors are three-dimensional tissues where close contacts between cancer cells, intercellular interactions between cancer and stromal cells, adhesion of cancer cells to the extracellular matrix, and signaling of soluble factors modulate functions of cancer cells and their response to therapeutics. Three-dimensional cultures of cancer cells overcome limitations of traditionally used monolayer cultures and recreate essential characteristics of tumors such as spatial gradients of oxygen, growth factors, and metabolites and presence of necrotic, hypoxic, quiescent, and proliferative cells. As such, three-dimensional tumor models provide a valuable tool for cancer research and oncology drug discovery. Here, we describe different tumor models and primarily focus on a model known as tumor spheroid. We summarize different technologies of spheroid formation, and discuss the use of spheroids to address the influence of stromal fibroblasts and immune cells on cancer cells in tumor microenvironment, study cancer stem cells, and facilitate compound screening in the drug discovery process. We review major techniques for quantification of cellular responses to drugs and discuss challenges ahead to enable broad utility of tumor spheroids in research laboratories, integrate spheroid models into drug development and discovery pipeline, and use primary tumor cells for drug screening studies to realize personalized cancer treatment.
Keywords: 3D tumor models, cancer cell spheroids, co-culture spheroids, tumor microenvironment, cancer stem cells, anti-cancer drug discovery
Introduction
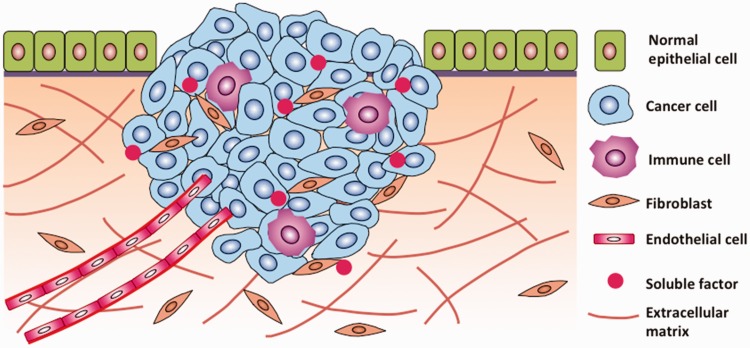
Solid tumors develop and grow in three-dimensional (3D) microenvironments comprising of cancer cells, stromal cells, extracellular matrix proteins, and soluble factors (Figure 1). In poorly vascularized and avascular tumors, transport of oxygen, nutrients, metabolites, and growth factors to cancer cells and removal of waste materials is limited to and determined by molecular diffusion. When tumors exceed a few hundreds of micrometers in diameter, cancer cells residing in peripheral layers of the tumor consume most of available nutrients, resulting in preferential proliferation of border cells. Insufficient oxygen and nutrients in the tumor core render cancer cells hypoxic and non-proliferating, and potentially necrotic. Additionally, cancer cells in a tumor form homotypic contacts and heterotypic adhesions with stromal cells such as fibroblasts.1 Close intercellular contacts modulate a broad range of functions of cancer cells including growth and proliferation,2 motility,3 and response to cytotoxic drugs.4 Antibody-mediated disruption of cell–cell contacts sensitizes cells to chemotherapeutics.5,6 Altogether, differential exposure of cells in a solid tumor to soluble factors and intimate cell–cell contacts have major regulatory roles on gene and protein expression of cancer cells that cannot be captured with traditionally used, simplistic monolayer cultures.7 In vitro sphere-like cancer models more faithfully represent various aspects of solid tumors.8–10 In addition to a compact morphology resulting from close intercellular contacts, 3D cancer models reproduce certain properties of tumors including exposure of cells to gradients of soluble factors, preferential growth of border cells, hypoxia and necrosis in the core, deposition of matrix proteins, expression of pro-angiogenic proteins, and upregulation of ABC transporter efflux pumps implicated in multidrug resistance (MDR) of cancers.11–19 As such, they provide a unique tool to study biology of cancers and develop novel anti-cancer therapeutics.
Figure 1.
Tumor is a complex heterocellular microenvironment where cancer cells are in constant communications with the stromal cells, the extracellular matrix, and biochemical signaling molecules. Tumor stroma plays a major role in regulating functions of cancer cells and their responses to therapeutic compounds. (Adapted from Joyce JA and Pollard JW, Nat Rev Cancer 2009). (A color version of this figure is available in the online journal.)
Tumor models
There are generally four different forms of 3D cancer models:20 (a) Cancer cell spheroids, also known as tumor spheroids or multicellular spheroids, are generated from suspension of single cells of immortalized cell lines in the presence of serum.21 Spheroids with a multitude of cancer cells including breast, skin, ovarian, prostate, brain, lung, and liver have been successfully formed. Spheroids are the primary topic of this review and will be discussed in detail below. (b) Tumorspheres are produced from suspension of single cells under serum-free conditions.22–24 A subset of cancer cells capable of surviving a serum-free culture is first sorted out from a population of cancer cells and subsequently used for tumorsphere formation. These cells are capable of proliferation and clonal expansion without serum supplements and thus suggested to possess stem cell-like properties. However, recent literature indicates that tumorsphere culture-mediated enrichment in stem cell markers is cell line dependent and resulting cells may not possess stemness.25 Tumorspheres have been formed with a variety of cancer cells including skin, colon, breast, and prostate. (c) Tissue-derived tumorspheres are generated from tumor tissue by fine slicing of the tissue and partially dissociating it to primarily contain tumor cells.26,27 Importantly, it has been shown that tissue-derived tumorspheres represent the parent tumor in terms of histological features, gene expression profiles and mutations, and tumorigenicity.26–29 Tissue-derived tumorspheres have been successfully generated from colon, lung, bladder, breast, prostate, and melanoma. (d) Organotypic spheroids are obtained by cutting a tumor tissue fragment in sub-millimeter pieces and maintaining them in a non-adherent vessel in the presence of serum and other supplements.30,31 Incubation results in round cellular structures that can be further cultured or frozen. The lack of dissociation contrasts this approach with the tissue-derived tumorsphere culture technique. Organotypic spheroids of different cancers such as lung, head and neck, bladder, colorectal, and glioblastoma have been successfully generated.20
Major benefits of the former two approaches are the ease of initiation and maintenance of cultures with various cell lines from the same cancer, compatibility with high-throughput drug screening against cancer cells, the ability to study a number of processes such as growth, migration, invasion, and drug resistance of cancer cells under defined conditions, and the possibility of creating a more complex solid tumor model by including other components of tumor microenvironment such as cancer-associated fibroblasts (CAFs), immune cells, and extracellular matrix proteins in culture. These approaches facilitate various mechanistic studies of cancers prior to validation with animal models. The latter two approaches provide a better representation of tumors in vivo; nevertheless, initiation and expansion of tissue-derived tumorspheres and organotypic spheroids are challenging, preventing their use in certain applications such as compound screening for anti-cancer drug discovery. Developing standard techniques and protocols to maintain and expand these cultures will enable the use of patient-derived cells in cancer research and help develop personalized cancer therapies.32 Conditionally reprogrammed cells (CRCs) provide a potential solution.33 Primary tumor epithelial cells maintained in the presence of a Rho-associated kinase inhibitor and on irradiated feeder fibroblast cells proliferate indefinitely in vitro, without a need for transduction of exogenous viral or cellular genes.34 This approach was successfully used to expand tumor cells of a patient with recurrent respiratory papillomatosis and bilateral tumor invasion of the lung parenchyma, perform chemosensitivity tests, and identify a therapeutic agent that stabilized the tumor.35
Liquid-based cancer cell spheroid models of avascular tumors
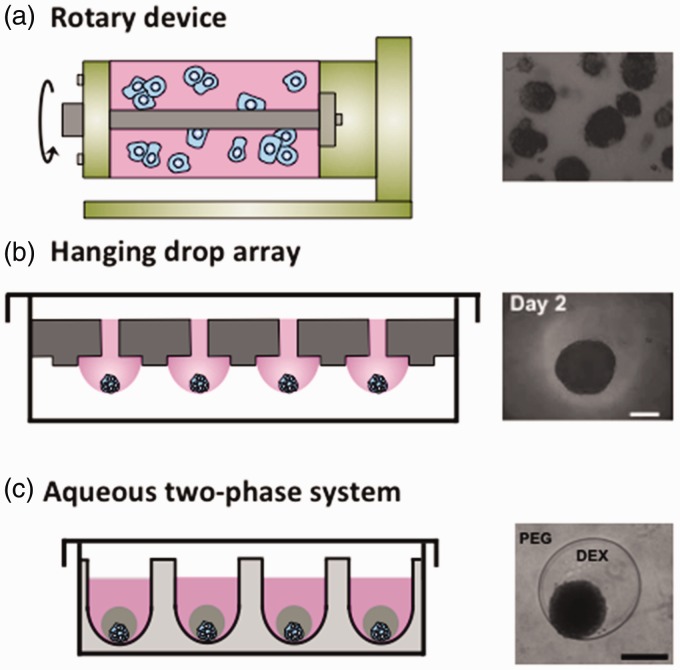
Over the past few decades, several methods were developed to culture cancer cell spheroids. These approaches can broadly be represented by liquid-based suspension cultures and scaffold-based cultures. Liquid-based cultures maintain cancer cells in suspension to facilitate self-assembly of cells into a compact 3D aggregate commonly known as a cancer cell spheroid, tumor spheroid, or multicellular spheroid. There are currently several major liquid-based spheroid culture methods: (a) Rotary vessel/spinner flask devices continuously spin cells in a liquid-filled chamber and rely on random collisions between cells to assemble into a large number of varying size spheroids (Figure 2a).36–38 (b) Hanging drop technique relies on gravity-mediated aggregation of cells close to the apex region of a pendant drop suspended from a flat surface, such as the lid of a Petri dish, to form a spheroid.39 A recent extension of this approach uses a special plate containing an array of through-holes surrounded by micro-rings for high-throughput spheroid generation (Figure 2b).40 Cell suspension is injected through the holes to form hanging drops bounded and stabilized by the micro-ring structures. The hanging drop method can additionally be used to culture embryonic stem cells into embryoid bodies.41 (c) Liquid overlay method involves maintaining suspension of cancer cells on a non-adherent surface to allow aggregation of cells into varying size spheroids. Adapting this method to multi-well plates with a round or v-shaped bottom provides better control over the size of resulting spheroids.42 Ultra-low attachment plates with non-adherent surfaces have been utilized to culture patient-derived ovarian cancer cells as spheres.43 (d) Polymeric aqueous two-phase system (ATPS) technology relies on confining cancer cells in a sub-microliter aqueous drop immersed within a second immersion aqueous phase (Figure 3c).44 The drop and immersion phases remain immiscible due to the presence of small amounts of two biopolymers such as polyethylene glycol and dextran.45–47 Cancer cells remain partitioned within the drop phase and aggregate to form a spheroid of well-defined size that is nourished through free diffusion of nutrients from the immersion phase.48 This approach enables spheroid formation in standard microwell plates with robotic tools and allows convenient high-throughput drug testing.49 (e) Multilayer polymeric microfluidic devices and microfabricated microwell arrays primarily fabricated using soft lithography allow flowing a suspension of cells through a channel or over a free surface containing an array of chambers or wells.50–53 Under sufficiently slow flow rates, cells follow laminar flow streamlines, enter the chambers, and become trapped. Subsequently, under static conditions and due to the non-adherent surface of devices, cancer cells aggregate and form a spheroid in each chamber. Microfabricated devices also provide opportunities for tissue modeling as organs-on-chips.54 Advantages and shortcomings of these major techniques of spheroid culture are summarized in Table 1. We note that several vascularized tumor models have been developed, such as a prevascularized tumor (PVT) model that promotes capillary formation by inserting spheroids of endothelial and tumor cells into a fibrin matrix containing fibroblasts.55 However, this review discusses models of avascular tumors, which can develop and grow up to ∼1 mm, and acquire necrotic and hypoxic zones, leading to signaling events that initiates vascularization.56
Figure 2.
Three major liquid-based methods of culture of cancer cell spheroids are shown (a) Rotary wall vessel and spinner flask systems use rotary devices to constantly keep cells in suspension to aggregate into spheroids of random size. (b) Hanging drop array method uses gravitational-mediated aggregation of cells at the apex region of drops hanging from a plate containing through-holes and micro-rings to result in one spheroid per drop. (c) Aqueous two-phase system (ATPS) method uses two immiscible polymeric aqueous phases, where cancer cells are confined in the drop phase surrounded by the immiscible immersion phase. Cells spontaneously aggregate to form a compact spheroid of well-defined size. Panel a is reproduced from Molecular & Cancer Therapeutics 2007;6:2505–14. (A color version of this figure is available in the online journal.)
Figure 3.
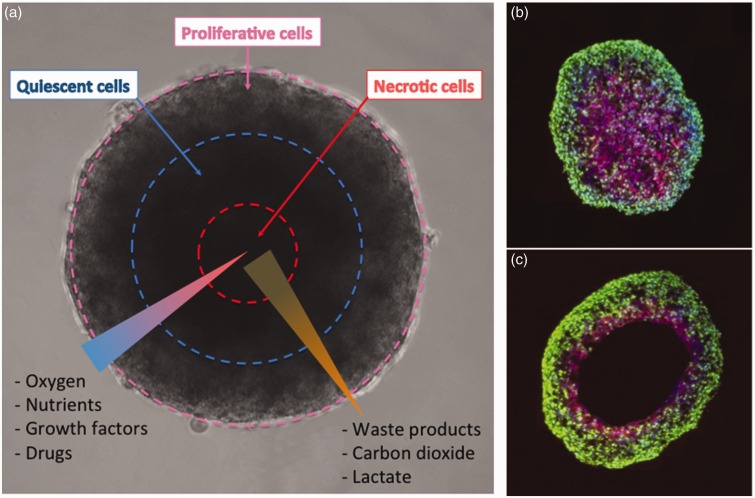
(a) Concentration gradients of oxygen, nutrients, and metabolites generate distinct concentric zones in spheroids: An outer zone containing proliferative cells, a middle zone with quiescent cells, and an inner zone containing necrotic cells. Abundance of oxygen and glucose at the outer zone and efficient removal of waste products facilitate cell proliferation, whereas low oxygen levels and a buildup of toxic metabolites such as carbon dioxide and lactate generate a necrotic core. (b,c) Development of hypoxia and anoxia is shown in spheroids. Sections taken from spheroids grown for (b) four days and (c) six days stained for the proliferation marker Ki-67 (green) and hypoxia (purple). On day 4 of growth, central hypoxia is observed and on day 6 of culture, an anoxic core develops. Panels b and c are reproduced from Journal of The Royal Society Interface, 20146;11: 20131124. (A color version of this figure is available in the online journal.)
Table 1.
Major liquid-based spheroid culture approaches
| Spheroid forming technique | Advantages | Disadvantages |
|---|---|---|
| Rotary vessel/spinner flask | - Large number of spheroids - Convenient culture and harvesting | - Non-uniformly sized spheroids - Incompatible with high-throughput screening - Incompatible with defined heterotypic culture conditions |
| Hanging drop array | - Uniformly sized spheroids - One spheroid per drop - High throughput - Compatible with robotics - Compatible with heterotypic cultures | - Difficult to handle cultures - Need for custom plates - Incompatible with plate readers - Lack of temporal control |
| Liquid overlay microplate Aqueous two-phase system (ATPS) Microfabricated microwell array | - Convenient culture and harvesting - High throughput - Compatible with robotics - Compatible with plate readers - Uniformly sized spheroids - One spheroid per well - High throughput - Compatible with robotics - Compatible with heterotypic cultures - Compatible with plate readers - Temporal control - Uniformly sized spheroids - One spheroid per well - High throughput | - Variations in size of spheroids - May give more than one spheroid per well - Need to optimize consistency of liquid handling dispensing of sub-microliter volumes - Need for microfabrication - Incompatible with robotics - Incompatible with plate readers - Exposes all spheroids to a single treatment condition - Lack of temporal control |
Using the spheroid formation techniques described above, it has been demonstrated that spheroids reproduce key aspects of avascular tumors (Figure 3). For example, cancer cell spheroids displayed tumor diffusion limitations.16,57 Prostate tumor cell spheroids were found to have more proliferative cells at the boundary of spheroids when compared to the spheroids’ core, which also contained necrotic cells.8 Spheroids formed with a hepatocellular carcinoma cell line revealed hypoxia in their interior and a gradient of oxygen concentration from the periphery to the center.58,59 This additionally correlated with proliferative cells favorably located at the periphery of the spheroids as well as limited diffusion of doxorubicin when compared to monolayer cultures.58 Breast cancer cell spheroids expressed low nutrition-responsive genes when compared to monolayer cultures and contained dormant inner core regions, causing cisplatin and paclitaxel resistance.60 The proliferation and oxygen gradients present in tumors and core of spheroids promote an acidic environment which can alter cellular functions.61 Lower pH levels in spheroids of cervical cancer cells were predicted to be hypoxia-induced.61 The pH distribution found in cervical cancer spheroids was similar to those found in mouse tumors.61
The ability to model diffusion limitations of avascular tumors using spheroids allows studies to improve delivery of chemotherapeutics. For example, pulsed ultrasounds increased the diffusion and concentration of nanoparticles into breast cancer spheroids 6–20 folds compared to control spheroids not exposed to ultrasound, providing a potential method for resolving limited penetration of chemotherapies and drug-loaded nanoparticles into tumors.62 Additionally, spheroids enable investigating efficacy of drugs that become active under hypoxic conditions. TH-302, which is a hypoxia-selective drug, showed 650-fold greater activity in hypoxic H460 lung cancer cell spheroids than in monolayer cells with IC50 values of 0.1 and 65 µmol/L, respectively.63 Furthermore, hypoxic spheroids can be utilized to investigate hypoxia regulation of cancer stem cells (CSCs), which are suggested as key drivers of tumor metastasis.64,65 A recent study using cancer cell spheroids showed that CSCs prefer a hypoxic environment and that the hypoxic environment correlates with tumor metastasis.66
Spheroids mimicking the complexity of tumor microenvironment
Cancer is traditionally considered a disease of a group of cells that turn malignant due to accumulated mutations. It is now recognized that this definition is too simplistic. In essence, a tumor is an organ, composed of multiple cell types, soluble factors, and a structural framework that are interdependent and intimately connected (Figure 1).67–69 Physical and biochemical interactions between cancer cells and stromal components including fibroblast cells, immune cells, vascular cells, and extracellular matrix proteins regulate phenotypes of cancer cells such as growth, proliferation, migration, and drug response.70–73 CAFs and immune cells constitute two major components of tumor stroma supporting tumor progression.74 Existing studies primarily use co-culture models of cancer with fibroblast and/or immune cells in the presence of defined chemical factors. Following is a brief summary of some of these models.
Regulation of cancer cells by stromal components
CAFs are the most prominent cell type in most cancers;75,76 they originate primarily from resident normal fibroblasts through signaling of cancer cell-derived factors such as transforming growth factor-β (TGF-β) as well as from bone-marrow-derived progenitor cells and transdifferentiated epithelial cells.75–81 CAFs support diverse functions of cancer cells.82 Paracrine signaling of CAFs-derived factors including hepatocyte growth factor (HGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and cytokines stromal-derived factor-1α (SDF-1α) and interleukin-6 (IL-6) promotes tumor initiation and proliferation,83–85 induces epithelial-mesenchymal transition (EMT),86,87 and fosters the ability of transformed epithelial cells to invade into the ECM,88 inhibits chemotherapy-induced apoptosis of cancer cells,89–95 and causes poor prognosis.96–98 It is also postulated that CAFs cohort exerts mechanical pressure on tumor cells and promotes cancer cell invasiveness.99 Genetic alterations, i.e. activation of oncogenes or inactivation of tumor-suppressor genes, in cancer cells drive activities of transcription factors (mainly NF-κB, STAT3, and HIF-1α) that result in production of inflammatory mediators (e.g. chemokines, cytokines, and prostaglandins) and subsequently, recruitment of inflammatory cells to tumor microenvironment. Presence of inflammatory cells in tumor tissue correlates with carcinogenesis, tumor invasion and metastasis, and poor patient prognosis.100–104 Considering the dramatic role of stroma in cancer, it is critical to understand how stromal components, individually and collectively, modulate functions of cancer cells, and develop novel therapeutics for targeting tumor stroma. Spheroid culture technologies provide a unique tool to recreate various aspects of tumor microenvironment. A major advantage of spheroid technology over animal models is the flexibility of modulating cellular constituents and complexity of the model, and dissecting the influence of specific stromal components on cancer cells.
Co-culture spheroid models of fibroblasts and cancer cells
In a co-culture spheroid model, breast cancer cells induced myofibroblast differentiation of tumor-derived fibroblasts, but not normal fibroblasts, resembling advanced desmoplastic carcinomas.105 Compared to spheroids of breast cancer cells, the growth of co-culture spheroids significantly slowed down during long-term culture, suggesting a negative correlation between myofibroblast differentiation and growth of cancer cells. This observation could not be captured using a two-chamber co-culture system, indicating a role for direct intercellular contacts. Epithelial cancer cells can also recruit fibroblasts to serve their growth, survival, and malignancy. Using a co-culture spheroid model of PC-3 prostate cancer cells and normal fibroblasts,36 it was shown that PC-3 cells induced expression of tenascin in fibroblasts to stimulate growth of cancer cells and serve as an immunosuppressive molecule.106,107 In a co-culture system of human mammary tumor epithelial cells and fibroblasts, outgrowth and increased matrix invasion of cancer cells was attributed to the lack of expression of a Rac exchange factor, Tiam1, in tumor fibroblasts,108 suggesting a major role for stromal factors in modulating activities of cancer cells. Similarly, NIH/3T3 fibroblasts enhanced proliferation and matrix invasiveness of 4T1 triple negative breast cancer cells.109 However, including macrophages in this model system disrupted these phenotypes.
Fibroblasts have also been implicated in neoplastic development of ovarian epithelial cells. Senescent fibroblasts, resembling old ovarian stroma, in co-culture spheroids increased ovarian epithelial cell proliferation 5-folds and induced nuclear atypia.110 These observations could not be made with presenescent fibroblasts. Considering that ovarian cancers are often diagnosed postmenopausally, this finding suggests that age-related changes in ovarian stroma may be responsible for development of epithelial ovarian cancers possibly due to loss of a carcinogenesis inhibitory factor in old fibroblasts.110 Analysis of human ovarian tumors showed a large number of senescent fibroblasts within the stroma, supporting this finding. A co-culture spheroid model of pancreatic cancer and fibroblast cells resulted in a significant increase in cancer cell survival than monolayer cultures in seven out of nine cell lines tested.111 It was also shown that co-culture spheroids of pancreatic cancer and fibroblast cells display greater resistance to an EGFR inhibitor (Erbitux) compared to mono-culture cancer cell spheroids.111 Similarly, co-culture spheroids of lung cancer cells and fibroblasts showed resistance to Erbitux treatment by upregulating fibroblast-derived HGFR expression and activation compared to cancer cell spheroids.111 These data agree with clinical treatment of lung cancer patients that only show a positive response when treated with both HGF and EGFR inhibitors. When non-small cell lung carcinoma cells were co-cultured with fibroblasts in spheroids, fibroblasts promoted spheroids growth and prevented cell death from paclitaxel treatment through paracrine signaling.112 In a spheroid model of urothelial cancer cells and fibroblasts, cancer cells preferentially oriented around the fibroblast cells when forming spheroids, resembling the morphology of urothelial tumors.113
Co-culture spheroid models of immune and cancer cells
Presence of macrophage cells in tumors is often an indicator of poorer patient prognosis.104 Macrophages increased spheroid formation capability of breast cancer cells and resulted in a significant increase in the number of spheroids compared to mono-culture breast cancer spheroids.109 It was shown with co-culture spheroids that macrophages may suppress tumor proliferation for certain cancers such as colorectal, stomach, and skin cancers.104 For example, co-culture spheroids of human colorectal cancer and macrophages resulted in reduced expression of proliferation-related genes and elevated expression of apoptosis-associated genes, indicating that inflammatory pathway activation results in anti-tumor immune responses.104 Cancer cell spheroids and suspension of immune cells were utilized to study immune cell migration into spheroids and their activation.114 Heterotypic spheroids of cancer and immune cells provide a unique platform to replicate the immunomodulatory effects of potential therapeutic compounds that activate and strengthen immune cell responses. When colon carcinoma cell spheroids were co-cultured with macrophages, treatment with a heteroglycan led to macrophage-mediated toxicity and lysis of proliferative cancer cells residing in the peripheral zone of spheroids.115 Spheroids of head and neck squamous carcinoma cells in the presence of peripheral blood mononuclear cells (PBMCs) in suspension were treated with a tri-functional antibody, Catumaxomab.116 This resulted in a significant decrease in spheroid volume, followed by disintegration of spheroids to single cells, PBMCs-mediated lysis, and antibody-induced toxicity of cancer cells.
Tri-culture spheroid models containing cancer cells, macrophages, and fibroblasts can replicate the tumor microenvironment more closely. The co-existence of the three cell types augmented the frequency of breast cancer spheroid formation, indicating the influence of the intercellular interactions on stroma formation and maintenance.109 A tri-culture of HT-29 colon carcinoma with monocytes and fibroblasts in a spheroid model resulted in higher expression of a proteolytic enzyme (cathepsin B) and subsequently 5-fold increase in invasiveness of the cancer cells compared to mono-culture HT-29 spheroids.72 These findings exemplify that spheroids recreate the physiological complexity of tumors and enable better understanding of tumor biology. Existing evidence based on heterotypic spheroid models strongly indicates a major role for fibroblasts and immune cells in modulating broad functions of cancer cells in the primary tumor including proliferation, survival and drug response, and matrix invasion and demonstrates the usefulness of these systems to investigate co-evolution of tumors and their microenvironment due to bidirectional communications between tumor cells and tumor-associated stroma.117–120
Spheroid cultures to study CSCs
CSCs are a subset of tumor cells that exhibit stem cell-like properties. There are two main theories about origin of CSCs. Adult stem cells serve as target for carcinogenesis and suffer oncogenic mutations to generate CSCs . The mutations accumulate and amplify over generations of differentiated progenies to yield tumor cells.121 Alternatively, CSCs originate from differentiated somatic cells or the mortal progenies that undergo de-differentiation and acquire stemness as well as malignancy over generations through genetic and epigenetic mutations.122 Similar to normal stem cells, CSCs are defined by specific functional traits: (1) CSCs can undergo infinite number of symmetric divisions, i.e. they can self-renew indefinitely to produce more daughter cells.123 (2) CSCs can undergo asymmetric divisions to produce non-CSCs or heterogeneous progenies that comprise the mass of tumors.123 (3) CSCs can switch between quiescent and active states. Since conventional chemotherapeutics mainly target proliferative cells, dormant CSCs may escape therapy and become activated post-therapy to proliferate and differentiate, causing cancer relapse.124–126
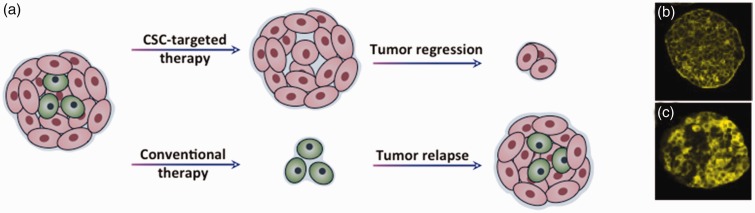
Genomic and proteomic analysis of primary patient-derived tumor tissues shows the presence of cells that display stem cell markers such as ALDH1 activity, CD44, CD133, CD166, and that expression of these markers correlates with tumorigenicity and inversely correlates with patient survival.22,127–129 Serial transplantation of CSC-positive acute myeloid leukemia (AML) patient-derived xenograft cells into immunodeficient mice developed tumors resembling primary patient tumors.130,131 PDX models transduced with a reporter gene to track cells showed that CD44+ breast CSCs metastasize to lungs and lymph nodes, suggesting a metastatic role for CSCs apart from primary tumor growth.132 A clonal analysis of squamous skin tumors using genetic lineage tracing showed presence of a small fraction of cells that persists long term and gives rise to a terminally differentiated progenies that occupy the tumor mass.133 Therefore, available evidence strongly suggests a major role for CSCs in driving tumor initiation, progression, and recurrence (Figure 4).
Figure 4.
(a) Tumors contain a subpopulation of cells, cancer stem cells (CSCs), with the ability to initiate and regenerate tumors. Conventional chemotherapy targets rapidly dividing cells and considerably diminishes the tumor. However, unaffected CSCs repopulate the tumor growth and result in recurrence of cancer, which is often resistant to therapies. Novel therapies that target CSCs are critical to eliminate tumorigenic cells and potentially eradicate the cancer. (b,c) Expression of CSCs markers CD44 and ALDH1 in HT29 colorectal tumor spheroids detected using immunostaining of sections of spheroids. Panels b and c are reproduced with permission from BMC Cancer, 2010;10:106. (A color version of this figure is available in the online journal.)
3D cancer cell cultures have been employed to study CSCs. Patient-derived colon cancer cells expressing an embryonic protein Cripto-1 (CR-1) exhibited increased clonogenic capacity and expression of stem cell-related markers CD133 and cytokeratin-20.134 While CD-133 was constantly expressed over time in spheroids, CR-1 expression showed fluctuations with the peak corresponding to an increased clonogenic capacity of the whole spheroid population and generation of large colonies. CR-1 positive cells showed increased levels of Nanog expression and nuclear β-catenin, suggesting CSCs enrichment. CR-1 silencing inhibited spheroid proliferation and growth of tumor xenografts, and decreased CSCs growth in primary and metastatic tumors. Tissue analysis of 28 breast cancer patients showed presence of poorly differentiated cells with ∼3-fold higher sphere forming efficiency and formation of spheroids almost twice as large compared to well-differentiated cancer cells, in agreement with 3- to 4-fold increase in CSCs markers from IHC analysis of tumor tissues.135 Primary ovarian cancer cells maintained as spheroids generated tumors in immunodeficient mice that fully recapitulated the original tumor.136 Compared to parent cells, spheroid cells were enriched for CSC markers, were more aggressive in growth, migration, invasion, and clonogenic survival, and displayed resistance to cisplatin. Pancreatic CSCs often show aberrant activity of PI3K/mTOR and Sonic Hedgehog (Shh) pathways. It was shown that a combination therapy of pancreatic spheroids using molecular inhibitors of these pathways suppressed the expression of pluripotency markers Nanog, Oct-4, Sox-2, and c-Myc and transcription factors mediating EMT, elevated the expression of apoptotic genes, and dose-dependently reduced colony forming capacity of cells.137 Spheroids generated from brain tumors showed tumor-initiating populations positive for CSCs markers and displayed cell growth patterns similar to xenograft studies.138 An invasion study using spheroids of a primary glioma tumor and a glioblastoma cell line showed that primary tumor spheroids were more invasive and contained cells expressing CSCs markers such as CD133 and Sox2, whereas cell line spheroids were more proliferative.139,140
This brief summary highlights that spheroid cultures of cancer cells enrich CSCs and reproduce important phenotypes of tumors in vivo. Therefore, spheroids provide a unique tool to understand the biology of CSCs and identify chemical compounds to effectively eliminate them. For example, a study reported screening ∼16,000 compounds including several diverse commercial libraries and collections of natural extracts against E-cadherin knockdown human breast cancer cells that displayed various CSCs activities such as high capacity for spheroid formation and seeding tumors in mice.141 This screening led to a single drug, Salinomycin, that effectively abolished CSCs phenotypes and showed selective toxicity against CSCs. Salinomycin treatment induced a 10-fold decrease in the number of tumorspheres relative to controls, decreased the proportion of CD44high/ CD24low cells by 4-fold compared to vehicle-treated controls, and downregulated or blocked the expression of CSCs-marker genes. In contrast, paclitaxel did not change sphere formation capacity of cells, increased CD44high/ CD24low cells by 4-fold, and did not cause major changes in CSCs-marker genes. This suggested that while Salinomycin inhibits CSCs, paclitaxel treatment enriches the sphere-forming population. Importantly, Salinomycin did not affect cell proliferation in monolayer, indicating its selectivity to CSCs in 3D culture.
Spheroid cultures for compound screening and drug discovery
Drug discovery and development is a costly and complex process (Figure 5). Developing a new drug approved by the US Food and Drug Administration (FDA) often takes about 10–15 years and costs about $2b over the development period.142–144 At the preclinical stage, a collection of thousands of compounds in the drug research phase undergoes target identification and lead optimization. This yields a library of several hundred compounds for subsequent tests including biodistribution, pharmacokinetics, and toxicity using laboratory assays and animal models. Based on the results of these tests, a few compounds are selected for clinical trials to conduct tests for safety, efficacy, human pharmacokinetics, availability, and dose adjustments. About one compound usually receives regulatory approval by the FDA at the end of the process.145 Unfortunately, this tremendously inefficient process makes oncology drug discovery very costly. For example, during 1997–2011, 12 major pharmaceutical companies spent a combined $802,468 million on drug research and development. Pfizer, Inc. led the pack spending a total of $108,178 million over this period. While there were almost 900 anti-cancer drugs in clinical trials or under FDA review in 2011, only 12 were actually approved that year. A recent report shows that global oncology market hit $100b in 2015.146 Despite considerably increased investments, the rate of introduction of novel drugs has remained relatively constant over the past 40 years and only two to three agents in new drug classes per year eventually make it to the market.145,147
Figure 5.
Workflow of anti-cancer drug discovery. (A color version of this figure is available in the online journal.)
Cell cultures introduced as a tool for compound screening in the 1950s have remained an integral part of drug discovery process in the pre-clinical stage.148 Traditionally, cancer cells are grown as a monolayer (2D) in standard microwell plates and treated with hundreds of candidate drugs for initial characterization of efficacy and toxicity of compounds. This high-throughput screening leads to compounds for subsequent tests with animal models and use in clinical trials. Ease of maintenance of 2D cultures in microwell plates, and the compatibility of these culture platforms with robotic liquid handlers for automated addition of drug compounds and biochemical analysis reagents have made 2D cell cultures indispensable to oncology drug discovery. However, the use of 2D cancer cell cultures is believed to be a major contributor to this inefficiency. Despite their simplicity and compatibility with high-throughput screening instruments, 2D cell assays often fail to predict the efficacy of compounds in vivo. Disparity between 2D cultures and the complex 3D environment of cancer cells in tumors is the major shortcoming of monolayer culture systems.149–152
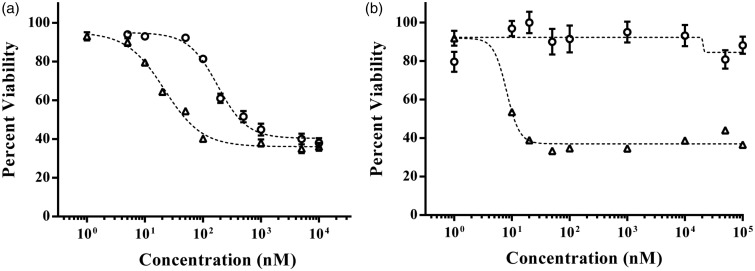
Spheroid cancer cell cultures have gained increasing attention in cancer research and drug discovery in order to bridge the existing gap between simplistic monolayer cultures and complex, expensive animal models. Cells cultured in 3D closely reproduce morphology, gradients of oxygen and nutrients, cell proliferation patterns, and gene expression profiles of tumors.14,153–157 Comprehensive gene expression analyses of various cancer cell lines show significant differences in expression profiles of hundreds of genes in the same cells under 2D and 3D cultures, including regulators of proliferation, invasiveness, apoptosis, hypoxia, and markers of CSCs.153–155,158–160 Compared to monolayer cultures, cancer cells in 3D spheroid cultures generally show greater resistance to cytotoxic drugs such as taxol, cisplatin, 5-fluorouracil, and doxorubicin (Figure 6(a)). 44,161–163 Interestingly, it was recently shown that spheroids of MDA-MB-157 triple negative breast cancer cells display complete resistance to paclitaxel but produce a dose-dependent response with an LD50 of ∼8.0 nM in 2D culture (Figure 6(b)).164 Major factors that contribute to drug resistance in 3D are insufficient drug penetration and distribution within the tumor cell mass,165,166 slowly cycling cells in the border zone and non-proliferative cells in the central zone,60 close cell–cell contacts,5 and production of extracellular matrix proteins and expression of drug transporter proteins.15,19,167,168 While it is often observed that a 3D environment causes resistance to most drugs, cells in spheroid cultures show greater toxicity towards certain compounds such as the hypoxia-activated drug TH-30263, tirapazamine169, and a HER-2 targeting agent trastuzumab152 compared to monolayer cultures. Available molecular and phenotypic studies of cancer cell lines and primary tumor cells strongly support the use of 3D cultures in drug screening applications to enhance the process of anti-cancer drug discovery and development by reducing attrition rates and costs.170
Figure 6.
High throughput dose-dependent testing of paclitaxel against monolayer and spheroids of (a) A431.H9 skin cancer cells and (b) MDA-MB-157 triple negative breast cancer cells generated using the aqueous two-phase technology. (a) 2D cultures of A431.H9 cells (triangles) show a sigmoidal response with an LD50 value of 22.1 nM, whereas cells in spheroid culture (circles) show greater resistance and result in an LD50 value of 178.5 nM. (b) Monolayer of MDA-MB-157 cells (triangles) also shows a sigmoidal response with an LD50 value of 8.0 nM. In contrast, these cells in spheroid culture (circles) show complete resistance to paclitaxel. Error bars indicate standard error of the mean. Dashed lines represent sigmoidal fit generated using GraphPad Prism
Considering the important role of stroma on drug response of cancer cells, more complex spheroid models can be developed by incorporating stromal cells such as fibroblasts and macrophages to facilitate intercellular communications present in tumor tissues. This will enable both delineating the influence of stromal cells on functions of cancer cells and allow identifying drug compounds that target stromal cells to abrogate intratumoral intercellular interactions. Heterotypic spheroids provide a useful tool for such applications. A co-culture spheroid model of liver cancer and fibroblast cells showed greater drug resistance than mono-culture spheroids of cancer cells.171 A tri-culture of tumorigenic and normal breast epithelial cells with normal human endothelial cells was utilized to identify drug compounds that selectively eliminate cancer cells. Among a panel of drugs with different mechanisms, microtubule-targeting agents and EGFR inhibitors showed selective toxicity against cancer cells only.172 Such studies identifying differential drug responses of cancer and normal cells will help reduce deleterious side effects frequently observed with cytotoxic agents. The amount of stroma in colorectal cancer173,174 and the tumor-stroma ratio in breast cancers175 are considered a prognostic factor. Cancers with higher stromal content correlate with poor prognosis and increased risk of relapse. The ability to recapitulate such events using spheroid cultures may lead to drugs that reduce or abolish stromal effects on drug response of cancer cells.
Quantification of drug responses of spheroids
A critical step in high-throughput compound screening is quantification of cellular responses to chemical compounds. The most commonly used techniques to determine toxicity of compounds are optical-based and include colorimetric, luminescence, and fluorescence assays.176
Colorimetric assays
Colorimetric assays rely on the metabolic activity of cells to biochemically convert a dye reagent to a specific color that can be measured at a defined wavelength. The level of signal correlates with the number of live cells. Several assays such as MTT, MTS, and XTT use tetrazolium salts to produce a colored insoluble formazan dye that can be detected with a spectrophotometer or a plate reader.177 These assays are routinely used in research laboratories; however, they involve multiple steps of reagent addition and wash, are labor-intensive and time consuming, and thus are incompatible with high-throughput screening applications to evaluate toxicity of libraries of compounds. Alternative colorimetric assays are AlamarBlue and PrestoBlue. The former contains an active reagent, resazurin, which is reduced by live cells to resorufin detectable at fluorescence and absorbance modes and representative of the number of live cells. Obtaining sufficient sensitivity with monolayer cultures requires several hours of incubation. The PrestoBlue assay uses a similar principle but can resolve viability of 2D cell cultures in about 10 min. Both these assays involve only a single addition step and are compatible with high-throughput applications. The colorimetric assays have been developed, optimized, and tested for monolayer cell cultures. Adapting them to 3D cultures requires assay optimization. In a recent study, the possibility of using PrestoBlue with 3D cultures was studied by incubating spheroids of MDA-MB-157 breast cancer cells and A431.H9 skin cancer cells, made with different densities of 1 × 103 – 100 × 103 cells, with the assay reagent. Statistical analysis of the fluorescent signal measured over time showed that 3–4 h incubation is necessary to confidently measure differences in the signal intensity between spheroids of different cell densities as well as between different time points for spheroids of the same cell density.44,164 The substantially longer incubation time of spheroids with the PrestoBlue reagent compared to monolayer cultures is likely due to reduced diffusion of the assay dye into spheroids.178
Luminescence assays
The principle behind luminescence assays is production and emission of light by live cells due to a chemical reaction that converts chemical energy to light.179 Oxidation of luciferin catalyzed by the enzyme luciferase and mediated by cellular ATP produces light that can be detected by instruments such as a luminometer. The luminescence signal level correlates with the number of live cells. CellTiter-Glo is a luminescent cell viability assay that requires a one-step reagent addition to cells and mixing to result in cell lysis and generation of luminescent signal. The utility of the assay for the detection of cell viability following drug treatment of spheroids of glioblastoma, breast cancer, and oral squamous carcinoma was demonstrated.42 After drug treatment of spheroids, the assay reagent was added for 10 min, contents of wells were mixed and then transferred into opaque, flat-bottom plates for luminescent measurements. This assay was also used to determine the viability and drug resistance of spheroids of ovarian cancer cells treated with cisplatin136 and to study drugs with cytotoxic and cytostatic effects on various colon cancer cell spheroids.178 Although the short incubation with the assay reagent is an advantage, the need for transfer of contents of wells from the original plate to an opaque plate makes this approach less attractive for high-throughput applications.
Fluorescence assays
Fluorescence assays offer an alternative approach to determine drug response of cancer cells in 3D cultures. The most common type is based on the use of fluorescent dyes specific to live and dead cells. For example, Calcein AM and Ethidium homodimer-1 (EthD-1) are two fluorescent probes that stain cells with intracellular esterase activity (live) and compromised plasma membrane (dead). Other fluorescent dyes include DiOC18, SYBR 14, and SYTOX Green for live cells and propidium iodide and C12-resazurin for dead cells. These assays only require a one-step addition of the dyes and typically no wash. Stained cells can be imaged with a fluorescent microscope, counted with a flow cytometer, or detected using a microplate reader. Fluorescent probes have been used in various studies to quantify effects of drugs on spheroids. 60,169,171,180–185 The major challenge associated with using fluorescent probes is slow penetration of dyes into spheroids, especially when spheroids are compact, requiring longer incubation than 2D cultures (4 h vs. 20 min).186 And despite a long incubation of spheroids with dyes, cells in the core of compact spheroids are usually not stained well.164 In addition, quantification of cell viability of spheroids using these assays requires a sensitive instrument to collect the fluorescent signal from a 3D mass of cells. A useful approach to circumvent such problems is using cells with endogenous expression of a reporter fluorescent protein such as gfp or dsRed. Prior to spheroid assay, cells are transduced to constitutively express a fluorescent protein. Live cells can then be quantified by measuring the fluorescent signal intensity of spheroids.42,187
Cellular responses in heterocellular spheroid models
Using heterocellular spheroid models, e.g. co-culture of cancer cells and fibroblasts, poses a major challenge when the response of defined cell types to therapeutics must be determined. Detection methods such as colorimetric assays or fluorescent probes result in a global measure of cell viability in a spheroid and cannot resolve the viability of each cell type. Using specific reporter proteins in each cell type provides a viable solution to this problem. Alternatively, individual cell types can be induced to express a different luciferase such as firefly luciferase, green click beetle luciferase, or red click beetle luciferase.188,189 These luciferases are ATP-dependent enzymes that sensitively quantify relative numbers of viable cells using bioluminescence imaging. This approach was recently used in a co-culture spheroid of breast cancer cells and bone marrow stromal cells to model metastatic bone marrow where quiescent disseminated tumor cells persist for many years before proliferating as recurrent metastases.190 Using dual-color bioluminescence imaging to quantify viability of cancer cells and stromal cells in the same spheroid, single and combination treatments that preferentially eliminated quiescent breast cancer cells but not stromal cells were identified. A treatment combination of doxorubicin and trametinib effective against malignant breast cells in spheroids also eliminated breast cancer cells from bone marrow in a mouse xenograft model, indicating the reliability of cellular response to therapy in the spheroid model. In contrast to colorimetric and luminescence assays, this approach allowed live, non-invasive monitoring of cells.
Challenges and outlook
The need for physiologic cell-based models has led to the development of various 3D cell culture systems to help understand tumor biology and develop more effective anti-cancer drugs by predicting drug efficacy prior to expensive animal tests and clinical trials. This review highlighted available platforms of liquid-based culture of spheroids and their applications in research and drug discovery. It is now well documented that spheroids provide a reliable model that recapitulates key properties of solid tumors at molecular and cellular levels, not reproducible with traditionally used monolayer cultures. Nevertheless, there are still major challenges to be addressed for spheroid cultures to be widely adapted and used both in research laboratories and pharmaceutical industries.
A critically important application of 3D culture models is for oncology drug development and discovery. Despite the use of spheroid cultures in research laboratories for more than four decades and their inherent power to predict drug efficacy, their routine use in the mainstream drug development process is hindered by complex and expensive methodological requirements for the formation, maintenance, and drug treatment of spheroids. Recognizing this problem has prompted design of new platforms that simplify culture and maintenance of spheroids. Commercially available standard round- and V-bottom microwell plates, a new design of hanging drop array plate in the format of microwell plates, and the ATPS-mediated approach in standard microwell plates are compatible with robotic liquid handling operations and automated imaging and detection systems such as plate readers and can potentially streamline drug screening against spheroids. The ATPS technology eliminates shortcomings of the other techniques such as possibility of formation of more than one spheroid in round- and V-bottom plates, and drug treatment, media exchange, and need for transfer of spheroids from the custom hanging drop array plate to a standard plate for downstream analysis of drug response of cells using standard plate readers.
Analysis of drug-treated spheroids primarily relies on colorimetric, luminescence, and fluorescence assays that have been originally developed for monolayer cell cultures. As such, adapting them to spheroid cultures requires assay optimization such as those discussed above for PrestoBlue and CellTiter Glo. It is crucial to ensure that spheroids are incubated with analysis reagents sufficiently long to allow adequate reagent diffusion to cells residing in the core region of spheroids. Additionally, simplifying these assays to a single step of addition of analysis reagents and detection of cellular responses using standard instruments will streamline their use in high-throughput screening applications, minimize labor, and reduce inconsistencies associated with multiple addition and wash steps involved in certain biochemical assays. Standard microplate-based detection methods are preferable over fluorescence-based imaging for spheroid viability quantification. Limited diffusion of some cell staining molecules into spheroids, difficulty with quantitative determination of cellular viability from imaging of a fluorescently labeled, compact, multilayer mass of cells, and time consuming nature of imaging make this approach not desirable for high-throughput screening applications.
Cancer cell spheroids provide a major advantage over monolayer cultures to mimic morphology of solid tumors, gradients of oxygen and soluble factors, and non-uniform proliferation of cells from core to border; however, they do not fully represent the tumor in its native microenvironment due to the lack of stroma. Considering dramatic effects of stroma on cancer cells and that high stromal content in tumors is a poor prognostic factor and correlates with relapse, it is critical to incorporate stromal components in spheroid cultures and delineate how they regulate functions of cancer cells. Major components that co-exist with cancer cells are fibroblasts, immune cells, endothelial cells, and extracellular matrix proteins. One strategy would be to separately include each component to elucidate its modulation of cancer cells and subsequently increase the complexity by adding other factors. Addition of stromal cells can be achieved by co-culturing cancer cells and tissue-specific stromal cells using the ATPS spheroid approach or the hanging drop method. The proportion of cancer and stromal cells can be varied to generate tumor models at different stages of the disease. Embedding of spheroids in an extracellular matrix is more challenging. This is an inherent limitation of the hanging drop method but conveniently achievable with the ATPS approach, which is modular in the sense that it allows both simultaneous and sequential addition of stromal components. Complex spheroid models better represent a tumor in its entirety but also introduce new challenges. For example, analyzing specific phenotypes, such as proliferation and drug response of cancer cells in spheroids containing stromal cells is not feasible with colorimetric assays as they provide a measure of activity of all cells. The use of fluorescent or luminescent reporter proteins can circumvent this problem and allow distinguishing cancer cells from stromal cells in real-time. This strategy can also help with sorting of cancer cells dissociated from complex spheroids for subsequent molecular analyses such as gene and protein expression.
Majority of studies in cancer research and drug discovery use lines of cancer cells due to the ease of maintaining and expanding them as a 2D culture with minimal costs. However, cell lines may not accurately reflect the initial disease. Availability of patient-derived cells from tumor tissue biopsies will increase the relevance of data; however, the use of patient-derived cells in spheroid cultures, especially for drug screening applications, requires maintaining and expanding them to generate sufficient number of cancer cells. This has proved difficult and currently, primary cancer cells are expanded in xenograft models. Strategies to enable cultures of primary cells, such as the approach of conditionally reprogramming cells (CRC), will be extremely useful and help realize personalized cancer therapy.
Acknowledgements
This work is supported by grants from the NIH (R21CA182333) and Ohio Third Frontier (TECG20140954).
Authors’ contribution
SLH, RJ, PST, and HT have jointly planned and written the manuscript. HT approved the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fujita H, Ohuchida K, Mizumoto K, Egami T, Miyoshi K, Moriyama T, Cui L, Yu J, Zhao M, Manabe T, Tanaka M. Tumor stromal interactions with direct cell contacts enhance proliferation of human pancreatic carcinoma cells. Cancer Sci 2009; 100: 2309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta G, Hsiao AY, Takayama S, Luker GD, Ngram M. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 2012; 164: 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choe C, Shin Y-S, Kim S-H, Jeon M-J, Choi S-J, Lee J, Kim J. Tumor–stromal interactions with direct cell contacts enhance motility of non-small cell lung cancer cells through the hedgehog signaling pathway. Anticancer Res 2013; 33: 3715–23. [PubMed] [Google Scholar]
- 4.Nakamura T, Kato Y, Fuji H, Horiuchi T, Chiba Y, Tanaka K. E-cadherin-dependent intercellular adhesion enhances chemoresistance. Int J Mol Med 2003; 12: 693–700. [PubMed] [Google Scholar]
- 5.Green SK, Francia G, Isidoro C, Kerbel RS. Antiadhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer Ther 2004; 3: 149–59. [PubMed] [Google Scholar]
- 6.Green SK, Karlsson MCI, Ravetch JV, Kerbel RS. Disruption of cell-cell adhesion enhances antibody-dependent cellular cytotoxicity: Implications for antibody-based therapeutics of cancer. Cancer Res 2002; 62: 6891–900. [PubMed] [Google Scholar]
- 7.Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal 2011; 5: 239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi A, Watanabe M, Ishii Y, Morita J, Hirokawa Y, Matsuzaki T, Shiraishi T. Three-dimensional cellular spheroid formation provides human prostate tumor cells with tissue-like features. Anticancer Res 2007; 27: 45–54. [PubMed] [Google Scholar]
- 9.Szade K, Zukowska M, Szade A, Collet G, Kloska D, Kieda C, Jozkowicz A, Dulak J. Spheroid-plug model as a tool to study tumor development, angiogenesis, and heterogeneity in vivo. Tumour Biol. Epub ahead of print. 2015. DOI: 10.1007/s13277-015-4065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zietarska M, Maugard C, Filali-Mouhim A, Alam-Fahmy M, Tonin P, Provencher D, Mes-Masson A. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol Carcinog 2007; 46: 872–85. [DOI] [PubMed] [Google Scholar]
- 11.Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol 1997; 273: C1109–23. [DOI] [PubMed] [Google Scholar]
- 12.Ballangrud A, Yang W, Dnistrian A, Lampen N, Sqouros G. Growth and characterization of LNCaP prostate cancer cell spheroids. Clin Cancer Res 1999; 5: 3171s–3176s. [PubMed] [Google Scholar]
- 13.Dolznig H, Rupp C, Puri C, Haslinger C, Schweifer N, Wieser E, Kerjaschki D, Garin-Chesa P. Modeling colon adenocarcinomas in vitro. Am J Pathol 2011; 179: 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Mhawech-Fauceglia P, Lee N, Parsanian LC, Lin YG, Gayther SA, Lawrenson K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab Invest 2013; 93: 528–42. [DOI] [PubMed] [Google Scholar]
- 15.Oshikata A, Matsushita T, Ueoka R. Enhancement of drug efflux activity via MDR1 protein by spheroid culture of human hepatic cancer cells. J Biosci Bioeng 2011; 111: 590–3. [DOI] [PubMed] [Google Scholar]
- 16.Ma HL, Jiang Q, Han S, Wu Y, Tomshine JC, Wang D, Gan Y, Zou G, Liang XJ. Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Mol Imaging 2012; 11: 487–98. [PubMed] [Google Scholar]
- 17.Correa de Sampaio P, Auslaender D, Krubasik D, Failla AV, Skepper JN, Murphy G, English WR. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS One 2012; 7: e30753.–e30753.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loessner D, Little JP, Pettet GJ, Hutmacher DW. A multiscale road map of cancer spheroids – incorporating experimental and mathematical modelling to understand cancer progression. J Cell Sci 2013; 126: 2761–71. [DOI] [PubMed] [Google Scholar]
- 19.Koshkin V, Ailles LE, Liu G, Krylov SN. Metabolic suppression of a drug resistant subpopulation in cancer spheroid cells. J Cell Biochem 2016; 117: 59–65. [DOI] [PubMed] [Google Scholar]
- 20.Weiswald L-B, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia 2015; 17: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland R, Inch W, McCredie J, Kruuv J. A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med 1970; 18: 491–5. [DOI] [PubMed] [Google Scholar]
- 22.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445: 111–5. [DOI] [PubMed] [Google Scholar]
- 23.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506–11. [DOI] [PubMed] [Google Scholar]
- 24.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem / progenitor cells. Genes Dev 2003; 17: 1253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvet CY, André FM, Mir LM. The culture of cancer cell lines as tumorspheres does not systematically result in cancer stem cell enrichment. PLoS One 2014; 9: e89644.–e89644.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiswald L-B, Richon S, Validire P, Briffod M, Lai-Kuen R, Cordelières FP, Bertrand F, Dargere D, Massonnet G, Marangoni E, Gayet B, Pocard M, Bieche I, Poupon M-F, Bellet D, Dangles-Marie V. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br J Cancer 2009; 101: 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, Ohue M, Inoue M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A 2011; 108: 6235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiswald LB, Richon S, Massonnet G, Guinebretiere JM, Vacher S, Laurendeau I, Cottu P, Marangoni E, Nemati F, Validire P, Bellet D, Bieche I, Dangles-Marie V. A short-term colorectal cancer sphere culture as a relevant tool for human cancer biology investigation. Br J Cancer 2013; 108: 1720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales J, Alpaugh ML. Gain in cellular organization of inflammatory breast cancer: a 3D in vitro model that mimics the in vivo metastasis. BMC Cancer 2009; 9: 462.–462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonn J, Ott M, Meixensberger J, Paulus W, Roosen K. Progesterone receptors are detectable in tumor fragment spheroids of meningiomas in vitro. Anticancer Res 1994; 14: 2453–6. [PubMed] [Google Scholar]
- 31.Rajcevic U, Knol JC, Piersma S, Bougnaud S, Fack F, Sundlisaeter E, Søndenaa K, Myklebust R, Pham TV, Niclou SP, Jiménez CR. Colorectal cancer derived organotypic spheroids maintain essential tissue characteristics but adapt their metabolism in culture. Proteome Sci 2014; 12: 39.–39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S, Rameshwar P. Tumorsphere passage for breast cancer stem cells. Protoc Exch. Epub ahaed of print. 2013.DOI:10.1038/protex.2013.023. [Google Scholar]
- 33.Chapman S, Liu X, Meyers C, Schlegel R, Mcbride AA. Technical advance Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest 2010; 120: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol 2012; 180: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan H, Myers S, Wang J, Zhou D, Woo JA, Kallakury B, Ju A, Bazylewicz M, Carter YM, Albanese C, Grant N, Shad A, Dritschilo A, Liu X, Schlegel R. Use of reprogrammed cells to identify therapy for respiratory papillomatosis. N Engl J Med 2012; 367: 1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram M, Techy GB, Saroufeem R, Yazan O, Narayan KS, Goodwin TJ, Spaulding GF. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. Vitr Cell Dev Biol Anim 1997; 33: 459–66. [DOI] [PubMed] [Google Scholar]
- 37.Zhau HE, Goodwin TJ, Chang SM, Baker TL, Chung LW. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: evaluation of androgen-induced growth and PSA expression. In Vitro Cell Dev Biol Anim 1997; 33: 375–80. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Yamamoto M, Yamaguchi T, Sugiyama S. Development of multicellular spheroids of HeLa cells cocultured with fibroblasts and their response to X-irradiation. Cancer Res 1984; 44: 345–51. [PubMed] [Google Scholar]
- 39.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 2003; 83: 173–80. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao AY, Tung Y, Kuo C, Mosadegh B, Bedenis R, Pienta KJ, Takayama S. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed Microdevices 2012; 14: 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 2001; 497: 15–19. [DOI] [PubMed] [Google Scholar]
- 42.Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 2012; 10: 29.–29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Balch C, Chan MW, Lai H, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 2009; 68: 4311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atefi E, Lemmo S, Fyffe D, Luker GD, Tavana H. High throughput, polymeric aqueous two-phase printing of tumor spheroids. Adv Funct Mater 2014; 24: 6509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavana H, Jovic A, Mosadegh B, Lee Q, Liu X, Luker K, Luker G, Weiss S, Takayama S. Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells. Nat Mater 2009; 8: 736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavana H, Mosadegh B, Takayama S. Polymeric aqueous biphasic systems for non-contact cell printing on cells: engineering heterocellular embryonic stem cell niches. Adv Mater 2010; 22: 2628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavana H, Kaylan K, Bersano-Begey T, Luker KE, Luker GD, Takayama S. Polymeric aqueous biphasic system rehydration facilitates high throughput cell exclusion patterning for cell migration studies. Adv Funct Mater 2011; 21: 2920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atefi E, Joshi R, Mann JA, Tavana H. Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Appl Mater Interfaces 2015; 7: 21305–14. [DOI] [PubMed] [Google Scholar]
- 49.Ham SL, Atefi E, Fyffe D, Tavana H. Robotic production of cancer cell spheroids with an aqueous two-phase system for drug testing. J Vis Exp 2015; e52754: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiao AY, Torisawa Y, Tung Y-C, Sud S, Taichman RS, Pienta KJ, Takayama S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009; 30: 3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S-YC, Hung PJ, Lee PJ. Microfluidic array for three-dimensional perfusion culture of human mammary epithelial cells. Biomed Microdevices 2011; 13: 753–8. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Wang JC, Wang J. Controllable organization and high throughput production of recoverable 3D tumors using pneumatic microfluidics. Lab Chip 2015; 15: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 53.Wong SF, No DY, Choi YY, Kim DS, Chung BG, Lee SH. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011; 32: 8087–96. [DOI] [PubMed] [Google Scholar]
- 54.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol 2011; 21: 745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CCW, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol 2014; 6: 603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirinifard A, Gens JS, Zaitlen BL, Poplawski NJ, Swat M, Glazier JA. 3D multi-cell simulation of tumor growth and angiogenesis. PLoS One 2009; 4: e7190.–e7190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Achilli T, Mccalla S, Meyer J, Tripathi A, Morgan R. Multilayer spheroids to quantify drug uptake and diffusion in 3D. Mol Pharm 2014; 11: 2071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ong S-M, Zhao Z, Arooz T, Zhao D, Zhang S, Du T, Wasser M, van Noort D, Yu H. Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials 2010; 31: 1180–90. [DOI] [PubMed] [Google Scholar]
- 59.Kim JW, Ho WJ, Wu BM. The role of the 3D environment in hypoxia-induced drug and apoptosis resistance. Anticancer Res 2011; 31: 3237–45. [PubMed] [Google Scholar]
- 60.Wenzel C, Riefke B, Gründemann S, Krebs A, Christian S, Prinz F, Osterland M, Golfier S, Räse S, Ansari N, Esner M, Bickle M, Pampaloni F, Mattheyer C, Stelzer EH, Parczyk K, Prechtl S, Steigemann P. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp Cell Res 2014; 323: 131–43. [DOI] [PubMed] [Google Scholar]
- 61.Shirmanova MV, Druzhkova IN, Lukina MM, Matlashov ME, Belousov VV, Snopova LB, Prodanetz NN, Dudenkova VV, Lukyanov SA, Zagaynova EV. Intracellular pH imaging in cancer cells in vitro and tumors in vivo using the new genetically encoded sensor SypHer2. Biochim Biophys Acta 2015; 1850: 1905–11. [DOI] [PubMed] [Google Scholar]
- 62.Grainger SJ, Serna JV, Sunny S, Zhou Y, Deng CX, El-sayed MEH. Pulsed ultrasound enhance nanoparticle penetration into breast cancer spheroids. 2011; 7: 2006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, Maroz A, Anderson RF, Patterson AV, Stachelek GC, Glazer PM, Matteucci MD, Hart CP. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther 2012; 11: 740–51. [DOI] [PubMed] [Google Scholar]
- 64.Gilbertson R, Graham T. Cancer: resolving the stem-cell debate. Nature 2012; 488: 462–3. [DOI] [PubMed] [Google Scholar]
- 65.Borah A, Raveendran S, Rochani A, Maekawa T, Kumar DS. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis 2015; 4: e177.–e177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denes V, Lakk M, Makarovskiy A, Jakso P, Szappanos S, Graf L, Mandel L, Karadi I, Geck P. Metastasis blood test by flow cytometry: in vivo cancer spheroids and the role of hypoxia. Int J Cancer 2015; 136: 1528–36. [DOI] [PubMed] [Google Scholar]
- 67.Stadler M, Walter S, Walzl A, Kramer N, Unger C, Scherzer M, Unterleuthner D, Hengstschläger M, Krupitza G, Dolznig H. Increased complexity in carcinomas: analyzing and modeling the interaction of human cancer cells with their microenvironment. Semin Cancer Biol 2015; 35: 107–24. [DOI] [PubMed] [Google Scholar]
- 68.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv 2014; 32: 1256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 2003; 83: 173–80. [DOI] [PubMed] [Google Scholar]
- 70.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat 2012; 15: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 2006; 5: 1597–1601. [DOI] [PubMed] [Google Scholar]
- 72.Krueger S, Kalinski T, Wolf H, Kellner U, Roessner A. Interactions between human colon carcinoma cells, fibroblasts and monocytic cells in coculture – regulation of cathepsin B expression and invasiveness. Cancer Lett 2005; 223: 313–22. [DOI] [PubMed] [Google Scholar]
- 73.Paraiso KHT, Smalley KSM. Fibroblast-mediated drug resistance in cancer. Biochem Pharmacol 2013; 85: 1033–41. [DOI] [PubMed] [Google Scholar]
- 74.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol Mech Dis 2006; 1: 119–50. [DOI] [PubMed] [Google Scholar]
- 75.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 76.Pietras K, Östman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 2010; 316: 1324–31. [DOI] [PubMed] [Google Scholar]
- 77.Bhowmick NA. TGF – signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303: 848–51. [DOI] [PubMed] [Google Scholar]
- 78.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth – bystanders turning into key players. Curr Opin Genet Dev 2009; 19: 67–73. [DOI] [PubMed] [Google Scholar]
- 79.Sugimoto H, Mundel T, Kieran M, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 2006; 5: 1640–6. [DOI] [PubMed] [Google Scholar]
- 80.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle 2009; 8: 1461–2. [DOI] [PubMed] [Google Scholar]
- 81.Heneberg P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit Rev Oncol Hematol 2016; 97: 303–11. [DOI] [PubMed] [Google Scholar]
- 82.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 2011; 1: 482–97. [PMC free article] [PubMed] [Google Scholar]
- 83.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyan S-W, Kuo W-H, Huang C-K, Pan C-C, Shew J-Y, Chang K-J, Lee EY-HP, Lee W-H. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One 2011; 6: e15313.–e15313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu C, Liu Y, Xiao L, Guo C, Deng S, Zheng S, Zeng E. The involvement of anterior gradient 2 in the stromal cell-derived factor 1-induced epithelial-mesenchymal transition of glioblastoma. Tumor Biol 2015. Epub ahead of print 25 November. DOI: 10/1007/s13277-015-4481-0. [DOI] [PubMed] [Google Scholar]
- 86.Yu Y, Zhang X, Hong S, Zhang M, Cai Q, Zhang M, Jiang W, Xu C. The expression of platelet-activating factor receptor modulates the cisplatin sensitivity of ovarian cancer cells: a novel target for combination therapy. Br J Cancer 2014; 111: 515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soon P, Kiaris H. MicroRNAs in the tumour microenvironment: big role for small players. Endocr Relat Cancer 2013; 20: R257–67. [DOI] [PubMed] [Google Scholar]
- 88.Grugan KD, Miller CG, Yao Y, Michaylira CZ, Ohashi S, Klein-Szanto AJ, Diehl JA, Herlyn M, Han M, Nakagawa H, Rustgi AK. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A 2010; 107: 11026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johansson A-C, Ansell A, Jerhammar F, Lindh MB, Grenman R, Munck-Wikland E, Ostman A, Roberg K. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res 2012; 10: 1158–68. [DOI] [PubMed] [Google Scholar]
- 90.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012; 487: 500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Busch S, Ryden L, Stal O, Jirstrom K, Landberg G. Low ERK phosphorylation in cancer-associated fibroblasts is associated with tamoxifen resistance in pre-menopausal breast cancer. PLoS One 2012; 7: e45669.–e45669.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinugasa Y, Matsui T, Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells 2014; 32: 145–56. [DOI] [PubMed] [Google Scholar]
- 93.Li X, Zhu W, Chen Z, Luo L, Huang J, Zhang F, Li M, Guo Y, Guo L. Fibroblast growth factor-inducible 14 regulates cell growth and multidrug resistance of small-cell lung cancer through the nuclear factor-κB pathway. Anticancer Drugs 2014; 25: 1152–64. [DOI] [PubMed] [Google Scholar]
- 94.Ying L, Zhu Z, Xu Z, He T, Li E, Guo Z, Liu F, Jiang C, Wang Q. Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PLoS One 2015; 10: e0129593.–e0129593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leyh B, Dittmer A, Lange T, Martens JWM. Stromal cells promote anti-estrogen resistance of breast cancer cells through an insulin-like growth factor binding protein 5 (IGFBP5)/ B-cell leukemia/lymphoma 3 (Bcl-3) axis. Oncotarget 2015; 5: 39307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou A, Lambert D, Yeh H, Yasukawa K, Behbod F, Fan F, Cheng N. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins. BMC Cancer 2014; 14: 781.–781.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasebe T, Mukai K, Tsuda H, Ochiai A. New prognostic histological parameter of invasive ductal carcinoma of the breast: clinicopathological significance of fibrotic focus. Pathol Int 2000; 50: 263–72. [DOI] [PubMed] [Google Scholar]
- 98.Anastassiades OT, Pryce DM. Fibrosis as in indication of time in infiltrating breast cancer and its importance in prognosis. Br J Cancer 1974; 29: 232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res 2012; 10: 1403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today 1992; 13: 265–70. [DOI] [PubMed] [Google Scholar]
- 101.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 2007; 67: 10019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193: 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ong S-M, Tan Y-C, Beretta O, Jiang D, Yeap W-H, Tai JJY, Wong W-C, Yang H, Schwarz H, Lim K-H, Koh P-K, Ling K-L, Wong S-C. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol 2012; 42: 89–100. [DOI] [PubMed] [Google Scholar]
- 105.Kunz-Schughart LA, Heyder P, Schroeder J, Knuechel R. A heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp Cell Res 2001; 266: 74–86. [DOI] [PubMed] [Google Scholar]
- 106.Erickson HP, Bourdon MA. Tenascin: An extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol 1989;5:71–92. [DOI] [PubMed]
- 107.Kato K. 9th international congress of immunology, San Francisco, CA: Elsevier Ltd, 1995, pp. 305–62. [PubMed] [Google Scholar]
- 108.Xu K, Rajagopal S, Klebba I, Dong S, Ji Y, Liu J, Kuperwasser C, Garlick JA, Naber SP, Buchsbaum RJ. The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene 2010; 29: 6533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rama-Esendagli D, Esendagli G, Yilmaz G, Guc D. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Mol Biol Rep 2014; 41: 2885–92. [DOI] [PubMed] [Google Scholar]
- 110.Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia 2010; 12: 317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Majety M, Pradel LP, Gies M, Ries CH. Fibroblasts influence survival and therapeutic response in a 3D co-culture model. PLoS One 2015; 10: e0127948.–e0127948.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bartling B, Hofmann H-S, Silber R-E, Simm A. Differential impact of fibroblasts on the efficient cell death of lung cancer cells induced by paclitaxel and cisplatin. Cancer Biol Ther 2008; 7: 1250–61. [DOI] [PubMed] [Google Scholar]
- 113.Kilani RT, Tamimi Y, Hanel EG, Wong KK, Karmali S, Lee PW, Moore RB. Selective reovirus killing of bladder cancer in a co-culture spheroid model. Virus Res 2003; 93: 1–12. [DOI] [PubMed] [Google Scholar]
- 114.Konur A, Kreutz M, Knüchel R, Krause SW, Andreesen R. Three-dimensional co-culture of human monocytes and macrophages with tumor cells: analysis of macrophage differentiation and activation. Int J cancer 1996; 66: 645–52. [DOI] [PubMed] [Google Scholar]
- 115.Devi KSP, Mishra D, Roy B, Ghosh SK, Maiti TK. Assessing the immunomodulatory role of heteroglycan in a tumor spheroid and macrophage co-culture model system. Carbohydr Polym 2015; 127: 1–10. [DOI] [PubMed] [Google Scholar]
- 116.Hirschhaeuser F, Leidig T, Rodday B, Lindemann C, Mueller-Klieser W. Test system for trifunctional antibodies in 3D MCTS culture. J Biomol Screen 2009; 14: 980–90. [DOI] [PubMed] [Google Scholar]
- 117.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, Csizmadia E, Mariani O, Zhu C, Campagne A, Toner M, Bhatia SN, Irimia D, Vincent-Salomon A, Karnoub AE. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci 2012; 109: 17460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513: 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bronte V. Tumor cells hijack macrophages via lactic acid. Immunol Cell Biol 2014; 92: 647–9. [DOI] [PubMed] [Google Scholar]
- 121.Tai M-H, Chang C-C, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005; 26: 495–502. [DOI] [PubMed] [Google Scholar]
- 122.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 123.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells – perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res 2006; 66: 9339–44. [DOI] [PubMed] [Google Scholar]
- 124.Tannishtha R, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–11. [DOI] [PubMed] [Google Scholar]
- 125.Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol 2009; 19: 96–105. [DOI] [PubMed] [Google Scholar]
- 126.Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol 2012; 13: e83–9. [DOI] [PubMed] [Google Scholar]
- 127.Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, Stass SA, Jiang F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest 2010; 90: 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K, Koch M, Weitz J, Schmidt M, von Kalle C, Glimm H. Distinct types of tumor-initiating cells form human colon Cancer tumors and metastases. Cell Stem Cell 2011; 9: 357–65. [DOI] [PubMed] [Google Scholar]
- 129.Gao Y-J, Li B, Wu X-Y, Cui J, Han J-K. Thyroid tumor-initiating cells: Increasing evidence and opportunities for anticancer therapy (review). Oncol Rep 2014; 31: 1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sarry J, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki A, Cavelier C, Récher C, Mas VM, Delabesse E, Carroll M. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD / SCID / IL2R γ c-deficient mice. J Clin Invest 2011; 121: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vick B, Rothenberg M, Sandhöfer N, Carlet M, Finkenzeller C, Krupka C, Grunert M, Trumpp A, Corbacioglu S, Ebinger M, André MC, Hiddemann W, Schneider S, Subklewe M, Metzeler KH, Spiekermann K, Jeremias I. An advanced preclinical mouse model for acute myeloid leukemia using patients’ cells of various genetic subgroups and in vivo bioluminescence imaging. PLoS One 2015; 10: e0120925.–e0120925.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang Y-F, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, Clarke MF. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci 2010;107:18115–20. [DOI] [PMC free article] [PubMed]
- 133.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature 2012; 488: 527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Francescangeli F, Contavalli P, De Angelis ML, Baiocchi M, Gambara G, Pagliuca A, Fiorenzano A, Prezioso C, Boe A, Todaro M, Stassi G, Castro NP, Watanabe K, Salomon DS, De Maria R, Minchiotti G, Zeuner A. Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death Differ 2015; 22: 1700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010; 140: 62–73. [DOI] [PubMed] [Google Scholar]
- 136.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One 2014; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharma N, Nanta R, Sharma J, Gunewardena S. PI3K / AKT / mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 2015; 6: 32039–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakariassen PØ, Prestegarden L, Wang J, Skaftnesmo K-O, Mahesparan R, Molthoff C, Sminia P, Sundlisaeter E, Misra A, Tysnes BB, Chekenya M, Peters H, Lende G, Kalland KH, Øyan AM, Petersen K, Jonassen I, van der Kogel A, Feuerstein BG, Terzis AJA, Bjerkvig R, Enger PØ. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci U S A 2006; 103: 16466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006; 9: 391–403. [DOI] [PubMed] [Google Scholar]
- 140.Aaberg-Jessen C, Nørregaard A, Christensen K, Pedersen CB, Andersen C, Kristensen BW. Invasion of primary glioma- and cell line-derived spheroids implanted into corticostriatal slice cultures. Int J Clin Exp Pathol 2013; 6: 546–60. [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138: 645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dimasi J. New drug development in the United States from 1963 to 1999. Clin Pharamcol Ther 2001; 69: 286–96. [DOI] [PubMed] [Google Scholar]
- 143.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003; 22: 151–85. [DOI] [PubMed] [Google Scholar]
- 144.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov 2008; 7: 591–607. [DOI] [PubMed] [Google Scholar]
- 145.Zambrowicz B, Sands A. Knockouts model the 100 best-selling drugs – will they model the next 100? Nat Rev Drug Discov 2003; 2: 38–51. [DOI] [PubMed] [Google Scholar]
- 146.Mullard A. Cancer market hits $100 billion. Nat Rev Drug Discov 2015; 14: 373.–373.. [Google Scholar]
- 147.Lindsay M. Target discovery. Nat Rev Drug Discov 2003; 2: 831–8. [DOI] [PubMed] [Google Scholar]
- 148.Eagle H, Foley GE. Cytotoxicity in human cell cultures as a primary screen for the detection of anti-tumor agents. Cancer Res 1958; 18: 1017–25. [PubMed] [Google Scholar]
- 149.Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep 2015; 33: 1837–43. [DOI] [PubMed] [Google Scholar]
- 150.Ingeson-Carlsson C, Nilsson M. Switching from MAPK-dependent to MAPK-independent repression of the sodium-iodide symporter in 2D and 3D cultured normal thyroid cells. Mol Cell Endocrinol 2013; 381: 241–54. [DOI] [PubMed] [Google Scholar]
- 151.Chitcholtan K, Sykes PH, Evans JJ. The resistance of intracellular mediators to doxorubicin and cisplatin are distinct in 3D and 2D endometrial cancer. J Transl Med 2012; 10: 38.–38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009; 28: 461–8. [DOI] [PubMed] [Google Scholar]
- 153.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci 2007; 12: 3468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 2007; 1: 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.De Witt Hamer PC, Van Tilborg AAG, Eijk PP, Sminia P, Troost D, Van Noorden CJF, Ylstra B, Leenstra S. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 2008; 27: 2091–6. [DOI] [PubMed] [Google Scholar]
- 156.Lee S-H, Hong JH, Park HK, Park JS, Kim B-K, Lee J-Y, Jeong JY, Yoon GS, Inoue M, Choi G-S, Lee I., -K Colorectal cancer-derived tumor spheroids retain the characteristics of original tumors. Cancer Lett 2015; 367: 34–42. [DOI] [PubMed] [Google Scholar]
- 157.Lovitt CJ, Shelper TB, Avery VM. Evaluation of chemotherapeutics in a three-dimensional breast cancer model. J Cancer Res Clin Oncol 2015; 141: 951–9. [DOI] [PubMed] [Google Scholar]
- 158.Le Beyec J, Xu R, Lee S-Y, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res 2007; 313: 3066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S-I, Nakashima S, Kunisada T, Iwama T. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem 2008; 283: 10958–66. [DOI] [PubMed] [Google Scholar]
- 160.Kim H, Phung Y, Ho M. Changes in global gene expression associated with 3D structure of tumors: an ex vivo matrix-free mesothelioma spheroid model. PLoS One 2012; 7: e39556.–e39556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.a Frankel, Buckman R, Kerbel RS. Abrogation of taxol-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res 1997; 57: 2388–93. [PubMed] [Google Scholar]
- 162.Godugu C, Patel AR, Desai U, Andey T, Sams A, Singh M. AlgiMatrix™ based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One 2013; 8: e53708.–e53708.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao Y, Foster R, Yang X, Feng Y, Shen J, Mankin H, Hornicek F, Amiji M, Duan Z. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 2015; 6: 9313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lemmo S, Atefi E, Luker GD, Tavana H. Optimization of aqueous biphasic tumor spheroid microtechnology for anti-cancer drug testing in 3D culture. Cell Mol Bioeng 2014; 7: 344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tunggal JK, Cowan DSM, Shaikh H, Tannock IF. Penetration of anticancer drugs through solid tissue: A factor that limits the effectiveness of chemotherapy for solid tumors. Clin Cancer Res 1999; 5: 1583–6. [PubMed] [Google Scholar]
- 166.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6: 583–92. [DOI] [PubMed] [Google Scholar]
- 167.Desoize B. Contribution of three-dimensional culture to cancer research. Crit Rev Oncol Hematol 2000; 36: 59–60. [DOI] [PubMed] [Google Scholar]
- 168.Longati P, Jia X, Eimer J, Wagman A, Witt M-R, Rehnmark S, Verbeke C, Toftgård R, Löhr M, Heuchel RL. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer 2013; 13: 95.–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tung Y-C, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011; 136: 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD, Wang J, Wallace O, Weir A. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov 2015; 14: 475–86. [DOI] [PubMed] [Google Scholar]
- 171.Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun 2013; 433: 327–32. [DOI] [PubMed] [Google Scholar]
- 172.Howes AL, Richardson RD, Finlay D, Vuori K. 3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PLoS One 2014; 9: e108283.–e108283.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mesker W, Junggeburt J, Szuhai K, de Heer P, Morreau H, Tanke H, Tollenaar R. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 2007; 29: 387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mesker W, Liefers G, Junggeburt J, van Pelt G, Alberici P, Kuppen P, Miranda N, van Leeuwen K, Morreau H, Szuhai K, Tollenaar R, Tanke H. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol 2009; 31: 169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Kruijf EM, van Nes JGH, van de Velde CJH, Putter H, Smit VTHBM, Liefers GJ, Kuppen PJK, Tollenaar RAEM, Mesker WE. Tumor–stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 2011; 125: 687–96. [DOI] [PubMed] [Google Scholar]
- 176.Zang R, Li D, Tang I, -C, Wang J, Yang S-T. Cell-based assays in high-throughput screening for drug discovery. Int J Biotechnol Wellness Ind 2012; 1: 31–51. [Google Scholar]
- 177.Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997; 124: 179–192. [DOI] [PubMed] [Google Scholar]
- 178.Walzl A, Unger C, Kramer N, Unterleuthner D, Scherzer M, Hengstschläger M, Schwanzer-Pfeiffer D, Dolznig H. The resazurin reduction assay can distinguish cytotoxic from cytostatic compounds in spheroid screening assays. J Biomol Screen 2014; 19: 1047–59. [DOI] [PubMed] [Google Scholar]
- 179.Crouch S, Kozlowski R, Slater K, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods 1993; 160: 81–8. [DOI] [PubMed] [Google Scholar]
- 180.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A 2008; 14: 227–36. [DOI] [PubMed] [Google Scholar]
- 181.Young SR, Saar M, Santos J, Nguyen HM, Vessella RL, Peehl DM. Establishment and serial passage of cell cultures derived from LuCaP xenografts. Prostate 2013; 73: 1251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sakai Y, Hattori K, Yanagawa F, Sugiura S, Kanamori T, Nakazawa K. Detachably assembled microfluidic device for perfusion culture and post-culture analysis of a spheroid array. Biotechnol J 2014; 9: 971–9. [DOI] [PubMed] [Google Scholar]
- 183.Tseng H, Gage JA, Shen T, Haisler WL, Neeley SK, Shiao S, Chen J, Desai PK, Liao A, Hebel C, Raphael RM, Becker JL, Souza GR. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci Rep 2015; 5: 13987.–13987.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo Y, Liu Q, Yang Y, Guo X, Lian R, Li S, Wang C, Zhang S, Chen J. The effects of ROCK inhibitor Y-27632 on injectable spheroids of bovine corneal endothelial cells. Cell Reprogr 2015; 17: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beauchamp P, Moritz W, Kelm JM, Ullrich ND, Agarkova I, Anson B, Suter TM, Zuppinger C. Development and characterization of a scaffold-free 3D spheroid model of iPSC-derived human cardiomyocytes. Tissue Eng Part C Methods 2015; 21: 852–61. [DOI] [PubMed] [Google Scholar]
- 186.Sirenko O, Mitlo T, Hesley J, Luke S, Owens W, Cromwell EF. High-content assays for characterizing the viability and morphology of 3D cancer spheroid cultures. Assay Drug Dev Technol 2015; 13: 402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muthana M, Scott SD, Farrow N, Morrow F, Murdoch C, Grubb S, Brown N, Dobson J, Lewis CE. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther 2008; 15: 902–10. [DOI] [PubMed] [Google Scholar]
- 188.Miloud T, Henrich C, Hämmerling GJ. Quantitative comparison of click beetle and firefly luciferases for in vivo bioluminescence imaging. J Biomed Opt 2007; 12: 54015–18. [DOI] [PubMed] [Google Scholar]
- 189.Smith MCP, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004; 3100: 8604–12. [DOI] [PubMed] [Google Scholar]
- 190.Cavnar SP, Rickelmann AD, Meguiar KF, Xiao A, Dosch J, Leung BM, Cai Lesher-Perez S, Chitta S, Luker KE, Takayama S, Luker GD. Modeling selective elimination of quiescent cancer cells from bone marrow. Neoplasia 2015; 17: 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]