Abstract
Biomaterials for tissue engineering provide scaffolds to support cells and guide tissue regeneration. Despite significant advances in biomaterials design and fabrication techniques, engineered tissue constructs remain functionally inferior to native tissues. This is largely due to the inability to recreate the complex and dynamic hierarchical organization of the extracellular matrix components, which is intimately linked to a tissue’s biological function. This review discusses current state-of-the-art strategies to control the spatial presentation of physical and biochemical cues within a biomaterial to recapitulate native tissue organization and function.
Keywords: Biomaterials, extracellular matrix, tissue engineering, regenerative medicine, translational, biomedical
Introduction
Tissue engineering is a multidisciplinary field that seeks to develop biological substitutes that replace or regenerate tissue function for lost or damaged tissues and organs.1 Biomaterials designed for tissue engineering have made a significant impact on the field serving as scaffolds to support cells and guide tissue regeneration. One of the key challenges limiting translation and clinical success is designing a scaffold that guides the regeneration of a functional tissue replacement with properties that match those of the biological counterpart. This challenge persists largely because of the inability to recreate the dynamic and complex hierarchical organization of native tissues that is intimately linked to the tissue’s function.2,3 Bone provides an excellent example of how hierarchical organization across length scales translates to function. At the nanoscale, bone is composed of cells surrounded by an abundance of biomolecules that regulate remodeling and reorganization of the tissue microenvironment.4 Collagen fibers are aligned according to the direction of stresses and reinforced by hydroxyapatite mineral, which is critical for the compressive strength and high fracture toughness of bone. Differences in porosity and anatomical shape at the macroscale influence the bulk mechanical properties and vary depending on the required loading conditions of the tissue.4–6 The extracellular matrix (ECM) components surrounding cells in other tissues are also arranged into distinct compositions and structures depending on the specific tissue or organ type and controls numerous processes such as cell migration, proliferation, and differentiation by mediating cell–cell and cell–ECM interactions.7 This spatial organization of physical and biochemical cues leads to higher levels of functionality that cannot be achieved by the individual factors alone. Tissue engineering scaffolds must therefore be designed to recreate the physical and biochemical organization of the ECM across scales in order to reestablish the functional properties of the native tissue.
The key properties of an ideal scaffold have evolved as advances in biomaterials and characterization techniques provide deeper understanding of cell–material interactions and how these interactions affect the resulting engineered tissue construct. Early generations of scaffolds focused primarily on providing structural support for cells and have progressed over time to mimic the ECM structure and mechanics as well as incorporating key bioactive moieties.8,9 Materials have been fabricated from synthetic and natural components using top-down and bottom-up approaches to tune properties such as matrix stiffness, scaffold morphology, surface topography, and chemistry, and incorporation of bioactive molecules in a myriad of ways.10 Similar to biological tissues, the spatial arrangement of these properties adds an additional level of functionality that influences cell behavior. Recent advances in biofabrication techniques and the development of new chemistries have significantly improved strategies to control the spatial arrangement of multiple features and components within a single scaffold.8,11–14 For example, photopatterning techniques can be used to create stiffness gradients within 3D hydrogels by tuning the degree of UV cross-linking, which can modulate cell spreading and proliferation on a single sample.15 A similar technique utilizing multiphoton laser light spatially patterned multiple proteins to guide cell migration and differentiation within a single hydrogel.16 Multiphoton patterning can also be combined with cytocompatible bioorthogonal chemistries to dynamically control both mechanical and biochemical signals spatially within a hydrogel in the presence of cells.17 Combining technologies in this fashion introduces the potential to generate complex biomaterials with spatially organized functionalities that mimic native tissues.
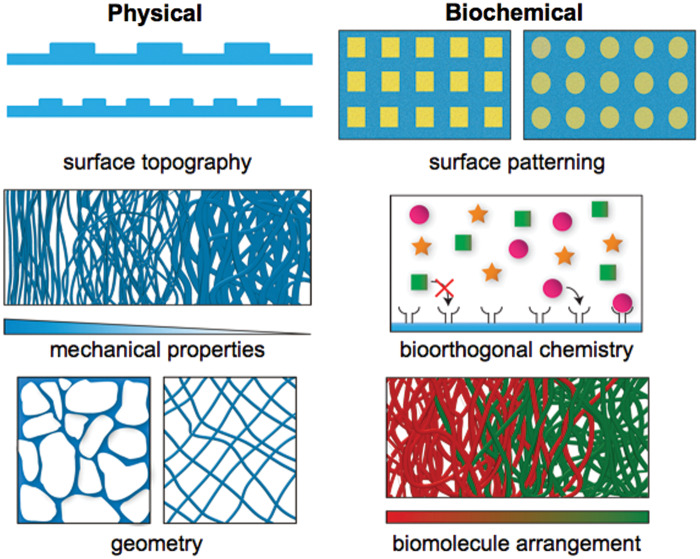
Considering the vast complexity and dynamic nature of biological tissues, it would be impossible to recreate all of their properties in a synthetic scaffold. Biomaterials designed to harness the remarkable regenerative capacity of the human body can provide templates to guide or jumpstart the initial stages of spatial tissue regeneration and then allow the body to regenerate itself.18,19 This raises key research questions to identify which features are critical for each tissue application and how to generate relatively simple yet effective biomaterials.20 Biomaterial-based platforms designed to study these questions will have a significant impact on the field and increase the potential for the clinical success of translational biomaterials. This requires a combination of techniques to create biomimetic scaffolds that mimic both physical and biochemical features of native tissues (Figure 1). Developing such scaffolds will also provide a deeper understanding of how tissue organization is linked to function and introduce versatile platforms for a wide range of applications. This review focuses on some of the state-of-the-art techniques used to spatially control functionality in biomaterials and highlights recent work where both physical and biochemical cues are controlled within a single construct.
Figure 1.
Strategies to control the spatial presentation of physical and biochemical cues in biomaterials for tissue engineering. (A color version of this figure is available in the online journal.)
Physical organization
The ECM provides structural support for cells through hierarchically organized physical and geometric cues that influence cellular processes such as cell adhesion, migration, proliferation, and differentiation.7,21,22 Mechanical properties vary widely across different tissues in vivo, and ECM elasticity has been shown to directly influence cell migration23 and stem cell differentiation.24–26 Cells are able to react to gradual changes in their physical environment as gradients in ECM stiffness can guide cell migration, a process known as durotaxis. Hydrogels with elasticity gradients formed by photopatterning induced fibroblasts to migrate to regions of increasing modulus in vitro.27 Stem cell differentiation is also affected by changes to the rigidity of the cellular microenvironment.24–26 Engler et al. showed that the modulus of a 2D substrate influenced cell traction, morphology, and differentiation of mesenchymal stem cells (MSCs).24 Interestingly, MSCs encapsulated within 3D hydrogels were shown to differentiate in response to matrix stiffness independently of cell morphology, and the matrix stiffness regulated integrin binding and cellular reorganization of the bound adhesion ligands.25 This work demonstrates how cells sense changes in physical cues through changes in the spatial presentation of adhesion ligands at the nanoscale.25 Hydrogels can also be designed to allow cells to degrade their microenvironment, mediating cellular traction that consequently directs stem cell fate.26 Therefore, the physical properties of the scaffold must be spatially and hierarchically controlled in order to mediate desired cell behavior and template functional tissue regeneration. This is particularly useful for directing multiple cell types and directing the formation of heterogeneous tissues within the same construct to more closely mimic native tissues.
Scaffold surface topography, stiffness, and geometry/morphology have been shown to influence cells independently of biochemical signaling.24,28,29 Topographical patterns such as grooves, channels, holes, pillars, and fibers ranging from the nano- to microscale can be created on biomaterial surfaces using fabrication techniques such as photolithography, microcontact printing, and electrospinning.12,30–33 Changing the topographical features is known to induce significant changes to the morphology, orientation, migration, proliferation, and differentiation of various cell types.32,34 Cells are able to sense very subtle changes in topography, such as rectangular channels with sharp corners (microgrooves) versus wavy channels with smooth edges (microwaves) of similar dimensions.35 Techniques that create different topographies with variable local density and anisotropic organization within the same system can be used to understand and control biological processes. For example, Guvendiren and Burdick demonstrated methods to control patterns spatially and dynamically using strain-responsive buckling patterns on poly(dimethylsiloxane) (PDMS) substrates that switch between patterned to flat topographies. These substrates dynamically influenced human MSC shape and alignment and also created anisotropic patterns across a single surface, spatially controlling cell shape and orientation.34 These methods could be used to organize topographical cues that mimic native ECM patterns onto biomaterial surfaces to guide desired cell behavior.
Scaffold geometry also influences cell behavior and should be tailored to the appropriate tissue organization. The structural organization of different tissues is critical for tissue function independent of cells. For example, the depth-dependent density and orientation of collagen fibers in articular cartilage create discrete zones with anisotropic mechanical properties that is necessary for the biomechanical function. Various fabrication techniques such as electrospinning, freeze drying, and porogen leaching have been widely used to generate scaffolds with fibrous, aligned, and porous geometries. Electrospinning is a popular technique that uses electrostatic forces to create fibers with nano- to micrometer diameters in random or aligned orientations.30 Anisotropically structured scaffolds can be formed using advanced electrospinning techniques to sequentially electrospin different fiber sizes and orientations in a continuous construct to mimic the structural organization and mechanical properties of cartilage’s collagen network.36 The resulting tissue engineered constructs mimicked the mechanical properties of native cartilage, illustrating the impact of physical organization on scaffold properties and function. Freeze drying is another technique that can be used to create scaffolds with varying structures. This process, also known as lyophilization, involves cooling a polymer solution below its freezing point to solidify the solvent and force the polymer to aggregate in the interstitial spaces around the frozen solvent. The solvent is then removed by sublimation, and the remaining polymer forms a highly porous scaffold with interconnected pores.10 The scaffold pore size and geometry can be modified by controlling the temperature and solvent conditions. Caliari and Harley have shown that varying the freezing temperature during directional freeze drying can be used to generate anisotropic pore geometries to mimic the native architecture of tendon.37,38 Porogen leaching also creates porosity in scaffolds by distributing a solid porogen within a polymer solution and then removing the solvent to leave behind a polymer scaffold with porogens that can be selectively removed with a different solvent (typically water). Pore size and scaffold geometry can be easily controlled by changing the size and distribution of the porogen.10 Recently, a new technique used rapidly degrading sacrificial gels as porogens within hydrogels to form pores after injection into the host tissue.39 This strategy allowed the elasticity of the hydrogel to be decoupled from pore formation and significantly improved in vivo bone formation. Multiple fabrication techniques can also be combined to create hierarchical structures similar to native tissues. For example, Steele et al. utilized a combination of different methods to create bilayered scaffolds that mimicked structural characteristics of native cartilage.40 Aligned electrospun fibers were directly deposited onto scaffolds formed by porogen leaching, which allowed ample space for chondrocyte ECM production within the scaffold depth and enhanced mechanical properties on the aligned surface.40
Additive manufacturing, or 3D printing, has become a key player for generating more complex scaffold geometries that more closely mimic anatomical tissue structures. This strategy has been discussed extensively in recent reviews.41,42 Briefly, this method involves computer-aided design to control the layer-by-layer direct deposition of materials, allowing for precise control over scaffold architecture with high reproducibility. Scaffolds can be printed into anatomical-like structures, such as ears, noses, meniscuses, and vertebral disks, using computer tomography data or 3D models.43
Biochemical organization
The spatial distribution of ECM biomolecules provides cues to direct tissue organization by mediating cell–cell and cell–ECM interactions that guide migration, growth, and differentiation. Gradients of growth factors, for example, regulate morphogenesis of tissues during development and template ECM organization to create different tissues and organs.44,45 The ECM composition is essential for physiological function and varies in organization depending on the specific tissue. For example, gradients of glycosaminoglycans (GAGs) in articular cartilage significantly affect the tissue’s mechanical and biological function.46,47 Strategies to incorporate biomolecules found in the ECM have been extensively developed and shown to improve a scaffolds biological function. Recent advances in top-down (e.g. photopatterning and microcontact printing) and bottom-up (e.g. microfluidics, self-assembly, electrospinning, and bioprinting) approaches have significantly improved efforts to spatially organize biochemical cues during and/or after scaffold fabrication.
Biofabrication technologies such as microfluidics and photolithography have been used extensively to control the location and density of ECM biomolecules in 2D and 3D.48 Microfluidics involve manipulating small volumes of liquid at the micrometer level and has been widely used to pattern biomolecules.44,49,50 This technique allows for the creation of biomolecule gradients across a surface or within a material. Gradients of photocrosslinkable monomers and signaling molecules can be formed within microfluidic channels and then gelled to immobilize a bioactive gradient to guide cell behavior.51 Recently, Pedron et al. developed a microfluidic-based approach to control spatial gradients of matrix molecules and cells by integrating microfluidic-mixing technologies with hydrogel functionalization approaches.52 This allowed for overlapping patterns of cell, matrix, and biomolecule signals to be formed in a single hydrogel.
Photolithography-based techniques such as microcontact printing and photopatterning can be used to define specific biomolecular patterns, shapes, and gradients. Microcontact printing utilizes physical stamps made from photolithography-made molds to pattern biomolecules onto a substrate. For example, Chen et al. used this technique to control the size and shape of fibronectin on substrates to observe the effect on cell morphology, viability, and proliferation.53 MSCs seeded on patterns with different sizes and shapes had marked effects on differentiation into adipocytes and osteoblasts.29,54 Microcontact printing can also be applied to 3D biomaterials to control cell response and organization on their surfaces.55 These studies illustrate how cells are able to sense and respond to the spatial presentation of ECM molecules at the micrometer level, which is an important consideration for surface functionalizing biomaterials.
Photopatterning offers higher spatial resolution in 2D and 3D than microcontact printing by using light, which can be precisely focused to encapsulate cells and/or immobilize biomolecules of interest into desired patterns or gradients. Mask-based photopatterning involves exposing the material to light through a mask to confine photo-based reactions to specific and desired regions within the sample. Mask-based strategies, however, are generally limited to producing 2D patterns through the depth of a 3D material. An approach involving conventional fluorescent microscopy and a simple photomask was developed to form multilayers of hydrogels that encapsulate multiple cell types within spatially defined 3D structures.56 Cells with a precursor polymer were exposed to a pattern of light to polymerize the hydrogel, and subsequent patterns and cell types were incorporated to create complex and diverse constructs.56 Photopatterning with focused laser light provides greater control to spatial patterning throughout the volume of a 3D material. This technique can be combined with novel photo-based chemistries to control the spatial location of specific cues.16,57,58 The Shoichet laboratory used multiphoton laser light to spatially pattern multiple growth factors within a single hydrogel.16,59 In this work, coumarin-based protecting groups were used as photolabile protecting groups, which were cleaved to expose reactive sulfhydryls in desired locations.16 Recently, a strategy to spatiotemporally control the in vivo presentation of adhesive cues was developed using bioadhesive ligands protected with a novel photolabile cage that could be removed using transdermal light to activate adhesion.58 These methods can easily be customized by incorporating chosen bioactive moieties and arranging them in the desired patterns.
Spatial organization of biochemical cues has been greatly enhanced with the development of bioorthogonal chemistries that provide a versatile and biocompatible technique to organize multiple cues dynamically, selectively, and simultaneously. Proteins and other biomolecules contain a variety of functional groups including amines, alcohols, carboxylic acids, and thiols that can participate in a wide range of reactions, making it difficult to tightly control their specific organization with traditional chemistries. Over the past decade, a set of chemical reactions commonly referred to as “click chemistry” have been developed that can be performed in the presence of cells without unwanted side reactions with native biomolecules.17,60–64 For example, DeForest and Anseth demonstrated that combining two bioorthogonal photochemical reactions, which enabled the reversible spatial presentation of a biological cue, could be performed in the presence of cells to create dynamic, well-defined biochemical gradients within a single hydrogel.17,63 Non-covalent bioorthogonal chemistries can also be utilized for specific and dynamic methods to organize functionality. For example, guest–host pairs involve a larger “host” molecule with a hydrophobic pocket that interacts specifically with a smaller “guest” molecule through molecular recognition.65 These bioorthogonal groups have been used to dynamically control the display of an adhesive peptide sequence within a hydrogel.66 The rapidly growing toolbox of bioorthogonal chemistries, which have been recently and extensively reviewed by others,14,60,61,65 introduces promising and versatile methods to improve control over the spatiotemporal organization of biomolecules in biomaterials.
Bottom-up approaches also offer improved control over biochemical organization in 3D by combining building blocks to construct biomaterials into larger structures. One major benefit to this strategy is that modular building blocks can be modified, interchanged, and assembled to form constructs with diverse compositions and arrangements. Self-assembling systems based on peptides, carbohydrates, and nucleic acids building blocks can also be exploited to form dynamic scaffolds displaying these functional moieties.67–69 These building blocks can be rationally designed to form various structures ranging from the nano- to micrometer scale. A class of peptide amphiphiles (PAs) developed in the Stupp laboratory self-assemble into nanofibers displaying the peptide sequence and can network into self-supporting hydrogels.70,71 Changing the amino acid sequence and mixing different PAs together allow this platform to be tailored for a wide range of tissue engineering and regenerative medicine applications.67,69 Interestingly, these PA molecules have been further manipulated to generate structures and architectures with hierarchical ordering, such as highly aligned nanofiber hydrogels.72,73 PA nanofibers can also be co-assembled with polyelectrolytes of opposite charge, such as GAGs, to form hierarchically ordered, hybrid component hydrogels.74–78 The structural and biochemical organization of these hydrogels can be altered by controlling the interaction between the different components77 or applying an electric field,78 which also affect the bulk mechanical properties.76
Self-assembling systems can also be rationally designed to precisely control intermixing and composition of multiple components. The Collier laboratory developed a platform based on peptides with propensity to form β-sheet-rich fibrils that could be manipulated to control the degree of intermixing of different self-assembling peptides. This strategy introduces the potential to create hydrogels with discrete or intermixed fibrils to control the spatial display of bioactive cues.79 Different functional proteins modified with the β-sheet-peptide could be inserted into peptide nanofibers alone or in combination at controllable concentrations, resulting in the ability to form controlled and predictable protein compositions.80 These strategies illustrate potential methods to create well-defined dynamic systems with hierarchical structures.
Combining physical and biochemical cues
Simultaneously controlling the spatial organization of both physical and chemical cues creates a powerful platform to study how these signals can synergistically affect cell behavior and function. Many of the strategies discussed involve versatile techniques that can be modified or combined in various ways to generate both physical and biochemical cues within a single construct. For example, photopatterning techniques can be used to spatially control the cross-linking density and confine matrix compositions to generate mechanical and biomolecule gradients in a hydrogel to mimic the zones of articular cartilage.46 Directional freeze drying can also be combined with layering methods to create coincident gradients of mineralization and geometric anisotropy within a single collagen–GAG scaffold to recreate the native osteotendinous junction.81
In particular, bottom-up approaches merged with top-down techniques exploit the advantages of each strategy at multiple length scales. Multiple building blocks can be integrated together using various fabrication methods to control the spatial organization of the components and the scaffold architecture. The following examples demonstrate only a few of the vast number of combinations that could be utilized to generate hierarchically organized biomaterial: collagen–chitosan microbeads assembled into larger scale constructs using vacuum molding and centrifugation to control their spatial organization throughout the scaffold volume;82 self-assembling peptides bioprinted83 or electrospun84 to form dynamic nanofibrous scaffolds with greater complexity; and pre-functionalized polymers electrospun to spatially pattern biochemical signals while generating nanofibrous geometries.85 In the last example, Wade et al. electrospun a norbornene-functionalized hyaluronic acid, which was later spatially cross-linked and functionalized with biomolecules via thiol-ene-based chemistry using mask-based photolithography.85 This concept could be expanded with bioorthogonal chemistries to add additional levels of complexity and control.
The spatial organization of pre-functionalized polymers can also be directly controlled using sequential electrospinning techniques to create fibrous scaffolds with defined gradients. By modulating the flow rate of syringe pumps, distinct solutions of different polymer precursor solutions can be cross-linked to create mechanical and adhesive gradients within one electrospun scaffold.86 Recently, polymers functionalized with two different GAG-binding peptides were electrospun into opposing gradients that dynamically guided the different GAGs into gradients throughout the scaffold.47 This same platform was used to create scaffolds with zonally discrete functions.87 Polymers functionalized with either a cell adhesive cyclic Arg-Gly-Asp-Ser (RGDS) peptide or a polymerization-initiating group were sequentially electrospun into a single scaffold. The polymerization-initiating group was used to graft an antifouling polymer bottle brush based on poly(ethylene glycol) using controlled radical polymerization to create dual functionality.87 This strategy offers a versatile platform to spatially present multiple functional cues within a single scaffold and could be combined with other techniques to organize other functionalities. For example, different peptide–polymer conjugates could be incorporated while changing the fiber morphology to form a scaffold with structural, mechanical, and biomolecule gradients.
Three-dimensional printing has recently expanded to more cell and biomolecule-friendly approaches to print cells and biomolecules into desired structures with high precision. This strategy, also known as bioprinting, has rapidly emerged as a promising technology to produce complex scaffold structures with spatial control over cell and biomolecule distribution.41 Novel decellularized ECM bioinks and cells were bioprinted into well-defined, cell-laden constructs, creating optimal 3D tissue templates.88 Combining bioprinting with sophisticated materials can further expand its potential for printing organ-like structures. For example, shear-thinning supramolecular hydrogels can be used as inks for continuous and direct writing into a self-healing support hydrogel.89 This technique combines dynamic supramolecular chemistries with advanced bioprinting to create precisely organized heterogeneous 3D structures that could be modified temporally.89 Hinton et al. recently developed a novel method to bioprint complex 3D biological structures composed of soft protein and polysaccharide hydrogels. Their technique termed freeform reversible embedding of suspended hydrogels (FRESH) uses a thermoreversible support hydrogel to template the 3D geometry of an embedded hydrogel. FRESH enables spatial printing of multiple biologically relevant materials into a 3D hydrogel.90 In addition, integrating microfluidic technology with bioprinting allows for cell-laden bioinks to be printed and gelled rapidly and precisely to create heterogeneous 3D structures.91
Current limitations and future outlook
As discussed throughout this review, the spatial presentation of physical and chemical features across length scales can significantly influence cell behavior and tissue regeneration. An ideal biomaterial should provide hierarchical cues that specifically guide cell and tissue organization to enhance biological function. However, the ability to achieve well-defined spatial control of functionality across these dimensions within the same 3D construct remains a considerable challenge. For example, some of the techniques that offer high spatial resolution in 2D cannot be implemented as successfully in 3D. Recent and ongoing advances in 3D printing and novel printable materials show great promise for spatially controlling structural, mechanical, and biochemical properties. Creating increasingly realistic tissue architectures allows us to shift the focus to identifying the essential properties that must be mimicked in order to generate functional tissues. The challenge for the biomaterials field is therefore to develop strategies that combine different materials, chemistries, and techniques in new ways to create the most effective scaffold construct for each application. Biomaterial platforms designed to investigate such questions will deepen understanding of how tissue organization is related to biological function and improve the potential for clinically relevant applications.
Conclusion
The versatile strategies described in this review have been used to form 3D biomaterials for tissue engineering with a wide range of structures and functions. The combination of different top-down and bottom-up technologies offer a promising approach to control the spatial organization of both physical and biochemical cues within a single construct. Biomaterials that can template the physical architecture and chemical composition of biological tissues introduce powerful platforms to engineer functional tissues with clinical potential. These modular systems can also be used to investigate the structure–function relationships in biological tissues to deepen understanding about the ECM and identify key features critical for regenerating different tissues. The knowledge gained can be applied to the rational design of the next generation of biomaterials to push the field of tissue engineering forward and open new opportunities to exploit the regenerative potential of the human body.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by startup funds from Lehigh University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. The authors acknowledge Dr E. Thomas Pashuck for useful discussions and editing the manuscript.
Authors’ contributions
LWC and JFF participated in the design and writing of the manuscript. LWC had primary responsibility for its final content.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science Adv 1993; 260: 920–6. [DOI] [PubMed] [Google Scholar]
- 2.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science 2005; 310: 1135–8. [DOI] [PubMed] [Google Scholar]
- 3.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater 2009; 8: 457–70. [DOI] [PubMed] [Google Scholar]
- 4.Stevens MM. Biomaterials for bone tissue engineering. Mater Today 2008; 11: 18–25. [Google Scholar]
- 5.Bonzani IC, George JH, Stevens MM. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol 2006; 10: 568–75. [DOI] [PubMed] [Google Scholar]
- 6.Leong KF, Chua CK, Sudarmadji N, Yeong WY. Engineering functionally graded tissue engineering scaffolds. J Mech Behav Biomed Mater 2008; 1: 140–52. [DOI] [PubMed] [Google Scholar]
- 7.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 2014; 15: 771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tibbitt MW, Rodell CB, Burdick JA, Anseth KS. Progress in material design for biomedical applications. Proc Natl Acad Sci USA 2015; 112: 14444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005; 23: 47–55. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 2014; 16: 247–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun 2012; 3: 1269–1269. [DOI] [PubMed] [Google Scholar]
- 12.Abbah SA, Delgado LM, Azeem A, Fuller K, Shologu N, Keeney M, Biggs MJ, Pandit A, Zeugolis DI. Harnessing hierarchical nano- and micro-fabrication technologies for musculoskeletal tissue engineering. Adv Healthcare Mater 2015; 4: 2488–99. [DOI] [PubMed] [Google Scholar]
- 13.Jia X, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol Biosci 2009; 9: 140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Dicker KT, Jia X. Modular and orthogonal synthesis of hybrid polymers and networks. Chem Commun (Camb) 2015; 51: 5218–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marklein RA, Burdick JA. Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter 2009; 6: 136–136. [Google Scholar]
- 16.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater 2011; 10: 799–806. [DOI] [PubMed] [Google Scholar]
- 17.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem 2011; 3: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med 2012; 4: 160sr4–160sr4. [DOI] [PubMed] [Google Scholar]
- 19.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthcare Mater 2012; 2: 57–71. [DOI] [PubMed] [Google Scholar]
- 20.Kyburz KA, Anseth KS. Synthetic mimics of the extracellular matrix: how simple is complex enough? Ann Biomed Eng 2015; 43: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janson IA, Putnam AJ. Extracellular matrix elasticity and topography: material-based cues that affect cell function via conserved mechanisms. J Biomed Mater Res 2015; 103: 1246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol 2014; 15: 813–24. [DOI] [PubMed] [Google Scholar]
- 23.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 1997; 94: 13661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–89. [DOI] [PubMed] [Google Scholar]
- 25.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010; 9: 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013; 12: 458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidoaki S, Matsuda T. Microelastic gradient gelatinous gels to induce cellular mechanotaxis. J Biotechnol 2008; 133: 225–30. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Staunton JR, Tanner K. Independent control of topography for 3D patterning of the ECM microenvironment. Adv Mater Weinheim 2015; 28: 132–37. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6: 483–95. [DOI] [PubMed] [Google Scholar]
- 30.Sill TJ, Recum von HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 2008; 29: 1989–2006. [DOI] [PubMed] [Google Scholar]
- 31.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nature Nanotechnol 2011; 6: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae H, Chu H, Edalat F, Cha JM, Sant S, Kashyap A, Ahari AF, Kwon CH, Nichol JW, Manoucheri S, Zamanian B, Wang Y, Khademhosseini A. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J Tissue Eng Regen Med 2014; 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D-H, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol 2012; 197: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guvendiren M, Burdick JA. Stem cell response to spatially and temporally displayed and reversible surface topography. Adv Healthcare Mater 2013; 2: 155–64. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Hardy C, Chen C-M, Yang S, Voloshin AS, Liu Y. Enhanced cell adhesion and alignment on micro-wavy patterned surfaces. PLoS One 2014; 9: e104502–e104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCullen SD, Autefage H, Callanan A, Gentleman E, Stevens MM. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng Part A 2012; 18: 2073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caliari SR, Harley BAC. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011; 32: 5330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caliari SR, Harley BAC. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv Healthcare Mater 2014; 3: 1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater 2015; 14: 1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steele JAM, McCullen SD, Callanan A, Autefage H, Accardi MA, Dini D, Stevens MM. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater 2014; 10: 2065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandrycky C, Wang Z, Kim K, Kim D-H. 3D bioprinting for engineering complex tissues. Biotechnol Adv –2015. Epub ahead of print 23 December. DOI: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandyopadhyay A, Bose S, Das S. 3D printing of biomaterials. MRS Bull 2015; 40: 108–15. [Google Scholar]
- 43.Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, Zenobi-Wong M. Bioprinting: bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater 2015; 25: 7397–7. [Google Scholar]
- 44.Custódio CA, Reis RL, Mano JF. Engineering biomolecular microenvironments for cell instructive biomaterials. Adv Healthcare Mater 2014; 3: 797–810. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira SM, Santo VE, Gomes ME, Reis RL, Mano JF. Layer-by-layer assembled cell instructive nanocoatings containing platelet lysate. Biomaterials 2015; 48: 56–65. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011; 32: 1–7. [DOI] [PubMed] [Google Scholar]
- 47.Chow LW, Armgarth A, St-Pierre J-P, Bertazzo S, Gentilini C, Aurisicchio C, McCullen SD, Steele JAM, Stevens MM. Peptide-directed spatial organization of biomolecules in dynamic gradient scaffolds. Adv Healthcare Mater 2014; 3: 1381–6. [DOI] [PubMed] [Google Scholar]
- 48.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA 2006; 103: 2480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S, Khademhosseini A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater 2011; 7: 1441–51. [DOI] [PubMed] [Google Scholar]
- 50.Ahadian S, Sadeghian RB, Salehi S, Ostrovidov S, Bae H, Ramalingam M, Khademhosseini A. Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjugate Chem 2015; 26: 1984–2001. [DOI] [PubMed] [Google Scholar]
- 51.Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 2004; 20: 5153–6. [DOI] [PubMed] [Google Scholar]
- 52.Pedron S, Becka E, Harley BA. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv Mater Weinheim 2015; 27: 1567–72. [DOI] [PubMed] [Google Scholar]
- 53.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 1997; 276: 1425–8. [DOI] [PubMed] [Google Scholar]
- 54.Shukla A, Slater JH, Culver JC, Dickinson ME, West JL. Biomimetic surface patterning promotes mesenchymal stem cell differentiation. ACS Appl Mater Interfaces -2015. Epub ahead of print 17 December. DOI: 10.1021/acsami.5b08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Custódio CA, Frias AM, del Campo A, Reis RL, Mano JF. Selective cell recruitment and spatially controlled cell attachment on instructive chitosan surfaces functionalized with antibodies. Biointerphases 2012; 7: 1–9. [DOI] [PubMed] [Google Scholar]
- 56.Davey SK, Aung A, Agrawal G, Lim HL, Kar M, Varghese S. Embedded 3D photopatterning of hydrogels with diverse and complex architectures for tissue engineering and disease models. Tissue Eng Part C Methods 2015; 21: 1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M, Lutolf MP. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater 2013; 12: 1072–8. [DOI] [PubMed] [Google Scholar]
- 58.Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, del Campo A, GarcIa AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater 2015; 14: 352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aizawa Y, Wylie R, Shoichet M. Endothelial cell guidance in 3D patterned scaffolds. Adv Mater Weinheim 2010; 22: 4831–5. [DOI] [PubMed] [Google Scholar]
- 60.Azagarsamy MA, Anseth KS. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett 2012; 2: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang W, Becker ML. “Click” reactions: a versatile toolbox for the synthesis of peptide-conjugates. Chem Soc Rev 2014; 43: 7013–39. [DOI] [PubMed] [Google Scholar]
- 62.DeForest CA, Sims EA, Anseth KS. Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chem Mater 2010; 22: 4783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeForest CA, Anseth KS. Photoreversible patterning of biomolecules within click-based hydrogels. Angew Chem Int Ed Engl 2012; 51: 1816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Dicker KT, Xu X, Jia X, Fox JM. Interfacial bioorthogonal cross-linking. ACS Macro Lett 2014; 3: 727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodell CB, Mealy JE, Burdick JA. Supramolecular guest-host interactions for the preparation of biomedical materials. Bioconjugate Chem 2015; 26: 2279–89. [DOI] [PubMed] [Google Scholar]
- 66.Boekhoven J, Rubert Pérez CM, Sur S, Worthy A, Stupp SI. Dynamic display of bioactivity through host-guest chemistry. Angew Chem Int Ed 2013; 52: 12077–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matson JB, Zha RH, Stupp SI. Peptide self-assembly for crafting functional biological materials. Curr Opin Solid State Mater Sci 2011; 15: 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guven S, Chen P, Inci F, Tasoglu S, Erkmen B, Demirci U. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol 2015; 33: 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matson JB, Stupp SI. Self-assembling peptide scaffolds for regenerative medicine. Chem Commun 2011; 48: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartgerink JD. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001; 294: 1684–8. [DOI] [PubMed] [Google Scholar]
- 71.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA 2002; 99: 5133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, la Cruz de MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat Mater 2010; 9: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClendon MT, Stupp SI. Tubular hydrogels of circumferentially aligned nanofibers to encapsulate and orient vascular cells. Biomaterials 2012; 33: 5713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 2008; 319: 1812–6. [DOI] [PubMed] [Google Scholar]
- 75.Carvajal D, Bitton R, Mantei JR, Velichko YS, Stupp SI, Shull KR. Physical properties of hierarchically ordered self-assembled planar and spherical membranes. Soft Matter 2010; 6: 1816–1816. [Google Scholar]
- 76.Chow LW, Bitton R, Webber MJ, Carvajal D, Shull KR, Sharma AK, Stupp SI. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials 2011; 32: 1574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bitton R, Chow LW, Zha RH, Velichko YS, Pashuck ET, Stupp SI. Electrostatic control of structure in self-assembled membranes. Small 2013; 10: 500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Velichko YS, Mantei JR, Bitton R, Carvajal D, Shull KR, Stupp SI. Electric field controlled self-assembly of hierarchically ordered membranes. Adv Funct Mater 2011; 22: 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gasiorowski JZ, Collier JH. Directed intermixing in multicomponent self-assembling biomaterials. Biomacromolecules 2011; 12: 3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hudalla GA, Sun T, Gasiorowski JZ, Han H, Tian YF, Chong AS, Collier JH. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat Mater 2014; 13: 829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caliari SR, Weisgerber DW, Grier WK, Mahmassani Z, Boppart MD, Harley BAC. Collagen scaffolds incorporating coincident gradations of instructive structural and biochemical cues for osteotendinous junction engineering. Adv Healthcare Mater 2015; 4: 831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caldwell DJ, Rao RR, Stegemann JP. Assembly of discrete collagen-chitosan microenvironments into multiphase tissue constructs. Adv Healthcare Mater 2013; 2: 673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loo Y, Lakshmanan A, Ni M, Toh LL, Wang S, Hauser CAE. Peptide bioink: self-assembling nanofibrous scaffolds for three-dimensional organotypic cultures. Nano Lett 2015; 15: 6919–25. [DOI] [PubMed] [Google Scholar]
- 84.Tayi AS, Pashuck ET, Newcomb CJ, McClendon MT, Stupp SI. Electrospinning bioactive supramolecular polymers from water. Biomacromolecules 2014; 15: 1323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wade RJ, Bassin EJ, Gramlich WM, Burdick JA. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv Mater Weinheim 2015; 27: 1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sundararaghavan HG, Burdick JA. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules 2011; 12: 2344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrison RH, Steele J, Chapman R, Gormley AJ, Chow LW, Mahat MM, Podhorska L, Palgrave RG, Payne DJ, Hettiaratchy SP, Dunlop IE, Stevens MM. Modular and versatile spatial functionalization of tissue engineering scaffolds through fiber-initiated controlled radical polymerization. Adv Funct Mater 2015; 25: 5748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pati F, Jang J, Ha D-H, Kim SW, Rhie J-W, Shim J-H, Kim D-H, Cho D-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014; 5: 3935–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Highley CB, Rodell CB, Burdick JA. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater Weinheim 2015; 27: 5075–9. [DOI] [PubMed] [Google Scholar]
- 90.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 2015; 1: e1500758–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater Weinheim 2016; 28: 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]