Abstract
Galectins, a 15-member family of soluble carbohydrate-binding proteins, are receiving increasing interest as therapeutic targets for immunotherapy and immunomodulation due to their role as extracellular signals that regulate innate and adaptive immune cell phenotype and function. However, different galectins can have redundant, synergistic, or antagonistic signaling activity in normal immunological responses, such as resolution of inflammation and induction of antigen-specific tolerance. In addition, certain galectins can be hijacked to promote progression of immunopathologies, such as tumor immune privilege, metastasis, and viral infection, while others can inhibit these processes. Thus, eliciting a desired immunological outcome will likely necessitate therapeutics that can precisely enhance or inhibit particular galectin–glycan interactions. Multivalency is an important determinant of the affinity and specificity of natural galectin–glycan interactions, and is emerging as a key design element for therapeutics that can effectively manipulate galectin bioactivity. This minireview surveys current molecular and biomaterial engineering approaches to create therapeutics that can stabilize galectin multivalency or recapitulate natural glycan multivalency (i.e. “the glycocluster effect”). In particular, we highlight examples of using natural and engineered multivalent galectins for immunosuppression and immune tolerance, with a particular emphasis on treating autoimmune diseases or avoiding transplant rejection. In addition, we present examples of multivalent inhibitors of galectin–glycan interactions to maintain or restore T-cell function, with a particular emphasis on promoting antitumor immunity. Finally, we discuss emerging opportunities to further engineer galectin–glycan interactions for immunotherapy and immunomodulation.
Keywords: Biomaterial, bionanoscience, engineering, immunology, immunobiology, glycan
Introduction: Lectins in biology and medicine
Lectins are proteins that non-covalently bind to glycans, carbohydrates that are covalently linked via glycosidic bonds to other carbohydrates, proteins (i.e. “glycoproteins”), or lipids (i.e. “glycolipids”). Lectins are ubiquitous in nature, with hundreds of variants identified in bacteria, plants, and animals since the discovery of ricin from castor oil plants by Peter Hermann Stillmark in the early 19th century.1 Mammalian lectins can act as signals, receptors, and structural elements within various intra- and extracellular microenvironments. For example, calnexins within the endoplasmic reticulum aid in glycoprotein folding,2,3 while P-type lectins regulate trafficking of acid hydrolases to lysosomes for antigen processing.4 Extracellular S- and C-type lectins can mediate homo- and heterotypic cell–cell adhesion by engaging glycoproteins or glycolipids protruding from adjacent cells, as well as cell adhesion to the extracellular matrix via binding to matrix glycoproteins (e.g. laminin, fibronectin, and collagen type IV).5 Extracellular lectins can also act as non-covalent cross-linkers that organize membrane-anchored glycoproteins into clusters, lattices, and arrays6,7 and are involved in ECM glycoprotein organization and assembly.8 As a result, lectin–glycan binding can initiate, amplify, attenuate, or inhibit transmembrane signal transduction to modulate cell proliferation, differentiation, migration, and apoptosis in various normal and pathological processes. Of particular current interest is the role of S-, C-, and I-type lectins as regulators of immune cell function and phenotype during innate and adaptive immune responses, including pathogen recognition, inflammation, induction of antigen-specific immunity, and immunological tolerance.9–11 The primary emphasis of this minireview is to highlight recent developments in therapeutics that can modulate T-cell-dependent immune responses by enhancing or inhibiting the biological activity of S-type lectins.
To design therapeutics that can precisely alter the bioactivity of a particular lectin one can gain important insights from an understanding of the biochemistry of lectin–glycan interactions. Carbohydrate-recognition domains (CRDs) of lectins can be highly selective for their cognate glycans, approaching binding specificities that are comparable to those of “enzyme-substrate” and “antibody–antigen” interactions.12 For example, concanavalin A preferentially recognizes α-d-glucose and α-d-mannose, wheat germ agglutinin binds to N-acetylglucosamine (GlcNAc), and ricin is specific for α- or β-d-galactose.13 Similarly, mammalian lectins can be categorized into families according to their glycan-binding specificity, with C-type lectins generally recognizing mannosides and requiring calcium ions for binding, while I-, P-, and S-type lectins preferentially bind to sialic acids, mannose-6-phosphate, and β-galactosides, respectively.14 In contrast to antibody-antigen interactions, however, lectin–glycan binding is often of low affinity, with dissociation constants (KD) in the µM to mM range.15 Lectins are typically multivalent, either due to the association of different subunits into quaternary structures or via multiple CRDs encoded within a single polypeptide chain, which can stabilize lectin–glycan binding and mediate non-covalent cross-linking of glycoproteins into higher ordered structures.14 In addition, lectin–glycan binding affinity is enhanced by the “glycocluster effect,” the natural presentation of glycans in dense clusters that establish local avidity effects, such as chelation and statistical rebinding.16 This minireview is largely focused on therapeutics that can manipulate lectin–glycan interactions by mimicking natural glycoclusters or stabilizing lectin CRD multivalency.
In accordance with the natural abundance of lectins and glycans, and their diverse roles within natural microenvironments, therapeutics that can promote, mimic, or interfere with lectin–glycan interactions are receiving increasing attention. A number of excellent recent reviews document the rapidly expanding landscape of therapeutics that can leverage lectins or glycans for immunomodulation. For instance, we recently surveyed synthetic glycomaterials for immunomodulation, immunotherapy, and infection prophylaxis.17 Other excellent recent reviews highlight the state-of-the-art of glycovaccines for cancer and infection prophylaxis,18–20 as well as glycotherapeutics to inhibit bacterial adhesion, biofilm formation, and the action of bacterial toxins.21–24 Within this special issue, Huang et al.112 discuss glycomaterials that can modulate C- and I-type lectins, with a particular emphasis on therapeutics for viral infection prophylaxis. Thus, this minireview focuses exclusively on molecular and biomaterial engineering approaches to harness or inhibit the biological activity of S-type lectins, or “galectins”, as extracellular signals in T-cell-dependent immune responses.
Galectins as extracellular signals within the immune system
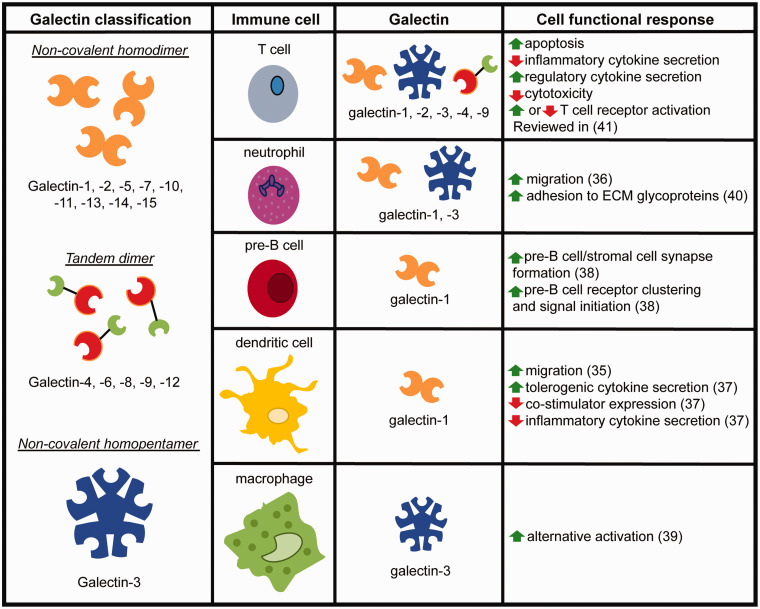
Mammalian galectins are a 15-member family of soluble β-galactoside-binding lectins that can be further subdivided into non-covalent homodimers with identical CRDs (galectin-1, -2, -5, -7, -10, -11, -13, -14, and -15); tandem dimers with distinct CRDs (galectin-4, -6, -8, -9, and -12); or a non-covalent homopentamer (galectin-3)25,26 (Figure 1). Galectins can be secreted into the extracellular space, where they influence cell behavior in various normal and pathological processes, including inflammation and its resolution,27 immunity and tolerance,28–30 cancer progression and metastasis,31 angiogenesis,32,33 and wound healing.34 Within the innate and adaptive immune systems, for example, galectin-1 can enhance migration of dendritic cells (DCs) and neutrophils,35,36 bias activation of DCs towards a tolerogenic phenotype,37 and mediate pre-B cell/stromal cell synapse formation leading to pre-B cell receptor clustering and signal initiation.38 Galectin-3 drives alternative activation of macrophages,39 and also mediates neutrophil adhesion to ECM glycoproteins.40 In addition, galectin-1, -2, -3, -4, and -9 modulate various functions of thymic, naïve, effector, and regulatory T cells, including apoptosis, activation, and cytokine expression.41 For greater depth on galectins in the context of innate and adaptive immune responses, we direct the reader to an excellent recent review by Thiemann and Baum.11 Owing to the diverse roles of galectins as signals that modulate immune cell behavior, and the centrality of these signaling events to various normal and pathological processes, there is increasing interest in galectins as therapeutic targets. In the following sections, we highlight recent advances, emerging opportunities, and challenges in applying molecular and materials engineering to create therapeutics that can harness or disrupt galectin–glycan interactions for immunomodulation (Figure 2).
Figure 1.
Classification of galectins by structure and an overview of their modulation of immune cell function. (A color version of this figure is available in the online journal.)
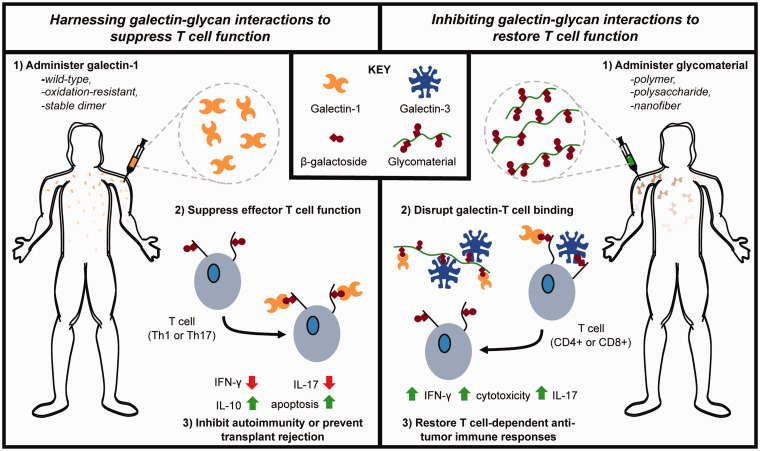
Figure 2.
Schematic overview of therapeutic approaches to harness galectin–glycan interactions to suppress T-cell function (left) or inhibit galectin–glycan interactions to restore T-cell function (right). (A color version of this figure is available in the online journal.)
Galectin-1 delivery for immunosuppression and immune tolerance
Galectin-1 is expressed at sites of immunosuppression during development and homeostasis, such as the fetal–maternal interface, retina, and testis,42–44 where it can act as an extracellular signal to downregulate adaptive immune responses and inflammation.45 For example, galectin-1 induces apoptosis of activated effector CD4 + T-helper 1 and 17 cells (Th1 and Th17), but not naïve T cells, T-helper 2 lymphocytes (Th2), or regulatory T cells.46,47 In addition, galectin-1 can also modulate T-cell expression of inflammatory and anti-inflammatory cytokines.48–50 In light of these observations, galectin-1 is gaining interest as a therapeutic for treating T-cell-dependent immunopathologies. Toward this end, delivery of galectin-1 prevented the onset of hyperglycemia in non-obese diabetic (NOD) mice, a preclinical model for the T-cell-mediated autoimmune disease, Type 1 diabetes (T1D).51 In particular, galectin-1 delivery reduced the number of Th1 cells, increased the number of T cells secreting anti-inflammatory cytokines (interleukin (IL)-4 and IL-10), and caused peripheral deletion of T cells reactive towards insulin-producing pancreatic β-cells. As a result, galectin-1 therapy prevented onset of hyperglycemia in NOD mice at early and subclinical stages of T1D, and also reversed β-cell autoimmunity and hyperglycemia in NOD mice with on-going T1D. Galectin-1 delivery has also proven effective in suppressing or reversing other autoimmune and autoinflammatory diseases, preventing rejection of allogeneic transplants, and inhibiting graft-versus-host disease following allogeneic hematopoietic stem cell transplantation52–57 (Table 1).
Table 1.
Summary of preclinical assessments of galectin-1 delivery to treat T-cell-dependent immunopathologies
| Immunopathology model | Host | Outcome | References |
|---|---|---|---|
| Graft-versus-host disease | Mouse | Increased numbers of splenic B cells and CD4 + T cells, decreased IL-2 and IFN-y release, decreased host alloreactivity | Baum et al.52 |
| Collagen-induced osteoarthritis | Mouse | Decreased proinflammatory cytokine release, decreased anticollagen IgG titers, Th2-skewed antigen immune response | Rabinovich et al.53 |
| Experimental colitis | Mouse | Decreased numbers of hapten-activated T cells, decreased proinflammatory cytokine release, increased numbers of apoptotic mononuclear cells within colon | Santucci et al.54 |
| Experimental autoimmune encephalomyelitis | Mouse | Increased microglia deactivation, decreased axonal damage, decreased demyelination, decreased neuronal degeneration | Starossom et al.55 |
| Experimental autoimmune uveitis | Mouse | Increased T cell apoptosis, decreased antigen-specific IgG titers, decreased leukocyte infiltrate, Th2 orTreg-skewed immune response | Toscano et al.56 |
| Renal allogeneic transplant | Rat | Increased recipient animal survival, decreased serum IFN-y and soluble CD30, decreased CD8 + T cell-mediated cytotoxicity | Xu et al.57 |
Despite these preclinical successes, however, translation of galectin-1 delivery is likely to be challenged by the rapid inactivation of the protein under physiological conditions due to covalent dimerization via cysteine oxidation.58 Ligands that bind galectin-1 can prevent oxidative dimerization,58,59 but are nonetheless impractical for enhancing the therapeutic efficacy of galectin-1 because they are likely to interfere with cell-surface or ECM glycoprotein binding. Alternatively, galectin-1 oxidative dimerization can be inhibited via protein engineering or chemical approaches. For example, an engineered mutant of galectin-1 having all cysteine residues replaced with serine residues demonstrated similar glycan-binding properties as the wild-type protein, and inhibited Jurkat T-cell growth with equal or greater efficacy than the wild-type protein.60 Notably, the cysteine-less mutant retained hemagluttination activity during storage for more than 400 days, while activity of the wild-type protein was greatly diminished by 10 days in the absence of reducing agents.60 Alkylation of cysteine residues with iodoacetamide or maleimide can also inhibit oxidative dimerization, thereby eliminating the need to modify the primary structure of galectin-1.61 Similar to the cysteine-less mutant, alkylated galectin-1 did not undergo oxidative dimerization over prolonged periods of time and retained the glycan-binding and biological properties of the wild-type protein. Further studies are warranted to assess the effectiveness of galectin-1 variants that resist oxidative dimerization for suppressing or reversing T-cell-dependent immunopathologies.
The therapeutic efficacy of galectin-1 delivery may also be dependent on CRD valency. Galectin-1 exists as a non-covalent homodimer,62 which dissociates into monomers at low concentrations (KD ∼1–7 µM, depending on species).58,63 Monomeric and dimeric galectin-1 have similar glycan binding specificities; however, dimeric galectin-1 has higher glycan binding affinity.64 T-cell apoptosis is induced by galectin-1 at concentrations greater than 7 µM, suggesting a dependence on the homodimeric quaternary structure.47 Consistent with this, a galectin-1 mutant with impaired dimerization lacked the ability to induce phosphatidylserine exposure by T cells, an early marker of apoptosis, despite retaining the ability to bind carbohydrates and induce intracellular calcium flux.59 Similarly, a truncated monomeric form of galectin-1 failed to induce apoptosis of Jurkat T cells, despite having profound effects on axonal regeneration.65 Thus, suppressing T-cell-dependent immune responses via systemic delivery of galectin-1 will likely require administration of relatively high doses that maintain homodimerization.
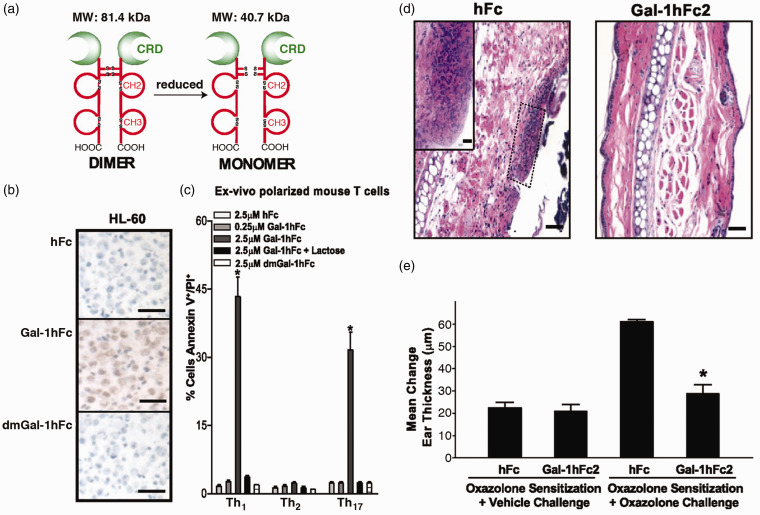
Alternatively, protein engineering approaches can be used to create galectin-1 homodimers with greater stability and, in turn, enhanced biological activity. For example, Visser and coworkers66 created a recombinant fusion of galectin-1 and a cysteine-terminated variant of the FBJ osteosarcoma viral oncogene homolog (FOS) leucine zipper, which forms disulfide-linked dimers. This fusion protein induced T-cell apoptosis with a minimum effective concentration that was 20-fold lower than that of wild-type galectin-1. In addition, the fusion protein enhanced peripheral blood mononuclear cell expression of the anti-inflammatory cytokine IL-10 with a 100-fold lower minimum effective dose than wild-type galectin-1, while also downregulating expression of the inflammatory cytokine IFN-γ. Together, these observations suggest that a stable dimeric fusion of galectin-1 may inhibit effector T-cell function more effectively than wild-type galectin-1. Similarly, Dimitroff and coworkers67 created a recombinant fusion of galectin-1 and the Fc region of immunoglobulin G1 (IgG1), referred to as Gal-1hFc, which forms stable homodimers via covalent Fc dimerization (Figure 3). Gal-1hFc induced apoptosis of Th1 and Th17 cells, similar to wild-type galectin-1, upregulated expression of IL-10 and other Th2 cytokines in activated T cells, and suppressed T-cell-dependent inflammation in a model of contact hypersensitivity by increasing the number of IL-4+/IL-10+/transforming growth factor-β+/CD25high/FoxP3 + regulatory T cells and decreasing the number of interferon-γ+/IL-17 + T cells. It remains to be seen if manipulating galectin-1 monomer-dimer equilibrium can enhance the efficacy of galectin-1 for treating T-cell-dependent immunopathologies.
Figure 3.
A stable dimeric variant of galectin-1 based on an Fc fusion protein. (a) The dimeric and monomeric states of the Gal-1hFc fusion protein. (b) Binding of Gal1-hFc to HL-60 cells. (c) Gal-1hFc induced apoptosis of Th1 and Th17, but not Th2 effector T cell subsets in a carbohydrate-dependent manner, similar to wild-type galectin-1. (d-e) In a murine skin hypersensitivity model, Gal-1hFc delivery (d) significantly decreased mononuclear and granulocytic infiltrates and (e) suppressed changes in ear thickness resulting from inflammation. Adapted from Cedeno-Laurent et al.67 (A color version of this figure is available in the online journal.)
Inhibiting galectin-T-cell interactions to maintain or restore T-cell function
Various galectins can also act as extracellular signals and structural elements during the onset or progression of pathologies, such as cancer and viral infection.52,68,69 Focusing specifically on T-cell function in cancer, binding of galectin-3 to antigen-specific activated CD8 + T cells inhibited their effector function within the tumor microenvironment.70 Galectin-9 within the tumor microenvironment induced apoptosis of Tim-3+CD8 + T cells infiltrating colon tumors.71 Galectin-1 expression is up-regulated in human pancreatic cancer cells (hPCC), and isolated hPCC induced higher levels of T cell apoptosis, increased secretion of IL-6 and IL-10, and decreased secretion of IFN-γ in ex vivo T-cell-hPCC co-cultures.72 In the context of viral infection, galectin-1 can enhance HIV infectivity by non-covalently cross-linking the viral coat glycoprotein GP120 and CD4 expressed by T cells.73 Similarly, galectin-1 increased the efficiency of human T-cell leukemia virus type 1 (HTLV-1) infection by stabilizing virus attachment to human T cells.74 Galectin-1 and -8 also promoted binding of influenza A virus (IAV) to target cells in a dose-dependent manner, while having no effect on internalization, and restored the ability of IAV to infect de-sialylated cells at levels comparable to native cells.75 In addition to viral entry, galectin-9 systemically overexpressed during acute and chronic stages of HIV infection likely contributes to persistent inflammation and systemic T-cell dysfunction.76
Owing to the importance of galectins as extracellular signals in pathological T-cell dysfunction, there is increasing interest in therapeutics that can inhibit galectin–T-cell interactions. One approach is to eliminate galectin-1 or its cognate glycans. For example, silencing galectin-1 gene expression enhanced antitumor immunity in various murine cancer models.77–79 Alternatively, inhibiting biosynthesis of N-acetyllactosamine (LacNAc) glycans via systemic delivery of a non-natural carbohydrate increased the number of infiltrating tumor-specific CD8 + T cells and intratumoral IFN-γ expression.80 However, efficient, targeted delivery of small-interfering RNA to tumors remains an unmet need,81 while systemic inhibition of glycan biosynthesis may broadly disrupt immune system function, giving way to onset or exacerbation of secondary disease and opportunistic infections.
To address these practical challenges, there is growing interest in therapeutics that can inhibit galectin bioactivity by disrupting galectin–glycan binding. Toward this end, a wide variety of natural and modified carbohydrates have been explored as galectin inhibitors,82 with increasing attention given to their efficacy in disrupting galectin–T-cell interactions that are integral to cancer and viral infection. For example, intratumoral injection of thiodigalactoside (TDG), a galectin-binding variant of lactose having enhanced glycolytic stability, increased the number of tumor-infiltrating CD8 + T cells and reduced tumor growth in murine melanoma and breast cancer models.83 Administering TDG following prophylactic vaccination with a tumor-specific antigen improved survival following tumor challenge in a murine breast cancer model.84 In addition, delivery of TDG increased the number of CD4 + and CD8 + T cells in peripheral blood, as well as the number of CD3 + T cells within metastases, ultimately leading to a reduction in pulmonary metastasis in murine breast and colon cancer models.85 Alternatively, lactoside derivatives can reduce HIV binding to target cells in vitro by inhibiting galectin-1 binding to CD4 + T cells,86 suggesting their potential for disrupting host–virus interactions that are a rate-limiting step in HIV infection.
Despite these successes, however, one persistent challenge in the design of effective small molecule galectin inhibitors is the low binding affinity of CRDs for monovalent carbohydrates. Within natural microenvironments, galectin–glycan binding affinity is enhanced via the “glycocluster effect,” in which high-density, multivalent display of carbohydrates provides avidity effects that stabilize galectin–glycan interactions.16 Inspired by these observations, naturally derived and synthetic polyvalent carbohydrates are receiving increasing attention as galectin inhibitors. For example, synthetic glycopolymers, such as glycodendrimers and pseudo-polyrotaxanes, can disrupt galectin binding to cell surface glycoproteins.87–89 However, their efficacy for treating cancer has largely focused on inhibiting galectin-mediated cell adhesion,90,91 with little emphasis on enhancing antitumor immunity to date. Alternatively, naturally derived glycopolymers are showing significant promise for restoring the function of tumor-infiltrating lymphocytes (TIL). For example, a modified citrus pectin (GCS-100) released galectin-3 from the surface of human tumor-derived TIL, upregulated expression of inflammatory cytokines (IFN-γ, IL-2, and TNF-α), and restored CD8 + TIL cytotoxicity in vitro, while also enhancing rejection of tumors in mice vaccinated with a tumor-specific antigen.92 Similarly, GM-CT-01 (Davanat™), a galactomannan from guar gum, enhanced IFN-γ secretion by CD8 + and CD4 + T cells from patients with various cancers by disorganizing the formation of galectin-glycoprotein lattices.93 In addition, Galectin Therapeutics is currently investigating a galactorhamnogalacturonate glycan derived from citrus pectin, GR-MD-02,94 in clinical trials as an adjuvant to enhance the efficacy of cancer immunotherapy.
Another significant challenge is creating therapeutics to selectively inhibit the bioactivity of specific galectins, given increasing evidence that different galectins can have contrasting activities within pathological microenvironments. For example, galectin-4 inhibits pancreatic cancer cell metastasis by interfering with cell migration,95 while galectin-3 mediates aberrant cytosolic redistribution of a membrane-bound epithelial mucin that is overexpressed in many cancer cells, MUC1.96 Similarly, galectin-1 promotes HIV infectivity of macrophages by stabilizing virus-cell binding, while galectin-3 does not mediate virus adsorption onto host cells.97 Thus, therapeutics that broadly recognize galectin CRDs may have limited efficacy because of competitive binding that decreases effective inhibitor dose or off-site activity inhibiting signaling events that suppress disease progression.
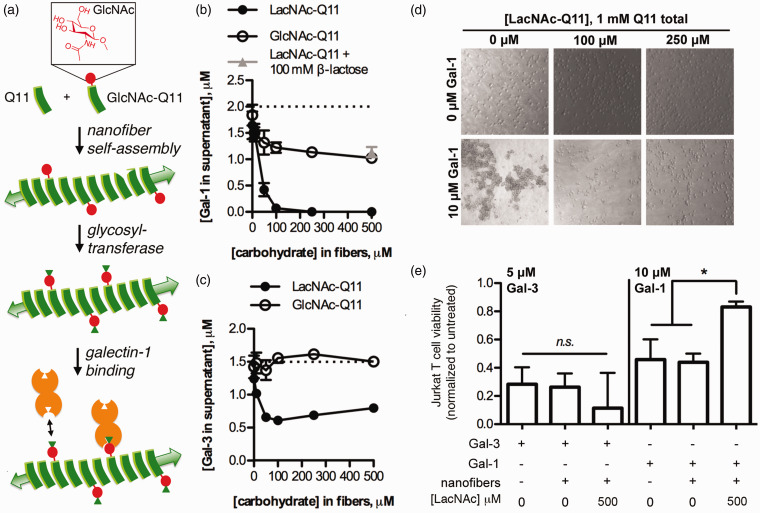
Different galectins demonstrate binding selectivity for subtle changes in carbohydrate chemistry, such as terminal versus internal repeated disaccharides, sialylation, and fucosylation.98–100 Coupled with increasing understanding of CRD architecture via galectin structure determination, these insights have informed on-going efforts to rationally design carbohydrate analogs as selective galectin inhibitors.82 In addition to glycochemistry, however, it is also becoming apparent that physical attributes of glycoclusters can dictate galectin-binding specificity. For example, clustering of complex-type glycans on the HIV coat protein GP120 in its native conformation imparts structural constraints that prevent galectin-3 binding, yet permit galectin-1 binding.73,101 Thus, synthetic glycoclusters with fine control of glycan chemistry and physical display may provide new opportunities for creating more robust galectin inhibitors. Toward this end, we have recently developed a synthetic glycopeptide, GlcNAc-QQKFQFQFEQQ (GlcNAc-Q11), which self-assembles into β-sheet nanofibers under aqueous conditions to provide highly multivalent glycoclusters101 (Figure 4). Carbohydrate concentration can be easily and precisely varied by simply mixing GlcNAc-Q11 and non-glycosylated Q11 together at different molar ratios in the preassembled state, while carbohydrate chemistry can be tailored by glycosyltransferase enzymes, together allowing for fine-tuning of nanofiber lectin binding specificity and affinity. For example, nanofibers bearing the galectin-binding disaccharide LacNAc have significantly higher binding affinity for galectin-1 than galectin-3. As a result, LacNAc-Q11 nanofibers robustly inhibited apoptosis of Jurkat T cells by galectin-1, while having no inhibitory effect on galectin-3. Notably, LacNAc-Q11 nanofibers had a significantly lower effective dose for inhibiting galectin-1 than TDG, a stable LacNAc analog with demonstrated efficacy for enhancing anti-tumor immunity as discussed above, further highlighting the potential of self-assembled glycopeptide nanofibers as robust and selective inhibitors of galectin-1.
Figure 4.
Self-assembled glycopeptide nanofibers as inhibitors of galectin-1. (a) Galectin-1 binding nanofibers fabricated via self-assembly of the glycopeptide, GlcNAc-Q11, and its non-glycosylated analog, followed by conversion of nanofibrillar GlcNAc to LacNAc via a glycosyltransferase enzyme. (b,c) Data demonstrating that LacNAc-Q11 nanofibers have higher affinity for galectin-1 than galectin-3, and that LacNAc-Q11 nanofibers have higher affinity for galectins than GlcNAc-Q11 nanofibers. (d) LacNAc-Q11 nanofibers inhibited Jurkat T cell agglutination via galectin-1, an early marker of apoptosis. (e) LacNAc-Q11 nanofibers inhibited apoptosis of Jurkat T cells via galectin-1, but failed to inhibit galectin-3, likely due to observed differences in nanofiber-galectin binding affinity (b,c). Adapted from Restuccia et al.101 (A color version of this figure is available in the online journal.)
Future opportunities
Many aspects of galectin–glycan interactions remain under-explored as therapeutic targets. For example, galectins often regulate outside-in signaling by cross-linking membrane glycoproteins into “lattices” via their multivalent CRDs, which play important roles in amplifying or inhibiting signal transmission at the DC-T-cell synapse.102–106 Recently, Belardi et al.107 developed lactosylated glycopolymers that can perturb galectin-glycoprotein lattice formation by inserting into cell membranes. Engineering the cell membrane glycosylation profile in this way has already offered unique insights into galectin-mediated cross-linking and the dynamics of lattice formation at the surface of cells. Moving forward, we anticipate that these glycomaterials may lead to new therapeutics that can modulate DC-T-cell crosstalk to enhance or suppress induction of antigen-specific immunity for infection prophylaxis, immunotherapy, and treatment of autoimmune diseases. Another area of potential interest is mimicking the ability of ECM glycoproteins to locally maintain galectin-1 bioactivity by inhibiting oxidative dimerization, which has implications in directing dendritic cell migration and inducing T-cell apoptosis,35,108,109 and may therefore provide unique opportunities for immunomodulation. Toward this end, Groll and coworkers110 developed poly(LacNAc) polymers that can mediate selective adsorption of ECM glycoproteins onto the surface of materials via galectin-1 binding, which may be useful for recapitulating galectin-1 signaling to DCs and T cells within natural microenvironments. In addition, we have recently created micron-sized hydrated gels (i.e. “microgels”) from self-assembled glycopeptide nanofibers that can release lectin payloads with tunable kinetics,111 which may provide the basis for vehicles for localized delivery of bioactive galectin-1. As appreciation of the role of galectins as extracellular signals in various normal and pathological immunological processes continues to increase, and the ‘sugar code’ relating galectin–glycan binding becomes more clearly defined, so too will efforts to engineer galectin–glycan interactions for immunotherapy and immunomodulation.
Acknowledgements
This work was supported by a National Science Foundation CAREER award to G.A.H. (DMR-1455201).
Authors' contributions
SAF and GAH contributed to the writing and preparation of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 2004; 14: 53R–62R. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K. Intracellular lectins involved in folding and transport in the endoplasmic reticulum. Biol Pharm Bull 2009; 32: 767–73. [DOI] [PubMed] [Google Scholar]
- 3.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science 2001; 291: 2364–9. [DOI] [PubMed] [Google Scholar]
- 4.Dahms NM, Olson LJ, Kim JJ. Strategies for carbohydrate recognition by the mannose 6-phosphate receptors. Glycobiology 2008; 18: 664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaltner H, Stierstorfer B. Animal lectins as cell adhesion molecules. Acta Anat (Basel) 1998; 161: 162–79. [DOI] [PubMed] [Google Scholar]
- 6.Elola MT, Blidner AG, Ferragut F, Bracalente C, Rabinovich GA. Assembly, organization and regulation of cell-surface receptors by lectin–glycan complexes. Biochem J 2015; 469: 1–16. [DOI] [PubMed] [Google Scholar]
- 7.Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci 2015; 128: 2213–9. [DOI] [PubMed] [Google Scholar]
- 8.Moiseeva EP, Williams B, Samani NJ. Galectin 1 inhibits incorporation of vitronectin and chondroitin sulfate B into the extracellular matrix of human vascular smooth muscle cells. Biochim Biophys Acta 2003; 1619: 125–32. [DOI] [PubMed] [Google Scholar]
- 9.Drickamer K, Taylor ME. Recent insights into structures and functions of C-type lectins in the immune system. Curr Opin Struct Biol 2015; 34: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14: 653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiemann S, Baum LG. Galectins and immune responses – just how do they do those things they do?. Annu Rev Immunol 2016; 34: 9.1–9.22. [DOI] [PubMed] [Google Scholar]
- 12.Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv Drug Deliv Rev 2004; 56: 491–509. [DOI] [PubMed] [Google Scholar]
- 13.Fontaniella B, Millanes AM, Vicente C, Legaz ME. Concanavalin A binds to a mannose-containing ligand in the cell wall of some lichen phycobionts. Plant Physiol Biochem 2004; 42: 773–9. [DOI] [PubMed] [Google Scholar]
- 14.Crocker PR. Mammalian carbohydrate recognition systems, Berlin: Springer, 2001. [Google Scholar]
- 15.Collins BE, Paulson JC. Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr Opin Chem Biol 2004; 8: 617–25. [DOI] [PubMed] [Google Scholar]
- 16.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev 2002; 102: 555–78. [DOI] [PubMed] [Google Scholar]
- 17.Restuccia A, Fettis MM, Hudalla GA. Glycomaterials for immunomodulation, immunotherapy, and infection prophylaxis. J Mater Chem B 2016; 4: 1569–1585. [DOI] [PubMed] [Google Scholar]
- 18.Morelli L, Poletti L, Lay L. Carbohydrates and immunology: synthetic oligosaccharide antigens for vaccine formulation. Eur J Org Chem 2011; 29: 5723–77. [Google Scholar]
- 19.McDonald DM, Byrne SN, Payne RJ. Synthetic self-adjuvanting glycopeptide cancer vaccines. Front Chem 2015; 3: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vella M, Pace D. Glycoconjugate vaccines: an update. Expert Opin Biol Ther 2015; 15: 529–46. [DOI] [PubMed] [Google Scholar]
- 21.Branson TR, Turnbull WB. Bacterial toxin inhibitors based on multivalent scaffolds. Chem Soc Rev 2013; 42: 4613–22. [DOI] [PubMed] [Google Scholar]
- 22.Yan XB, Sivignon A, Yamakawa N, Crepet A, Travelet C, Borsali R, Dumych T, Li ZL, Bilyy R, Deniaud D, Feury E, Barnich N, Darfeuille-Michaud A, Gouin SG, Bouckaert J, Bernard J. Glycopolymers as antiadhesives of E. coli strains inducing inflammatory bowel diseases. Biomacromolecules 2015; 16: 1827–36. [DOI] [PubMed] [Google Scholar]
- 23.Bernardi A, Jimenez-Barbero J, Casnati A, De Castro C, Darbre T, Fieschi F, Finne J, Funken H, Jaeger KE, Lahmann M, Lindhorst TK, Marradi M, Messner P, Molinaro A, Murphy PV, Nativi C, Oscarson S, Penades S, Peri F, Pieters RJ, Renaudet O, Reymond JL, Richichi B, Rojo J, Sansone F, Schaffer C, Turnbull WB, Velasco-Torrijos T, Vidal S, Vincent S, Wennekes T, Zuilhof H, Imberty A. Multivalent glycoconjugates as anti-pathogenic agents. Chem Soc Rev 2013; 42: 4709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisman A, Chou B, O'Brien J, Shea KJ. Polymer antidotes for toxin sequestration. Adv Drug Del Rev 2015; 90: 81–100. [DOI] [PubMed] [Google Scholar]
- 25.Compagno D, Jaworski FM, Gentilini L, Contrufo G, Gonzalez Perez I, Elola MT, Pregi N, Rabinovich GA, Laderach DJ. Galectins: major signaling modulators inside and outside the cell. Curr Mol Med 2014; 14: 630–51. [DOI] [PubMed] [Google Scholar]
- 26.Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol 2012; 946: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci 2012; 1253: 80–91. [DOI] [PubMed] [Google Scholar]
- 28.Vasta GR, Ahmed H, Nita-Lazar M, Banerjee A, Pasek M, Shridhar S, Guha P, Fernandez-Robledo JA. Galectins as self/non-self recognition receptors in innate and adaptive immunity: an unresolved paradox. Front Immunol 2012; 3: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascanfroni ID, Cerliani JP, Dergan-Dylon S, Croci DO, Ilarregui JM, Rabinovich GA. Endogenous lectins shape the function of dendritic cells and tailor adaptive immunity: mechanisms and biomedical applications. Int Immunopharmacol 2011; 11: 833–41. [DOI] [PubMed] [Google Scholar]
- 30.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol 2012; 142: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebrahim AH, Alalawi Z, Mirandola L, Rakhshanda R, Dahlbeck S, Nguyen D, Jenkins M, Grizzi F, Cobos E, Figueroa JA, Chiriva-Internati M. Galectins in cancer: carcinogenesis, diagnosis and therapy. Ann Transl Med 2014; 2: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thijssen VL, Griffioen AW. Galectin-1 and -9 in angiogenesis: a sweet couple. Glycobiology 2014; 24: 915–20. [DOI] [PubMed] [Google Scholar]
- 33.Blois SM, Conrad ML, Freitag N, Barrientos G. Galectins in angiogenesis: consequences for gestation. J Reprod Immunol 2015; 108: 33–41. [DOI] [PubMed] [Google Scholar]
- 34.Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transl Med 2014; 2: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol 2006; 177: 216–26. [DOI] [PubMed] [Google Scholar]
- 36.Auvynet C, Moreno S, Melchy E, Coronado-Martinez I, Montiel JL, Aguilar-Delfin I, Rosenstein Y. Galectin-1 promotes human neutrophil migration. Glycobiology 2013; 23: 32–42. [DOI] [PubMed] [Google Scholar]
- 37.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol 2009; 10: 981–91. [DOI] [PubMed] [Google Scholar]
- 38.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA 2002; 99: 13014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol 2008; 180: 2650–8. [DOI] [PubMed] [Google Scholar]
- 40.Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol 1996; 156: 3939–44. [PubMed] [Google Scholar]
- 41.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 2009; 9: 338–52. [DOI] [PubMed] [Google Scholar]
- 42.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 2007; 13: 1450–7. [DOI] [PubMed] [Google Scholar]
- 43.Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm 2003; 11: 91–105. [DOI] [PubMed] [Google Scholar]
- 44.Dettin L, Rubinstein N, Aoki A, Rabinovich GA, Maldonado CA. Regulated expression and ultrastructural localization of galectin-1, a proapoptotic beta-galactoside-binding lectin, during spermatogenesis in rat testis. Biol Reprod 2003; 68: 51–9. [DOI] [PubMed] [Google Scholar]
- 45.Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J Endocrinol 2009; 201: 169–84. [DOI] [PubMed] [Google Scholar]
- 46.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol 2007; 8: 825–34. [DOI] [PubMed] [Google Scholar]
- 47.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature 1995; 378: 736–9. [DOI] [PubMed] [Google Scholar]
- 48.Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol 2008; 38: 3015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cedeno-Laurent F, Watanabe R, Teague JE, Kupper TS, Clark RA, Dimitroff CJ. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood 2012; 119: 3534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol 2012; 188: 3127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perone MJ, Bertera S, Shufesky WJ, Divito SJ, Montecalvo A, Mathers AR, Larregina AT, Pang M, Seth N, Wucherpfennig KW, Trucco M, Baum LG, Morelli AE. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol 2009; 182: 2641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol 2003; 109: 295–307. [DOI] [PubMed] [Google Scholar]
- 53.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med 1999; 190: 385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003; 124: 1381–94. [DOI] [PubMed] [Google Scholar]
- 55.Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, Bassil R, Croci DO, Cerliani JP, Delacour D, Wang Y, Elyaman W, Khoury SJ, Rabinovich GA. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 2012; 37: 249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol 2006; 176: 6323–32. [DOI] [PubMed] [Google Scholar]
- 57.Xu G, Tu W, Xu C. Immunological tolerance induced by galectin-1 in rat allogeneic renal transplantation. Int Immunopharmacol 2010; 10: 643–7. [DOI] [PubMed] [Google Scholar]
- 58.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem 1995; 270: 5207–12. [DOI] [PubMed] [Google Scholar]
- 59.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem 2009; 284: 4989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishi N, Abe A, Iwaki J, Yoshida H, Itoh A, Shoji H, Kamitori S, Hirabayashi J, Nakamura T. Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology 2008; 18: 1065–73. [DOI] [PubMed] [Google Scholar]
- 61.Stowell SR, Arthur CM, Cummings RD, Feasley CL. Alkylation of galectin-1 with iodoacetamide and mass spectrometric mapping of the sites of incorporation. Methods Mol Biol 2015;1207:51-62. [DOI] [PMC free article] [PubMed]
- 62.Giudicelli V, Lutomski D, Levi-Strauss M, Bladier D, Joubert-Caron R, Caron M. Is human galectin-1 activity modulated by monomer/dimer equilibrium?. Glycobiology 1997; 7: viii–x. [DOI] [PubMed] [Google Scholar]
- 63.Cooper DN, Barondes SH. God must love galectins; he made so many of them. Glycobiology 1999; 9: 979–84. [DOI] [PubMed] [Google Scholar]
- 64.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem 2005; 280: 5549–62. [DOI] [PubMed] [Google Scholar]
- 65.Miura T, Takahashi M, Horie H, Kurushima H, Tsuchimoto D, Sakumi K, Nakabeppu Y. Galectin-1beta, a natural monomeric form of galectin-1 lacking its six amino-terminal residues promotes axonal regeneration but not cell death. Cell Death Differ 2004; 11: 1076–83. [DOI] [PubMed] [Google Scholar]
- 66.van der Leij J, van den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol 2007; 44: 506–13. [DOI] [PubMed] [Google Scholar]
- 67.Cedeno-Laurent F, Barthel SR, Opperman MJ, Lee DM, Clark RA, Dimitroff CJ. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol 2010; 185: 4659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruvolo PP, Ruvolo VR, Benton CB, AlRawi A, Burks JK, Schober W, Rolke J, Tidmarsh G, Hail N, Jr., Eric Davis R, Andreeff M. Combination of galectin inhibitor GCS-100 and BH3 mimetics eliminates both p53 wild type and p53 null AML cells. Biochim Biophys Acta 2015; 1863: 562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito K, Stannard K, Gabutero E, Clark AM, Neo SY, Onturk S, Blanchard H, Ralph SJ. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev 2012; 31: 763–78. [DOI] [PubMed] [Google Scholar]
- 70.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8 + T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res 2015; 3: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, Hsu CY, Huang CT, Su WT, Chu YY, Lin CY. Apoptosis of tumor infiltrating effector TIM-3+CD8 + T cells in colon cancer. Sci Rep 2015; 5: 15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y, Xu C, Jiang X, Li B, Yin W, Miao Y, Wang D, Jiang K. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumour Biol 2015; 36: 5617–26. [DOI] [PubMed] [Google Scholar]
- 73.St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol 2011; 85: 11742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gauthier S, Pelletier I, Ouellet M, Vargas A, Tremblay MJ, Sato S, Barbeau B. Induction of galectin-1 expression by HTLV-I tax and its impact on HTLV-I infectivity. Retrovirology 2008; 5: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chernyy ES, Rapoport EM, Andre S, Kaltner H, Gabius HJ, Bovin NV. Galectins promote the interaction of influenza virus with its target cell. Biochemistry (Mosc) 2011; 76: 958–67. [DOI] [PubMed] [Google Scholar]
- 76.Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M, Barbour JD, Norris PJ, Lanteri MC, Martin JN, Deeks SG, Ndhlovu LC. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retroviruses 2014; 30: 654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; a potential mechanism of tumor-immune privilege. Cancer Cell 2004; 5: 241–51. [DOI] [PubMed] [Google Scholar]
- 78.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, Sundblad V, Rabinovich GA, Salatino M. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res 2013; 73: 1107–17. [DOI] [PubMed] [Google Scholar]
- 79.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res 2011; 71: 4423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cedeno-Laurent F, Opperman MJ, Barthel SR, Hays D, Schatton T, Zhan Q, He X, Matta KL, Supko JG, Frank MH, Murphy GF, Dimitroff CJ. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. J Invest Dermatol 2012; 132: 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013; 12: 967–77. [DOI] [PubMed] [Google Scholar]
- 82.Blanchard H, Bum-Erdene K, Hugo MW. Inhibitors of galectins and implications for structure-based design of galectin-specific therapeutics. Aust J Chem 2014; 67: 1763. [Google Scholar]
- 83.Ito K, Scott SA, Cutler S, Dong LF, Neuzil J, Blanchard H, Ralph SJ. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis 2011; 14: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stannard KA, Collins PM, Ito K, Sullivan EM, Scott SA, Gabutero E, Darren Grice I, Low P, Nilsson UJ, Leffler H, Blanchard H, Ralph SJ. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett 2010; 299: 95–110. [DOI] [PubMed] [Google Scholar]
- 85.Ito K, Ralph SJ. Inhibiting galectin-1 reduces murine lung metastasis with increased CD4(+) and CD8 (+) T cells and reduced cancer cell adherence. Clin Exp Metastasis 2012; 29: 561–72. [DOI] [PubMed] [Google Scholar]
- 86.St-Pierre C, Ouellet M, Giguere D, Ohtake R, Roy R, Sato S, Tremblay MJ. Galectin-1-specific inhibitors as a new class of compounds to treat HIV-1 infection. Antimicrob Agents Chemother 2012; 56: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belitsky JM, Nelson A, Hernandez JD, Baum LG, Stoddart JF. Multivalent interactions between lectins and supramolecular complexes: galectin-1 and self-assembled pseudopolyrotaxanes. Chem Biol 2007; 14: 1140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson A, Belitsky JM, Vidal S, Joiner CS, Baum LG, Stoddart JF. A self-assembled multivalent pseudopolyrotaxane for binding galectin-1. J Am Chem Soc 2004; 126: 11914–22. [DOI] [PubMed] [Google Scholar]
- 89.Andre S, Pieters RJ, Vrasidas I, Kaltner H, Kuwabara I, Liu FT, Liskamp RM, Gabius HJ. Wedgelike glycodendrimers as inhibitors of binding of mammalian galectins to glycoproteins, lactose maxiclusters, and cell surface glycoconjugates. Chembiochem 2001; 2: 822–30. [DOI] [PubMed] [Google Scholar]
- 90.Michel AK, Nangia-Makker P, Raz A, Cloninger MJ. Lactose-functionalized dendrimers arbitrate the interaction of galectin-3/MUC1 mediated cancer cellular aggregation. Chembiochem 2014; 15: 2106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cousin JM, Cloninger MJ. Glycodendrimers: tools to explore multivalent galectin-1 interactions. Beilstein J Org Chem 2015; 11: 739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Demotte N, Wieers G, Van Der Smissen P, Moser M, Schmidt C, Thielemans K, Squifflet JL, Weynand B, Carrasco J, Lurquin C, Courtoy PJ, van der Bruggen P. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res 2010; 70: 7476–88. [DOI] [PubMed] [Google Scholar]
- 93.Demotte N, Bigirimana R, Wieers G, Stroobant V, Squifflet JL, Carrasco J, Thielemans K, Baurain JF, Van Der Smissen P, Courtoy PJ, van der Bruggen P. A short treatment with galactomannan GM-CT-01 corrects the functions of freshly isolated human tumor-infiltrating lymphocytes. Clin Cancer Res 2014; 20: 1823–33. [DOI] [PubMed] [Google Scholar]
- 94.Miller MC, Nesmelova IV, Platt D, Klyosov A, Mayo KH. The carbohydrate-binding domain on galectin-1 is more extensive for a complex glycan than for simple saccharides: implications for galectin–glycan interactions at the cell surface. Biochem J 2009; 421: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Belo AI, van der Sar AM, Tefsen B, van Die I. Galectin-4 reduces migration and metastasis formation of pancreatic cancer cells. PLoS One 2013; 8: e65957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merlin J, Stechly L, de Beauce S, Monte D, Leteurtre E, van Seuningen I, Huet G, Pigny P. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene 2011; 30: 2514–25. [DOI] [PubMed] [Google Scholar]
- 97.Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 2008; 371: 121–9. [DOI] [PubMed] [Google Scholar]
- 98.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 2008; 283: 10109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horlacher T, Oberli MA, Werz DB, Krock L, Bufali S, Mishra R, Sobek J, Simons K, Hirashima M, Niki T, Seeberger PH. Determination of carbohydrate-binding preferences of human galectins with carbohydrate microarrays. Chembiochem 2010; 11: 1563–73. [DOI] [PubMed] [Google Scholar]
- 100.Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 2011; 50: 7842–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Restuccia A, Tian YF, Collier JH, Hudalla GA. Self-assembled glycopeptide nanofibers as modulators of galectin-1 bioactivity. Cell Mol Bioeng 2015; 8: 471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML, Liu FT. Galectin-3 negatively regulates TCR-mediated CD4 + T-cell activation at the immunological synapse. Proc Natl Acad Sci USA 2009; 106: 14496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev 2009; 230: 232–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vespa GN, Lewis LA, Kozak KR, Moran M, Nguyen JT, Baum LG, Miceli MC. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J Immunol 1999; 162: 799–806. [PubMed] [Google Scholar]
- 105.Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol 2000; 165: 3722–9. [DOI] [PubMed] [Google Scholar]
- 106.Liu SD, Whiting CC, Tomassian T, Pang M, Bissel SJ, Baum LG, Mossine VV, Poirier F, Huflejt ME, Miceli MC. Endogenous galectin-1 enforces class I-restricted TCR functional fate decisions in thymocytes. Blood 2008; 112: 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Belardi B, O'Donoghue GP, Smith AW, Groves JT, Bertozzi CR. Investigating cell surface galectin-mediated cross-linking on glycoengineered cells. J Am Chem Soc 2012; 134: 9549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem 2004; 279: 4705–12. [DOI] [PubMed] [Google Scholar]
- 109.Thiemann S, Man JH, Chang MH, Lee B, Baum LG. Galectin-1 regulates tissue exit of specific dendritic cell populations. J Biol Chem 2015; 290: 22662–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beer MV, Rech C, Gasteier P, Sauerzapfe B, Salber J, Ewald A, Moller M, Elling L, Groll J. The next step in biomimetic material design: poly-LacNAc-mediated reversible exposure of extra cellular matrix components. Adv Healthc Mater 2013; 2: 306–11. [DOI] [PubMed] [Google Scholar]
- 111.Fettis MM, Wei Y, Restuccia A, Kurian JJ, Wallet SM, Hudalla GA. Microgels with tunable affinity-controlled protein release via desolvation of self-assembled peptide nanofibers. J Mater Chem B 2016;4:3054–64. [DOI] [PubMed]
- 112.Huang ML, Fisher CJ, Godula K. Glycomaterials for probing host-pathogen interactions and the immune response. Exp Biol Med 2016;241:1042–53. [DOI] [PMC free article] [PubMed]