Abstract
The initial engagement of host cells by pathogens is often mediated by glycan structures presented on the cell surface. Various components of the glycocalyx can be targeted by pathogens for adhesion to facilitate infection. Glycans also play integral roles in the modulation of the host immune response to infection. Therefore, understanding the parameters that define glycan interactions with both pathogens and the various components of the host immune system can aid in the development of strategies to prevent, interrupt, or manage infection. Glycomaterials provide a unique and powerful tool with which to interrogate the compositional and functional complexity of the glycocalyx. The objective of this review is to highlight some key contributions from this area of research in deciphering the mechanisms of pathogenesis and the associated host response.
Keywords: Glycomaterials, glycobiology, glycocalyx, pathogenesis, immunomodulation, immunology/microbiology/virology
Introduction
Carbohydrates, or glycans, are ubiquitous constituents of the cell surface, serving in a variety of roles that range from establishing protective physical barriers against the outside environment, mediating cell–cell and cell–matrix interactions, or regulating intracellular signaling through organization of membrane receptors.1 Throughout evolution, opportunistic pathogens have developed both the ability to target glycan structures on host cells to facilitate infection, as well as to adopt the host glycosylation machinery to acquire stealth, enabling them to evade immune surveillance.2 As such, glycans present attractive drug targets for infectious disease prevention and treatment.
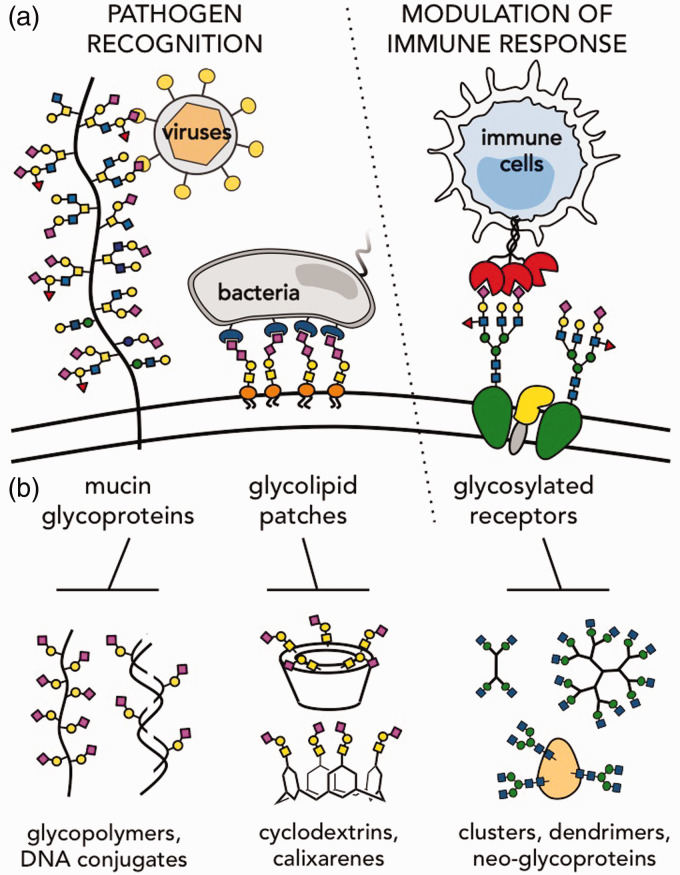
Despite their promise, glycan-based intervention strategies have proved difficult to attain. This is due to a combination of challenges posed by the glycome. Among those are the complexity and diversity of glycan structures and their non-template-driven biosynthesis. Another crucial factor is the typically weak binding affinity of protein receptors toward individual carbohydrates, which, in a biological setting, is augmented through multivalent presentation of glycans on cell surface proteins or through the dynamic assembly of glycolipid patches in the plasma membrane. This gives rise to a carbohydrate-rich, hierarchical macromolecular system, called the glycocalyx (Figure 1(a)), which glycan-recognizing pathogens must navigate in a highly coordinated manner, to engage receptors on host cells, leading to the initiation of infection.
Figure 1.
The cellular glycocalyx is a key mediator of pathogenesis. (a) Various components of the glycocalyx can be targeted by opportunistic pathogens to gain entry into host cells. At the same time, surface glycoconjugates participate in regulation of host immune responses to pathogenic threats. (b) Synthetic glycomaterials approximating the dimensions and architectures of various cell-surface glycoconjugates have been developed as experimental tools to probe the functional role of the glycocalyx in infection. (A color version of this figure is available in the online journal.)
The nanoscale dimensions and organization of the glycocalyx have confounded determination of the activity of the relevant glycan structures and necessitated the development of material-based approaches to elucidate their biological functions. Advances in synthetic macromolecular chemistry have greatly advanced this field.3 A few themes have emerged as important considerations in the design of materials for probing glycan-mediated interactions such as glycan structure, valency, and macromolecular scaffold architecture (Figure 1(b)). This review highlights some recent contributions from the area of materials science in deciphering the identity and mechanisms of action of host glycans targeted by pathogens to initiate infection and in elucidating the roles of host glycans in regulating the ensuing immune response.
Defining glycan-mediated host–pathogen interactions
A large assortment of viral,4–7 bacterial,8–10 and protozoan11,12 pathogens employ the recognition of carbohydrates displayed on surfaces of epithelial cells to engage, colonize, and infect their target organisms. Therefore, considerable efforts have been directed toward developing technologies for cataloging the complexity of carbohydrate recognition by pathogen receptors. Over the last 15 years, the glycan array has emerged as one of the most general and versatile tools for determining the ligand specificities of glycan binding proteins. Although vital to many divisions of glycobiology, this technology has greatly contributed to the identification of glycan receptors targeted by viruses and microbes alike. In their traditional format, glycan arrays are constructed through immobilization of individual carbohydrate structures, either synthetic or derived from natural sources, onto solid supports to generate two-dimensional multivalent glycan assemblies at sufficient densities to elicit high-avidity interactions with target proteins. This straightforward technological platform has helped unearth a great deal of information regarding the specific roles of glycans within the life cycle of many pathogens. Considering the immense number of glycan binding pathogens, a select few are highlighted below to demonstrate the utility of these arrays alongside current material-based improvements geared toward enhancing our understanding of host–pathogen interactions beyond their recognition of individual glycan structures and in the context of their three-dimensional presentation in the milieu of the cellular glycocalyx.
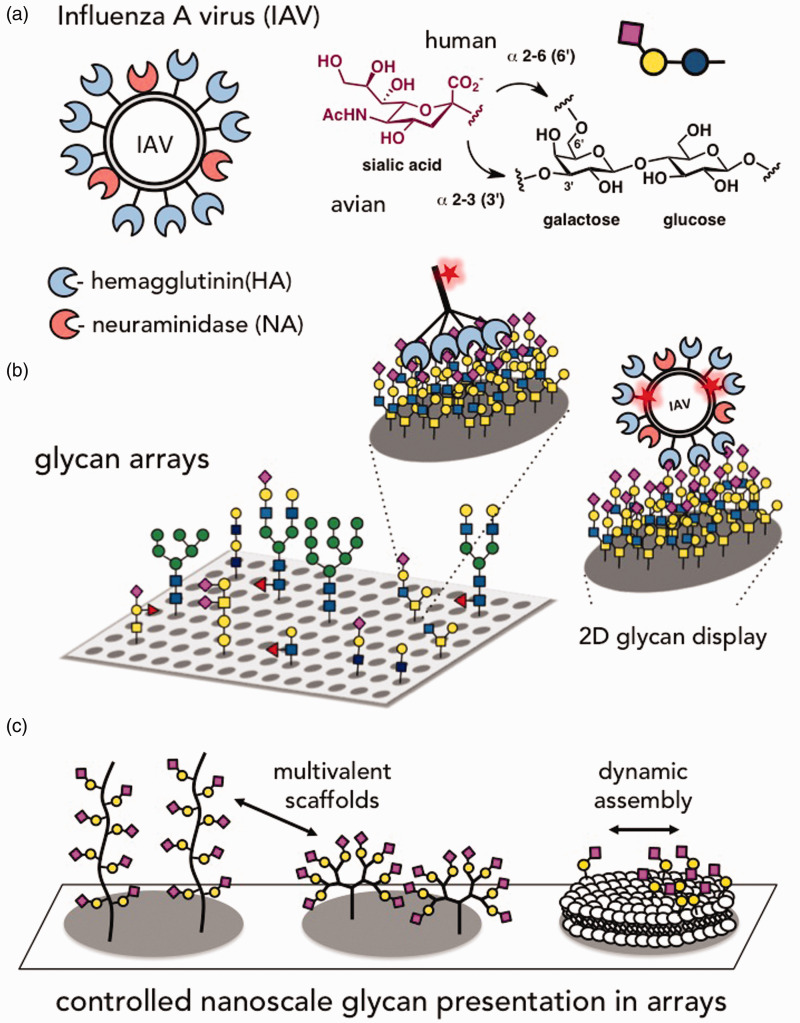
Glycan microarrays have been extensively used to scrutinize the glycan specificity of the Influenza A virus (IAV, Figure 2). The glycan specificity of IAV has been of particular interest, due to its rapidly evolving nature, and the role of recognition toward determining host specificity.13 IAV employs two sialic acid (5-N-acetylneuraminic acid, Neu5Ac)-specific surface proteins, the receptor-binding hemagglutinin (HA) and the receptor-destroying neuraminidase (NA) to, respectively, initiate and complete infection (Figure 2(a)). Avian IAVs generally recognize sialic acid with a α(2,3) glycosidic linkage to an adjacent galactose residue, whereas human viruses bind sialic acid with a α(2,6) glycosidic linkage.7,14 A switch in HA receptor specificity from α(2,3)- to α(2,6)-linked sialic acid is considered a common prerequisite for human transmission,14–16 although not in every case.17–19 Array-based analysis of the glycan-binding phenotype is routinely used as part of IAV surveillance (Figure 2(b))14,20,21; however, discrepancies between the predictions of transmission risks based on glycan array analysis and those determined in infection assays7,22 help motivate continued development of array platforms, with the aim of constructing a more biologically representative array.
Figure 2.
The Influenza A virus (IAV) is an example of an opportunistic pathogen exploiting host glycans to initiate infection. (a) IAV employs sialic acid-specific surface proteins, the receptor-binding hemagglutinin (HA) and the receptor-destroying neuraminidase (NA) to, respectively, initiate and complete infection. (b) Glycan arrays are routinely used to assess changes in host-specificity of IAV strains. (c) Array platforms are emerging that utilize glycomaterials to determine how sialoglycan presentation on host cells influences IAV binding and specificity. (A color version of this figure is available in the online journal.)
Whereas numerous approaches have been explored to optimize the array construction with respect to glycan structure, linker length, grafting chemistry, and surface functionality (these studies have been the subject of several extensive reviews23–26), definite advantageous platforms for accurately probing glycan-binding specificity of pathogen-associated proteins have yet to distinguish themselves. Multiple groups have recently begun to investigate the effects of array design and immobilization strategy by comparing microarray binding results across multiple platforms utilized in various laboratories.27,28 These results demonstrated the variability of carbohydrate recognition across array platforms and the necessity to understand how array construction affects glycan presentation, and, more to the point, how the presentation of these molecules influences the specificity of interactions with protein receptors. Currently, efforts utilizing the principles of macromolecular design are underway to recapitulate the nanoscale three-dimensional presentation of glycans at the cell surface in arrays in order to carefully curate and preserve the contributions of parameters, such as glycan valency and density, on the specificity of glycan recognition (Figure 2(c)).24,29
Bovin and colleagues provided an early demonstration of this approach in their study of glycan-binding specificities of model lectins using a 3D hydrogel array.30,31 Recently, Liu and co-workers adapted this technology to capture distinct bacterial species in carbohydrate-modified hydrogel microarrays.32 The binding specificity of Pseudomonas aeruginosa lectins (e.g. LecA) has also been explored using multivalent glycoconjugate array formats to investigate how both the scaffold itself and the glycan presentation along the scaffold affect lectin binding. The Pieters lab explored galactosyl glycodendrimer arrays to obtain binding profiles of LecA,33 demonstrating a 16-fold increase in potency with a tetravalent β-galactoside dendrimer over a monovalent scaffold, a result similarly echoed while investigating the binding of cholera toxin, another bacterial protein with glycan-binding lectin domains.34 Gildersleeve and his co-workers used synthetically glycosylated bovine serum albumin (BSA) ligands,35 with well-defined glycan valencies, arranged on the array at increasing surface densities to identify high-affinity inhibitors of LecA. In a differing approach, Novoa et al. constructed a library of 625 monovalent and divalent galactose-containing glycans with varying linker components presented on peptide nucleic acid (PNA) scaffolds to screen for LecA recognition. This targeted array aimed to optimize binding of the pathogenic protein by enhancing the ability of LecA to interact with adjacent glycan binding sites separated by 30 Å.36 This array addressed the molecular geometry37 and conformational constrains of glycan recognition, while simultaneously identifying soluble inhibitors capable of inhibiting P. aeruginosa invasion of human lung cells.
While IAV specificity has been routinely examined in the traditional glycan array platform, several groups have begun to consider the effects of valency, density, and spatial organization of cell-surface glycans on IAV recognition by utilizing polymeric glycomaterials grafted to solid surfaces to survey viral specificity. Outside the context of microarrays, surface plasmon resonance (SPR) analysis of the binding of HA proteins to surface-immobilized sialylated glycopolymers confirmed the specificity of avian (A/Anhui/1/2005(H5N1)) and human (A/Brisbane/10/2007(H3N2)) IAV forms for the characteristic α(2,3) and α(2,6) sialic acid linkages, respectively.38 Our own group has also explored the use of linear glycopolymers mimicking the architecture of epithelial mucin glycoproteins to explore the effects of sialoglycan presentation on whole IAV binding (Figure 2(c)).39
Glycan mobility within the fluid environment of the cell membrane is also believed to be an important factor in mediating multivalent interactions.40 Current glycan arrays, in which glycans are immobilized to the surface in fixed positions, fail to recreate the dynamic nature of glycan membrane organization. Building on an early report by Disney and Seeberger demonstrating the capture of whole ORN178 Escherichia coli (E. coli) on oligomannose arrays41 through the mannose-specific FimH adhesion protein42 presented at the tips of the bacteria’s type 1 fimbriae, Barth et al. created a fluidic microarray to address concerns of glycan mobility as well as the challenge of tightly controlling the glycan density (Figure 2(c)).43 Utilizing a supported lipid bilayer (SLB) containing mannosylated lipids,44 the researchers observed that a critical mannose density was required for a switch in the avidity of FimH in the ORN178 strain from a monovalent to a trivalent interaction. This result led the authors to hypothesize that each fimbriae binds to a cluster of three mannose residues, in agreement with the known affinity of FimH for a covalently linked trimannose ligand,45 and the enhanced avidity of the resulting multivalent interaction triggers the anchoring of additional fimbriae. Similarly in later work, Shen et al. made use of SPR imaging of SLB arrays46 to investigate the kinetic effects of the membrane surface on the recognition of sialylated glycolipid receptors by recombinant HA proteins from an H5N1 IAV strain.47 In this work, the authors measured the formation of HA–glycan complexes while maintaining the concentration of the preferred receptor, α(2,3) sialyl-N-acetyllactosamine glycolipid and varying a secondary lipid/glycolipid component within the membrane. Receptor binding could be significantly enhanced with increasing amounts of accessible hydrophobic elements; an effect quantitatively attributed to the kinetic formation of a weak HA-membrane interaction prior to glycan recognition. Taken together, these results provide further evidence for the importance of membrane and dynamics in carbohydrate recognition by pathogens.
Inhibiting glycan-mediated host–pathogen interactions
Naturally, inquiries into glycan receptor specificity are ultimately motivated by the desire to neutralize the disease causing agents, and as such, the development of glycomaterials as inhibitors of pathogen binding has had an enduring presence. The Whitesides group was among the earliest to consider the fitness of multivalent glycan ligands as inhibitors of pathogens. Although not alone in this pursuit,48–52 much of their work focused on the creation of polyvalent IAV inhibitors presenting sialic acid on both liposomal53 and polyacrylamide54–58 scaffolds. The principles that govern multivalent glycan interactions, as formulated by Whitesides in the course of these studies, still remain a vital component of pathogen inhibitor design. In particular, good inhibitors make use of additive enthalpic gains stemming from each successful glycan–protein interaction balanced against any entropic penalty caused by restricting the conformational freedom of the polymer scaffold. Progress in this field59,60 has been accelerated by the development of modern synthetic techniques yielding novel scaffolds with ever increasing complexity and control over macromolecular architecture. For instance, reaching beyond the linear architectures of glycopolymers, Papp et al. synthesized sialic acid presenting polyglycerol nanoparticles of varying size and showed that particles roughly the same size as IAV (50–100 nm) were the most effective scaffold for inhibiting IAV infection of Madin–Darby canine kidney cells.61 The diversity in this field is ever growing and is further elaborated by techniques that can create newer diversity in multivalent structure,62 including but not limited to chemoenzymatic,63 gold nanoparticle,64 fullerenes,65 and synthetic peptide carrier66 strategies. Some consideration has also been paid to the use of naturally isolated mucin glycoproteins, which are heavily decorated with pathogen receptor decoys that viruses such as IAV must negotiate en route to their cellular target,67 as inhibitors of infection. One such example demonstrated that a solution of purified porcine gastric mucins could inhibit infection of epithelial cells by human papilloma virus type 16, Merkel cell polyoma-virus, and the A/WSN/1933 (H1N1) IAV strain.68
Soluble glycomaterials are of particular interest as inhibitors of pathogenic bacteria in part because they offer an alternative to current antibiotic treatment, circumventing the pressing challenges associated with the development of antibacterial drug resistance. Designing treatments for bacterial infections with antiadhesive agents offers an attractive alternative to antibiotics by reducing the selective pressure on the pathogen to evolve resistance.69 Being that antiadhesion multivalent materials have been reviewed extensively,70–73 this review will only briefly highlight a few recent examples.
A number of labs have paid particular attention to inhibition of the P. aeruginosa lectins by exploring the use of diverse ligand architectures. In particular, calix-[4]-arenes74 have been ranked among the most effective architectures against LecA (Figure 1(b)).75,76 In a recent report, Boukerb et al. synthesized tetravalent galactosyl and fucosyl calixarenes for the purpose of inhibiting both major P. aeruginosa lectins, LecA and LecB.77 These glycoconjugates were capable of inducing bacterial aggregation, inhibiting adhesion, and protecting against lung injury in a mouse lung model. Importantly, the prevention of biofilm formation was dependent on the multivalency of the glycosylated calixarenes. In addition, other synthetic architectures have been utilized, including carbohydrate-functionalized goldnanoclusters,78 pillararenes,79 dendrimers,80–83 and fullerenes (Figure 1(b)).84 Furthermore, like with viruses,68 the Ribbeck group was also able to inhibit adhesion and biofilm formation of the bacteria by once more utilizing native gastric porcine mucins.85
Although the neutralization of P. aeruginosa with soluble glycopolymers is arguably the most explored, similar approaches have been applied to other bacteria, including E. coli. Multivalent N-heptyl-α-d-mannosylated glycopolymers86 were capable of disrupting binding of invasive E. coli to intestinal epithelial cells by 102–106 fold, ultimately protecting ex vivo mouse colonic loops from adhesion of these cells. In another example, Ryu et al. demonstrated that the dynamic assembly of multivalent nanofibers from amphiphilic glycosylated building blocks could induce aggregation of ORN178 E. coli, by engaging FimH, in a length-dependent fashion. The self-assembly of these nanofibers could be tuned to define lengths87 as well as dynamically shift morphology from linear fibers to spherical micelles.88
Inhibitor strategies can be expanded for effective use in many distinct forms of pathogenic organisms, including the bacterial AB5 toxins,89 as demonstrated in a study by Polizzotti and Kiick on the inhibition of cholera toxin with galactosylated recombinant peptides.90 Similarly, Gram-positive bacteria, such as Streptococcus suis, could be effectively inhibited with tetra- and octavalent galabiose dendrimers91,92 and even the protozoan Entamoeba histolytica could be targeted with linear polyvalent N-acetylgalactosaminides.93,94 These studies nicely illustrate the applicability of soluble glycomaterials as therapeutic and diagnostic tools for pathogen research.
Glycan-mediated regulation of the host immune response
The immune system, including innate and adaptive components, serves as the host defense to combat infection and invasion by foreign pathogens.95 Host glycans play integral roles in mounting host defense to pathogenic threats and, in some cases, their functions can be subverted by pathogens (and even cancer cells) to escape immune surveillance. The burgeoning field of synthetic immunology,96,97 which aims to employ synthetic systems to modulate immune responses, has profoundly impacted this field of study. Among the tools that have been instrumental to its cause are glycomaterials, reflecting the centrality of glycans in orchestrating host immunological responses. The following sections describe several applications of glycomaterials to reveal the mechanisms through which glycans influence the activity of the various components of the immune response and to harness these functions to combat pathogen infections.
Probing glycan functions in innate immunity with glycomaterials
The complement system
A key component of innate immunity is the complement system, which consists of ∼ 30 soluble and membrane-bound proteins. Activation of the complement cascade is the hallmark innate immune effector mechanism present in plasma, cerebral spinal fluid, and mucosa. The complement system can be activated by three initiation pathways: the lectin, classical, and alternative pathways. Glycopolymers with pendant glucose or galactose monosaccharides grafted onto polystyrene nanoparticles have previously been used to assess glycan-mediated complement activation.98 In this study, the nanoparticles were tested for their ability to activate complement by its consumption in normal human serum. In another example, Geng et al. generated well-defined neoglycopolymer-protein biohybrid materials to modulate complement activation through the lectin pathway.99 Complement lectins can bind to mannose-containing structures on the surface of bacteria, fungal pathogens, and viruses. Taking advantage of a single free cysteine residue in BSA, maleimide-terminated synthetic neoglycopolymers decorated with pendant mannose monosaccharide ligands (valency = 74), were conjugated to a single site in the BSA protein. The resulting glycomaterials were used to investigate binding to the complement lectin, MBL (mannose-binding lectin) in an SPR assay. The authors demonstrate that the hybrid mannoglycopolymer–BSA conjugates bound MBL with a much higher affinity (KD = 7.9 + 10-9) and activated complement to a higher extent compared to BSA alone in both SPR and ELISA assays.
Phagocytic immune components (neutrophils, monocytes, macrophages)
Neutrophils, white blood cells, which can engulf, or phagocytose, foreign pathogens present in the bloodstream, were once thought to be non-discriminatory components of the immune system. Recent studies have shown that neutrophils can distinguish between structurally similar glucan components of the fungal cell wall. In a landmark study, Rubin-Bejearno et al. coated polystyrene beads with either β(1–3) or β(1–6) glucans and evaluated the activation of human neutrophils in response to their exposure.100 Although both structural isomers are present in the fungal cell wall, β(1–6) glucans comprise only 9–20% of the total glucan composition. Notably, the 6 µm polystyrene beads used in this study, match the size of Candida albicans yeast form cells (5 µm). The authors demonstrate that the β(1–6) beads led to significantly enhanced production of heat shock proteins, and that the presentation on particles (versus soluble polysaccharides) was necessary to elicit this effect. These beads also caused greater ingestion and reactive oxygen species (ROS) production by neutrophils. The authors go on to demonstrate that the β(1–6) beads recruited the complement factor C3d (proteolytic fragements of C3b) to a larger extent compared to the β(1–3) beads, and that this recruitment led to the binding of the neutrophil cell surface complement receptor CR3. Finally, the authors show that selectively glycan-digested whole C. albicans cells also exhibited this behavior, wherein cells exposed to an endo β(1–6) glucanase exhibited a 50% reduction in phagocytosis ROS production and HSP expression. Similar polystyrene beads have also been used to decode the mechanism through which human monocyte-derived macrophages phagocytose virulent strains of Mycobacterium tuberculosis (Erdman and H37Rv).101 The authors demonstrate that additional terminal (single, di-, or tri-) mannosyl units on lipoarabinomannan glycolipids, which are somewhat unusual for mycobacteria, but are present on virulent strains, are directly responsible for binding to the macrophage mannose receptor.
Dendritic cells (DCs)
DCs play critical roles in the rapid response toward the presence of foreign pathogens and also bridge the innate and adaptive immune systems. The molecular signature of pathogens, often referred to as pathogen-associated molecular patterns (PAMPs), is recognized by immune pattern recognition receptors (PRRs) on DCs. Sugar-complexed PAMPs102 are the largest constituents of PAMPs and include lipopolysaccharide (LPS), N-acetylglucosamine (GlcNAc), peptidoglycan, and glucan-containing cell walls. Activation of PRRs results in the expression of antigen-presenting molecules (MHCII), co-stimulatory factors (CD80/86, CD40), and pro-inflammatory cytokines. Various glycoconjugates have been used to modulate DC responses. Hotaling et al. recently conducted a study on BSA conjugates of various glycans to clarify the molecular determinants (glycan composition, density, carrier cationicity) that influence DC responses.103
The LPS capsule of Gram-negative bacterial pathogens, which includes a glucosamine disaccharide moiety, is a potent activator of Toll-like receptor-4 s (TLR4s), a type of PRR present on DCs. The interaction of LPS and TLR4 can be beneficial, as it signals the presence of a foreign pathogen, but it can also be harmful as it can lead to potentially lethal septic shock. Thus, LPS or TLR4 antagonists have long been proposed as therapeutic agents to treat septic shock. Shaunak et al. have developed partially glycosylated dendrimers as TLR4 antagonists that can inhibit the inflammation response.104 Anionic carboxylic acid-terminated polyamidoamine dendrimers modified with ∼ 8 surface glucosamine residues inhibited LPS-induced TLR4 responses with µM inhibitory activities (e.g. production of inflammatory cytokines and chemokines) in immature human DCs and macrophages. In a follow-up study, the authors provide computation-based guidelines for the design of other types of macromolecular structures for the inhibition of TLR4.105
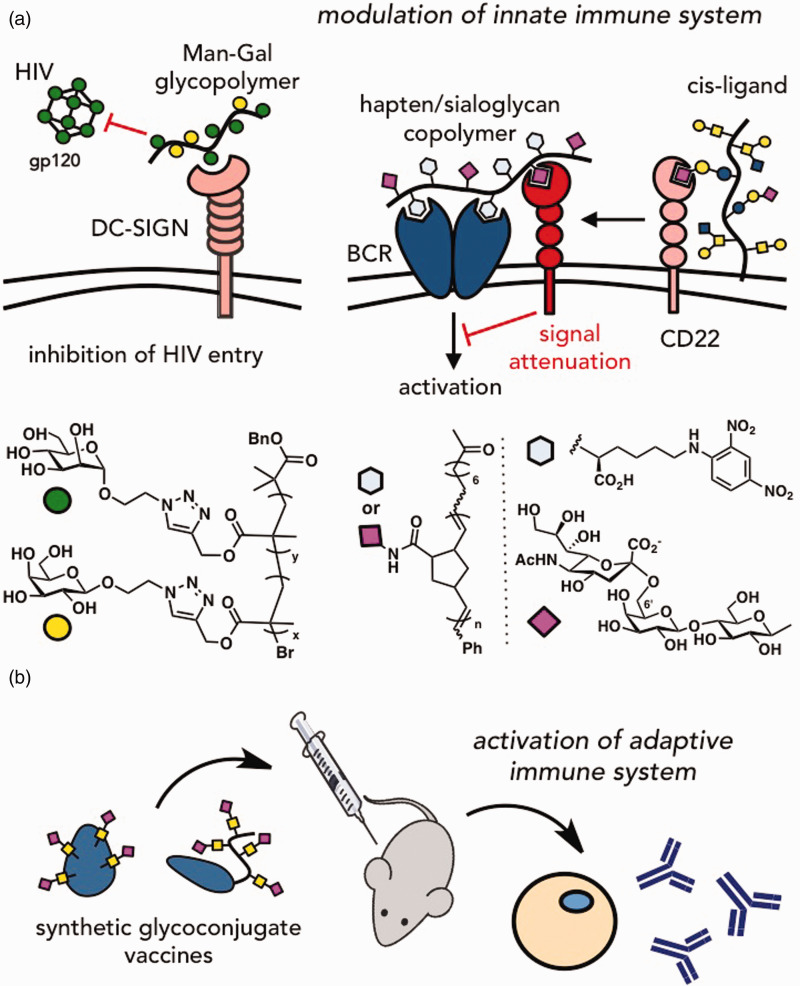
Another PRR that has been a popular target is DC-SIGN (specific intercellular adhesion molecule-3-grabbing non-integrin), a C-type (or calcium-dependent) lectin,106 which binds to terminal fucose or mannose saccharides. DC-SIGN is often exploited by pathogens to facilitate infection and transmission, as in the cases of HIV, Ebola, or Dengue viruses. Thus, targeting or disrupting the pathogen–DC-SIGN interaction has been proposed as a route to vaccines and therapies (Figure 3(a)). Varga and co-workers demonstrated that glycodendrons incorporating multiple copies of mannose saccharides inhibited DC-SIGN binding to mannose-conjugated BSA in an SPR assay, as well as inhibited HIV and Dengue infection of DC-SIGN transfected cells with activities in the low micromolar range.107 Lewis X oligosaccharides conjugated to ovalbumin have also been evaluated for their activities to inhibit DC-SIGN and stimulate immune responses.108 Recently, glycofullerenes have generated considerable interest as inhibitors of Ebola virus infection.109 Using hexakis adducts of [60]fullerene as building blocks, Munoz et al. generated water-soluble tridecafullerenes decorated with 120 peripheral mannose subunits with subnanomolar inhibitory activity against Ebola virus.
Figure 3.
Glycomaterials have found widespread use as tools to interrogate glycan functions in immunomodulation. (a) Soluble glycoconjugates can serve as inhibitors of pathogen binding to receptors of the immune system or as probes for elucidating the mechanisms of immune response regulation. (b) Synthetic glycoconjugates have been explored as immunogens to activate adaptive immune responses. (A color version of this figure is available in the online journal.)
Sialic acid-binding immunoglobin-like lectins (Siglecs) and B cell activation
Siglecs are a family of cell surface transmembrane receptors present on immune cells (e.g. NK, DCs).110 Various members of the Siglec family have served as targets for the development of immunotherapies, because of their restricted expression patterns on immune cell types, high expression, and the ability to modulate receptor signaling. Due to their ability to bind sialic acids, numerous scaffolds bearing various sialoside glycans have been developed as Siglec ligands. Historically, polyacrylamide conjugates of α(2–6) sialyllactose glycans have been used to reveal the sialic-acid binding lectin activity of Siglec-2 (CD22) on B lymphocytes.111 As competitive trans ligands of Siglec-2, these multivalent probes possess sufficient avidity to overcome “masking” of the Siglec binding site by endogenous cis ligands. Much work has been devoted to targeting Siglecs as anticancer therapies (e.g. B-cell lymphomas). Toxins conjugated to the sialoglycan probes are efficiently taken up by B-cells, resulting in toxin-mediated killing.112 Rillahan and co-workers have developed glycan microarrays as a high-throughput screen for synthetic structural analogs of sialoglycans against Siglecs-7, 9, and 10.113,114 They then conjugated the high affinity ligand to PEG lipids to generate liposomal nanoparticles and evaluated targeting of Siglec-expressing cells in human blood. The liposomal nanoparticle strategy has also been used to deliver antigens to macrophages expressing Siglec-1.115
Interestingly, Siglec-2 is also an inhibitory co-receptor of B-cell receptors (BCRs), which recognize dinitrophenyl (DNP) antigens. Upon engagement of the BCR, a signaling cascade ensues to endocytose the BCR–hapten complex and facilitate antigen uptake for processing and display on the cell surface. Siglec-2 functions to prevent erroneous B cell activation by down-regulating signaling and inhibits the BCR signaling cascade. Courtney and co-workers have previously generated homopolymers of DNP (stimulatory antigen) and its co-polymers with pendant α(2–6) sialyllactose groups (inhibitory antigen) to modulate BCR signaling and establish the relationship between signaling and endocytosis116 (Figure 3(a)). Hudak et al. have shown that sialic acid glycopolymers end-functionalized with phospholipids can passively incorporate into cell membranes of tumor cells to engage, in a trans interaction, Siglec-7 presented on natural killer (NK) cells, resulting in the remodeled cell’s altered susceptibility to NK killing.117 This study provided an insight into the functional role of the hypersialylated phenotype acquired by many adenocarcinoma cells to evade immune surveillance by NK cells.
Selectins
Upon encountering a pathogen, macrophages express pro-inflammatory cytokines that induce cell surface expression of selectins, which are also members of the C-type lectin family. Interactions between selectins expressed on the surfaces of endothelial cells and their sialylated (and often sulfated) ligands presented on circulating leukocytes induce “leukocyte rolling,” a phenomenon, which allows leukocytes to scan a blood vessel and enter an infection site. Competitive inhibitors of selectin in the form of glycoconjugates (polymers of synthetic glycoproteins) have been proposed as anti-inflammatory agents. Sanders and co-workers have designed glycopolymers generated by ring-opening metathesis that efficiently inhibited L-selectin binding.118 This study revealed that inhibition is dependent on the specific sulfation pattern of the glycan moieties, as well as multivalency. Importantly, this study points to the need to consider the effects of shear forces influencing glycan binding under physiologically relevant flow conditions. A subsequent study by Rele and co-workers used poly(ethylene)-oxide dendrimers of sulfated lactose to inhibit L-selectin binding, as well as reduce inflammatory cell recruitment.119
Glycomaterials for the activation of the adaptive immune system
Prophylactic and/or vaccine treatments have been poised as solutions to infectious diseases that have been difficult to diagnose and treat, due to the lack of specific clinical symptoms and rapid emergence of antibiotic resistance. A number of glycan-based vaccines have been developed to immunize against pathogenic microbes, such as bacteria, fungi, and viruses.120 Often, these approaches employ protein glycoconjugates121 to elicit stronger immunogenic responses from otherwise weakly immunogenic glycans isolated from pathogenic cell surfaces (Figure 3(b)). In many cases, the carrier protein, often itself immunogenic, can also serve as a scaffold for the multivalent display of glycans. Such conjugate vaccines, including Prevnar (Wyeth Pharmaceuticals, against Streptococcus pneumoniae) and Pentacel (Sanofi Pasteur, against Haemophilus influenzae), are now commercially available, or in development, and have been proven to produce long-lasting protection against pathogens.
Perhaps the most prominent example of antibodies combating infection via a glycan-mediated mechanism is that of the broadly neutralizing antibody 2G12. 2G12 is a human monoclonal antibody that specifically recognizes the HIV envelope glycoprotein, gp120, via its mannose oligosaccharides.122,123 The discovery of 2G12 and other glycan-targeting antibodies was remarkable, as the dense canopy of glycans on the HIV envelope, often called the “glycan shield” for its defensive ability to obscure peptide epitopes from antibody recognition, was now breachable and could even be used as a targeting moiety.124,125 Over the years, several groups have created various multivalent constructs of this oligosaccharide to generate high-avidity 2G12 binders that could serve as haptens for the development of anti-HIV antibodies. Such constructs include protein or immunogen conjugates of a cyclic glycopeptide mimotope of the oligomannose saccharides,126,127 dendrons,128 viral capsids,129,130 and even nucleic acid-based displays.131 The latter have especially garnered considerable interest, due to the ability for combinatorial screening and the capability to precisely tune interligand distances and valencies. Using PNA conjugates of mannose oligosaccharides to display the glycan epitopes, Gorska et al. demonstrated that carbohydrate spacing matching the binding sites of the dimeric 2G12 antibody is crucial for avidity.132 Because DC-SIGN also has affinity for the same oligomannose saccharides, anti-HIV therapeutics with the inhibition of the DC-SIGN-gp120 interaction in mind have also been proposed. Dendrimers,133,134 glycopolymers,135 and other platforms that do so have previously been developed.
Liposomal adjuvants generated by incorporation of glycolipid components of pathogens into nanoparticles have also been developed. Lipid A, a common component of Gram-negative bacterial cell walls, and trehalose 6,6′-dimycolate (TDM), a cord factor present in mycobacterial cell walls are prominent in adjuvant formulation. Monophosphoryl lipid A, a TLR4 agonist, has been shown to elicit CD8 ( + ) T-cell responses in vivo.136 A synthetic analog of TDM, trehalose 6,6′-dibehenate (TDB) has been inserted into cationic liposomes of the quaternary ammonium lipid N,N′-dimethyl-N,N′-dioctadecylammonium, to generate the adjuvant CAF01.137 Using ovalbumin as a model vaccine antigen, the authors show that CAF01 primed complex immune responses, and these responses were above those obtained with currently used adjuvants (e.g. monophosphoryl lipid A, Alum). They also demonstrate that vaccines based on CAF01 elicited significant immunity against three models of infection, M. tuberculosis, Chlamydia trachomatis, and malaria (Plasmodium yoelii), as well as HIV.138,139 In addition to its immunoprotective effects, TDB has also been shown to play a critical role in promoting the stability of such cationic liposomes.140 Due to its capability to retain water and stabilize membranes, the trehalose disaccharide has been implicated in endowing organisms to survive anhydrous and freezing conditions.141 Indeed, trehalose glycopolymers have been shown to promote stability of protein conjugates.142
Torosantucci et al. have generated laminarin conjugates of the diphtheria toxoid CRM197 as antifungal prophylactic vaccines.143 Laminarin is a β-glucan with β(1,3) and some β(1,6) branching, (isolated from plant algae) that mimics the viability-critical β-glucan polysaccharides present on the cell walls of all human pathogenic fungi. The authors show that mice immunized with the laminarin–CRM conjugates produced significant titers of IgG and IgM antibodies against β(1,3) and β(1,6) glucans. They show that the laminarin–CRM conjugates effectively reduced fungal burden and conferred significant protection against lethal systemic Candida albicans and Aspergillus fumigatus infections. Importantly, they also show that the protection was mediated mostly (if not all) via the elicited anti-β-glucan (not anti-CRM) IgG antibodies. These antiglucan antibodies inhibited fungal growth by directly binding to the fungal hyphae cell surfaces. In another study, Paulovicova et al. demonstrated that mice immunized with heptamannoside BSA conjugates induced Th1, Th2, and Th17 immune responses to induce candidacidal activity.144
Recently, researchers at GlaxoSmithKline, Novartis, and the University of Oxford developed a glycoconjugate vaccine against Salmonella enterica serovar typhimurium, for which none is currently available.145 The authors conjugated the Salmonella LPS O-antigen (OAg) to CRM197 and evaluated the production of anti-OAg antibodies in mice after immunization, as well as the serum bactericidal activity against Salmonella. Glycoconjugates with single or double attachments were found to elicit significant levels of anti-OAg antibodies with serum bactericidal activity. Notably, the researchers explored various site-selective conjugation methods and found that the conjugation site of the antigen to CRM197 greatly affected immunogenicity. These findings are consistent with the model proposed by Avci et al.,146 wherein carbohydrate presentation within peptides can differentially affect immune responses by influencing the communication between B-cells and T-cells.
Summary
The elegant studies described in this review clearly illustrate the central role of materials science in providing key insights into how the organization of glycans at the cell surface influences host–pathogen interactions, and how glycans may be targeted to prevent infection or to modulate the host response to pathogenic threats. There is no doubt that future innovation in the design and synthesis of ever more sophisticated glycomaterials will yield novel macromolecular architectures, providing advanced tools enabling the interrogation of glycan function. The staggering compositional and functional complexity of the glycocalyx remains one of the most exciting, yet largely unexplored, frontiers in glycobiology research. While the contributions of its individual components have increasingly come into focus, the glycocalyx in its entirety, as a dynamic hierarchically organized biomolecular system, still harbors many discoveries to be made. New avenues of glycobiology research are emerging that integrate chemical, materials, and molecular biological approaches aimed at defining how the nanoscale organization of the glycocalyx instructs biological events occurring at the cellular boundary. A particular challenge in this field is the translation of information obtained from various analytical platforms within the complexity of biological systems. Cell surface glycan engineering strategies that integrate concepts of materials design are beginning to emerge and are likely to play a central role in revealing the mechanisms through which the glycocalyx influences pathogenesis and to provide new paradigms for the formulation of therapeutic interventions.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CJF was supported in part by the UCSD Graduate Training Program in Molecular Biophysics through an institutional training grant from the National Institute of General Medical Sciences, T32 GM08326. This work was supported in part by the National Institutes of Health through grants 5 R00 EB013446-05 (NIBIB) and 1DP2HD087954-01 (NICHD).
Authors’ contributions
MLH, CJF, and KG designed and wrote the manuscript. KG had primary responsibility for its final content.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.s
References
- 1.Varki A, Lowe JB. Biological roles of glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (eds). Essentials of Glycobiology, 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009. Chapter 6. [PubMed] [Google Scholar]
- 2.Springer SA, Gagneux P. Glycan evolution in response to collaboration, conflict, and constraint. J Biol Chem 2013; 288: 6904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coullerez G, Seeberger PH, Textor M. Merging organic and polymer chemistries to create glycomaterials for glycomics applications. Macromol Biosci 2006; 6: 634–47. [DOI] [PubMed] [Google Scholar]
- 4.Ströh LJ, Stehle T. Glycan engagement by viruses: receptor switches and specificity. Annu Rev Virol 2014; 1: 285–306. [DOI] [PubMed] [Google Scholar]
- 5.Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS. The sweet spot: defining virus-sialic acid interactions. Nat Rev Microbiol 2014; 12: 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Air GM. The role of carbohydrates in viral infections. In: Wang B, Boons G-J. (eds). Carbohydrate recognition: biological problems, methods, and applications, 1st ed Hoboken, NJ: John Wiley & Sons, Inc., 2011. Chapter 3. [Google Scholar]
- 7.Smith DF, Cummings RD. Investigating virus-glycan interactions using glycan microarrays. Curr Opin Virol 2014; 7: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieters RJ. The role of carbohydrates in bacterial infections. In: Wang B, Boons G-J. (eds). Carbohydrate recognition: biological problems, methods, and applications, 1st ed Hoboken, NJ: John Wiley & Sons, Inc., 2011. Chapter 4. [Google Scholar]
- 9.Audfray A, Varrot A, Imberty A. Bacteria love our sugars: interaction between soluble lectins and human fucosylated glycans, structures, thermodynamics and design of competing glycocompounds. Comptes Rendus Chimie 2013; 16: 482–90. [Google Scholar]
- 10.Pieters RJ. Carbohydrate mediated bacterial adhesion. Adv Exp Med Biol 2011; 715: 227–40. [DOI] [PubMed] [Google Scholar]
- 11.Cummings RD, Turco S. Parasidic infections. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (eds). Essentials of Glycobiology, 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009. Chapter 40. [PubMed] [Google Scholar]
- 12.Sinnis P, Coppi A. A long and winding road: the Plasmodium sporozoite’s journey in the mammalian host. Parasitol Int 2007; 56: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai M, Kawaoka Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol 2012; 2: 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Microbiol 2006; 4: 857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y. Sialobiology of influenza molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull 2005; 28: 399–408. [DOI] [PubMed] [Google Scholar]
- 16.Rogers GN, Paulson JC, Daniels RS, Skehel JJ, Wilson IA, Wiley DC. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983; 304: 76–8. [DOI] [PubMed] [Google Scholar]
- 17.Sutton TC, Finch C, Shao H, Angel M, Chen H, Capua I, Cattoli G, Monne I, Perez DR. Airborne transmission of highly pathogenic H7N1 influenza in ferrets. J Virol 2014; 88: 6623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, de Vries RP, Tzarum N, Zhu X, Yu W, McBride R, Paulson JC, Wilson IA. A human-infecting H10N8 influenza virus retains a strong preference for avian-type receptors. Cell Host Microbe 2015; 17: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R, de Vries RP, Zhu X, Nycholat CM, McBride R, Yu W, Paulson JC, Wilson IA. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science 2013; 342: 1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Air GM. Influenza virus-glycan interactions. Curr Opin Virol 2014; 7: 128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman R, Tharakaraman K, Shriver Z, Jayaraman A, Sasisekharan V, Sasisekharan R. Glycan receptor specificity as a useful tool for characterization and surveillance of influenza A virus. Trends Microbiol 2014; 22: 632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther T, Karamanska R, Chan RWY, Chan MCW, Jia N, Air G, Hopton C, Wong MP, Dell A, Malik Peiris JS, Haslam SM, Nicholls JM. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog 2013; 9: e1003223–e1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu Rev Biochem 2011; 80: 797–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Gildersleeve JC, Blixt O, Shin I. Carbohydrate microarrays. Chem Soc Rev 2013; 42: 4310–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Heimburg-Molinaro J, Cummings RD, Smith DF. Chemistry of natural glycan microarrays. Curr Opin Chem Biol 2014; 18: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L, Chen X, Varki A. Exploration of sialic acid diversity and biology using sialoglycan microarrays. Biopolymers 2013; 99: 650–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padler-Karavani V, Song X, Yu H, Hurtado-Ziola N, Huang S, Muthana S, Chokhawala HA, Cheng J, Verhagen A, Langereis MA, Kleene R, Schachner M, de Groot RJ, Lasanajak Y, Matsuda H, Schwab R, Chen X, Smith DF, Cummings RD, Varki A. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J Biol Chem 2012; 287: 22593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Cummings RD, Smith DF, Huflejt M, Campbell CT, Gildersleeve JC, Gerlach JQ, Kilcoyne M, Joshi L, Serna S, Reichardt N-C, Parera Pera N, Pieters RJ, Eng W, Mahal LK. Cross-platform comparison of glycan microarray formats. Glycobiology 2014; 24: 507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narla SN, Nie H, Li Y, Sun X-L. Multi-dimensional glycan microarrays with glyco-macroligands. Glycoconj J 2015; 32: 483–95. [DOI] [PubMed] [Google Scholar]
- 30.Dyukova VI, Shilova NV, Galanina OE, Rubina AY, Bovin NV. Design of carbohydrate multiarrays. Biochim Biophys Acta 2006; 1760: 603–9. [DOI] [PubMed] [Google Scholar]
- 31.Dyukova VI, Dementieva EI, Zubtsov DA, Galanina OE, Bovin NV, Rubina AY. Hydrogel glycan microarrays. Anal Biochem 2005; 347: 94–105. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Lei Z, Liu F, Liu D, Wang Z. Fabricating three-dimensional carbohydrate hydrogel microarray for lectin-mediated bacterium capturing. Biosens Bioelectron 2014; 58: 92–100. [DOI] [PubMed] [Google Scholar]
- 33.Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. Multivalent carbohydrate recognition on a glycodendrimer-functionalized flow-through chip. ChemBioChem 2008; 9: 1836–44. [DOI] [PubMed] [Google Scholar]
- 34.Parera Pera N, Branderhorst HM, Kooij R, Maierhofer C, van der Kaaden M, Liskamp RMJ, Wittman V, Ruijtenbeek R, Pieters RJ. Rapid screening of lectins for multivalency effects with a glycodendrimer microarray. ChemBioChem 2010; 11: 1896–904. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Gildersleeve JC. General procedure for the synthesis of neoglycoproteins and immobilization on epoxide-modified glass slides. Methods Mol Biol 2012; 808: 155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novoa A, Eierhoff T, Topin J, Varrot A, Barluenga S, Imberty A, Römer W, Winssinger N. A LecA ligand identified from a galactoside-conjugate array inhibits host cell invasion by Pseudomonas aeruginosa. Angew Chemie Int Ed Engl 2014; 53: 8885–9. [DOI] [PubMed] [Google Scholar]
- 37.Wittman V, Pieters RJ. Bridging lectin binding sites by multivalent carbohydrates. Chem Soc Rev 2013; 42: 4492–503. [DOI] [PubMed] [Google Scholar]
- 38.Narla SN, Sun X-L. Immobilized sialyloligo-macroligand and its protein binding specificity. Biomacromolecules 2012; 13: 1675–82. [DOI] [PubMed] [Google Scholar]
- 39.Huang ML, Cohen M, Fisher CJ, Schooley RT, Gagneux P, Godula K. Determination of receptor specificities for whole influenza viruses using multivalent glycan arrays. Chem Commun 2015; 51: 5326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed Engl 1998; 37: 2754–94. [DOI] [PubMed] [Google Scholar]
- 41.Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol 2004; 11: 1701–7. [DOI] [PubMed] [Google Scholar]
- 42.Soto GE, Hultgren SJ. Bacterial adhesions: common themes and variation in architecture and assembly. J Bacteriol 1999; 181: 1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barth KA, Coullerez G, Nilsson LM, Castelli R, Seeberger PH, Vogel V, Textor M. An engineered mannoside presenting platform: Escherichia coli adhesion under static and dynamic conditions. Adv Funct Mater 2008; 18: 1459–69. [Google Scholar]
- 44.Zhu X-Y, Holtz B, Wang Y, Wang L-X, Orndorff PE, Guo A. Quantitative glycomics from fluidic glycan microarrays. J Am Chem Soc 2009; 131: 13646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsson LM, Thomas WE, Trintchina E, Vogel V, Sokurenko EV. Catch bond-mediated adhesion without a shear threshold: trimannose versus monomannose interactions with the FimH adhesin of Escherichia coli. J Biol Chem 2006; 281: 16656–63. [DOI] [PubMed] [Google Scholar]
- 46.Gaunitz S, Liu J, Nilsson A, Karlsson N, Holgersson J. Avian influenza H5 hemagglutinin binds with high avidity to sialic acid on different O-linked core structures on mucin-type fusion proteins. Glycoconj J 2014; 31: 145–59. [DOI] [PubMed] [Google Scholar]
- 47.Shen L, Wang Y, Lin C-I, Liu H-W, Guo A, Zhu X-Y. Membrane environment can enhance the interaction of glycan binding protein to cell surface glycan receptors. ACS Chem Biol 2014; 9: 1877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spevak W, Nagy JO, Charych DH, Schaefer ME, Gibert JH, Bednarski MD. Polymerized liposomes containing C-glycosides of sialic acid: potent inhibitors of influenza virus in vitro infectivity. J Am Chem Soc 1993; 115: 1146–7. [Google Scholar]
- 49.Gamian A, Chomik M, Laferriere GA, Roy R. Inhibition of influenza A virus hemagglutinin and induction of interferon by synthetic sialylated glycoconjugates. Can J Microbiol 1991; 37: 233–7. [DOI] [PubMed] [Google Scholar]
- 50.Nagy JO, Wang P, Gilbert JH, Schaefer ME, Hill TG, Callstrom MR, Bednarski MD. Carbohydrate materials bearing neuraminidase-resistant C-glycosides of sialic acid strongly inhibit the in vitro infectivity of influenza virus. J Med Chem 1992; 35: 4501–2. [DOI] [PubMed] [Google Scholar]
- 51.Mochalova LV, Tuzikov AB, Marinia VP, Gambaryan AS, Byramovin NE, Bovin NV, Matrosovich MN. Synthetic polymeric inhibitors of influenza virus receptor-binding activity suppress virus replication. Antiviral Res 1994; 23: 179–190. [DOI] [PubMed] [Google Scholar]
- 52.Matrosovich MN, Mochalova LV, Marinina VP, Bryamova NE, Tuzikov AB, Bovin NV. Synthetic polymeric sialoside inhibitors of Influenza virus receptor-binding activity. FEBS Lett 1990; 272: 209–12. [DOI] [PubMed] [Google Scholar]
- 53.Kingergy-Wood JE, Williams KW, Sigal GB, Whitesides GM. The agglutination of erythrocytes by influenza virus is strongly inhibited by liposomes incorporating an analog of sialyl gangliosides. J Am Chem Soc 1992; 114: 7303–5. [Google Scholar]
- 54.Spaltenstein A, Whitesides GM. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus. J Am Chem Soc 1991; 113: 686–7. [DOI] [PubMed] [Google Scholar]
- 55.Mammen M, Dahmann G, Whitesides GM. Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups: Insight into mechanism of inhibition. J Med Chem 1995; 38: 4179–90. [DOI] [PubMed] [Google Scholar]
- 56.Lees WJ, Spaltenstein A, Kingery-Wood JE, Whitesides GM. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: multivalency and steric stabilization of particulate biological systems. J Med Chem 1994; 37: 3419–33. [DOI] [PubMed] [Google Scholar]
- 57.Sparks MA, Williams KW, Whitesides GM. Neuraminidase-resistant hemagglutination inhibitors: acrylamide copolymers containing a C-glycoside of N-acetylneuraminic acid. J Med Chem 1993; 36: 778–83. [DOI] [PubMed] [Google Scholar]
- 58.Sigal GB, Mammen M, Dahmann G, Whitesides GM. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus—the strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J Am Chem Soc 1996; 118: 3789–800. [Google Scholar]
- 59.Matrosovich M, Klenk H-D. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev Med Virol 2003; 13: 85–97. [DOI] [PubMed] [Google Scholar]
- 60.Carlescu I, Scutaru D, Popa M, Uglea CV. Synthetic sialic-acid-containing polyvalent antiviral inhibitors. Med Chem Res 2009; 18: 477–94. [Google Scholar]
- 61.Papp I, Sieben C, Sisson AL, Kostka J, Böttcher C, Ludwig K, Herrmann A, Haag R. Inhibition of influenza virus activity by multivalent glycoarchitectures with matched sizes. ChemBioChem 2011; 12: 887–95. [DOI] [PubMed] [Google Scholar]
- 62.Waldmann M, Jirmann R, Hoelscher K, Wienke M, Niemeyer FC, Rehders D, Meyers B. A nanomolar multivalent ligand as entry inhibitor of the hemagglutinin of avian influenza. J Am Chem Soc 2014; 136: 783–8. [DOI] [PubMed] [Google Scholar]
- 63.Hidari KI, Murata T, Yoshida K, Takahashi Y, Minamijima Y-H, Miwa Y, Adachi S, Ogata M, Usui T, Suzuki Y, Suzuki T. Chemoenzymatic synthesis, characterization, and application of glycopolymers carrying lactosamine repeats as entry inhibitors against influenza virus infection. Glycobiology 2008; 18: 779–88. [DOI] [PubMed] [Google Scholar]
- 64.Papp I, Sieben C, Ludwig K, Roskamp M, Böttcher C, Schlect S, Herrmann A. Inhibition of influenza A virus infection by multivalent sialic acid functionalized gold particles. Small 2010; 6: 2900–6. [DOI] [PubMed] [Google Scholar]
- 65.Tollas S, Bereczki I, Borbas A, Batta G, Vanderlinden E, Naesens L, Herczegh P. Synthesis of a cluster-forming sialylthio-D-galactose fullerene conjugate and evaluation of its interaction with influenza virus hemagglutinin and neuraminidase. Bioorg Med Chem Lett 2014; 24: 2420–3. [DOI] [PubMed] [Google Scholar]
- 66.Zevgiti S, Zabala JG, Darji A, Dietrich U, Panou-Pomonis E, Sakarellos-Daitsiotis M. Sialic acid and sialyl-lactose glycol-conjugates: design, synthesis and binding assays to lectins and swine influenza H1N1 virus. J Pept Sci 2012; 18: 52–8. [DOI] [PubMed] [Google Scholar]
- 67.Cohen M, Zhang X-Q, Senaati H, Chen H-W, Varki NM, Schooley RT, Gagneux Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 2013; 10: 321–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 2012; 13: 1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta 2006; 1760: 527–37. [DOI] [PubMed] [Google Scholar]
- 70.Imberty A, Chabre YM, Roy R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chem Eur J 2008; 14: 7490–9. [DOI] [PubMed] [Google Scholar]
- 71.Bernardi A, Jimenez-Barbero J, Casnati A, De Castro C, Darbre T, Fieschi F, Finne J, Funken H, Jaeger K-E, Lahmann M, Lindhorst TK, Marradi M, Messner P, Molinaro A, Murphy PV, Nativi C, Oscarson S, Penades S, Peri F, Pieters RJ, Renaudet O, Reymond J-L, Richichi B, Rojo J, Sansone F, Schaffer C, Turnbull WB, Velasco-Torrijos T, Vidal S, Vincent S, Wennekes T, Zuilhof H, Imberty A. Multivalent glycoconjugates as anti-pathogenic agents. Chem Soc Rev 2013; 42: 4709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cecioni S, Imberty A, Vidal S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem Rev 2015; 115: 525–61. [DOI] [PubMed] [Google Scholar]
- 73.Chabre YM, Giguère D, Blanchard B, Rodrigue J, Rocheleau S, Neault M, Rauthu S, Papadopoulos A, Arnold AA, Imberty A, Roy R. Combining glycomimetic and multivalent strategies toward designing potent bacterial lectin inhibitors. Chem Eur J 2011; 17: 6545–62. [DOI] [PubMed] [Google Scholar]
- 74.Dondoni A, Marra A. Calixarene and calixresorcarene glycosides: their synthesis and biological applications. Chem Rev 2010; 110: 4949−77. [DOI] [PubMed] [Google Scholar]
- 75.Cecioni S, Lalor R, Blanchard B, Praly JP, Imberty A, Matthews SE, Vidal S. Achieving high affinity towards a bacterial lectin through multivalent topological isomers of calix[4]arene glycoconjugate. Chem Eur J 2009; 15: 13232−40. [DOI] [PubMed] [Google Scholar]
- 76.Cecioni S, Praly JP, Matthews SE, Wimmerová M, Imberty A, Vidal S. Rational design and synthesis of optimized glycoclusters for multivalent lectin carbohydrate interactions: influence of the linker arm. Chem Eur J 2012; 18: 6250−63. [DOI] [PubMed] [Google Scholar]
- 77.Boukerb AM, Rousset A, Galanos N, Méar J-B, Thépaut M, Grandjean T, Gillon E, Cecioni S, Abderrahmen C, Faure K, Redelberger D, Kipnis E, Dessein R, Havet S, Darblade B, Matthews SE, de Bentzmann S, Guéry B, Cournoyer B, Imberty A, Vidal S. Antiadhesive properties of glycoclusters against Pseudomonas aeruginosa lung infection. J Med Chem 2014; 57: 10275–89. [DOI] [PubMed] [Google Scholar]
- 78.Reynolds M, Marradi M, Imberty A, Penadés S, Pérez S. Multivalent gold glycoclusters: high affinity molecular recognition by bacterial lectin PA-IL. Chemistry 2012; 18: 4264–73. [DOI] [PubMed] [Google Scholar]
- 79.Vincent SP, Buffet K, Nierengarten I, Imberty A, Nierengarten JF. Biologically active hetero-glycoclusters constructed on a pillar[5]arene-containing [2]rotaxane scaffold. Chem Eur J 2016; 4: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy R, Shiao TC, Rittenhouse-Olson K. Glycodendrimers—versatile tools for nanotechnology. BJPS 2013; 49: 85–108. [Google Scholar]
- 81.Bergmann M, Michaud G, Visini R, Jin X, Gillon E, Stocker A, Imberty A, Darbre T, Reymond J-L. Multivalency effects on Pseudomonas aeruginosa biofilm inhibition and dispersal by glycopeptide dendrimers targeting lectin LecA. Org Biomol Chem 2016; 14: 138–48. [DOI] [PubMed] [Google Scholar]
- 82.Michaud G, Visini R, Bergmann M, Salerno G, Bosco R, Gillon E, Richichi B, Nativi C, Imberty A, Stocker A, Darbre T, Reymond J-L. Overcoming antibiotic resistance in Pseudomonas aeruginosa biofilms using glycopeptide dendrimers. Chem Sci 2016; 7: 166–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reymond JL, Bergmann M, Darbre T. Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors. Chem Soc Rev 2013; 42: 4814–22. [DOI] [PubMed] [Google Scholar]
- 84.Buffet K, Gillon E, Holler M, Nierengarten JF, Imberty A, Vincent SP. Fucofullerenes as tight ligands of RSL and LecB, two bacterial lectins. Org Biomol Chem 2015; 13: 6482–92. [DOI] [PubMed] [Google Scholar]
- 85.Caldara M, Friedlander RS, Kavanaugh NL, Aizenberg J, Foster KR, Ribbeck K. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr Biol 2012; 22: 2325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan X, Sivignon A, Yamakawa N, Crepet A, Travelet C, Borsali R, Dumych T, Li Z, Bilyy R, Deniaud D, Fleury E, Barnich N, Darfeuille-Michaud A, Gouin SG, Bouckaert J, Bernard J. Glycopolymers as antiadhesives of E. coli strains inducing inflammatory bowel diseases. Biomacromolecules 2015; 16: 1827–36. [DOI] [PubMed] [Google Scholar]
- 87.Lee D-W, Kim T, Park I, -S, Huang Z, Lee M. Multivalent nanofibers of a controlled length: regulation of bacterial cell agglutination. J Am Chem Soc 2012; 134: 14722–25. [DOI] [PubMed] [Google Scholar]
- 88.Ryu J-H, Lee E, Lim Y-B, Lee M. Carbohydrate-coated supramolecular structures: transformation of nanofibers into spherical micelles triggered by guest encapsulation. J Am Chem Soc 2007; 129: 4808–14. [DOI] [PubMed] [Google Scholar]
- 89.Branson TR, Turnbull WB. Bacterial toxin inhibitors based on multivalent scaffolds. Chem Soc Rev 2013; 42: 4613–22. [DOI] [PubMed] [Google Scholar]
- 90.Polizzotti BD, Kiick KL. Effects of polymer structure on the inhibition of cholera toxin by linear polypeptide-based glycopolymers. Biomacromolecules 2006; 7: 483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joosten JAF, Loimaranta V, Appeldoorn CCM, Haataja S, El Maate FA, Liskamp RMJ, Finne J, Pieters PJ. Inhibition of Streptococcus suis adhesion by dendritic galabiose compounds at low nanomolar concentration. J Med Chem 2004; 47: 6499–6508. [DOI] [PubMed] [Google Scholar]
- 92.Branderhorst HM, Kooij R, Salminen A, Jongeneel LH, Arnusch CJ, Liskamp RM, Finne J, Pieters RJ. Synthesis of multivalent Streptococcus suis adhesion inhibitors by enzymatic cleavage of polygalacturonic acid and ‘click’ conjugation. Org Biomol Chem 2008; 6: 1425–34. [DOI] [PubMed] [Google Scholar]
- 93.Ravdin JI, Stanley P, Murphy CF, Petri WA., Jr Characterization of cell surface carbohydrate receptors for Entamoeba histolytica adherence lectin. Infect Immun 1989; 57: 2179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adler P, Woods SJ, Lee YC, Lee RT, Petri WA, Jr, Schnaar RL. High affinity binding of the Entamoeba histolytica lectin to polyvalent N-acetylgalactosaminides. J Biol Chem 1995; 270: 5164–71. [DOI] [PubMed] [Google Scholar]
- 95.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 96.Spiegel DA. Synthetic immunology to engineer human immunity. Nat Chem Biol 2010; 6: 871–2. [DOI] [PubMed] [Google Scholar]
- 97.Geering B, Fussenegger M. Synthetic immunology: modulating the human immune system. Trends Biotechnol 2015; 33: 65–79. [DOI] [PubMed] [Google Scholar]
- 98.Yu K, Lai BF, Foley JH, Krisinger MJ, Conway EM, Kizzhakkedathu JN. Modulation of complement activation and amplification on nanoparticle surfaces by glycopolymer conformation and chemistry. ACS Nano 2014; 8: 7687–703. [DOI] [PubMed] [Google Scholar]
- 99.Geng J, Mantovani G, Tao L, Nicolas J, Chen G, Wallis R, Mitchell DA, Johnson BR, Evans SD, Haddleton DM. Site-directed conjugation of “clicked” glycopolymers to form glycoprotein mimics: binding to mammalian lectin and induction of immunological function. J Am Chem Soc 2007; 129: 15156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi R, Fink GR. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2007; 2: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol 1994; 152: 4070–9. [PubMed] [Google Scholar]
- 102.Mahla RS, Reddy MC, Prasad DV, Kumar H. Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol 2013; 4: 248–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotaling NA, Cummings RD, Ratner DM, Babensee JE. Molecular factors in dendritic cell responses to adsorbed glycoconjugates. Biomaterials 2014; 35: 5862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I, Mireskandri K, Luthret P, Duncan R, Patterson S, Khaw P, Brocchini S. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol 2004; 22: 977–84. [DOI] [PubMed] [Google Scholar]
- 105.Barata TS, Teo I, Brocchini S, Zloh M, Shaunak S. Partially glycosylated dendrimers block MD-2 and prevent TLR4-MD-2-LPS complex mediated cytokine responses. PLOS Comput Biol 2011; 7: e1002095–e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cummings RD, McEver RP. C-type lectins. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (eds). Essentials of Glycobiology, 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2009. Chapter 31. [PubMed] [Google Scholar]
- 107.Varga N, Sutkeviciute I, Ribeiro-Viana R, Berzi A, Ramdasi R, Daghetti A, Veloretti G, Amara A, Clerici M, Rojo J, Fieschi F, Bernardi A. A multivalent inhibitor of the DC-SIGN dependent uptake of HIV-1 and Dengue virus. Biomaterials 2014; 35: 4175–84. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Zhang Y, Wei J, Zhang X, Zhang B, Zhu Z, Zou W, Wang Y, Mou Z, Ni B, Wu Y. Lewis X oligosaccharides targeting to DC-SIGN enhanced antigen-specific immune response. Immunology 2007; 121: 174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munoz A, Sigwalt D, Illescas BM, Luczkowiak J, Rodriguez-Perez L, Nierengarten I, Holler M, Remy J-S, Buffet K, Vincent SP, Rojo J, Delgado R, Nierengarten J-F, Martin N. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat Chem 2016; 8: 50–7. [DOI] [PubMed] [Google Scholar]
- 110.Angata T, Nycholat CM, Macauley MS. Therapeutic targeting of Siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci 2015; 36: 645–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA 1998; 95: 7469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, Bovin N, Paulson JC. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol 2006; 177: 2994–3003. [DOI] [PubMed] [Google Scholar]
- 113.Rillahan CD, Schwartz E, McBride R, Fokin VV, Paulson JC. Click and pick: identification of sialoside analogues for siglec-based cell targeting. Angew Chem Int Ed Engl 2012; 51: 11014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rillahan CD, Schwartz E, Rademacher C, McBride R, Rangarajan J, Fokin VV, Paulson JC. On-chip synthesis and screening of a sialoside library yields a high affinity ligand for Siglec-7. ACS Chem Biol 2013; 8: 1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One 2012; 7: e39039–e39039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Courtney AH, Bennett NR, Zwick DB, Hudon J, Kiessling LL. Synthetic antigens reveal dynamics of BCR endocytosis during inhibitory signaling. ACS Chem Biol 2014; 9: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoinvasion. Nat Chem Biol 2014; 10: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanders WJ, Gordon EJ, Dwir O, Beck PJ, Alon R, Kiessling LL. Inhibition of L-selectin-mediated leukocyte rolling by synthetic glycoprotein mimics. J Biol Chem 1999; 274: 5271–8. [DOI] [PubMed] [Google Scholar]
- 119.Rele SM, Cui W, Wang L, Hou S, Barr-Zarse G, Tatton D, Gnanou Y, Esko JD, Chaikof EL. Dendrimer-like PEO glycopolymers exhibit anti-inflammatory properties. J Am Chem Soc 2005; 127: 10132–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations. Nat Rev Drug Discov 2010; 9: 308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III Pneumococcus with foreign protein. J Exp Med 1931; 54: 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus. J Virol 1996; 70: 1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 2003; 300: 2065–71. [DOI] [PubMed] [Google Scholar]
- 124.Horiya S, MacPherson IS, Krauss IJ. Recent strategies targeting HIV glycans in vaccine design. Nat Chem Biol 2014; 10: 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang L-X. Synthetic carbohydrate antigens for HIV vaccine design. Curr Opin Chem Biol 2013; 17: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krauss IJ, Joyce JG, Finnefrock AC, Song HC, Dudkin VY, Geng X, Warren JD, Chastain M, Shiver JW, Danishefsky SJ. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J Am Chem Soc 2007; 129: 11042–4. [DOI] [PubMed] [Google Scholar]
- 127.Wang J, Li H, Zou G, Wang LX. Novel template-assembled oligosaccharide clusters as epitope mimics for HIV-neutralizing antibody 2G12. Design, synthesis, and antibody binding study. Org Biomol Chem 2007; 5: 1529–40. [DOI] [PubMed] [Google Scholar]
- 128.Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, Wong CH. Targeting the carbohydrates on HIV-1: interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci USA 2008; 105: 3690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Doores KJ, Fulton Z, Hong V, Patel MK, Scanlan CN, Wormald MR, Finn MG, Burton DR, Wilson IA, Davis BG. A nonself sugar mimic of the HIV glycan shield shows enhanced antigenicity. Proc Natl Acad Sci USA 2010; 107: 17107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Astronomo RD, Kaltgrad E, Udit AK, Wang SK, Doores KJ, Huang CY, Pantophlet R, Paulson JC, Wong CH, Finn MG, Burton DR. Defining criteria for oligomannose immunosense for HIV using icosahedral virus capsid scaffolds. Chem Biol 2010; 17: 357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Novoa A, Winssinger N. DNA display of glycoconjugates to emulate oligomeric interactions of glycans. Beilstein J Org Chem 2015; 11: 707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gorska K, Huang K-T, Chaloin O, Winssinger N. DNA-templated homo- and heterodimerization of peptide nucleic acid encoded oligosaccharides that mimick the carbohydrate epitope of HIV. Angew Chem Int Ed 2009; 48: 7695–700. [DOI] [PubMed] [Google Scholar]
- 133.Ribero-Viana R, García-Vallejo JJ, Collado D, Pérez-Inestrosa E, Bloem K, van Kooyk Y, Rojo J. BODIPY-labeled DC-SIGN-targeting glycodendrons efficiently internalize and route to lysosomes in human dendritic cells. Biomacromolecules 2012; 13: 3209–19. [DOI] [PubMed] [Google Scholar]
- 134.Ciobanu M, Huang K-T, Daguer J-P, Barluenga S, Chaloin O, Schaeffer E, Mueller CG, Mitchell DA, Winssinger N. Selection of a synthetic glycan oligomer from a library of DNA-templated fragments against DC-SIGN and inhibition of HIV gp120 binding to dendritic cells. Chem Commun 2011; 47: 9321–3. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Q, Collins J, Anastasaki A, Wallis R, Mitchell DA, Becer CR, Haddleton DM. Sequence-controlled multi-block glycopolymers to inhibit DC-SIGN-gp120 binding. Angew Chem Int Ed 2013; 52: 4435–9. [DOI] [PubMed] [Google Scholar]
- 136.Nordly P, Agger EM, Andersen P, Nielsen HM, Foged C. Incorporation of the TLR4 agonist monophosporyl lipid A into the bilayer of DDA/TDB liposomes: physicochemical characterization and induction of CD8+ T-cell responses in vivo. Pharm Res 2011; 28: 553–62. [DOI] [PubMed] [Google Scholar]
- 137.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 2008; 3: e3116–e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gram GJ, Karlsson I, Agger EM, Andersen P, Fomsgaard A. A novel liposome-based adjuvant CAF01 for induction of CD8(+) cytotoxic T-lymphocytes (CTL) to HIV-1 minimal CTL peptides in HLA-A*0201 transgenic mice. PLoS One 2009; 4: e6950–e6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fomsgaard A, Karlsson I, Gram G, Schou C, Tang S, Bang P, Kromann I, Andersen P, Andreasen LV. Development and preclinical safety evaluation of a new therapeutic HIV-1 vaccine based on 18 T-cell minimal epitope peptides applying a novel cationic adjuvant CAF01. Vaccine 2011; 29: 7067–74. [DOI] [PubMed] [Google Scholar]
- 140.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, Agger EM, Andersen P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—a novel adjuvant inducing both strong CMI and antibody responses. Biochem Biophys Acta 2005; 1718: 22–31. [DOI] [PubMed] [Google Scholar]
- 141.Teramoto N, Sachnivala ND, Shibata M. Trehalose and trehalose-based polymers for environmentally benign, biocompatible and bioactive materials. Molecules 2008; 13: 1773–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mancini RJ, Lee J, Maynard HD. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J Am Chem Soc 2012; 134: 8474–9. [DOI] [PubMed] [Google Scholar]
- 143.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005; 202: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paulovicova L, Paulovicova E, Karelin AA, Tsvetkov YE, Nifantiev NE, Bystricky S. Humoral and cell-mediated immunity following vaccination with synthetic Candida cell wall mannan derived heptamannoside-protein conjugate: immunomodulatory properties of heptamannoside-BSA conjugate. Int Immunopharmacol 2012; 14: 179–87. [DOI] [PubMed] [Google Scholar]
- 145.Stefanetti G, Hu Q-Y, Usera A, Robinson Z, Allan M, Singh A, Imase H, Cobb J, Zhai H, Quinn D, Lei M, Saul A, Adamo R, MacLennan CA, Micoli F. Sugar-protein connectivity impacts on the immunogenicity of site-selective Salmonella O-antigen glycoconjugate vaccines. Angew Chem Int Ed 2015; 54: 13198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 2011; 17: 1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]