Abstract
Sickle cell disease, a common single gene disorder, has a complex pathophysiology that at its root is initiated by the polymerization of deoxy sickle hemoglobin. Sickle vasoocclusion and hemolytic anemia drive the development of disease complications. In this review, we focus on the genetic modifiers of disease heterogeneity. The phenotypic heterogeneity of disease is only partially explained by genetic variability of fetal hemoglobin gene expression and co-inheritance of α thalassemia. Given the complexity of pathophysiology, many different definitions of severity are possible complicating a full understanding of its genetic foundation. The pathophysiological complexity and the interlocking nature of the biological processes underpinning disease severity are becoming better understood. Nevertheless, useful genetic signatures of severity, regardless of how this is defined, are insufficiently developed to be used for treatment decisions and for counseling.
Keywords: Severity in sickle cell disease, subphenotypes of sickle cell disease, single nucleotide polymorphisms, genome-wide association study, hemolysis, genotype–phenotype correlation
Introduction
Sickle cell disease, one of the world’s commonest single gene disorders, was first described by Herrick in 1910, who linked his patient’s symptoms to abnormally shaped erythrocytes in the blood. Pauling and colleagues in 1949 detected abnormal hemoglobin, that has subsequently called sickle hemoglobin (HbS), and was shown by Ingram to contain a valine residue in place of glutamic acid as the 6th amino acid of the β-hemoglobin chain; the mutation was subsequently confirmed as GAG to GTG in codon 6 (rs334).1,2
An understanding of pathophysiology is a prerequisite to appreciating and measuring disease severity. One interpretation of the phenotype of sickle cell disease dichotomizes its pathophysiology into two interrelated branches (Table 1): viscosity-vasoocclusion (Figure 1); hemolysis-endothelial dysfunction (Figure 2). The events depicted within these branches occur simultaneously and neither branch should be considered in isolation from the other. Solely for the purpose of edifying the disease pathophysiology and envisaging the mechanistic basis of disease complications, sickle vasoocclusion and hemolytic anemia can be thought of as discrete pathophysiological entities. Each of these pathophysiologic branches has been associated with certain clinical features.3 Although clearly an oversimplification of the pathophysiology of sickle cell disease—one also subjected to some criticism—this scheme is useful for understanding the pathobiology, estimating the severity of disease and useful for considering how certain targeted treatments might affect one but not the other pathway. Recent studies have further validated the role of hemolytic anemia as a driver of some pathophysiologic features of sickle cell and other hemolytic anemias.6–11 As an example of the importance of carefully considering pathophysiology when formulating treatment, a Phase 3 clinical trial focused on reducing the frequency of sickle vasoocclusive events; however, the drug, a Gardos channel inhibitor, was known to ease hemolysis by reducing sickle erythrocyte density. The drug, a cation channel inhibitor, had the anticipated effect of increasing hemoglobin level. A higher hemoglobin level causes increased blood viscosity, unless the additional red cells have a high content of fetal hemoglobin (HbF) which they did not. The primary endpoint of the trial, sickle vasoocclusion, did not improve and might even have worsened, perhaps because of the increased hemoglobin levels. The trial was prematurely terminated.12
Table 1.
Clinical and laboratory features of the vasoocclusive and hemolytic subphenotypes of sickle cell disease.3–5
| Viscosity-vasoocclusion | Hemolysis-endothelial dysfunction | |
|---|---|---|
| Laboratory | Lower LDH Higher PCV | Higher LDH Lower PCV |
| HbF | High HbF protective | HbF less protective |
| α Thalassemia | Absence of α thalassemia associated with increased viscosity | α thalassemia protective by reducing MCHC, erythrocyte density and hemolysis |
| Common clinical complications | 1. Acute painful episodes. 2. Acute chest syndrome 3. Osteonecrosis. | 1. Leg ulcer 2. Pulmonary hypertension 3. Priapism. 4. Stroke |
Figure 1.
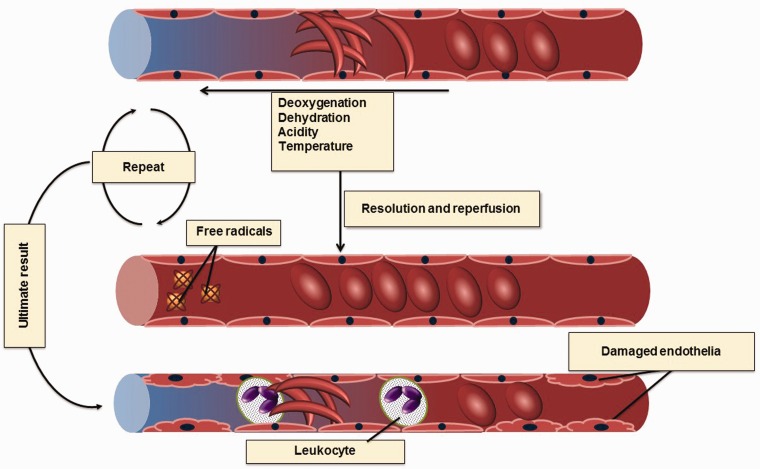
Mechanism of sickle vasoocclusion. Erythrocyte damage and deformation (sickling) occur as a result of polymerization of deoxyHbS and also high concentrations of unpolymerized oxidized HbS, modulated by cellular levels of HbF, erythrocyte cation and water content, pH, temperature, and mechanical stresses that result in membrane damage and eventual failure. Hemolytic anemia and vasoocclusion cause tissue hypoxia. When this occlusion resolved and perfusion is established in the hypoxic tissue, free radicals are produced. These free radicals cause damage to the endothelia making them sticky for RBCs and also for leucocytes. The vascular wall ultimately becomes more vulnerable to occlusion (A color version of this figure is available in the online journal)
Figure 2.
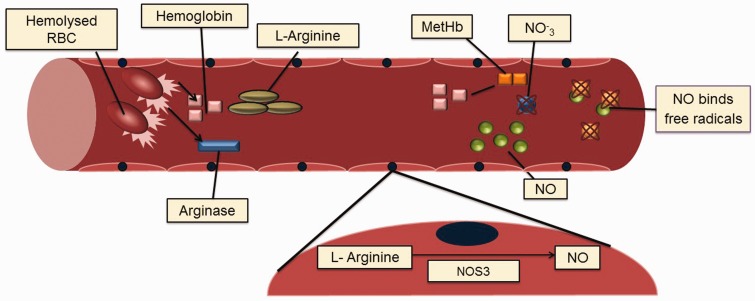
Hemolysis-endothelial dysfunction. With intravascular hemolysis, erythrocytes release hemoglobin and arginase. Arginine is the precursor for NO production by the endothelium via NOS3. Arginase degrades L-arginine, the NOS3 substrate causing reduced NO production. Free plasma hemoglobin interacts with NO producing methemoglobin and nitrate depleting NO. These mechanisms occur during steady state and the amount of intravascular hemolysis varies among patients. An additional mechanism which occurs commonly during vasoocclusive episodes relates to free radicals production with the oxidation of NO. NO depletions will disturb the vasodilatorvasoconstrictor balance, ultimately leading to vasoconstriction of the blood vessels which will complicate the VOC further (A color version of this figure is available in the online journal)
Sickle cell disease is composed of diverse genotypes (Table 2). Sickle cell anemia denotes homozygosity for the HbS mutation and is the most common, most clinically apparent and best studied form of this disease. Nearly all genetic studies of sickle cell disease have concentrated on this β-globin genotype. Other genotypes of sickle cell disease are due to compound heterozygosity for HbS and other hemoglobin variants like HbC, HbE, and HbD, or the many different genotypes of HbS-β thalassemia. These disorders, with the exception of HbS-β0 thalassemia, are when considering each genotype, usually less clinically severe than sickle cell anemia. Nevertheless, within each genotype there is great clinical and hematological heterogeneity that is poorly explained by the effects of the other variant hemoglobin. Usually, HbS-β+ thalassemia is a milder disorder than HbS-β0 thalassemia but this depends in part on the concentration of HbA found in HbS-β+ thalassemia. When assessing severity, all sickle cell genotypes should not be lumped together. Only a single genotype should be included in the analysis, otherwise severity differences are likely to be due to the clinical differences among sickle hemoglobinopathies. The clinical and laboratory features of the sickling hemoglobinopathies have been characterized in detail and some of the common complications are shown in Table 3.1,13
Table 2.
Clinical and hematological features of the most common genotypes of sickle cell disease
| Genotype | Incidence in African Americans | PCV | Retic | MCV | HbF | % Variant | Severity |
|---|---|---|---|---|---|---|---|
| Sickle cell anemia (HbS homozygotes) | 1/600 | 25 | 8 | 90 | 5 | >90% HbS | 4 |
| HbSC disease | 1/800 | 35 | 3 | 80 | 2 | 50% HbS and HbC | 2 |
| S-β0 thalassemia | 1/1500 | 27 | 7 | 82 | 7 | 90% HbS | 4 |
| S-β+ thalassemia | 38 | 2 | 70 | 2 | 5%-30% HbA | 2 | |
| HbSE disease | rare | 35 | 3 | 75 | 2 | ∼30% HbE | 2 |
| Sickle cell anemia-α thalassemia | 30% of all cases | 30 | 6 | 78 | 5 | >90% HbS | 3 |
Note: Shown are average findings for a young adult for each genotype, in the absence of transfusion or hydroxyurea treatment. Findings in young children will differ. Within any genotype of sickle cell disease, results in an individual patient can vary widely. Severity of disease, rated from most severe (4) to absence of clinical events (0) includes complications related to sickle vasoocclusion and hemolysis. Retic = reticulocyte count. Sickle cell trait is not included among sickle hemoglobinopathies because of its benignity although some complications are well known (1).
Table 3.
Some common complications of sickle cell anemia
| Complication | Comment |
|---|---|
| Hyposthenuria | Present in sickle cell trait. Without access to water can lead to dehydration. |
| Renal Papillary necrosis | Medullary hypertonicity and loss of vasculature. Sometimes NSAID related.14 |
| Acute painful episodes | Most common complication in sickle cell disease. |
| Acute chest syndrome | Second most common complication. Accounts for about 25% of deaths. Often present with or after a painful episode.15,16 |
| Pulmonary hypertension | Effect about 30% of patients with sickle cell disease and a major risk factor for near term death.17 |
| Priapism | Recurrent ischemic priapism can lead erectile dysfunction. |
| Leg ulceration | The most common site is the lateral and medial malleoli.18 |
| Osteonecrosis | Most common site is the femoral head. |
Genetic association studies
Sickle cell anemia is a prototypical monogenic Mendelian disease but one notorious for its clinical heterogeneity. Some patients are constantly ill and display most of the clinical and laboratory subphenotypes of this disease; others have few overt signs and symptoms. However, it is rare for an affected individual to escape the consequences of HbS polymerization that drives sickle vasoocclusion and the hemolytic anemia that continues relentlessly even when acute events are quiescent. In developed countries, the environment might explain some of the observed heterogeneity of disease but most often, genetic variation among patients, intrinsic, and extrinsic to the sickle erythrocyte, is more likely to account for the bulk of their phenotypic differences.19 The discovery of this genetic variation is the subject of genotype–phenotype association studies.
Genotype–phenotype association studies require a precise definition of the phenotype. This must be applicable to more than a single patient clinic cohort as individual clinics see small numbers of patients relative to the sample size needed for these studies. Phenotypes that can be expressed as quantitative traits, like HbF, have yielded the most robust and reproducible results. In contrast, sickle cell anemia subphenotypes like disease severity, acute chest syndrome or pain, which do not have well-defined heritability, have yet to be associated with consistent replication of the results of genetic association studies. This does not imply that these and other subphenotypes of disease are not genetically modulated. Many common quantitative traits like height and weight have been associated with dozens of quantitative trait loci or QTL, each one contributing a very small proportion of the variance of the trait. In many studies, the amount of variance explained is less than 10%. To find these associations, tens or hundreds of thousands of people must be studied, a near impossibility in a rare disease like sickle cell anemia. In the absence of assembling cohorts of many thousands of well-phenotyped cases, it is highly unlikely that additional variants with small effects on a subphenotype like acute chest syndrome or sickle cell pain, the most common disease complications, will be found. Even less likely to be discovered are genetic variants modulating the more rare common complications of disease where it is even more difficult to find sufficient numbers of cases to compare with controls.
The relationships between a genetic variant and a disease phenotype is ascertained by comparing the odds of a selected phenotype occurring in carriers of a genetic variant compared with those who do not carry the variant. In most sickle cell disease phenotypes, the definition of cases and controls can be confounded by age. For example, vasoocclusive stroke predominates in children and one might reasonably capture the majority of these strokes by studying adolescents and young adult case and control groups. In contrast, the incidence of leg ulcers increases with aging so that young controls might ultimately become cases and confound the distinction of case and control groups. Prerequisites for genetic association studies are evidence that the phenotype examined is heritable and that cases with the phenotype are distinct from controls lacking the phenotype. It is clear that HbF is a heritable trait and at least in the normal population stroke is at least partially a heritable trait; less clear is the heritability of acute painful episodes or acute chest syndrome.
Genetic association studies applied to sickle cell anemia have taken both focused candidate gene and agnostic genome-wide approaches. In the former, variants of candidate genes suspected of being associated with a phenotype and identified using prior knowledge are tested for their association with disease subphenotypes (Table 3). Candidate gene association studies have been criticized for their lack of robustness and replicability.20 With some exceptions, validation of much of this work is weak.20,21
In genome-wide association studies (GWAS), hundreds of thousands and even millions of known genetic variants, usually single nucleotide polymorphisms (SNPs), are queried for the possible association of one or more variants with a subphenotype. Usually when an SNP is associated with a phenotype, the functional locus or a putative mechanism whereby the disease is modified by the variant is unknown. This is because most variants associated with a trait are found in non-coding DNA and are likely to have regulatory functions that can in many instances affect genes far removed from the variant and might even be on a different chromosome. GWAS have been used in sickle cell anemia to study the genetic associations of HbF, bilirubin, cholelithiasis, hemolysis, HbA2 level, tricuspid regurgitation velocity, stroke, and systemic blood pressure. Genetic association studies are summarized in Table 4.
Table 4.
Genetic associations with subphenotypes of sickle cell anemia
| Subphenotype | Genes | References |
|---|---|---|
| Survival | Multiple including TGFBR3 | 22,23–26 |
| Stroke, silent infarction, TCD velocity | Multiple gene identified, VCAM1, ILR4, ADBR2, HLA, LDLR, but few have been validated (see text) | 27,28–30 |
| Painful episodes | GCH1-results reported in abstract only. Biologically plausible. MBL2 in children, low expression associated with increased pain PLA2G4A. | 31–34 35 |
| Acute chest syndrome | Many genes have been “identified” but no study has been validated. HMOX1 (GT)n S/S – Reduce incidence Intergenic region between DNMT3B –COMMD7 significant in children <age 5 years. | 36–39 40 35 |
| Bacteremia/Infection | MBL2-contradictory evidence in different populations that that low level protective. Other genes include CCL5, various HLA alleles, IGF1R, TGF-β/SMAD/BMP pathway HLA-E*0101 HLA class 1 Susceptibility for infection in homozygotes HLA-E*0103 HLA class 1 Protection against infection in heterozygotes HLA-DRB1*15 HLA class 2 Protection HLA-DQB1*03 HLA class 2 Increased risk IGF1, TGFβ/BMP | 41–43 44,41,45 |
| Osteonecrosis | Little evidence for MTHFR;BMP6-results validated in 2 different populations | 38,46–48 |
| Priapism | KL, TEK, TGFBR3, AQP1 | 49–51 |
| Leg ulcers | TGF-β/SMAD/BMP pathway, KL, possibly HLA alleles | 51–53 |
| Sickle vasculopathy/TRV velocity | BMP6, TGFBR3, ACVR1, BMP2 | 54 |
| Cholelithiasis | Promoter repeats in UGT1A1 associated with serum bilirubin | 55–57 |
| Renal function/albuminuria/Glomerular hyperfiltration | DARC FY- associated with proteinuria, TGF-β/Smad/BMP pathway, MYH9, APOL1 | 58–61 |
| Multiple subphenotypes | Duffy antigen receptor (DARC) No relationship to leg ulcers,? nephropathy, priapism, osteonecrosis, response to opioids | 62–64 |
| Hemolysis | NPRL3 VCAM1, CD36 NOS3 | 65,66 |
S/S: homozygous for the short GTn repeat allele for HMOX1
The results of GWAS of bilirubin and cholelithiasis were robust and identified the well-known UGT1A gene family as the major regulator of bilirubin metabolism in African Americans with sickle cell anemia, as it is in other ethnicities. Bilirubin levels increase with hemolysis, and the intensity of hemolytic anemia is a marker of severity in sickle cell disease. Another well-validated result was the association of an SNP in NPRL3 with hemolysis. This result was initially found in a discovery cohort and the association remained after adjustment for HbF level and the presence of gene deletion α thalassemia. The original result was then replicated using both GWAS and targeted genotyping in three additional cohorts. The HBA1/HBA2 regulatory elements, hypersensitive sites HS-33, HS-40, and HS-48 are located in introns of NPRL3. Perhaps by independently down-regulating expression of HBA1/HBA2, variants tagged by this NPRL3 SNP reduce hemolysis.
GWAS have not validated any of the associations found in candidate gene association studies. This might be due to the stringency needed to accept an association by GWAS where the widely accepted level of significance is 10−7 to 10−8 allowing for stringent correction of multiple testing. Achieving this level of significance is usually difficult without many thousands of subjects or where the associated SNP has a large effect on the phenotype of interest. It is easy to imagine that complex subphenotypes like pain, acute chest syndrome, and survival are effected by many genes, each with a small effect on the subphenotype, so that very careful experimental design and perhaps a touch of luck are required to achieve a positive result.
Other approaches to mining GWAS data include pathway analysis to discover possible interconnectivity among genes that might influence a subphenotype, inform as to the possible functions of involved genes and help define underlying mechanisms.
As discussed above, genetic association studies rarely detect the functional variant accounting for the phenotype. Association analysis alone cannot substitute for functional and mechanistic studies that are the ultimate test of causation that is needed to validate potential therapeutic targets. Genetic association studies can be used as one means of improving prognosis or identifying regions likely to harbor functional variants.27 The latter can then be examined functionally and identify promising therapeutic targets.67–69
Estimating severity of sickle cell anemia
Determining the severity of sickle cell anemia is difficult and many definitions of severity are possible. Any estimate of severity should control for HbF level, the presence of α thalassemia and age. Discrete clinical features of the disease and laboratory measurements have been used to gauge severity. Patients with HbF ≥8.6% survived longer than those with HbF <8.6%.22 The tricuspid regurgitant velocity predicted near-term death.22,70,71 Patients with high rates of pain had shorter survival. A score reflecting the intensity of hemolysis was associated with an increased risk of death.71,4 To develop a more integrated estimate of severity, a Bayesian network modeled 24 clinical events and laboratory tests to estimate disease severity deriving a score that predicted two-year mortality.72 In contrast to regression models, which can only represent the dependency of a single outcome variable on one or more predictor variables, a Bayesian network can represent the mutual and hierarchal relationships among many variables using probabilistic rules and thus, in many instances, is better suited to prognostic and diagnostic applications. The network can be used as an unbiased assessment of the clinical severity of patients with sickle cell disease, given any clinical and laboratory profile, and a simple web tool that is freely available has allowed the partial validation of the model in independent studies.73
Genetic basis of disease severity
HbF concentration and α thalassemia are the major genetic modifiers of disease but are unlikely to be the only ones.74,75 They have been discussed extensively and will not be further examined in this review. Their effects on different aspects of disease severity have also been reviewed.
Genetic association studies have used some estimates of severity as phenotypes to understand the genetic basis of their pathophysiology. Using this score derived from Bayesian network modelling as a phenotype, GWAS discovered 40 SNPs that were strongly associated with severity but none met the accepted definition of genome-wide significance. Thirty-two of these SNPs could be analyzed in a small independent cohort but this attempt at replication was only partially successful. Among the replicated associations were SNPs in KCNK6, a potassium channel gene but not the Gardos channel that is active in sickle erythrocytes and responsible for the erythrocyte dehydration that impacts the pathophysiology of disease. Other implicated genes had an even more tenuous connection with severity.21 While in the general population longevity is genetically modulated, survival in sickle cell anemia is likely to be driven by the adverse effects of the disease rather than longevity genes.
As a result of its inhibition of HbS polymerization, HbF is the major modulator of the clinical course of sickle cell anemia. HbF levels are highly heritable trait. The genetic basis of HbF gene regulation has been intensively studied and a substantial portion of the networks regulating HbF gene expression have been defined.76 HbF has been associated with protection from some of the complications of sickle cell anemia and in many studies has been associated with increased survival and a reduced incidence of some of the vasoocclusive complications of disease.76–78 The genetic modulators of HbF, because of the major effect of HbF on HbS polymerization, have been associated with painful episodes of sickle cell anemia,77,79 one determinant of survival.
Tricuspid regurgitant velocity and pulmonary hypertension are both associated with mortality in sickle cell disease.70,80,81 Candidate genes associated with tricuspid regurgitant velocity in sickle cell anemia have been reported and some of these genes have been associated with idiopathic pulmonary hypertension in the general population (Table 4).
Sickle cell vascular disease, exemplified by pulmonary hypertension, has been closely linked to the intensity of intravascular hemolysis (Figure 2) and hemolytic anemia has been associated with survival.4,82 NPRL3 was associated with hemolysis, perhaps by down-regulating expression of HBA1/HBA2, and producing an α-thalassemia-like effect.
Conclusions
The genetic basis of clinical heterogeneity of sickle cell anemia is incompletely understood. In some regions of the world, the environmental impact on the course of disease is likely to be dominant.83 Understanding the genetic basis of severity will not be simple given the pathophysiological complexity and interlocking nature of the biological processes culminating in a subphenotype. A good deal of work has been done to date but with insufficient progress to permit the use of this information prognostically or therapeutically. In addition to the biological discoveries made during genetic association studies—most vividly illustrated by the totally unexpected association of the role of BCL11A in HbF gene expression that has brought us to the cusp of a new approach to HbF induction—the rationale for these studies has been the use of their results for making treatment decisions and for patient counseling.68,69 The most likely future outcome will be the validation of multiple genetic variants that modulate some of the common subphenotypes of disease. Perhaps with the advent of more careful phenotyping, international collaborations permitting the assembly of larger patient cohorts than possible previously, newer high-throughput genotyping methods, coupled with analytical techniques like Bayesian networks, it might be possible to derive genetic signatures of survival that can be clinically useful.
Acknowledgements
Supported in part by grants from NHLBI: HL87681; HL101212; HL068970.
Authors’ contribution
Both the authors wrote and edited this manuscript and neither have any disclosures to report.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. 2009; 94: 602–603. [Google Scholar]
- 2.Ranney HM. Historical milestones. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH. (eds). Sickle cell disease: basic principles and clinical practice, New York, NY: Lippincott-Raven, 1994, pp. 1–5. [Google Scholar]
- 3.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007; 21: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, Oriss TB, Zeng Q, Kato GJ, Gibbs JS, Hildesheim ME. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013; 98: 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376: 2018–31. [DOI] [PubMed] [Google Scholar]
- 6.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 2010; 116: 687–92. [DOI] [PubMed] [Google Scholar]
- 7.Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood 2010; 116: 1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 2007; 109: 3088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida CB, Souza LE, Leonardo FC, Costa FT, Werneck CC, Covas DT, Costa FF, Conran N. Acute hemolytic vascular inflammatory processes are prevented by nitric oxide replacement or a single dose of hydroxyurea. Blood 2015; 126: 711–20. [DOI] [PubMed] [Google Scholar]
- 10.Caughey MC, Poole C, Ataga KI, Hinderliter AL. Estimated pulmonary artery systolic pressure and sickle cell disease: a meta-analysis and systematic review. Br J Haematol 2015; 170: 416–24. [DOI] [PubMed] [Google Scholar]
- 11.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest 2012; 122: 1205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH, Stocker JW. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso occlusive crises in patients with sickle cell disease: a phase III randomized, placebo controlled, double blind study of the gardos channel blocker senicapoc (ICA 17043). Br J Haematol 2011; 153: 92–104. [DOI] [PubMed] [Google Scholar]
- 13.Weatherall D, Clegg J. The molecular pathology of the thalassaemias. The Thalassaemia Syndromes, 4th ed Oxford: Wiley-Blackwell, 2001, pp. 133–91. [Google Scholar]
- 14.Kiryluk K, Jadoon A, Gupta M, Radhakrishnan J. Sickle cell trait and gross hematuria. Kidney Int 2007; 71: 706–10. [DOI] [PubMed] [Google Scholar]
- 15.Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, Godeau B, Galacteros F, Brun-Buisson C, Brochard L, Maitre B. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med 2008; 177: 646–53. [DOI] [PubMed] [Google Scholar]
- 16.Paul RN, Castro OL, Aggarwal A, Oneal PA. Acute chest syndrome: sickle cell disease. Eur J Haematol 2011; 87: 191–207. [DOI] [PubMed] [Google Scholar]
- 17.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007; 21: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol 2010; 85: 831–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewari S, Brousse V, Piel FB, Menzel S, Rees DC. Environmental determinants of severity in sickle cell disease. Haematologica 2015; 100: 1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lettre G. The search for genetic modifiers of disease severity in the β-hemoglobinopathies. Cold Spring Harb Perspect Med 2012; 2: a015032–a015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastiani P, Solovieff N, Hartley SW, Milton JN, Riva A, Dworkis DA, Melista E, Klings ES, Garrett ME, Telen MJ, Ashley-Koch A. Genetic modifiers of the severity of sickle cell anemia identified through a genome-wide association study. Am J Hematolo 2010; 85: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease – life expectancy and risk factors for early death. N Engl J Med 1994; 330: 1639–44. [DOI] [PubMed] [Google Scholar]
- 23.Sebastiani P, Wang L, Perls T, Terry DF, Montano M, Baldwin CT and Steinberg MH. A repertoire of genes modifying the risk of death in sickle cell anemia. Blood 2007;110:150.
- 24.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003; 289: 1645–51. [DOI] [PubMed] [Google Scholar]
- 25.Voskaridou E, Christoulas D, Bilalis A, Plata E, Varvagiannis K, Stamatopoulos G, Sinopoulou K, Balassopoulou A, Loukopoulos D, Terpos E. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 2010; 115: 2354–63. [DOI] [PubMed] [Google Scholar]
- 26.Bakanay SM, Dainer E, Clair B, Adekile A, Daitch L, Wells L, Holley L, Smith D, Kutlar A. Mortality in sickle cell patients on hydroxyurea therapy. Blood 2005; 105: 545–7. [DOI] [PubMed] [Google Scholar]
- 27.Sebastiani P, Ramoni MF, Nolan V, Baldwin CT, Steinberg MH. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nat Genet 2005; 37: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VI JG, Tang DC, Savage SA, Leitman SF, Heller SI, Serjeant GR, Rodgers GP, Chanock SJ. Variants in the VCAM1 gene and risk for symptomatic stroke in sickle cell disease. Blood 2002; 100: 4303–9. [DOI] [PubMed] [Google Scholar]
- 29.Hoppe C, Klitz W, Cheng S, Apple R, Steiner L, Robles L, Girard T, Vichinsky E, Styles L. Gene interactions and stroke risk in children with sickle cell anemia. Blood 2004; 103: 2391–6. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan JM, Frohlich DM, Howard TA, Schultz WH, Driscoll C, Nagasubramanian R, Mortier NA, Kimble AC, Aygun B, Adams RJ, Helms RW. Genetic predictors for stroke in children with sickle cell anemia. Blood 2011; 117: 6681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor JG, Belfer I, Desai K, Youngblood V, Freeman LA, Darbari DS, Kato GJ, Milton JN, Hartley SW, Steinberg MH, Goldman D. A GCH1 haplotype associated with susceptibility to vasoocclusive pain and impaired vascular function in sickle cell anemia. Blood 2009; 114: 575–575. [Google Scholar]
- 32.Mendonça TF, Oliveira MC, Vasconcelos LR, Pereira LM, Moura P, Bezerra MA, Santos MN, Araújo AS, Cavalcanti MS. Association of variant alleles of MBL2 gene with vasoocclusive crisis in children with sickle cell anemia. Blood Cells Mol Dis 2010; 44: 224–8. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira MC, Mendonça TF, Vasconcelos LR, Moura P, Bezerra MA, Santos MN, Araújo AS, Cavalcanti MS. Association of the MBL2 gene EXON1 polymorphism and vasoocclusive crisis in patients with sickle cell anemia. Acta Haematol 2009; 121: 212–5. [DOI] [PubMed] [Google Scholar]
- 34.Al-Subaie AM, Fawaz NA, Mahdi N, Al-Absi IK, Al-Ola K, Ameen G, Almawi WY. Human platelet alloantigens (HPA) 1, HPA2, HPA3, HPA4, and HPA5 polymorphisms in sickle cell anemia patients with vaso-occlusive crisis. Eur J Haemato 2009; 83: 579–85. [DOI] [PubMed] [Google Scholar]
- 35.Galarneau G, Coady S, Garrett ME, Jeffries N, Puggal M, Paltoo D, Soldano K, Guasch A, Ashley-Koch AE, Telen MJ, Kutlar A. Gene-centric association study of acute chest syndrome and painful crisis in sickle cell disease patients. Blood 2013; 122: 434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharan K, Surrey S, Ballas S, Borowski M, Devoto M, Wang KF, Sandler E, Keller M. Association of T-786C eNOS gene polymorphism with increased susceptibility to acute chest syndrome in females with sickle cell disease. Br J Haematol 2004; 124: 240–3. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan KJ, Kissoon N, Duckworth LJ, Sandler E, Freeman B, Bayne E, Sylvester JE, Lima JJ. Low exhaled nitric oxide and a polymorphism in the NOS I gene is associated with acute chest syndrome. Am J Respir Crit Care Med 2001; 164: 2186–90. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin C, Nolan VG, Wyszynski DF, Ma QL, Sebastiani P, Embury SH, Bisbee A, Farrell J, Farrer L, Steinberg MH. Association of klotho, bone morphogenic protein 6, and annexin A2 polymorphisms with sickle cell osteonecrosis. Blood 2005; 106: 372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Castaldi C, Nolan VG, Baldwin CT, Farrer LA, Steinberg MH, Klings ES. Association of genetic polymorphisms in the TGF-β pathway with the acute chest syndrome of sickle cell disease. Blood 2007; 110: 2247–2247. [Google Scholar]
- 40.Bean CJ, Boulet SL, Ellingsen D, Pyle ME, Barron-Casella EA, Casella JF, Payne AB, Driggers J, Trau HA, Yang G, Jones K. Heme oxygenase-1 gene promoter polymorphism is associated with reduced incidence of acute chest syndrome among children with sickle cell disease. Blood 2012; 120: 3822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adewoye AH, Nolan VG, Ma Q, Baldwin C, Wyszynski DF, Farrell JJ, Farrer LA, Steinberg MH. Association of polymorphisms of IGF1R and genes in the transforming growth factor- beta/bone morphogenetic protein pathway with bacteremia in sickle cell anemia. Clin Infect Dis 2006; 43: 593–8. [DOI] [PubMed] [Google Scholar]
- 42.Neonato MG, Lu CY, Guilloud-Bataille M, Lapoumeroulie C, Nabeel-Jassim H, Dabit D, Girot R, Krishnamoorthy R, Feingold J, Besmond C, Elion J. Genetic polymorphism of the mannose-binding protein gene in children with sickle cell disease: identification of three new variant alleles and relationship to infections. Eur J Hum Genet 1999; 7: 679–86. [DOI] [PubMed] [Google Scholar]
- 43.Dossou Yovo OP, Zaccaria I, Benkerrou M, Hauchecorne M, Alberti C, Rahimy MC, Elion J, Lapoumeroulie C. Effects of RANTES and MBL2 gene polymorphisms in sickle cell disease clinical outcomes: association of the g.In1.1T > C RANTES variant with protection against infections. Am J Hematol 2009; 84: 378–80. [DOI] [PubMed] [Google Scholar]
- 44.Tamouza R, Busson M, Fortier C, Diagne I, Diallo D, Sloma I, Contouris H, Krishnamoorthy R, Labie D, Girot R, Charron D. HLA-E* 0101 allele in homozygous state favors severe bacterial infections in sickle cell anemia. Hum Immunol 2007; 68: 849–53. [DOI] [PubMed] [Google Scholar]
- 45.Tamouza R, Neonato MG, Busson M, Marzais F, Girot R, Labie D, Elion J, Charron D. Infectious complications in sickle cell disease are influenced by HLA class II alleles. Hum Immunol 2002; 63: 194–9. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman SA, Ware RE. Inherited DNA mutations contributing to thrombotic complications in patients with sickle cell disease. Am J Hematol 1998; 59: 267–72. [DOI] [PubMed] [Google Scholar]
- 47.Ulug P, Vasavda N, Awogbade M, Cunningham J, Menzel S, Thein SL. Association of sickle avascular necrosis with bone morphogenic protein 6. Ann Hematol 2009; 88: 803–5. [DOI] [PubMed] [Google Scholar]
- 48.Castro V, Alberto FL, Costa RN, Lepikson-Neto J, Gualandro SF, Figueiredo MS, Annichino-Bizzacchi JM, Saad ST, Costa FF. Polymorphism of the human platelet antigen-5 system is a risk factor for occlusive vascular complications in patients with sickle cell anemia. Vox Sang 2004; 87: 118–23. [DOI] [PubMed] [Google Scholar]
- 49.Nolan VG, Baldwin C, Ma Q, Wyszynski DF, Amirault Y, Farrell JJ, Bisbee A, Embury SH, Farrer LA, Steinberg MH. Association of single nucleotide polymorphisms in KLOTHO with priapism in sickle cell anemia. Br J Haematol 2004; 128: 266–72. [DOI] [PubMed] [Google Scholar]
- 50.Elliott L, Ashley-Koch AE, Castro LD, Jonassaint J, Price J, Ataga KI, Levesque MC, Brice Weinberg J, Eckman JR, Orringer EP, Vance JM. Genetic polymorphisms associated with priapism in sickle cell disease. Br J Haematol 2007; 137: 262–7. [DOI] [PubMed] [Google Scholar]
- 51.Lustosa Souza CR, Azevedo Shimmoto MM, Vicari P, Arruda MM, Roizenblatt M, Cabanas Pedro AC, Hayashida DY, Mecabo G, Figueiredo MS. Klotho polymorphisms and priapism in sickle cell disease. Blood 2010; 116: 1653a–1653a. [Google Scholar]
- 52.Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, Farrell JJ, Sebastiani P, Farrer LA, Steinberg MH. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol 2006; 133: 570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ofosu MD, Castro O, Alarif L. Sickle cell leg ulcers are associated with HLA-B35 and Cw4. Arch Dermatol 1987; 123: 482–4. [PubMed] [Google Scholar]
- 54.Ashley-Koch AE, Elliott L, Kail ME, De Castro LM, Jonassaint J, Jackson TL, Price J, Ataga KI, Levesque MC, Weinberg JB, Orringer EP. Identification of genetic polymorphisms associated with risk for pulmonary hypertension in sickle cell disease. Blood 2008; 111: 5721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Passon RG, Howard TA, Zimmerman SA, Schultz WH, Ware RE. Influence of bilirubin uridine diphosphate-glucuronosyltransferase 1A promoter polymorphisms on serum bilirubin levels and cholelithiasis in children with sickle cell anemia. J Pediatr Hematol Oncol 2001; 23: 448–51. [DOI] [PubMed] [Google Scholar]
- 56.Fertrin KY, Melo MB, Assis AM, Saad ST, Costa FF. UDP-glucuronosyltransferase 1 gene promoter polymorphism is associated with increased serum bilirubin levels and cholecystectomy in patients with sickle cell anemia. Clin Genet 2003; 64: 160–2. [DOI] [PubMed] [Google Scholar]
- 57.Milton JN, Sebastiani P, Solovieff N, Hartley SW, Bhatnagar P, Arking DE, Dworkis DA, Casella JF, Barron-Casella E, Bean CJ, Hooper WC. A genome-wide association study of total bilirubin and cholelithiasis risk in sickle cell anemia. PLoS One 2012; 7: e34741–e34741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nolan VG, Ma Q, Cohen HT, Adewoye A, Rybicki AC, Baldwin C, Mahabir RN, Homan EP, Wyszynski DF, Fabry ME, Nagel RL. Estimated glomerular filtration rate in sickle cell anemia is associated with polymorphisms of bone morphogenetic protein receptor 1B. Am J Hematol 2007; 82: 179–84. [DOI] [PubMed] [Google Scholar]
- 59.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 2011; 155: 386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afenyi-Annan A, Kail M, Combs MR, Orringer EP, shley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion 2008; 48: 917–24. [DOI] [PubMed] [Google Scholar]
- 61.Saraf SL, Zhang X, Shah B, Kanias T, Gudehithlu KP, Kittles R, Machado RF, Arruda JA, Gladwin MT, Singh AK, Gordeuk VR. Genetic variants and cell-free hemoglobin processing in sickle cell nephropathy. Haematologica 2015; 100: 1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nebor D, Durpes MC, Mougenel D, Mukisi-Mukaza M, Elion J, Hardy-Dessources MD, Romana M. Association between Duffy antigen receptor for chemokines expression and levels of inflammation markers in sickle cell anemia patients. Clin Immunol 2010; 136: 116–22. [DOI] [PubMed] [Google Scholar]
- 63.Joly P, Gagnieu MC, Bardel C, Francina A, Pondarre C, Martin C. Genotypic screening of the main opiate-related polymorphisms in a cohort of 139 sickle cell disease patients. Am J Hematol 2012; 87: 534–6. [DOI] [PubMed] [Google Scholar]
- 64.Afenyi-Annan A, Kail M, Combs MR, Orringer EP, Ashley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion 2008; 48: 917–24. [DOI] [PubMed] [Google Scholar]
- 65.Milton JN, Rooks H, Drasar E, McCabe EL, Baldwin CT, Melista E, Gordeuk VR, Nouraie M, Kato GR, Minniti C, Taylor J. Genetic determinants of haemolysis in sickle cell anaemia. Br J Haematol 2013; 161: 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coelho A, Dias A, Morais A, Nunes B, Ferreira E, Picanço I, Faustino P, Lavinha J. Genetic variation in CD36, HBA, NOS3 and VCAM1 is associated with chronic haemolysis level in sickle cell anaemia: a longitudinal study. Eur J Haematol 2014; 92: 237–43. [DOI] [PubMed] [Google Scholar]
- 67.Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, Shao Z, Canver MC, Smith EC, Pinello L, Sabo PJ. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 2013; 342: 253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, Best S. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet 2007; 39: 1197–9. [DOI] [PubMed] [Google Scholar]
- 69.Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP, Luc S. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 2015; 527: 192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004; 350: 886–95. [DOI] [PubMed] [Google Scholar]
- 71.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991; 325: 11–6. [DOI] [PubMed] [Google Scholar]
- 72.Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH, McMahon LC, Farrer LA, Taylor JG, Kato GJ, Gladwin MT. A network model to predict the risk of death in sickle cell disease. Blood 2007; 110: 2727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Junior EB, Silva DGH, de Souza Torres L, Okumura JV, de Castro Lobo CL, Bonini-Domingos CR. Severity of Brazilian sickle cell disease patients: severity scores and feasibility of the Bayesian network model use. Blood Cells Mole Dis 2015; 54: 321–7. [DOI] [PubMed] [Google Scholar]
- 74.Steinberg MH, Embury SH. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. [Review]. Blood 1986; 68: 985–90. [PubMed] [Google Scholar]
- 75.Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. BrJ Haematol 2009; 145: 455–67. [DOI] [PubMed] [Google Scholar]
- 76.Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring HarbPerspect Med 2013; 3: a011643–a011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, Chui DH, Steinberg MH. Fetal hemoglobin in sickle cell anemia. Blood 2011; 118: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. Am J Hematol 2012; 87: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lettre G, Sankaran VG, Bezerra MA, Araújo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH. DNA polymorphisms at the BCL11A, HBS1L-MYB, and β-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci 2008; 105: 11869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA 2012; 307: 1254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol 2006; 134: 109–15. [DOI] [PubMed] [Google Scholar]
- 82.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One 2008; 3: e2095–e2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet 2013; 381: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]