Abstract
Parvovirus B19 infection causes transient aplastic crisis in sickle cell disease (SCD) due to a temporary interruption in the red blood cell production. Toxicity from hydroxyurea includes anemia and reticulocytopenia, both of which also occur during a transient aplastic crisis event. Hydroxyurea inhibits proliferation of hematopoietic cells and may be immunosuppressive. We postulated that hydroxyurea could exacerbate parvovirus B19-induced aplastic crisis and inhibit the development of specific immune responses in children with SCD. We conducted a retrospective review of parvovirus B19 infection in 330 children with SCD. Altogether there were 120 known cases of aplastic crisis attributed to parvovirus B19 infection, and 12% of children were on hydroxyurea treatment during the episode. We evaluated hematological and immune responses. Children with HbSS or HbSβ0-thalassemia treated with hydroxyurea, when compared with untreated children, required fewer transfusions and had higher Hb concentration nadir during transient aplastic crisis. Duration of hospital stays was no different between hydroxyurea-treated and untreated groups. Children tested within a week following aplastic crisis were positive for parvovirus-specific IgG. Immune responses lasted for the duration of the observation period, up to 13 years after transient aplastic crisis, and there were no repeat aplastic crisis episodes. The frequencies of parvovirus-specific antibodies in all children with SCD increased with age, as expected due to the increased likelihood of a parvovirus exposure, and were comparable to frequencies reported for healthy children. Approximately one-third of children had a positive parvovirus B19-specific IgG test without a documented history of transient aplastic crisis, and 64% of them were treated with hydroxyurea. Hydroxyurea may reduce requirements for blood transfusions and may attenuate symptoms during transient aplastic crisis episodes caused by parvovirus B19 infections. Children with SCD, whether treated or untreated with hydroxyurea, generate sustained and protective parvovirus B19-specific immune responses.
Keywords: Sickle cell anemia, hydroxycarbamide, immune response
Introduction
Parvovirus B19 causes “fifth” disease, a common illness of childhood that is usually associated with low grade fever, malaise, arthralgia and a characteristic facial exanthem that resolves about two weeks after infection.1 In patients with sickle cell disease (SCD), however, parvovirus B19 infection exacerbates anemia by temporarily suppressing bone marrow erythropoietic activity, leading to a transient aplastic crisis episode that often requires hospitalization and supportive red blood cell transfusions.2–5 This complication is particularly common in young children with SCD, and less frequent, but present, in adults.6–8 In SCD patients, an acute parvovirus B19 infection event may also precipitate prolonged vaso-occlusive crisis, myocarditis, splenic sequestration, and fatal bone marrow embolism.9–12 Long-term parvovirus B19-associated problems in SCD can include glomerulonephritis (which may lead to end-stage renal disease), cardiac dysfunction, and stroke with permanent neurologic deficits.13,14 Acute and chronic complications arising from parvovirus B19 infection affect all SCD genotypes. In most individuals, including those with SCD, parvovirus B19 infection promotes the production of specific serum IgM and IgG antibodies and IgG-specific antibodies provide lifetime immunity from reinfection. In immunocompromised patients, however, acute parvovirus B19 infection can fail to resolve and produces severe and refractory anemia.15 These clinical observations raise the question as to whether hydroxyurea, an S-phase acting agent that inhibits hematopoietic cell proliferation, can impair clinical recovery during acute parvovirus B19 infection in SCD patients.
Hydroxyurea is a myelosupressive agent used with increasing frequency in the pediatric SCD population. Hydroxyurea therapy increases fetal hemoglobin production, hemoglobin (Hb) concentration and reduces white blood cell count and hemolysis markers, thereby ameliorating disease-associated symptoms and complications.16–18 Toxicities of hydroxyurea therapy in SCD include reversible, dose-related reticulocytopenia, neutropenia, and thrombocytopenia.19,20 Indeed, hydroxyurea therapy is most effective when dose escalated to mild myelosuppression.21 Acute parvovirus B19 infection causes reticulocytopenia, and less commonly neutropenia and thrombocytopenia, all of which are also reported as hydroxyurea toxicities. Moreover, hydroxyurea can potentially alter the immune response. A recent report demonstrated that total lymphocyte, CD4, and memory T-cell counts were reduced in hydroxyurea-treated children compared to placebo-treated controls, although these values were still within the normal range.22
Little information exists in the literature regarding the hematopoietic effects and immune response during a natural parvovirus B19 infection in the context of hydroxyurea therapy. Defining the hematopoietic effects, immune response and degree of protection elicited by natural infection in children with SCD treated with hydroxyurea are necessary (i) to further evaluate the risk-benefit profile for hydroxyurea used in young children, (ii) to support vaccine development by defining a patient’s potential to respond to parvovirus B19 antigens while receiving hydroxyurea therapy, and (iii) to provide benchmarks against which new vaccine candidates may be judged. To address these issues, we conducted a retrospective review of clinical parameters during a transient aplastic crisis episode and the parvovirus B19-specific immune responses in a large pediatric SCD population.
Methods
All participants were children with SCD treated at St. Jude Children’s Research Hospital. The median age was 7.6 years (range 0.4–18 years). Inclusion criteria were: pediatric patients (age ≤18 years) with SCD (all genotypes) who were tested for parvovirus B19-specific antibody using the semi-quantitative commercially available enzyme-linked immunosorbent assay (ELISA, Focus Diagnostics, Cypress, CA, USA) between June 2006 and December 2013. Parvovirus-specific immune responses, as reported by the manufacturer, were negative <0.9, equivocal 0.9–1.1, and positive >1.1. Parvovirus B19 testing was performed due to suspicion of transient aplastic crisis, prior to initiation of hydroxyurea therapy, or as screening of prior viral exposure. In our program, we routinely screen young children for prior parvovirus B19 exposure (positive IgG) to help guide general management during a febrile event (e.g. help differentiate reticulocytopenia caused by transient aplastic crisis from a different virus) or hydroxyurea therapy (e.g. help differentiate reticulocytopenia caused by hydroxyurea toxicity from transient aplastic crisis).
The medical records of children with SCD who underwent a parvovirus ELISA test were reviewed for history of transient aplastic crisis and hydroxyurea therapy treatment. Transient aplastic crisis was defined as an exacerbation of anemia with severe reticulocytopenia (absolute reticulocyte count, ARC <50 × 109/L),23 or a positive IgM score for parvovirus B19 (denoting acute infection). Parvovirus B19-specific serology was examined as a function of time relative to the transient aplastic crisis episodes. Duration of hospital stay, laboratory parameters, and erythrocyte transfusions (number of packed red blood cell, PRBC, units transfused) during the transient aplastic crisis event were compared between hydroxyurea-treated and hydroxyurea-untreated participants using Fisher’s exact test or Wilcoxon–Mann-Whitney test, where appropriate. IRB approval was granted for this retrospective analysis, with a waiver of consent from participants.
Results
Transient aplastic crisis and hydroxyurea therapy
Of 330 eligible participants in our study, there were 120 who experienced an episode of transient aplastic crisis. The Hb genotype distribution of these 120 patients was HbSS (80), HbSC (30), HbSβ0-thalassemia (6), and HbSβ+-thalassemia (4). Fourteen (12%) patients who experienced aplastic crisis were treated with hydroxyurea for a median of 2.8 years (range 0.14–10.4 years) prior to this episode. In order to examine the severity of the transient aplastic crisis in relationship to hydroxyurea therapy, the initial and the nadir Hb concentration, ARC, duration of admission, and blood transfusion history were compared among the hydroxyurea-treated and untreated groups, including all sickle genotypes. The proportion of participants given erythrocyte transfusion (for a significant decline from baseline or clinical symptoms of anemia) during the episode of transient aplastic crisis was similar among patients receiving hydroxyurea therapy compared to those who were not on hydroxyurea (Table 1). Children who were not treated with hydroxyurea (median age = 7.1, range 0.5–17.6 years), however, were more likely to receive >1 unit of packed red blood cells than those treated with hydroxyurea (median age = 8.9, range 4.0–17.3 years) during the transient aplastic crisis (Table 1). There was no difference in Hb concentration and ARC at the onset or during nadir of the transient aplastic crisis episode (Table 1). The median duration of hospital stay between children receiving hydroxyurea and those who were not on this treatment was similar (two versus three days, respectively, p = 0.1). This analysis was repeated among hydroxyurea-treated and untreated children with HbSS or HbSβ0 thalassemia Hb genotypes only. The findings were similar, except that the group of hydroxyurea-treated patients exhibited a higher median Hb concentration nadir during aplastic crisis and were less likely to receive an erythrocyte transfusion in comparison with untreated patients (Table 1). The number of SCD-related complications (acute vaso-occlusive pain, acute chest syndrome and acute splenic sequestration) in the four weeks following the episode of transient aplastic crisis was similar among hydroxyurea-treated and untreated children. One child developed myocarditis during the episode of transient aplastic crisis, and she was not receiving hydroxyurea during this episode.
Table 1.
Comparison of hematologic indices and erythrocyte transfusions according to therapy during the transient aplastic crisis episode
| Hydroxyurea- treated | Hydroxyurea- untreated | P value | |
|---|---|---|---|
|
All genotypes |
|||
| (N = 14) | (N = 106) | ||
| Baseline Hb (g/dL) | 8.9 (7.3, 11.5) | 9.2 (6.1, 11.9) | 0.7a |
| Nadir Hb (g/dL) | 6.5 (4.4, 8.6) | 5.8 (2.1, 9.9) | 0.1a |
| Nadir ARC (×109/L) | 22.0 (1.3, 168.8) | 14.0 (0, 319.0) | 0.3a |
| No. of patients transfused (%) | 10/14 (71%) | 87/100 (87%) | 0.2B |
| No. of patients who received >1 PRBC (%) | 1/10 (10%) | 37/76 (49%) | 0.04b |
|
HbSS and HbSβ0 thalassemia |
|||
| (N = 14) | (N = 72) | ||
| Baseline Hb (g/dL) | 8.9 (7.3, 11.5) | 8.5 (6.1, 11.0) | 0.1a |
| Nadir Hb (g/dL) | 6.5 (4.4, 8.6) | 5.2 (2.1, 8.7) | 0.01a |
| Nadir ARC (×109/L) | 22.0 (1.3, 168.8) | 18.0 (0, 190.8) | 0.7a |
| No. of patients transfused (%) | 10/14 (71%) | 62/66 (94%) | 0.03b |
| No. of patients who received >1 PRBC (%) | 1/10 (10%) | 31/51 (61%) | 0.005b |
Note: Results presented as median (range) unless noted otherwise. Hb: hemoglobin concentration, ARC: absolute reticulocyte count, PRBC: packed red blood cell.
Wilcoxon–Mann-Whitney test was applied.
Fisher’s Exact Test was applied.
B19 Parvovirus-specific immune response following a known aplastic crisis event
We next analyzed the timing of parvovirus B19-specific responses after documenting transient aplastic crises. Parvovirus B19-specific IgM antibodies were not detected in IgG positive patients who exhibited no evidence of a current transient aplastic crisis, indicating a past infection. When tested prior to or greater than seven days from the aplastic crisis episodes, the IgM antibodies were not present; however, when tested at the time of diagnosis of aplastic crisis, patients exhibited parvovirus B19-specific IgM antibodies (index > 1.1), indicating a rapid primary response (Figure 1(a)). The IgG antibody response was evident at time periods shortly thereafter (Figure 1(b)). IgM responses quickly waned, whereas IgG responses were sustained for the duration of the observation period (up to 4976 days) in all patients who experienced transient aplastic crisis. Two individuals exhibited signs of transient aplastic crisis without a detectable IgG response; however, since the antibody measurement was conducted only four days after the onset of symptoms (fever and reticulocytopenia), it likely preceded the IgG rise. These two participants were not receiving hydroxyurea at the time of the transient aplastic crisis episode. There were no cases of recurrent aplastic crisis among any patients in this cohort.
Figure 1.
Parvovirus antibody scores in relationship with transient aplastic crisis events. Each dot represents a different blood sample tested for IgM (panel (a)) or IgG (panel (b)). IgM responses were observed immediately following the onset of symptoms and IgG responses were slightly delayed. IgG responses were long-lasting in all tested subjects
There were no differences in the magnitude or timing of parvovirus-specific sero-responses among participants who were treated with hydroxyurea or not (p = 0.38 for IgG; p = 0.47 for IgM). After the transient aplastic crisis, an additional 35 participants initiated hydroxyurea therapy, yielding a total of 49 hydroxyurea-treated children. At a median of 0.6 years (range 0–9.1) after the transient aplastic crisis, these hydroxyurea-treated children continued to exhibit positive IgG scores that did not significantly differ in magnitude from those of untreated patients.
Parvovirus B-19 specific immune responses among all 330 children with SCD
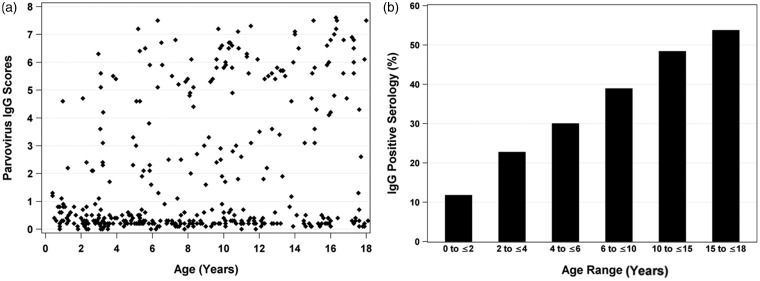
We further analyzed the frequencies of anti-parvovirus B19 antibody production in all 330 pediatric SCD patients, regardless of hydroxyurea treatment or prior aplastic crisis, over a 7.5-year interval. Overall, 124 of 330 (38%) patients exhibited a positive IgG result. There were 387 valid observations in total, because some patients were tested more than once (Figure 2(a)). Positive scores in the first year of life (0.6% of events) could have occurred via maternal antibody transmission. A steady rise in anti-parvovirus B19 seropositivity among children with SCD was observed with increasing age (range 12–53%) (Figure 2(b)), as would be expected due to an increased likelihood of parvovirus B19 exposure over time.
Figure 2.
IgG seropositivity rates among children with SCD. (a) Each point represents an individual sample. (b) The fraction of children of various age groups with positive parvovirus-specific antibody responses increases with age
Patients with parvovirus-specific IgG, but no record of parvovirus-induced aplastic crisis
Forty-four (35%) out of 124 participants with positive IgG did not have a documented history of prior transient aplastic crisis in their medical records. Of these, there were 23 patients for whom records were complete. Medical records were incomplete for the remaining 21 patients due to transfer from other programs or gaps in their care. Among the 23 patients with complete records at the time of documentation of a positive IgG, 16 had HbSS or HbSβ0 thalassemia (15 of whom received hydroxyurea or monthly transfusion therapy), and 7 had HbSC or HbSβ+ thalassemia (three of whom received hydroxyurea therapy). Of the remaining 21 patients for whom records were incomplete, 19 had HbSS or HbSβ0 thalassemia (17 of whom received hydroxyurea or monthly transfusion therapy), and 2 had HbSC or HbSβ+ thalassemia (none of them receiving any disease-modifying therapy). Overall, a greater proportion of children with IgG sero-positive tests without prior documented aplastic crises were treated with hydroxyurea than children with IgG sero-positive tests and prior documented aplastic crises (64% versus 12%).
Discussion
We studied 330 children with SCD including 120 who had suffered transient aplastic crisis, 12% of whom were treated with hydroxyurea. Hydroxyurea treatment was associated with a reduced requirement for transfused PRBCs and higher Hb concentration nadir during aplastic crisis events, although there was no difference in the duration of hospitalization. There was no difference in production of parvovirus B19-specific immunoglobulins with hydroxyurea therapy.
Because transient aplastic crisis and hydroxyurea toxicity both cause bone marrow suppression with ensuing reticulocytopenia and anemia, it is theoretically possible that children receiving hydroxyurea could experience worsened symptoms during transient aplastic crisis. Our findings refute this possibility as hematologic indices and duration of stay were not worse when accounting for hydroxyurea treatment. Indeed, our study suggests that concomitant hydroxyurea therapy may reduce the total volume of transfused red blood cells during aplastic crisis, which may be mediated by an increase in the lifespan of red blood cells in individuals with SCD, as reflected by a higher Hb concentration nadir among children with HbSS or HbSβ0 thalassemia. Red blood survival (as measured by 51Cr method) is indeed prolonged during therapy with hydroxyurea.24
In immunocompetent individuals, a first infection by parvovirus B19 usually provides lifelong protection against re-infection, although exceptions have been reported in SCD patients.25 In our cohort, no cases of re-infection were observed. The onset of aplastic crisis indicated the approximate time of parvovirus exposure for many patients and therefore provided a unique opportunity to monitor the kinetics of the humoral immune response. In our sample, IgM production was evident within a median of one day after clinical presentation and typically waned by one week. IgG responses were evident as early as the first day of symptom onset and were sustained for more than 13 years after infection. Among all patients with SCD, regardless of known exposure to parvovirus or hydroxyurea therapy, the frequency of parvovirus B19-specific antibody responses increased with age, as expected due to an increased likelihood of parvovirus B19 exposure over time. Frequencies of parvovirus-specific antibodies were similar to those of healthy children and other previously examined SCD pediatric populations.6,7,26,27
Positive IgG results were recognized in approximately one-third of patients who had no documentation of prior transient aplastic crisis. This indicates that infection with parvovirus B19 does not cause clinically evident transient aplastic crisis in all SCD patients. Prior reports have shown prevalence of absent prior documented transient aplastic crisis in patients with parvovirus B19 IgG ranging from 20 to 35%, which is in agreement with our findings.6,28 In our study, several patients with parvovirus-specific antibodies without a documented history of worsened anemia and reticulocytopenia were receiving chronic transfusion therapy or harbored HbSC or HbSβ+ thalassemia genotypes. These situations increase the red blood cell lifespan and are therefore predicted to reduce the severity of transient aplastic crisis. Furthermore, passive parvovirus B19 IgG may occur during transfusions, possibly explaining the absence of documented history of transient aplastic crisis. Finally, 64% of these patients were treated with hydroxyurea, possibly minimizing symptoms of worsened anemia during transient aplastic crisis. Because we may have underreported the occurrence of prior transient aplastic crisis in some patients (due to incomplete records in 21 subjects), it is possible that the true rate of asymptomatic parvovirus B19 infection was lower than 35% in our population.
Our findings indicate that concomitant hydroxyurea therapy does not worsen parvovirus B19-induced transient aplastic crisis nor does it compromise immune responses that protect against repeat infections. However, there are several limitations to data interpretation. First, our study was retrospective with a relatively small number of participants on hydroxyurea at the time of the transient aplastic crisis. In addition, full records were not available on patients who had positive anti-parvovirus B19 IgG without prior documentation of transient aplastic crisis, and we were not able to retrieve the total volume of transfused PRBCs in all participants (only total number of units). Finally, we could not exclude the possibility that clinicians’ preferences for post-transfusion target Hb played a role in our results. However, we have formally investigated the relationships between hydroxyurea therapy and severity of transient aplastic crisis and the parvovirus B19-specific immune response, both of which will aid in the development of future vaccines and help to better define the relative risks and benefits of the drug. This information is particularly important considering that recent guidelines encourage caregivers to offer hydroxyurea therapy to all HbSS and HbSβ0-thalassemia children over the age of nine months, regardless of severity of symptoms.29 Thus, use of this drug for SCD is likely to increase substantially in the near future.
In conclusion, we demonstrate that the responses to natural parvovirus B19 infection in children with SCD are predictable, protective, long-lasting and not worsened by concomitant hydroxyurea therapy. These data support other studies indicating that hydroxyurea therapy does not cause clinically significant immunosuppression22,30 and alleviate concerns that use of the drug could cause chronic or relapsed parvovirus B19 infection in SCD patients. Patients with SCD who are treated with hydroxyurea exhibit normal response to a natural parvovirus B19 infection and are likely to exhibit normal responses to candidate parvovirus B19 vaccines, and therefore should not be excluded from related clinical trials.
Acknowledgments
This work was supported in part by the Children’s Infectious Defense Center (CIDC), NIH R01 AI088729, NIH P30 CA21765, and the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to thank Mitch Weiss, MD, PhD for his critical editing of the manuscript, and Bertha Davis and Jola Dowdy, BS, MS for their support with data collection and regulatory procedures.
Authors’ Contributions
JH conceptualized and designed the study, performed data collection, drafted the initial manuscript, and approved the final manuscript as submitted; RRP interpreted data, reviewed and revised the manuscript, and approved the final manuscript as submitted; PL performed data collection, reviewed the manuscript, and approved the final manuscript as submitted; LT performed data analysis, interpreted data, reviewed and revised the manuscript, and approved the final manuscript as submitted; YS performed data analysis, interpreted data, reviewed and revised the manuscript, and approved the final manuscript as submitted; JLH conceptualized and designed the study, edited the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004; 350: 586–97. [DOI] [PubMed] [Google Scholar]
- 2.Sant'Anna AL, Garcia RC, Marzoche M, da Rocha HH, Paula MT, Lobo CC, Nascimento JP. Study of chronic hemolytic anaemia patients in Rio de Janeiro: prevalence of anti-human parvovirus B19 IgG antibodies and the development aplastic crises. Rev Inst Med Trop Sao Paulo 2002; 44: 187–90. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher JF, Jr., Luban NL, Cohen BJ, Mortimer PP. Human serum parvovirus as the cause of aplastic crisis in sickle cell disease. Am J Dis Child 1984; 138: 401–3. [DOI] [PubMed] [Google Scholar]
- 4.Serjeant GR, Topley JM, Mason K, Serjeant BE, Pattison JR, Jones SE, Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet 1981; 2: 595–7. [DOI] [PubMed] [Google Scholar]
- 5.Rao SP, Miller ST, Cohen BJ. Transient aplastic crisis in patients with sickle cell disease. B19 parvovirus studies during a 7-year period. Am J Dis Child 1992; 146: 1328–30. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Whitley K, Zhao H, Hodinka RL, Kwiakowski J, Cecil R, Cecil T, Cnaan A, Ohene-Frempong K. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood 2004; 103: 422–7. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman SA, Davis JS, Schultz WH, Ware RE. Subclinical parvovirus B19 infection in children with sickle cell anemia. J Pediatr Hematol Oncol 2003; 25: 387–9. [DOI] [PubMed] [Google Scholar]
- 8.Win N, Lee E, Needs M, Homeida S, Stasi R. Profound sustained reticulocytopenia and anaemia in an adult patient with sickle cell disease. Transfus Med 2014; 24: 418–20. [DOI] [PubMed] [Google Scholar]
- 9.Godeau B, Galacteros F, Schaeffer A, Morinet F, Bachir D, Rosa J, Portos JL. Aplastic crisis due to extensive bone marrow necrosis and human parvovirus infection in sickle cell disease. Am J Med 1991; 91: 557–8. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurti L, Lanford L, Munoz R. Life threatening parvovirus B19 and herpes simplex virus associated acute myocardial dysfunction in a child with homozygous sickle cell disease. Pediatr Blood Cancer 2007; 49: 1019–21. [DOI] [PubMed] [Google Scholar]
- 11.Yates AM, Hankins JS, Mortier NA, Aygun B, Ware RE. Simultaneous acute splenic sequestration and transient aplastic crisis in children with sickle cell disease. Pediatr Blood Cancer 2009; 53: 479–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayburg M, Kalinyak KA, Towbin AJ, Baker PB, Joiner CH. Fatal bone marrow embolism in a child with hemoglobin SE disease. Am J Hematol 2010; 85: 182–4. [DOI] [PubMed] [Google Scholar]
- 13.Quek L, Sharpe C, Dutt N, Height S, Allman M, Awogbade M, Rees DC, Zuckerman M, Thein SL. Acute human parvovirus B19 infection and nephrotic syndrome in patients with sickle cell disease. Br J Haematol 2010; 149: 289–91. [DOI] [PubMed] [Google Scholar]
- 14.Balkaran B, Char G, Morris JS, Thomas PW, Serjeant BE, Serjeant GR. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr 1992; 120: 360–6. [DOI] [PubMed] [Google Scholar]
- 15.Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis 2006; 43: 40–8. [DOI] [PubMed] [Google Scholar]
- 16.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest 1984; 74: 652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995; 332: 1317–22. [DOI] [PubMed] [Google Scholar]
- 18.Wang WC, Ware RE, Miller ST, Iver RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waslawiw MA, Huang X, Thompson BW. BABY HUG Investigators. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011; 377: 1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornburg CD, Files BA, Luo Z, Miller ST, Kalpatthi R, Lyer R, Seaman P, Lebensburger J, Alvarez O, Thompson B, Ware RE, Wang WC. BABY HUG Investigators. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012; 120: 4304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strouse JJ, Lanzkron S, Beach MC, Haywood C, Park H, Witkop C, Wilson RF, Bass ED, Segal JB. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics 2008; 122: 1332–42. [DOI] [PubMed] [Google Scholar]
- 21.Ware RE, Aygun B. Advances in the use of hydroxyurea. Hematology Am Soc Hematol Educ Program 2009; 53: 62–9. [DOI] [PubMed] [Google Scholar]
- 22.Lederman HM, Connolly MA, Kalpatthi R, Ware RE, Wang WC, Luchtman-Jones L, Waclawiw M, Goldsmith JC, Swift A, Casella JF, BABY HUG Investigators Immunologic effects of hydroxyurea in sickle cell anemia. Pediatrics 2014; 134: 686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, Johnson CS, Rogers ZR, Smith-Whitley K, Wang WC, Telen MJ, Investigators, Comprehensive Sickle Cell Centers Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol 2010; 85: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballas SK, Marcolina MJ, Dover GJ, Barton FB. Erythropoietic activity in patients with sickle cell anaemia before and after treatment with hydroxyurea. Br J Haematol 1999; 105: 491–6. [PubMed] [Google Scholar]
- 25.Anderson MJ, Davis LR, Hodgson J, Jones SE, Murtaza L, Pattison JR, Stroud CE, White JM. Occurrence of infection with a parvovirus-like agent in children with sickle cell anaemia during a two-year period. J Clin Pathol 1982; 35: 744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen BJ, Buckley MM. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol 1988; 25: 151–3. [DOI] [PubMed] [Google Scholar]
- 27.Huatuco EM, Durigon EL, Lebrun FL, Passos SD, Gazela RE, Azevedo Nelo RS, Massad E. Seroprevalence of human parvovirus B19 in a suburban population in Sao Paulo, Brazil. Rev Saude Publica 2008; 42: 443–9. [DOI] [PubMed] [Google Scholar]
- 28.Serjeant GR, Serjeant BE, Thomas PW, Anderson MJ, Palou G, Pattison JR. Human parvovirus infection in homozygous sickle cell disease. Lancet 1993; 341: 1237–40. [DOI] [PubMed] [Google Scholar]
- 29.Yawn BP, Buchanan GR, Afenyi-Annan AN. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014; 312: 1033–48. [DOI] [PubMed] [Google Scholar]
- 30.Nickel RS, Osunkwo I, Garrett A, Robertson J, Archer DR, Promislow DE, Horan JT, Hendrickson JE, Kean LS. Immune parameter analysis of children with sickle cell disease on hydroxycarbamide or chronic transfusion therapy. Br J Haematol 2015; 169(4): 574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]