Abstract
Hydroxyurea is an excellent therapeutic agent for the pharmacological induction of HbF in patients with sickle cell disease (SCD). However, all completed clinical trials of hydroxyurea have excluded patients with hemoglobin SC (HbSC) disease. HbSC differs significantly in pathophysiology from HbSS, as HbC does not sickle, but instead causes cellular dehydration which potentiates sickling of HbS. Many severely affected HbSC patients have been placed on hydroxyurea on a case by case basis, but there are no large scale prospective data on safety or efficacy of hydroxyurea in this subset of patients with SCD. Here, we report a case series of 14 pediatric patients with HbSC treated to maximum tolerated dose (MTD) with hydroxyurea. Those who failed to show clinical improvement after at least six months at MTD were offered phlebotomy in addition to hydroxyurea. Five out of 11 patients with HbSC who achieved MTD failed to demonstrate clinical improvement on hydroxyurea. Of the four placed on dual hydroxyurea and phlebotomy therapy, all showed at least partial clinical improvement. Percent dense red blood cells (%DRBC) were measured via an ADVIA hematology analyzer. A marked rise in percent dense cells preceded clinical complications in three patients. Dual therapy with hydroxyurea and phlebotomy may be an effective approach to patients with HbSC that do not experience improvement with hydroxyurea alone. Monitoring of %DRBC may predict adverse events and aid in assessing hydroxyurea compliance. Large scale clinical trials are needed to evaluate the safety and efficacy of hydroxyurea and hydroxyurea with phlebotomy in patients with HbSC disease.
Keywords: Hemoglobin SC disease, hydroxyurea, phlebotomy, red blood cell density
Introduction
Hemoglobin SC (HbSC) disease is one of the most common inherited diseases in the United States, affecting approximately 1 in 833 African-American live births.1 Patients with HbSC are compound heterozygotes. One beta globin gene contains the sickle mutation, glutamic acid to valine change at the sixth codon, and the other beta globin gene contains the C mutation, a change from glutamic acid to lysine, also at the sixth codon. HbS undergoes intracellular polymerization in the deoxygenated state, leading to deformation of the red cell membrane and alteration of cellular physiology. While HbC does not polymerize, it increases the mean corpuscular hemoglobin concentration (MCHC) of the cell via volume regulated K+ efflux, thereby potentiating the polymerization of HbS.2
Clinical manifestations of sickle cell anemia (SCA, specifically HbSS and HbSβ0 genotypes) result primarily from chronic severe hemolytic anemia and the effects of repeated intravascular sickling of erythrocytes within the capillaries and small venules. Hemolysis leads to chronic anemia, gallstone formation, and intimal damage/hyperplasia within the arterial vasculature. Red blood cell sickling leads to acute vaso-occlusive events with varied presentations, including painful events, priapism, splenic sequestration, acute chest syndrome, or stroke. These processes occur in HbSC disease as well, but at 25–50% the frequency.3 Patients with HbSC disease have similar rates of avascular necrosis (AVN), and higher rates of proliferative sickle retinopathy (PSR), than HbSS patients. While pain events and ACS occur more frequently in patients with HbSS, they are not uncommon in patients with HbSC, especially in adolescence. HbSC patients typically exhibit the sickle cell disease (SCD) phenotypes associated with high viscosity, rather than high hemolysis, consistent with their higher hemoglobin levels and increased red blood cell lifespans.4
In patients with HbSS, hydroxyurea provides an important therapeutic option for patients with SCD, since it increases the amount of HbF within circulating erythrocytes, can be administered orally with once-daily dosing, has minimal short-term adverse effects, and is clinically effective.5 While there is compelling evidence for the effectiveness of hydroxyurea in HbSS, HbSC patients were not included in randomized controlled trials of hydroxyurea in adult and pediatric sickle cell patients. It has been hypothesized that HbSC patients may not benefit from hydroxyurea because adult patients with HbSC have baseline HbF<5%, and exhibit little or no HbF induction on hydroxyurea.6 In a cohort of 10 adult patients with HbSC treated with hydroxyurea at doses ranging 500–1000 mg per day, a significant increase in MCV was noted. No significant change in HbF was noted for the group but one individual had a 6.8% rise in HbF.7
Case series of pediatric patients with HbSC disease treated with hydroxyurea found that MCV increased significantly, as did %HbF.8–10 However, the rise in %HbF was modest compared to the levels of induction seen in patients with SCA, and did not come close to the 20% threshold of %HbF suggested to be necessary to prevent pain events.11 There may be other benefits to hydroxyurea use outside of HbF induction; these benefits may be especially relevant to HbSC patients, who, as noted above, make very little HbF at baseline, and experience a very modest increase in HbF on hydroxyurea. Individuals with HbSS have been reported to experience a reduction in dense cells12 and whole blood viscosity when treated with hydroxyurea. The CHAMPS trial (Effects of Hydroxyurea and Magnesium Pidolate in HbSC Disease, NCT00532883) was a Phase II double-blinded multicenter trial that examined the efficacy of hydroxyurea and magnesium pidolate in individuals with HbSC over the age of 5 with at least one pain event in the last 12 months. CHAMPS participants who received hydroxyurea (±magnesium pidolate) had significant increases in MCV and HbF, without changes in hemoglobin level. Children on the study treated with hydroxyurea had greater increases in MCV and HbF after 24 weeks than adults. Unfortunately, clinical conclusions from this trial were limited because of its incomplete enrollment and early termination.13
Another possible therapy for HbSC patients, one targeted to the uniquely high viscosity of the whole blood from individuals with HbSC,14 is phlebotomy. The rationale behind phlebotomy in HbSC is that there is a linear relationship between hemoglobin levels and whole blood viscosity; therefore, reducing hemoglobin levels should reduce whole blood viscosity.15 While the use of phlebotomy has been described in adult HbSC patients, and reduction in incidence of pain events and retinopathy reported,16,17 this is the first report of its use in pediatric patients with HbSC, and the first of dual therapy of hydroxyurea and phlebotomy. Adult patients with HbSC treated with phlebotomy are allowed to become iron deficient, in order to reduce phlebotomy-induced erythropoiesis, thereby lowering the hemoglobin level, reducing whole blood viscosity, and permitting greater time intervals between phlebotomies.16 Induction of iron deficiency is not acceptable in pediatric patients, due to concerns for the effect on their neurocognitive development.18 One possible approach to counteracting the stimulatory effect of phlebotomy on erythropoiesis is to continue hydroxyurea while phlebotomizing the patient, using the known ability of hydroxyurea to suppress reticulocytosis, even in the absence of HbF induction.10 This approach should allow one to maintain iron stores through oral iron supplementation while lowering the hemoglobin set point, achieving the desired reduction in whole blood viscosity.
Finally, yet another question to be answered is a safe dosing regimen of hydroxyurea in patients with HbSC. These patients are known to have lower white blood cell counts (WBC) and absolute reticulocyte counts (ARC) than individuals with SCA. Many are within the laboratory range defined as maximum tolerated dose (ANC 2000-4000 × 106/L, ARC 70–150 × 109/L) before initiating therapy with hydroxyurea. Platelet counts are also lower on average compared to individuals with SCA, whether from splenic trapping or lower levels of chronic inflammation. It is, therefore, important to determine a safe starting dose for patients with HbSC, with review of case reports as a guide. Previously published case reports of hydroxyurea use in HbSC often did not escalate to MTD, 13,19,20 or describe average doses of 19.6–24 mg/kg/day,9,21 significantly lower than average doses of hydroxyurea in SCA.22
Materials and methods
Chart review
We reviewed the charts of all patients with HbSC disease receiving hydroxyurea therapy at Texas Children's Hospital Hematology Center between January 2007 and December 2014. Laboratory data, including complete blood count, percent fetal hemoglobin (determined by high performance liquid chromatography), and absolute reticulocyte count at baseline and at maximum tolerated dose (MTD) were collected. MTD was defined as previously described for SCA.22 Reasons for initiating or terminating therapy, clinical response to hydroxyurea, and where applicable, clinical response to dual therapy with hydroxyurea and phlebotomy were obtained through review of clinic notes. It is our institutional practice to offer hydroxyurea to HbSC patients with three or more pain events requiring acute care (urgent care, or emergency department visits, with or without inpatient admission), proliferative retinopathy, or avascular necrosis. Pain events requiring acute care usage were recorded for each patient placed on hydroxyurea for a pain indication. Clinical improvement of pain was assessed by calculating the average number of acute care visits for pain per year before initiating hydroxyurea and on hydroxyurea at MTD. Clinical improvement in retinopathy was assessed by reviewing ophthalmology reports, and defined as stabilization or improvement of retinopathy on dilated eye exam.
Hematological toxicity was defined as a platelet count <75 × 109/L, absolute neutrophil count (ANC) <1.0 × 109/L, or a drop in hemoglobin of ≥2g/dL. Hydroxyurea was decreased or temporarily discontinued for these toxicities. Hepatic or renal toxicity was defined as a twofold or greater increase in alanine aminotransferase or creatinine, respectively. Differences between pre-hydroxyurea and MTD hydroxyurea laboratory values were measured using a two-tailed t-test.
Phlebotomy was offered to patients who were at MTD for six months or more without clinical improvement, with Hb ≥10.5 g/dL beginning in May of 2012. Four patients received this therapy, with a phlebotomy goal of Hb 9.5–10 g/dL; 10 ml/kg was removed monthly from most patients, with a maximum volume of 600 ml per phlebotomy.
Percent dense red blood cells
%DRBC was measured using an ADVIA hematology analyzer (Siemens, Tarrytown, NY, USA) and is defined as the percentage of red blood cells (RBC) with a hemoglobin concentration of 41 g/dL or higher. Baseline %DRBC values were available for only one patient, although longitudinal values from serial clinic visits were available for 4 of the 14 patients.
Results
A total of 14 patients, 2 female and 12 male, all African-American, with HbSC disease, were prescribed hydroxyurea between 2007 and 2014 at Texas Children's Hospital Hematology Center. Eleven of the 14 reached MTD, defined as an ANC between 2000 and 4000 × 106/L. None experienced a significant increase in hemoglobin, and all had some increase in MCV (Table 1).
Table 1.
Patient demographics
| Pre‐HU, n = 14 | On HU at MTD, n = 11 | P value | |
|---|---|---|---|
| Age (years) | 11.0 ± 3.2 | ||
| Gender | 86% Male | ||
| HU dose (g/dL) | 16.5 ± 4.3 | ||
| WBC (× 109/L) | 9.2 ± 3.2 | 5.8 ± 2.1 | 0.004 |
| Hb (g/dL) | 11.4 ± 1.3 | 11.2 ± 1.2 | NS |
| MCV (fL) | 69.7 ± 4.4 | 85.8 ± 8.1 | <0.001 |
| MCHC (g/dL) | 36.4 ± 1.0 | 36.2 ± 0.87 | NS |
| Platelet count (× 109/L) | 225 ± 76 | 167 ± 80 | NS |
| ANC (× 106/L) | 4685 ± 2190 | 2304 ± 933 | 0.002 |
| ARC (× 109/L) | 150 ± 60 | 107 ± 30 | NS |
| %HbF | 1.7 ± 1.9 | 6.3 ± 6.7 | 0.02 |
HU, hydroxyurea; MTD, maximum tolerated dose. Pre‐HU values are laboratory values immediately preceding initiation of hydroxyurea. On‐HU at MTD values are laboratory values at least six months after initiating hydroxyurea, at MTD as defined in the text.
The most common indication for hydroxyurea treatment was frequent pain events, occurring in 12 of the 14 patients, followed by acute chest syndrome (ACS) in 4 patients, and retinopathy or avascular necrosis (AVN) in 2 patients each. Six patients had two separate SCD complications that resulted in implementation of hydroxyurea treatment (Table 2).
Table 2.
Demographic and clinical data for 14 HbSC patients treated with hydroxyurea between 2007 and 2014 at the Texas Children's Hospital Hematology Center.
| Patient no. | Age at HU start (years) | Gender | Indications for hydroxyurea | Adverse events | Splenomegaly | HU starting dose (mg/kg) | HU dose at MTD (mg/kg) | Clinical course |
|---|---|---|---|---|---|---|---|---|
| 1 | 10.60 | M | Pain | Neutropenia | Yes | 20 | 20.2 | Reduction in pain events |
| 2 | 12.88 | M | Pain | Abdominal pain | No | 19 | Not reached | Unable to assessb |
| 3 | 9.02 | M | AVN | None | No | 20 | 23.5 | AVN resolved |
| 4 | 5.67 | F | ACS, Pain | None | No | 21 | Not reached | Unable to assessb |
| 5 | 7.25 | M | Pain | Thrombocytopenia, | No | 20a | 15.6 | Reduction in pain events |
| 6 | 9.91 | M | Pain | Thrombocytopenia, abdominal pain | Yes | 20a | 9.5 | Reduction in pain events on dual therapy |
| 7 | 9.90 | M | Pain | Thrombocytopenia | No | 20a | 10 | Reduction in pain events |
| 8 | 11.46 | M | Pain | None | No | 20a | 17 | No improvement in pain events |
| 9 | 18.18 | M | Retinopathy | Thrombocytopenia | No | 18 | 18 | Stabilization of retinopathy on dual therapy |
| 10 | 7.71 | F | Pain, Retinopathy | Neutropenia, thrombocytopenia GI bleed | Yes | 15a | 12.7 | Stabilization of retinopathy on dual therapy; no improvement in pain events |
| 11 | 14.25 | M | Pain, AVN | Thrombocytopenia | Yes | 14 | 18 | 50% reduction in chronic narcotic use on dual therapy |
| 12 | 11.62 | M | ACS, pain | None | No | 20 | 20 | Reduction in pain events |
| 13 | 12.00 | M | Pain, ACS | None | Yes | 14 | Not reached | Unable to asssesb |
| 14 | 12.84 | M | Pain, ACS | None | Yes | 15 | 17.6 | Reduction in pain events |
ACS, acute chest syndrome; AVN, avascular necrosis; MTD, maximum tolerated dose, as defined in text.
Dual therapy refers to the combination of hydroxyurea dosed to MTD and phlebotomy with a goal Hb of 9.5–10 g/dL.
Some patients achieved MTD at doses below their starting dose.
Unable to assess effect of hydroxyurea on clinical course if MTD was not reached.
Of the 11 patients who achieved MTD of six months or greater duration, 5 did not exhibit signs of clinical improvement. Four of the five were placed on a combination of hydroxyurea and phlebotomy. Patient 8 refused phlebotomy. All phlebotomized patients had baseline hemoglobin values of 10.5 g/dL or higher. All four patients who received combined hydroxyurea and phlebotomy therapy experienced at least partial clinical improvement. Two experienced stabilization or improvement of severe retinopathy, determined by serial ophthalmology exams by a single provider, and two experienced improvement in pain events, either a reduction in need for acute care visits for pain, or a reduction in chronic opioid use (Tables 2 and 3). In the three patients without evidence of MTD, clinical impact of hydroxyurea could not be assessed.
Table 3.
Impact of hydroxyurea and phlebotomy on pain events requiring acute care
| Pre‐HU |
On HU at MTD |
On HU and phlebotomy |
||||
|---|---|---|---|---|---|---|
| Patient # | Pain events/yr | Years observed | Pain events/yr | Years observed | Pain events/yr | Years observed |
| 1 | 6 | 3 | 1.5 | 4 | n/a | n/a |
| 5 | 3 | 3 | 0 | 4 | n/a | n/a |
| 6 | 3 | 4 | 3 | 2 | 0 | 2 |
| 7 | 2 | 5 | 0 | 3 | n/a | n/a |
| 8 | 2 | 3 | 2 | 4 | n/a | n/a |
| 10 | 2.5 | 2 | 4 | 3 | 4 | 1 |
| 11 | 1 | 2 | 1 | 1 | 0 | 1 |
| 12 | 2 | 3 | 0.5 | 6 | n/a | n/a |
| 14 | 1 | 4 | 0 | 1 | n/a | n/a |
Table includes all patients placed on hydroxyurea for pain events with documented achievement of MTD. Patient 6 was lost to follow‐up after two years of monthly phlebotomy; during that year off dual therapy, acute care usage for pain events returned to 3 per year.
Of the phlebotomized patients, ARC suppression was achieved in three of the four. Patient 9 experienced significant thrombocytopenia before significant suppression of ARC (levels less than 150 × 109/L were not achieved, Table 4). He continued on monthly phlebotomy. Patients 6, 10, and 11 had significant suppression of ARC, all below 120 × 109/L (ARC range 50–119 × 109/L), and required phlebotomy only every two to three months.
Table 4.
Hematologic toxicities experienced on hydroxyurea therapy
| Patient no. | Age at toxicity (years) | Dose‐limiting toxicity | Hydroxyurea dose at toxicity (mg/kg) |
|---|---|---|---|
| 1 | 12.1 | ANC 580 × 106/L | n/a |
| 5 | 10.4 | Platelets 61 × 109/L | 15.6 |
| 6 | 11.7 | Platelets 52 × 109/L | 10 |
| 7 | 10.0 | Platelets 55 × 109/L | 12 |
| 9 | 18.7 | Platelets 22 × 109/L | 21 |
| 10 | 8.2 | ANC 730 × 106/L Platelets 59 × 109/L | 14 |
| 11 | 14.5 | Platelets 56 × 109/L | 20 |
n/a, not available.
Thrombocytopenia was the dose-limiting toxicity in 6 of our 11 patients who achieved MTD on hydroxyurea (Table 4). All six had abdominal ultrasound exams performed in the last three years. Three of the six had mild splenomegaly, three did not. In all six cases, platelet counts recovered within two to four weeks of holding hydroxyurea. Baseline platelet counts were not significantly different between patients who experienced dose-limiting thrombocytopenia and those who did not. Hydroxyurea doses needed to reach MTD were significantly lower than prior reports, averaging only 16.5 mg/kg. Hematologic toxicity was experienced at low doses, at or below 10 mg/kg/day in two patients. This is concerning, because it is our institutional practice to start patients at 20 mg/kg of hydroxyurea, and as a result, our pilot study of hydroxyurea in HbSC (SCYTHE, NCT02336373) has a starting dose of 10 mg/kg. Patient 10 experienced gastrointestinal bleeding during a period of thrombocytopenia. All four patients placed on phlebotomy had experienced dose-limiting thrombocytopenia during hydroxyurea therapy. The average dose of hydroxyurea tolerated was 14 mg/kg/day for these patients.
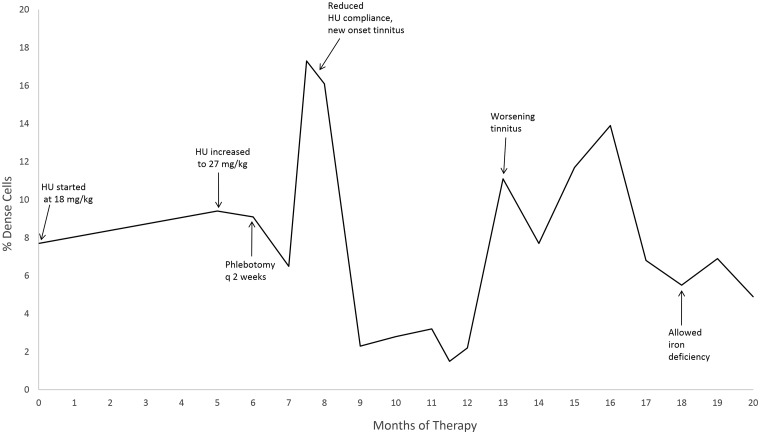
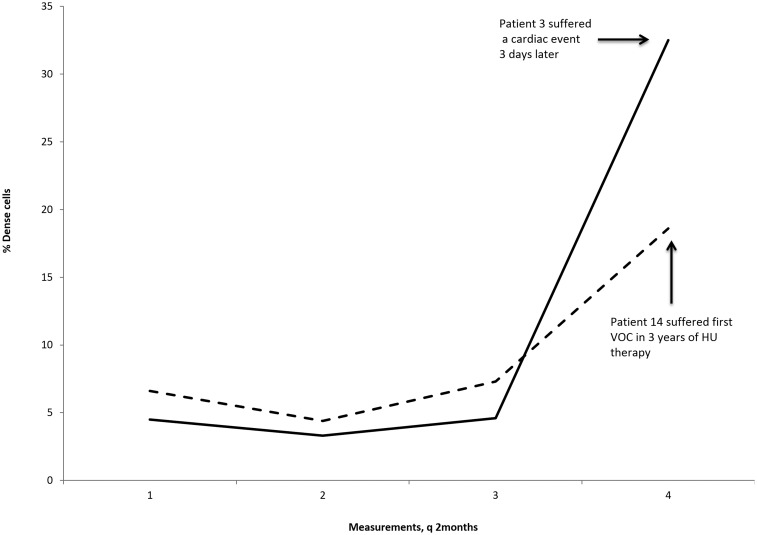
%DRBC was measured for 7 of the 14 patients on at least one occasion with an average value of 6.0 ± 2.8%. Four patients had serial measurements of four or more. Patient 9, placed on hydroxyurea for central retinal artery occlusion (CRAO) and phlebotomy, had 19 %DRBC measurements (Figure 1), with a rise in %DRBC to 17.3% during a period of hydroxyurea non-compliance associated with new onset tinnitus, which resolved concurrent with the subsequent decline in %DRBC. Patients 3 and 14 experienced rapid rises in %DRBC that corresponded with episodes of severe disease complications, cardiac arrest, and pain crisis, respectively (Figure 2). Patient 3 had a %DRBC of 32.5, compared to prior measurements averaging 4.5% (Figure 2). Patient 14 had prior measurements averaging 6.2%, with an increase to 18.6% (Figure 2). Patient 6 did not demonstrate marked variation in %DRBC, with four %DRBC measurements made over approximately one year ranging from 0.8% to 3.4%. For reference, we measured the %DRBC on 55 HbSC patients at steady state, not on hydroxyurea therapy, with an average value of 4.0% (SD 2.8%).
Figure 1.
Effects of hydroxyurea and phlebotomy on % dense cells in Patient 9. Reduced compliance with hydroxyurea was determined by patient interview and pill counts
Figure 2.
Sudden unexplained alterations in % dense cells may precede severe disease-related complications in HbSC patients on hydroxyurea. Patient 3 suffered a cardiac arrest three days after measurement no. 4. Patient 14 experienced his first pain crisis in his three years on hydroxyurea when measurement no. 4 was obtained. Both patients were at stable MTD
Discussion
Here, we report a case series of 14 patients with HbSC treated with hydroxyurea at a single institution, four of whom received novel dual treatment of phlebotomy and hydroxyurea when they failed to exhibit clinical improvement on hydroxyurea after at least six months at MTD. We continued hydroxyurea after initiating phlebotomy in order to suppress phlebotomy-induced reticulocytosis and lower the hemoglobin set point. All patients on phlebotomy were monitored for the development of iron deficiency, through traditional iron studies (iron levels, ferritin, total iron binding capacity) and in the cases of patients 6, 9, and 11, through measurement of the reticulocyte hemoglobin concentration (CHr), a highly sensitive and specific measure of early iron deficiency.23 Patient 6 was found to be iron deficient by CHr, and subsequently supplemented with oral iron, without an increase in pain events. There have been case reports of reduction of pain events with induction of iron deficiency in patients with HbSS, along with subsequent increase in pain episodes with iron replacement; it has been theorized that iron deficiency may be necessary for clinical improvement on phlebotomy.24 Since all dual therapy patients demonstrated at least a partial clinical improvement, and only one became iron deficient as a result of phlebotomy during the observed clinical improvement period, induction of iron deficiency does not appear to be essential for clinical response to phlebotomy in our patients with HbSC.
Not all of our dual therapy patients experienced enough suppression of their reticulocytosis via hydroxyurea therapy to permit spacing of their phlebotomy beyond monthly intervals. Patient 9 experienced significant thrombocytopenia before significant suppression of ARC (Table 4). He frequently had pre-phlebotomy hemoglobin levels greater than 10.5 g/dL despite at least monthly phlebotomy. At the patient's request, and given that he was 19 years of age, iron deficiency was permitted, by cessation of oral iron supplementation. Patient 9's iron deficiency was confirmed by serum iron levels, and his phlebotomy was performed every six to eight weeks, while still achieving target hemoglobin levels.
In contrast, patients 6, 10, and 11 had significant suppression of ARC on hydroxyurea and achieved the goal of hemoglobin reduction to 9.5–10 g/dL through the majority of the observation period without induction of iron deficiency. Further investigation into the viability of hydroxyurea-induced suppression of reticulocytosis to facilitate effective phlebotomy is needed, as well as alternative methods of reticulocyte suppression investigated for patients with toxicity in non-erythroid cell lines on hydroxyurea prior to reduction in ARC.
Another area of investigation is potential benefits of monitoring %DRBC, a laboratory value that can be obtained from a commonly used commercial hematology analyzer (Siemens ADVIA). Dense red blood cells, or DRBCs, have an intracellular hemoglobin concentration of 40–50 g/dL, compared to a normal cell, with a hemoglobin concentration of approximately 33 g/dL.2 DRBCs are highly prone to sickling, have increased rigidity, and decreased stability. In a recent report, %DRBC correlated significantly with skin ulcers, priapism, and renal dysfunction in patients with HbSS.25 Hydroxyurea has been shown to decrease %DRBCs by 34% after six months of therapy in patients with HbSS, independent of degree of HbF induction.25 The impact of hydroxyurea on %DRBC's in HbSC patients is unclear. A series of six adult HbSC patients treated with a fixed dose of hydroxyurea showed a reduction in dense cells as measured by a phalate gradient.19 In the CHAMPS study, %DRBC were measured by an ADVIA hematology analyzer and showed no change with hydroxyurea or magnesium pidolate therapy.13
Patient 9 suffered a central retinal artery occlusion at the age of 12, and a branch retinal artery occlusion (BRAO) four years later. While proliferative retinopathy, or vascular occlusion of the peripheral retina, is well described, there is only one case report of vascular occlusion of the posterior pole in HbSC, in a 31-year-old man.26 Patient 9 otherwise had no significant SCD complications, with no gallstones, pain events, avascular necrosis, or splenomegaly. His hypercoagulable evaluation was normal, and there was no family history of thrombosis. The cause for this unusual complication in an otherwise mildly affected HbSC patient is not clear. Our patient's only exceptional laboratory value was his %DRBC, which was significantly elevated after therapy with hydroxyurea and phlebotomy at 10.3%, and rose to 17.3% prior to scheduled phlebotomy, during a period of admitted hydroxyurea non-compliance (Figure 1). An increase in tinnitus was reported with the rise in %DRBC, but no other complications or events were reported. %DRBC were measured in subsequent clinic visits for Patient 9 and proved useful in identifying poor hydroxyurea compliance, as they rose markedly with missed doses, and close examination of the patient's CBC showed a concomitant subtle rise in ARC. As mentioned above, Patient 9 was allowed to become iron deficient; upon achieving iron deficiency, dense cells trended down (Figure 1), consistent with a previous report, 24 but were not lower than a preceding period of optimal hydroxyurea use.
Two of our patients who experienced severe clinical complications had an unusually high %DRBC immediately preceding their clinical event. A remarkable rise in %DRBC was noted on a routine clinic visit in Patient 3. His only complaint at that clinic visit was new onset tinnitus of one week's duration. However, three days later he suffered a cardiac arrest, resulting in a devastating hypoxic brain injury. A possible undiagnosed hypertrophic cardiomyopathy may have contributed to his cardiac arrest. He had been at stable MTD for over six years and was at MTD at the clinic visit prior to his cardiac arrest. He had exhibited the greatest HbF response of the group, with a peak of 22% HbF, which declined after three years of therapy to 14% despite maintenance of MCV, ANC, and ARC levels. Patient 14 also exhibited a rise in %DRBC prior to his first pain crisis in three years of hydroxyurea therapy, also at stable MTD (Figure 2). Further longitudinal data and clinical outcomes must be collected to validate the role of %DRBC measurements in assessing hydroxyurea compliance, and potentially predicting severe disease-related complications.
More than 25 years of clinical experience has established hydroxyurea as a safe and effective therapy for SCA. There is increasing interest in using hydroxyurea in HbSC disease, in hopes of achieving similar outcomes. However, there are several questions that must be addressed before initiating the widespread use of hydroxyurea in patients with HbSC, particularly significant safety issues surrounding the initial dosing and titration of hydroxyurea in HbSC. Our case series found a surprising number of patients experienced thrombocytopenia at low doses of hydroxyurea, which did not correlate with pre-drug levels, and therefore, could not be predicted by baseline laboratory values. It is possible that some HbSC patients cannot tolerate sufficient doses of hydroxyurea to experience clinical or laboratory improvement. Of the 11 patients who achieved MTD for at least six consecutive months, five did not experience clinical improvement. It seems likely that hydroxyurea is not a one size fits all therapy, and that a significant percentage will require the addition of phlebotomy to their treatment regimen.
In this case series, we have highlighted some of the challenges of hydroxyurea therapy in pediatric patients with HbSC and proposed novel clinical approaches to modify the risks and benefits, including monitoring of %DRBC during therapy, cautious titration and low starting doses, and the potential inclusion of phlebotomy in patients who do not respond clinically to hydroxyurea. These cases informed the design of an open clinical trial of hydroxyurea in pediatric patients with HbSC (SC Youth Treatment with Hydroxyurea Effects, NCT02336373), and a related trial for adult patients with HbSC that permits the use of phlebotomy in patients who do not experience improvement in quality of life with hydroxyurea alone.
Acknowledgements
We thank Dr. Donald Mahoney Jr. for his review of this manuscript, and Dr. Mohandas Narla for introducing us to the capabilities of the ADVIA hematology analyzer. We also thank Martin Huyhn and Loretta James for their data collection, and Sheryl Nelson for sample collection. The work described in this article was funded by the Baylor College of Medicine Department of Pediatrics, Division of Hematology/Oncology.
Author contributions
VS designed the study. VS and CGS performed the experiments and wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Motulsky AG. Frequency of sickling disorders in U.S. blacks. N Engl J Med 1973; 288: 31–3. [DOI] [PubMed] [Google Scholar]
- 2.Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982; 70: 1315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powars DR, Hiti A, Ramicone E, Johnson C, Chan L. Outcome in hemoglobin SC disease: a four-decade observational study of clinical, hematologic, and genetic factors. Am J Hematol 2002; 70: 206–15. [DOI] [PubMed] [Google Scholar]
- 4.Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003; 17: 167–78. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003; 289: 1645–51. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder WA, Powars DR, Kay LM, Chan LS, Huynh V, Shelton JB, Shelton JR. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989; 13: 325–53. [DOI] [PubMed] [Google Scholar]
- 7.Lee K, Prehu C, Merault G, Keclard L, Roudot-Thoraval F, Bachir D, Wajcman H, Denis L, Galacteros F. Genetic and hematological studies in a group of 114 adult patients with SC sickle cell disease. Am J Hematol 1998; 59: 15–21. [DOI] [PubMed] [Google Scholar]
- 8.Miller MK, Zimmerman SA, Schultz WH, Ware RE. Hydroxyurea therapy for pediatric patients with hemoglobin SC disease. J Pediatr Hematol Oncol 2001; 23: 306–8. [DOI] [PubMed] [Google Scholar]
- 9.Yates AM, Dedeken L, Smeltzer MP, Lebensburger JD, Wang WC, Robitaille N. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013; 60: 323–5. [DOI] [PubMed] [Google Scholar]
- 10.Luchtman-Jones L, Pressel S, Hilliard L, Brown RC, Smith MG, Thompson AA, Lee MT, Rothman J, Rogers ZR, Owen W, Imran H, Thornburg C, Kwiatkowski JL, Aygun B, Nelson S, Roberts C, Gauger C, Piccone C, Kalfa T, Alvarez O, Hassell K, Davis BR, Ware RE. Effects of hydroxyurea treatment for patients with hemoglobin SC disease. Am J Hematol 2016; 91: 238–42. [DOI] [PubMed] [Google Scholar]
- 11.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984; 63: 921–6. [PubMed] [Google Scholar]
- 12.Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989; 32: 104–11. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Brugnara C, Snyder C, Wynn L, Rogers Z, Kalinyak K, Brown C, Qureshi A, Bigelow C, Neumayr L, Smith-Whitley K, Chui DH, Delahunty M, Woolson R, Steinberg M, Telen M, Kesler K. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011; 152: 771–6. [DOI] [PubMed] [Google Scholar]
- 14.Lemonne N, Billaud M, Waltz X, Romana M, Hierso R, Etienne-Julan M, Connes P. Rheology of red blood cells in patients with HbC disease. Clin Hemorheol Microcirc 2016; 61: 571–7. [DOI] [PubMed] [Google Scholar]
- 15.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost 2003; 29: 435–50. [DOI] [PubMed] [Google Scholar]
- 16.Lionnet F, Hammoudi N, Stankovic Stojanovic K, Avellino V, Grateau G, Girot R, Haymann JP. Hemoglobin SC disease complications: a clinical study of 179 cases. Haematologica. 97: 1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markham MJ, Lottenberg R, Zumberg M. Role of phlebotomy in the management of hemoglobin SC disease: case report and review of the literature. Am J Hematol 2003; 73: 121–5. [DOI] [PubMed] [Google Scholar]
- 18.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr 2011; 2: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997; 98: 838–44. [DOI] [PubMed] [Google Scholar]
- 20.Iyer R, Baliga R, Nagel RL, Brugnara C, Kirchner K, Hogan S, Steinberg MH. Maximum urine concentrating ability in children with Hb SC disease: effects of hydroxyurea. Am J Hematol 2000; 64: 47–52. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood 2004; 103: 2039–45. [DOI] [PubMed] [Google Scholar]
- 22.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010; 115: 5300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes's original 1934 classification to the third millennium. Curr Opin Hematol 2013; 20: 222–30. [DOI] [PubMed] [Google Scholar]
- 24.Castro O, Poillon WN, Finke H, Massac E. Improvement of sickle cell anemia by iron-limited erythropoiesis. Am J Hematol 1994; 47: 74–81. [DOI] [PubMed] [Google Scholar]
- 25.Bartolucci P, Brugnara C, Teixeira-Pinto A, Pissard S, Moradkhani K, Jouault H, Galacteros F. Erythrocyte density in sickle cell syndromes is associated with specific clinical manifestations and hemolysis. Blood 2012; 120: 3136–41. [DOI] [PubMed] [Google Scholar]
- 26.Fine LC, Petrovic V, Irvine AR, Bhisitkul RB. Spontaneous central retinal artery occlusion in hemoglobin sickle cell disease. Am J Ophthalmol 2000; 129: 680–1. [DOI] [PubMed] [Google Scholar]