Abstract
The dihydropyridine receptor in the plasma membrane and the ryanodine receptor in the sarcoplasmic reticulum are known to physically interact in the process of excitation–contraction coupling. However, the mechanism for subsequent Ca2+ release through the ryanodine receptor is unknown. Our lab has previously presented evidence that the dihydropyridine receptor and ryanodine receptor combine as a channel for the entry of Ca2+ under resting conditions, known as store operated calcium entry. Here, we provide evidence that depolarization during excitation–contraction coupling causes the dihydropyridine receptor to disengage from the ryanodine receptor. The newly freed ryanodine receptor can then transport Ca2+ from the sarcoplasmic reticulum to the cytosol. Experimentally, this should more greatly expose the ryanodine receptor to exogenous ryanodine. To examine this hypothesis, we titrated L6 skeletal muscle cells with ryanodine in resting and excited (depolarized) states. When L6 muscle cells were depolarized with high potassium or exposed to the dihydropyridine receptor agonist BAYK-8644, known to induce dihydropyridine receptor movement within the membrane, ryanodine sensitivity was enhanced. However, ryanodine sensitivity was unaffected when Ca2+ was elevated without depolarization by the ryanodine receptor agonist chloromethylcresol, or by increasing Ca2+ concentration in the media. Ca2+ entry currents (from the extracellular space) during excitation were strongly inhibited by ryanodine, but Ca2+ entry currents in the resting state were not. We conclude that excitation releases the ryanodine receptor from occlusion by the dihydropyridine receptor, enabling Ca2+ release from the ryanodine receptor to the cytosol.
Keywords: Excitation–contraction coupling, skeletal muscle, dihydropyridine receptor, ryanodine receptor, calcium
Introduction
Ca2+ is the essential trigger for muscle contraction.1 During this process, Ca2+ is released from the interior lumen of the sarcoplasmic reticulum (SR) to the cytosol. Cytosolic Ca2+ activates contraction by binding troponin, allowing the engagement of actin and myosin for contraction. Termination of the signal is largely through re-entry of Ca2+ back to the SR lumen through the SR membrane protein CaATPase.
The overall process of excitation–contraction coupling (ECC) requires the depolarization of the sarcolemma (initiated by activation of cholinergic sodium channels in the endplate region, and propagated by voltage-gated Na channels), transmitted to the dihydropyridine receptor (DHPR), which physically interacts with the ryanodine receptor (RyR) embedded in the SR membrane.2,3 This leads to a release of Ca2+ from the lumen of the SR, which triggers muscle contraction. Thus, the RyR has two roles: a physical interaction with the DHPR to signal Ca2+ release, and as the conduit for Ca2+ release itself.4 How this is accomplished is at present unknown. It is established that there is commonly an excess of RyRs over DHPRs, often two-fold.5 Physical measurements of frozen sections of tissue using high resolution electron microscopy show regular arrangements of DHPRs and RyRs, and direct contact between them which is believed to be altered upon plasma membrane excitation.6–8 However, a mechanistic connection between biophysical studies and measurements of operational calcium flows that explains ECC remains elusive.
A related process to ECC is store operated calcium entry (SOCE),9,10 which refills Ca2+ stores (SR) from the extracellular space in the resting state. SOCE is required for overall Ca2+ homeostasis because a small amount of Ca2+ is lost to the cell exterior through the plasma membrane bound CaATPase and possibly the Na/Ca exchange protein during excitation-induced Ca2+ elevation. In previous studies, we have provided evidence for a direct interaction of the DHPR and the RyR to explain SOCE.11,12 We suggested that these two Ca2+ channels together form a combined channel for the direct entry of Ca2+ from the extracellular space to the SR lumen. As this process occurs in resting (polarized) conditions, the DHPR is static in location, directly facing (and occupying) an RyR. We had postulated11,13 that ECC might be explained if the DHPR moved during excitation, freeing a formerly occluded RyR to release Ca2+ to the cytosol.
Since displacing the DHPR should expose more RyRs to ryanodine, we reasoned that the sensitivity to ryanodine should increase under excitation conditions. We therefore conducted experiments to test this expectation.
Materials and methods
Materials
The subclone of the rat myogenic cell line L6 was obtained from ATCC (Manassas, VA). The calcium fluorescent indicators Fura-2 (AM) and MagFura-2 (AM),14 Dulbeco’s minimum essential media (DMEM), α-Minimum essential assay (MEM), antibiotic–antimycotic solution, and amino acids were obtained from Invitrogen Life Technologies (Norwalk, CT). The ACLAR™ embedding film was obtained from Ted Pella Inc. (Redding, CA). Cell culture plates and flasks were obtained from Corning Inc. (Corning, NY). BAYK-8644 (±) was purchased from Tocris Bioscience (Minneapolis, MN). Thapsigargin was purchased from Sigma Chemical Co. (St. Louis, MO). Ryanodine and BAPTA were purchased from Enzo Life Sciences (Farmingdale, NY).
Methods
Cell culture
L6 cells were cultured and grown in DMEM supplemented with 10% fetal calf serum and plated in 75 cm2 tissue culture flasks and incubated until the cells reached 80% confluency.15 All incubations were conducted at 37℃ at 5% CO2 tension. After an initial incubation, cells were trypsinized and plated on ACLAR sheets with dimensions of 10 × 12 mm in 12-well plates. On the second day of incubation in the plates, differentiation of the muscle cells was induced by replacing the media with MEM supplemented with 2% horse serum, and then with MEM supplemented with 1% horse serum on the third day until the cells were ready for use.
Cytosolic calcium measurement
Cytosolic calcium measurement in L6 cells were performed similarly to our prior studies.16 Cells were incubated with 4 µM of Fura-2 (AM) dye in Hank’s Balanced Salt Solution (HBSS) for 1 h. Probenecid and Pluronic acid were added at final concentrations of 0.03% and 2.7 mM, respectively. Cells were washed twice after the incubation with the dye and were re-incubated in HBSS for another hour to allow de-esterification. Subsequently, ACLAR sheets were placed in a quartz cuvette containing 1 ml of calcium buffer at an inclination of approximately 45°. Ca2+ measurements were done using a Hitachi F-2500 spectrofluorimeter at excitation wavelengths of 340/380 nm and the emission wavelength at 510 nm. Following intracellular Ca2+ measurements, the ACLAR strip was subjected to treatment with 0.1% Triton X100 and 20 mM of ethylene glycol tetraacetic acid (EGTA) in order to calculate maximum and minimum background calcium. Maximum calcium fluorescence (Fmax) was measured after Triton X100 treatment, and minimum calcium fluorescence (Fmin) after EGTA addition. Standard ratiometric analysis using the Grynkewicz equation17 was conducted as in our prior studies.11
Measurement of L-type calcium currents
Pipettes were fabricated from borosilicate glass (World Precision Instruments 150-4) and had resistances of ∼2.5 MΩ when filled with internal solution which contained (in mM): 140 Cs-Aspartate, 10 Cs2EGTA, 5 MgCl2 and 10 HEPES, pH 7.4. The standard external solution was composed of (in mM): 137.5 TEA-Cl, 10 CaCl2, 10 HEPES, pH 7.4.18 The concentration of calcium was varied in the external solution in some experiments. For recording SOCE, 20 mM of BAPTA was included in the internal solution instead of EGTA for faster chelation. Electronic compensation was used to reduce the effective series resistance (<5 MΩ). L-type currents were filtered at 2.9 KHz using a 4-pole Bessel filter. In most of the recordings, a 1 s prepulse to −20 mV followed by a 50 ms repolarization to −50 mV was administered before the test pulse to inactivate T-type calcium channels.19 I–V relationships were generated by applying 12 pulses from a holding potential of −60 mV in 10 mV increments. Pulse duration was fixed at 450 ms. In SOCE experiments, holding potential was fixed at −80 mV. Cslow capacitance settings of the Patchmaster 11.0 software by HEKA were used to determine capacitance and normalize current amplitudes (pA/pF). Averages of six pulses were taken. All electrophysiological measurements were made using an EPC 10 amplifier. All electrophysiological measurements were carried out at room temperature (∼25℃).
Statistics
Data are represented as average of 3 unless otherwise stated. Statistical evaluation was done using Student’s t-test and analysis of variance (ANOVA). Data are represented as average ± SEM. The level of significance was set at P < 0.05.
Results
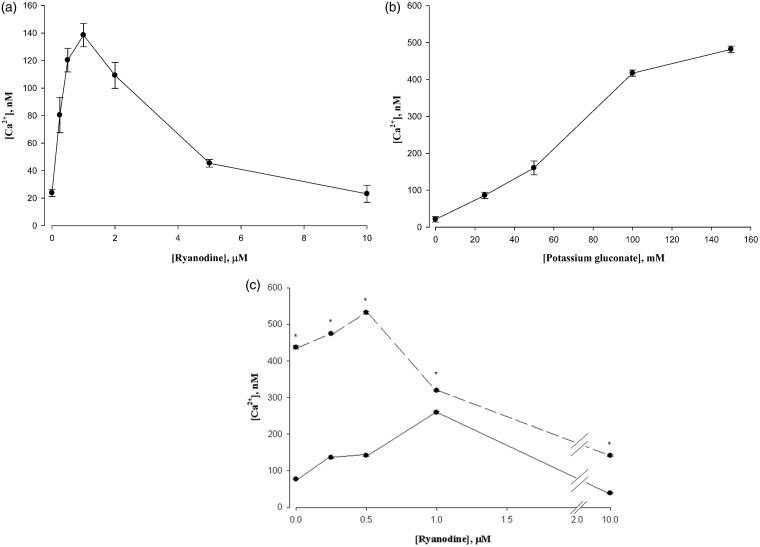
Ryanodine is known to exert a biphasic effect on the eponymous RyR responsible for SR Ca2+ release: stimulation at low concentrations and inhibition at high concentrations.15,20 This is replicated for the L6 cells of our studies in Figure 1(a). We observed peak stimulation at 1 µM and full inhibition at 10 µM. For all of the experiments presented here, the exposure to ryanodine prior to measurement was about 1 min. In some other studies,18,21 a much higher concentration of ryanodine than used here (100 µM or more) and longer incubation (1 h or more) was used. In preliminary studies (not shown), we found a loss of cell viability under those conditions.
Figure 1.
(a) Biphasic response of L6 cell cytosolic Ca2+ to ryanodine. Ryanodine was present for 2 min prior to Ca2+ measurement by Fura-2 fluorescence. Data are means ± SEM, n = 4. (b) Effect of potassium gluconate on cytosolic Ca2+ in L6 cells. Potassium gluconate was present for 2 min prior to Ca2+ measurement. Data are means ± SEM, n = 4. (c) High K+ sensitives L6 cells to ryanodine. The solid line indicates control cells. The dashed line indicates the presence of 150 mM potassium gluconate. The peak of ryanodine stimulated Ca2+ release was shifted to the left in the presence of potassium. *P < 0.05 versus Control, n = 4
Effects of ryanodine titration on cytosolic Ca2+ during conditions eliciting DHPR movement
We elicited an activation of the DHPR by adding high concentrations of potassium to the media to induce depolarization (Figure 1). Various concentrations of potassium gluconate increased cytosolic Ca2+ as shown in Figure 1(b). We then compared responses of control and K-depolarized cells to ryanodine; the result is shown in Figure 1(c). It is evident that the ryanodine titration curve is shifted to the left, indicating a greater sensitivity towards ryanodine for Ca2+ release in the presence of potassium. While both low concentrations of ryanodine as well as K-depolarization increase cytosolic Ca2+, the K-depolarization was responsible for most of the effect.
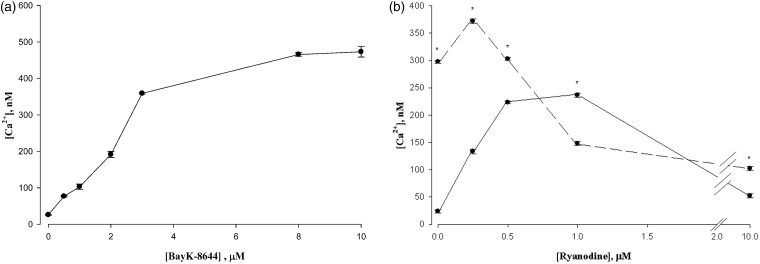
As a separate means of activating the L-channel, we exposed cells to the L-channel agonist BAYK-8644 (BayK). The stimulation of Ca2+ release is documented in Figure 2(a), showing full activation by 10 µM BayK. The influence of BayK on the ryanodine titration was to shift the titration curve strongly to lower concentrations of ryanodine both in stimulation as well as inhibition (Figure 2(b)). Thus, BayK caused an increase in ryanodine sensitivity in L6 cells.
Figure 2.
(a) Effect of BAYK on cytosolic Ca2+ in L6 Cells. BAYK produced a monotonic increase in cytosolic calcium. Data are means ± SEM, n = 4. (b) BAYK sensitives L6 cells to ryanodine. The solid line indicates control cells. The dashed line indicates the presence of 10 μM BAYK. The peak of ryanodine stimulated Ca2+ release was shifted to the left in the presence of BAYK. *P < 0.05 versus Control, n = 4
Stimulation of Ca2+ release without a change in ryanodine sensitivity
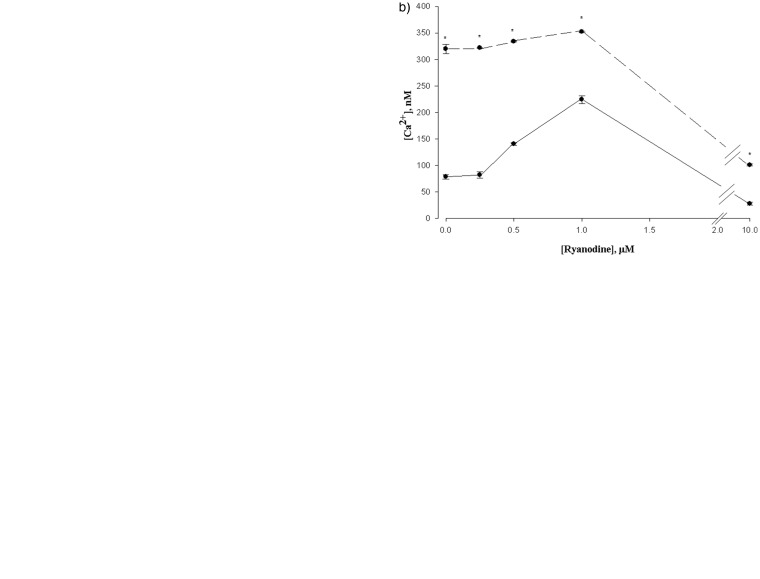
We examined two situations which are known to increase cytosolic Ca2+, but should not cause an activation or movement of the DHPR. The first is the RyR activator 4-chloro-m-cresol (CMC). Like caffeine, CMC elicits Ca2+ release by activation of the RyR.22 As expected, CMC stimulated Ca2+ release monotonically in L6 cells (Figure 3(a)). We next determined the response of cells to CMC at various concentrations of ryanodine (Figure 3(b)). It is apparent that CMC stimulated Ca2+ release at all concentrations of ryanodine, and did not alter position of the maximum ryanodine stimulation, which still occurred at 1 µM. Thus, CMC did not lead to a change in ryanodine sensitivity.
Figure 3.
(a) Effect of CMC on cytosolic Ca2+ in L6 cells. CMC maximally stimulated release of Ca2+ 1 mM. Data are means ± SEM, n = 4. (b) CMC does not alter ryanodine sensitivity. The solid line indicates control cells. The dashed line indicates the presence of 1 mM CMC. *P < 0.05 versus control, n = 4. (c) Variation of external calcium does not affect ryanodine sensitivity. The dotted line indicates control cells (with 1 mM externally added calcium). The dashed line indicates the presence of 2 mM externally added calcium. *P < 0.05 versus Control, n = 4.
CMC: 4-chloro-m-cresol
The second method of increasing cytosolic Ca2+ was simply an increase in the concentration of Ca2+ in the media for the cells. The results of this change on the ryanodine titration are shown in Figure 3(c). While increasing extracellular Ca2+ was able to increase cytosolic Ca2+ beyond that increased by ryanodine, the titration curve was not shifted, showing a maximal stimulation of 1 µM.
Effect of ryanodine on calcium currents measured by whole-cell voltage patch clamp
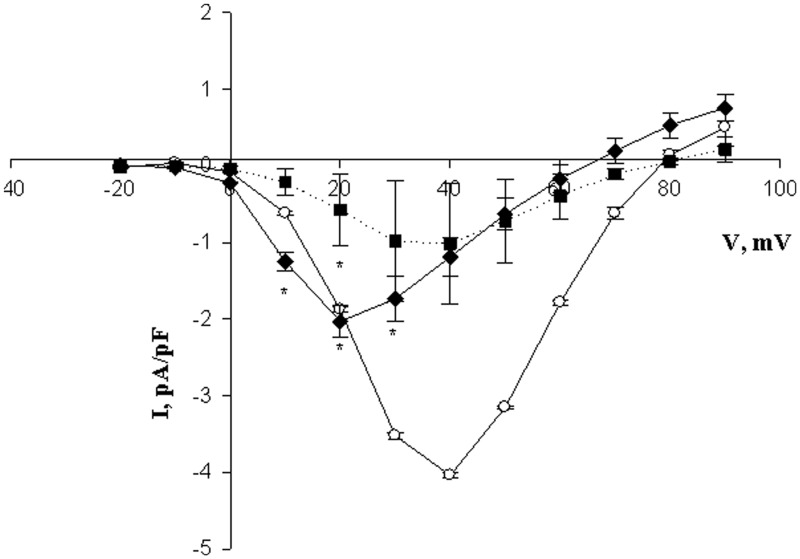
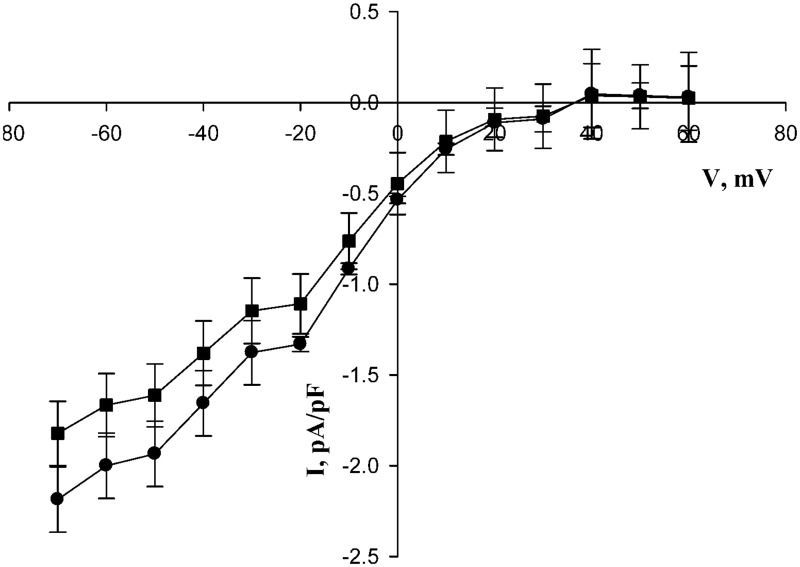
We measured Ca2+ currents by patch clamp electrophoresis under whole-cell voltage clamp conditions. As is evident from Figure 4, under control conditions, a peak of inward current was evident at about 35 mV, as expected from previous studies.23,24 However, the additional presence of ryanodine led to strong suppression of these currents.
Figure 4.
Ryanodine inhibition of constant-voltage currents under excitation conditions. L6 cells were measured in whole-cell voltage clamp. The holding potential was −60 mV. Voltage ramps were from −20 mV to +90 mV for 450 ms at 1 s intervals. Ryanodine was present at zero (circles), 5 μM (diamonds), or 10 μM (squares connected by dotted lines) concentration in the external solution. *P < 0.05 versus control with zero ryanodine, n = 6
We also measured Ca2+ currents after depleting SR Ca2+ by the prior addition of thapsigargin, i.e. under conditions of SOCE (Figure 5). As expected from the studies of others, a strong inward current was observed only at negative voltage values.9 This inward current was little affected by the presence of ryanodine.
Figure 5.
Effect of ryanodine on constant-voltage currents under SOCE conditions. Calcium was depleted by thapsigargin incubation (see Materials and methods section). The chelator BAPTA was also included in the internal pipet solution to ensure depletion of calcium. The holding potential was −80 mV and voltage ramps conducted from −70 mV to +60 mV for 450 ms at 1 s intervals. Ryanodine was present at zero (circles) or 10 μM (squares) concentrations in the external solution. No significant ryanodine inhibition was observed at P < 0.05, n = 6
Discussion
The mechanism for ECC for skeletal muscle remains unclear. It is established that it is distinct from cardiac muscle, the other type of striated muscle. For heart, the leading hypothesis is a “calcium-induced calcium release” (CICR). Originally, CICR was proposed to explain ECC of skeletal muscle,25,26 but skeletal muscle does not need extracellular Ca2+ for continued contraction.27 A modified form of CICR was proposed for the skeletal muscle to elicit a secondary Ca2+ release from excess RyRs, although this is not fully established.27 Thus, the CICR hypothesis may not account for ECC in skeletal muscle.
Extensive investigations have shown that a physical coupling between the DHPR and the RyR exists that triggers ECC in an unknown way. What has been uncovered are details of the portions of the two proteins that interact with each other.6,28,29 Channel mutation studies have demonstrated that an interaction between these two proteins is essential for the process of ECC. A large number of ancillary proteins may also play a role in the process,30,31 but none are clearly as central as the DHPR and the RyR.
Once the RyR is activated for Ca2+ release, a large flow of Ca2+ from the SR lumen to the cytosol ensues,32 and contraction is triggered by the binding of Ca2+ to troponin33 until Ca2+ concentration is lowered. Most of the decrease in Ca2+ to terminate contraction takes place by transport back in to the SR catalyzed by the CaATPase.33,34 However, a small amount of Ca2+ is lost to the exterior space through the plasma membrane bound CaATPase.35 That Ca2+ is restored to the cell by a process known as SOCE.36
The process of SOCE in non-muscle cells is known to involve two proteins also embedded in the plasma membrane and ER: the Orai1 and Stim1, respectively.34,37 The role of the Orai1 in the skeletal muscle was proposed recently,21,38 but Orai1 appears not to be essential for muscle Ca2+ cycling as its complete ablation led only to a selective muscle weakness rather than a prevention of complete Ca2+ refilling.39
We have previously proposed a mechanism for SOCE for both muscle and non-muscle cells that also involves a direct interaction between proteins in the plasma membrane and SR.11 The proteins we have proposed are the same proteins that are critical for ECC: the DHPR and RyR. Based on the finding that Ca2+ can enter SR directly even when entry from the cytosol is blocked by the SR CaATPase inhibitor thapsigargin, we proposed a direct entry for Ca2+ through both DHPR and RyR channels. We confirmed the dye method for SR Ca2+ measurement by incorporating a Ca2+ sensor protein into the SR by transfection.40
Experiments in the present study further support this view. Figure 5 shows that the SR lumen of the L6 cells accumulates extracellular Ca2+ even in the presence of both thapsigargin as well as high concentrations of Ry, so that no exchange with cytosolic Ca2+ is possible. Furthermore, the complementary experiment directly measuring SR Ca2+ contents (Figure S1) also shows that Ca2+ enters cells under these conditions without being affected by high concentrations of Ry.
In the present study, we reasoned that when RyR was bound to the DHPR, it would be unable to react with ryanodine. Thus, only the unbound RyR could respond to ryanodine. We anticipated that upon excitation of the plasma membrane, the RyR would be released from the DHPR and thereby exposed to ryanodine. Since ryanodine exerts a biphasic action on the RyR – activation at low concentrations, but inhibition at high concentrations,41 we expected that both phases would require a lower ryanodine concentration once more RyRs were exposed. Aside from depolarization, it is also established that BayK can displace the DHPR and initiate an increase in intracellular flow of Ca2+ from the sarcoplasmic reticular lumen to the cytosol.42 Note that while BayK also enables a flow of Ca2+ from the extracellular space to the cytosol through the DHPR, this is unlikely to be a major contributor to ECC in skeletal muscle, as no extracellular Ca2+ is required for skeletal muscle depolarization.43 In the heart, on the other hand, extracellular Ca2+ is required for the depolarization phase,44 and it has been argued that a CICR can explain flow through the RyR under similar conditions in this tissue.45
We modeled the two situations in our studies by K-depolarization and the presence of BayK, respectively. As demonstrated in Figure 1(c) (depolarization) and Figure 2(b) (BayK), there was a shift in the ryanodine titration curve. Thus, both activation and inactivation by ryanodine occurred at lower concentrations when the DHPR was shifted relative to the RyR measured by Ca2+ release to the cytosol.
As a control to test the hypothesis, we examined Ca2+ release elicited by the caffeine-like agent CMC. This compound is known to cause an oxidation of RyR sulphydryls, which opens the RyR calcium channel.46 CMC showed a greater release of Ca2+ to the cytosol, but no change in sensitivity to ryanodine (Figure 3(b)) was observed, indicating no relative movement of RyR and DHPRs. As a further control, we increased the concentration of Ca2+ in the medium. This also produced an increase in cytosolic Ca2+, but left the sensitivity to Ry unaffected (Figure 3(c)).
While CMC is a widely used compound to elicit Ca2+ release through its activation of the RyR, one study suggests it may also inhibit the CaATPase of the SR.47 It is not likely that it has this action in L6 cells, as the increase in Ca2+ elicited by the far more potent inhibitor thapsigargin is very small even if Ca2+ is removed from the media.9 Even if CaATPase inhibition did contribute to the increase in cytosolic Ca2+, it would not alter the interaction between the DHPR and the RyR, so that the conclusion to the experiment is unaltered: simply altering cytosolic Ca2+ does not alter the sensitivity to ryanodine.
As another test of our hypothesis we measured Ca2+ currents under whole-cell voltage clamp conditions. The finding that Ry greatly inhibited Ca2+ entry under ECC conditions is surprising since the current measured represents entry of Ca2+ from the extracellular space into the cytosol. The inhibitor does not affect the path of Ca2+ entry (DHPR), so it is difficult to account for this result under existing models. However, our proposed model is consistent with this finding, since disengagement of the DHPR and RyR to elicit Ca2+ release from the SR exposes the previously occupied RyR to ryanodine. Further excitation cycles would no longer be allowed, disrupting the process and inhibiting excitation coupled calcium entry (ECCE) along with ECC. While further experiments are clearly needed to fully explore this finding, it is likely that re-engagement of the DHPR with the RyR is necessary during excitation cycles to re-activate the DHPR since the latter is rapidly inactivated as a channel for Ca2+ flow.
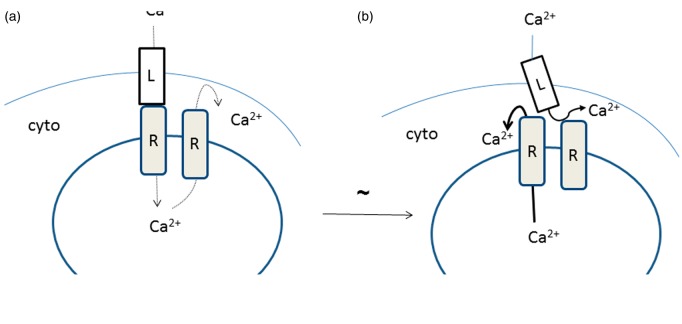
Model for ECC
Figure 6 illustrates our proposal for how excitation leads to Ca2+ release. Figure 6(a) represents the resting condition, and the major fluxes are Ca2+ entry through the DHPR (labelled as L in the figure to indicate the L-channel), and subsequently the RyR (labelled R). This is SOCE that enables refilling of the SR. Also indicated is a flux of Ca2+ through a separate RyR out to the cytosol. These fluxes are relatively modest, indicated by dotted arrows. Not shown are pumps, such as the SR CaATPase responsible for returning Ca2+ to the SR from the cytosol, and the plasma membrane ATPase, responsible for the loss of Ca2+ that necessitates SOCE.
Figure 6.
Proposed mechanism for excitation–contraction coupling. In the resting state (a), a slow inward flux of Ca2+ to the SR involves both the DHPR (labelled L in the figure) and an RyR (labelled R in the figure). There is also a slow exit of Ca2+ from the SR to the cytosol (since the Ca2+ channel is inactivated during the resting state (56)) this is indicated by the thin dashed lines. In the excited state (b), movement of the DHPR exposes the Ca2+ release site of the RyR, Ca2+ rapidly flows from the SR to the cytosol. (A color version of this figure is available in the online journal.)
Following depolarization, the situation shifts to that diagrammed as shown in Figure 6(b). Now the previously occluded RyR releases substantial Ca2+ from the SR, indicated in the figure as a bold arrow. This is possible because of the removal of the occluding DHPR. A movement of the DHPR in response to membrane depolarization is already established, experimentally observed as a virtual current called the “charge movement”.48,49
Comparing SOCE and ECCE currents
In our studies, the current–voltage curves for both Ca2+ currents during SOCE (“Calcium Release Activated Current ”) and ECCE are similar to what has been observed by other investigators.9,50 While SOCE was once believed to be an electrical signature unique to non-excitable cells, these are now established to occur in electrically excitable cells, including muscle.51 Clearly SOCE and ECCE represent separate processes, but the fundamental question of what the driving forces are has not been raised. We propose that the Ca2+ entry into the SR lumen for SOCE is driven by the electrical portion of the electrochemical gradient, while the Ca2+ entry into the cytosol for ECCE is driven by the chemical portion of the electrochemical gradient.
We have previously presented data showing that the pathway of Ca2+ flow during SOCE is a direct entry from extracellular space to the SR lumen.11 We have suggested that entry is through a superchannel composed of the DHPR and the RyR. The evidence includes the appearance of SR luminal Ca2+ even in the presence of both thapsigargin and ryanodine (also confirmed for this study, Figure S1), and the fact that kinetically Ca2+ entry appears first into the SR and then the cytosol.12 A relative lack of inhibition of SOCE currents by ryanodine in the present study (Figure 5) is consistent with a static DHPR:RyR structure. The driving force for Ca2+ entry during SOCE that is consistent with all of this data is almost entirely electrical. The chemical gradient is only about one order of magnitude rather than 4 (which would be the case if SOCE involved entry into the cytosol).
ECCE, by contrast, delivers Ca2+ into the cell cytosol. There is no entry below about 10 mV because the DHPR, being voltage dependent, is not open. Beyond that, it can conduct an inward Ca2+ to a maximum of about 35 mV, after which the direction is reversed. The driving force for Ca2+ entry must be exclusively the chemical component of the electrochemical gradient, as there is no favorable electrical component at any positive voltage value. It is already established that flow through the RyR is not only bidirectional but non-selective. For example, K+ is known to be transported to balance the electrical flows of Ca2+. In effect, the reason that the RyR is considered a Ca2+ channel is entirely due to the existing ion gradients.52
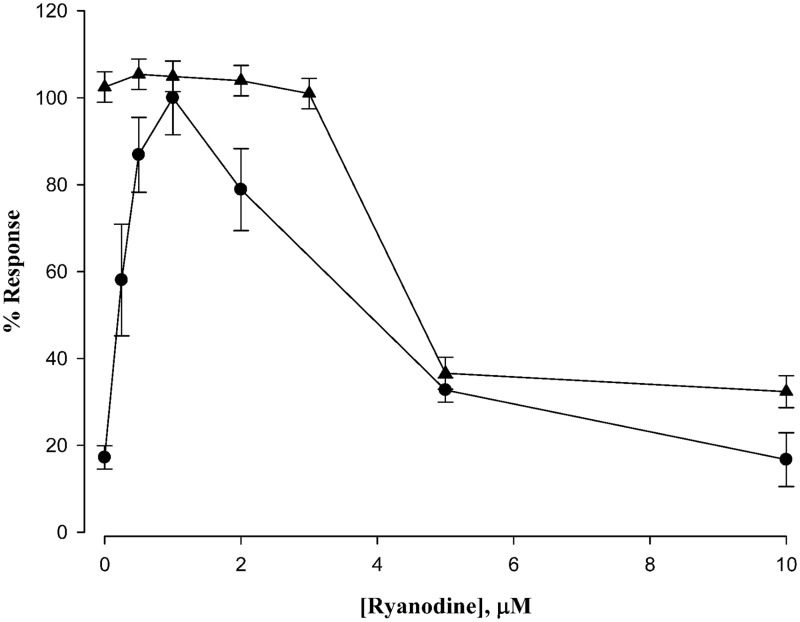
Figure 7 compares two processes measured in this study by recasting data from Figure 1(a) and Figure 4 (with further data points obtained). The cytosolic Ca2+ measurements of Figure 1(a) and the Ca2+ entry currents of Figure 4 were normalized (each maximum point was set at 100%) to facilitate comparisons. It is apparent that the same maximum inhibition by ryanodine is reached in both data sets. This is consistent with selective ryanodine inhibition of the RyR. The fact that ryanodine inhibits Ca2+ release more potently than Ca2+ entry current (which strictly employs the DHPR channel) suggests that more RyRs are involved during excitation than during rest.
Figure 7.
Comparison of ryanodine-induced calcium responses. The data from Figure 1(a) and an expanded series of data similar to that of Figure 4 were normalized for display on the same ordinate. Ryanodine sensitivity of cells under resting conditions (filled circles) shows a peak calcium release at 1 μM, which was set at 100% for that curve. The calcium currents (an expanded data set of Figure 4) under maximum excitation conditions (+30 mV) was set at 100% and also plotted as a function of ryanodine concentration filled triangles. Data are means ± SEM, n = 4
We speculate that one reason for the presence of approximately double the number of RyR to DHPR, and in strict geometric arrangement53,54 is that different RyR are engaged by the DHPR during depolarization. This might involve a shift of the two proteins within their respective membranes in a similar way to the sliding filament hypothesis.55 While further studies are required to test this and to define the interactions of these channels, the present report is the first to offer an explanation for the contact between the DHPR and the RyR in ECC.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Robert Dirksen (University of Rochester School of Medicine) for his valuable guidance in the electrophysiology setup and recording. The research was entirely funded by St. John’s University, with no extramural sources.
Authors’contributions
SP performed most of the experiments, assisted in their design, and participated in the writing of the manuscript. RO performed experimental design and manuscript writing.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1995; 1241: 59–116. [DOI] [PubMed] [Google Scholar]
- 2.Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 1970; 228: 34–6. [DOI] [PubMed] [Google Scholar]
- 3.Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol 1992; 450: 217–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorrentino V. Ryanodine receptors, Boca Raton, Florida: CRC Press, Inc., 1995. [Google Scholar]
- 5.Franzini-Armstrong C, Jorgensen AO. Structure and development of E–C coupling units in skeletal muscle. Annu Rev Physiol 1994; 56: 509–34. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, Wang Z, Wei R, Fan G, Wang Q, Zhang K, Yin CC. The molecular architecture of dihydropyrindine receptor/L-type Ca2+ channel complex. Sci Rep 2015; 5: 8370–8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van PF. Ryanodine receptors: allosteric ion channel giants. J Mol Biol 2015; 427: 31–53. [DOI] [PubMed] [Google Scholar]
- 8.Lai FA, Misra M, Xu L, Smith HA, Meissner G. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J Biol Chem 1989; 264: 16776–85. [PubMed] [Google Scholar]
- 9.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev 2005; 85: 757–810. [DOI] [PubMed] [Google Scholar]
- 10.Trebak M, Zhang W, Ruhle B, Henkel MM, Gonzalez-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X. What role for store-operated Ca2+ entry in muscle? Microcirculation 2013; 20: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam MN, Narayanan B, Ochs RS. A mechanism for both capacitative Ca2+ entry and excitation-contraction coupled Ca2+ release by the sarcoplasmic reticulum of skeletal muscle cells. Exp Biol Med (Maywood) 2002; 227: 425–31. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan B, Islam MN, Bartlelt D, Ochs RS. A direct mass-action mechanism explains capacitative calcium entry in jurkat and skeletal L6 muscle cells. J Biol Chem 2003; 278: 44188–96. [DOI] [PubMed] [Google Scholar]
- 13.Islam MN, Ochs RS. A new hypothesis for Ca2+ flows in skeletal muscle and its implication for other cells. Cell Biochem Biophys 2005; 44: 251–71. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama T, Goldman WF. Measurement of SR free Ca2+ and Mg2+ in permeablized smooth muscle cells with use of furaptra. Am J Physiol 1995; 269: C698–C705. [DOI] [PubMed] [Google Scholar]
- 15.Wingertzahn MA, Ochs RS. Changes in ryanodine receptor-mediated calcium release during skeletal muscle differentiation. Proc Soc Exp Biol Med 1999; 221: 234–41. [DOI] [PubMed] [Google Scholar]
- 16.Wingertzahn MA, Ochs RS. Changes in ryanodine receptor-mediated calcium release during skeletal muscle differentiation. II Resolution of caffeine-ryanodine paradox. Exp Biol Med 2001; 226: 119–26. [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440–50. [PubMed] [Google Scholar]
- 18.Bannister RA, Pessah IN, Beam KG. The Skeletal L-type Ca2+ Current is a major contributor to excitation-coupled Ca2+ entry. J Gen Physiol 2009; 133: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirksen RT, Beam KG. Single calcium channel behavior in native skeletal muscle. J Gen Physiol 1995; 105: 227–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem 1986; 261: 6300–6. [PubMed] [Google Scholar]
- 21.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol 2008; 586: 4815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez JR, Linares N, Pessah IN, Allen PD. Enhanced response to caffeine and 4-chloro-m-cresol in malignant hyperthermia-susceptible muscle is related in part to chronically elevated resting [Ca2+]. Am J Physiol Cell Physiol 2005; 288: C606–C12. [DOI] [PubMed] [Google Scholar]
- 23.Bannister RA, Beam KG. CaV1.1: the atypical prototypical voltage-gated Ca2+ channel. Biochim Biophys Acta 2013; 1828: 1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donaldson PL, Beam KG. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol 1983; 82: 449–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 1970; 228: 34–6. [DOI] [PubMed] [Google Scholar]
- 26.Ford LE, Podolsky RJ. Regenerative calcium release within muscle cells. Science 1970; 167: 58–9. [DOI] [PubMed] [Google Scholar]
- 27.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev 2009; 89: 1153–76. [DOI] [PubMed] [Google Scholar]
- 28.Protasi F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front Biosci 2002; 7: D650–D8. [DOI] [PubMed] [Google Scholar]
- 29.Sencer S, Papineni RV, Halling DB, Pate P, Krol J, Zhang JZ, Hamilton SL. Coupling of RYR1 and L-type calcium channels via calmodulin binding domains. J Biol Chem 2001; 276: 38237–41. [DOI] [PubMed] [Google Scholar]
- 30.Beard NA, Wei L, Dulhunty AF. Control of muscle ryanodine receptor calcium release channels by proteins in the sarcoplasmic reticulum lumen. Clin Exp Pharmacol Physiol 2009; 36: 340–5. [DOI] [PubMed] [Google Scholar]
- 31.Golini L, Chouabe C, Berthier C, Cusimano V, Fornaro M, Bonvallet R, Formoso L, Giacomello E, Jacquemond V, Sorrentino V. Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle. J Biol Chem 2011; 286: 43717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios E, Stern MD. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct 1997; 26: 47–82. [DOI] [PubMed] [Google Scholar]
- 33.Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol 1968; 18: 123–83. [DOI] [PubMed] [Google Scholar]
- 34.Putney JW. Capacitative calcium entry revisited. Cell Calcium 1990; 11: 611–24. [DOI] [PubMed] [Google Scholar]
- 35.Carafoli E. Calcium pump of the plasma membrane. Physiol Rev 1991; 71: 129–153. [DOI] [PubMed] [Google Scholar]
- 36.Putney JW, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci 2001; 114(Pt 12): 2223–9. [DOI] [PubMed] [Google Scholar]
- 37.Putney JW., Jr New molecular players in capacitative Ca2+ entry. J Cell Sci 2007; 120(Pt 12): 1959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 2006; 1763: 1147–60. [DOI] [PubMed] [Google Scholar]
- 39.Wei-Lapierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat Commun 2013; 4: 2805–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayanan B, Islam MN, Bartelt D, Ochs RS. A direct mass-action mechanism explains capacitative calcium entry in jurkat and skeletal L6 muscle cells. J Biol Chem 2003; 278: 44188–96. [DOI] [PubMed] [Google Scholar]
- 41.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum calcium channel/ryanodine receptor: modulation by endogenous effectors and disease states. Pharmacol Rev 1997; 49: 1–51. [PubMed] [Google Scholar]
- 42.Mackiewicz U, Emanuel K, Lewartowski B. Agonist of dihydropyridine receptors, BayK8644 depresses excitation-contraction coupling in myocytes of guinea pig heart. J Physiol Pharmacol 2001; 52: 459–69. [PubMed] [Google Scholar]
- 43.Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol 1979; 41: 473–84. [DOI] [PubMed] [Google Scholar]
- 44.Langer GA. Events at the cardiac sarcolemma: localization and movement of contractile-dependent calcium. Fed Proc 1976; 35: 1274–8. [PubMed] [Google Scholar]
- 45.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 1983; 245: C1–14. [DOI] [PubMed] [Google Scholar]
- 46.Xia R, Stangler T, Abramson JJ. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem 2000; 275: 36556–61. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mousa F, Michelangeli F. Commonly used ryanodine receptor activator, 4-chloro-m-cresol (4CmC), is also an inhibitor of SERCA Ca2+ pumps. Pharmacol Rep 2009; 61: 838–42. [DOI] [PubMed] [Google Scholar]
- 48.Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 1996; 380: 72–5. [DOI] [PubMed] [Google Scholar]
- 49.Polster A, Ohrtman JD, Beam KG, Papadopoulos S. Fluorescence resonance energy transfer (FRET) indicates that association with the type i ryanodine receptor (RyR1) causes reorientation of multiple cytoplasmic domains of the dihydropyridine receptor (DHPR) α1S subunit. J Biol Chem 2012; 287: 41560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avila G, Dirksen RT. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J Gen Physiol 2000; 115: 467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 2014; 47: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie D, Chen H, Fill M. Is ryanodine receptor a calcium or magnesium channel? Roles of K+ and Mg2+ during Ca2+ release. Cell Calcium 2012; 51: 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Protasi F, Paolini C, Nakai J, Beam KG, Franzini-Armstrong C, Allen PD. Multiple regions of RyR1 mediate functional and structural interactions with alpha(1S)-dihydropyridine receptors in skeletal muscle. Biophys J 2002; 83: 3230–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paolini C, Fessenden JD, Pessah IN, Franzini-Armstrong C. Evidence for conformational coupling between two calcium channels. Proc Natl Acad Sci U S A 2004; 101: 12748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huxley HE. Fifty years of muscle and the sliding filament hypothesis. Eur J Biochem 2004; 271: 1403–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.