Abstract
Evidence has revealed that vitamin D status is associated with the cardiometabolic risk factors. Moreover, few gender-specific analyses have been performed in the clinical trials regarding vitamin D supplementation. As a result, assessing gender differences regarding the effects of vitamin D supplementation on some cardiometabolic biomarkers in patients with non-alcoholic fatty liver disease (NAFLD) was the aim of present study. We conducted a post hoc subgroup analysis of a double blind placebo controlled study. Patients with NAFLD randomly allocated to receive one oral pearl consisting of 50,000 IU vitamin D3 (n = 27, 13 men and 14 women) or a placebo (n = 26, 13 men and 13 women) every 14 days for four months. Serum lipid profiles, aminotransferases, high-sensitive C-reactive protein (hs-CRP), adiponectin as well as insulin resistance and dietary intakes were assessed pre- and post-study. In both genders, serum 25(OH) D3 increased significantly (P < 0.001). This increase was accompanied by significant decrease in serum total cholesterol (TC) (% of change: −7% in vitamin D vs. + 0.4% in placebo, P = 0.04) and LDL-C (%of change: −9.6% in vitamin D vs. −4% in placebo, P = 0.006) in women. However, in men, vitamin D supplementation increased the levels of serum TC (% of change: +9.2% in vitamin D vs. −10% in placebo, P = 0.02) with no significant effects on LDL-C. Moreover, vitamin D significantly reduced serum hs-CRP in women. The median daily calcium intake in both genders was well below the dietary reference intake for adults. In conclusion, improved vitamin D status might decrease serum TC and LDL-C levels as well as hs-CRP in women with NAFLD. However, it might increase serum TC in men who have low daily calcium intake. Further studies with larger sample sizes are needed to confirm these results.
Keywords: Vitamin D, non-alcoholic fatty liver disease, cardiometabolic biomarkers, gender
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined as hepatic accumulation of fat in the absence of excess alcohol consumption that has a broad spectrum from steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis.1 One of the important risks that is threatening the health status of patients with NAFLD, as much as liver damage, is cardiovascular diseases (CVDs).2 The prevalence of CVDs in patients with NAFLD is estimated to be higher than the general population.3 Insulin resistance (IR), hyperlipidemia, and abdominal obesity are the major causes of the increased risk of CVDs in patients with NAFLD.4 Moreover, accumulation and oxidation of fatty acids in liver trigger the release of inflammatory mediators that enhance the risk of CVDs.5 As a result, finding strategies to reduce the risk factors of CVDs in patients with NAFLD has become important for investigators equally to the importance of finding therapeutic ways to decrease hepatic fat accumulation.
The results of previous studies have shown that vitamin D status is associated with the risk of CVDs.6 On the other hand, vitamin D deficiency is prevalent worldwide and estimated to be 92.4% in patients with chronic liver diseases.7 Furthermore, Targher et al. for the first time showed that patients with NAFLD have a significantly higher prevalence of vitamin D deficiency compared with healthy controls that may predispose them to increased risk of cardiovascular events.8 In addition, the results of previous studies have shown that supplementation with vitamin D may have beneficial effects on some of the cardiometabolic biomarkers such as IR, lipid profile, and inflammatory mediators in other diseases.9–11 Also, the results of the recent studies showed that impaired vitamin D signaling through polymorphisms of vitamin D receptor (VDR) could be involved in left ventricular hypertrophy in renal patients.12–16 However, there is a lack of evidence for the effects of vitamin D supplementation on cardiometabolic biomarkers in patients with NAFLD.
Interestingly, because of the differences in pharmacokinetics, pharmacodynamics, physiology, and hormonal influences, men and women may respond differently to cardiovascular medications.17 It is notable that few gender-specific analyses have been performed in the most applicable clinical trials. In order to individualize therapies, more specific studies are needed to determine what intervention strategies in the prevention or treatment of CVDs should be applied for men and women separately. Therefore, we conducted a post hoc subgroup analysis within a randomized controlled trial in patients with NAFLD to examine the hypothesis that men, who were supplemented with vitamin D, may respond different from women in terms of selected cardiometabolic biomarkers such as IR, lipid profiles, serum levels of high sensitive C-reactive protein (hs-CRP), and adiponectin.
Materials and methods
Study design and participants
Present study is a post hoc subgroup analysis that was performed within a randomized double blind placebo controlled trial of vitamin D intervention in patients with NAFLD.18 The design, sample size calculation, randomization, and concealment methods have been described completely elsewhere.18 Briefly, known cases of patients with NAFLD, aged 18–70 years who were diagnosed by ultrasonography scans and increased levels of alanine transaminase (ALT) were recruited from the outpatient clinic of Jundishapur University of Medical Sciences, Ahvaz, Iran. Serum levels of ALT higher than 19 U/L for women and 30 U/L for men were used as inclusion criteria.19 Patients were excluded from the study if any of the following conditions existed: 1) alcohol consumption greater than 20 g/day; 2) pregnancy and lactation; 3) any forms of hepatic disease; 4) history of intestinal bypass surgery; 5) using total parenteral nutrition in the past six months; and 6) taking hepatotoxic drugs. Patients with a history of hypothyroidism, renal failure, and kidney stones were also excluded. Moreover, serum calcium levels more than 10.6 mg/dL and the intake of vitamin D, vitamin E, and calcium supplements during the last six months were additional exclusion criteria. Twenty-five subjects were calculated to be the sample size for each arm of our trial to detect a change of 2.7 in the homeostasis model assessment of insulin resistance (HOMA-IR). We considered 0.05 and 0.2 (80% power) as type I and type II error, respectively.18 In order to cover possible dropouts, five subjects were added to each group to reach the 30 subjects for final sample size.
After giving complete description about the study protocol, all patients signed a written informed consent form. Then, 60 subjects were randomly assigned in a double blind manner to one of the two groups to receive either one oral pearl consisting of 50,000 IU vitamin D3 (D-Vitin 50,000; Zahravi Pharm Co, Tabriz, Iran) or a placebo (Zahravi Pharm Co) every 14 days for four months. Randomization and allocation were concealed from the researchers and participants until statistical analysis was completed. Computer-generated random numbers were used for random assignment. The trained person, who was not involved in the trial and not aware of random sequences, assigned and enrolled the patients. The study protocol was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (under the Registration No. ETH-605) and the trial was prospectively registered at IRCT.ir (IRCT2012071810333N1).
Clinical and biochemical measurements
At the beginning, demographic data and history of diseases, medications, and supplements were obtained from the patients. Height, weight, waist and hip circumferences, and body fat percentage for each participant were measured at baseline and at the end of study. Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2). Body fat percentage was measured using bioelectrical impedance analysis (BIA) (Quadscan 4000, Bodystat Ltd; Isle of Man, UK). Subjects were advised not to take any vitamin D, calcium, vitamin E, fish oil, and other supplements as well as statin medications during the study. Lifestyle modifications regarding restriction of high carbohydrate/high fat diet and increasing physical activity level were recommended to all study participants as standard care for NAFLD. To assess dietary intake, a two-day 24 h dietary recall (a weekday and a weekend) was obtained from each participant pre- and post-study. Moreover, the physical activity of each subject was assessed using the short version of International Physical Activity Questionnaire (IPAQ) at baseline and at the end of study.20 To measure the Metabolic Equivalent of Tasks per minute/per week (METs-min/week) for each subject, we multiplied the times (in minute per week) by its related METs coefficient which has been defined for each physical activity intensity level.20
A total of 10 mL of fasting blood samples were obtained from each participant after 8–12 h overnight fast at the study entry and at the end of research when one week had passed since taking the last pearl. The blood samples were centrifuged and serum samples were stored at −80℃ until further assays were completed. Serum 25-hydroxy vitamin D3 (25(OH)D3) levels were assayed using radioimmunoassay (Immunodiagnostic Systems, Boldon, UK). Serum calcium levels were measured using a photometric test (Arsenazo III Method; Pars Azmoon Co, Tehran, Iran). Serum ALT and aspartate transaminase (AST) enzyme activity were measured using the kinetic method (Pars Azmoon Co, Tehran, Iran). Fasting insulin concentrations were detected by electrochemiluminescence immunoassays (Roche Diagnostics, Mannheim, Germany). The HOMA-IR was calculated using the formula HOMA-IR = fasting glucose (mg/dL) × fasting insulin (µU/mL)/405.
Serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) concentrations were determined using enzymatic kits (Pars Azmoon Co, Tehran, Iran). High-sensitive C-reactive protein (hs-CRP) levels were measured using turbidimetric immunoassay (BioSystems Co, Barcelona, Spain). Serum levels of adiponectin were measured using enzyme-linked immunosorbent assay (ELISA) (Orgenium Co, Vantaa, Finland).
Serum levels of ALT and HOMA-IR were considered as the primary outcomes and serum lipid profiles, hs-CRP, and adiponectin were considered as the secondary outcomes.18
Statistical analysis
Both trial groups were divided into two subgroups according to the gender. Then, a subgroup analysis of data was performed for each arm. Data were reported as median with 25th and 75th percentile because the number of subjects in each subgroup was less than 30. The between-group comparisons for baseline characteristics were done using the Mann–Whitney U test. The Wilcoxon Paired Rank Test was used for within-group comparisons. Because all variables were non-normally distributed, the data were normalized by logarithmic transformation. Then, we used a repeated measure analysis of variances to assess time × group (vitamin D and placebo) interaction effects on all dependent variables in men and women, separately. To control the effect of confounding variables, baseline values, and season entered as covariates in the model. Differences in proportions were evaluated using chi-square or Fisher’s exact tests. Analysis was conducted using SPSS® (version 16) (SPSS Inc, Chicago, IL). Two sided P values < 0.05 were considered statistically significant.
Results
Of the 60 participants (30 men and 30 women) allocated to the trial groups, seven patients failed to complete the study (1 woman and 2 men in vitamin D and 2 women and 2 men in placebo group). Thus, 13 men and 14 women in the vitamin D group and 13 men and 13 women in the placebo group completed the study. Patients were recruited from November 2012 to May 2013 with the intervention period lasting until September 2013. Participants did not report any adverse or side effects such as hypercalcemia. The compliance rates were more than 90% in the vitamin D and placebo groups for both genders. The history of diseases and use of medications were comparable between the subgroups. Table 1 displays the baseline characteristics of the study groups. There were no significant differences in dietary variables and grades of NAFLD between men and women in both groups (Table 1). In men, variables including weight, waist circumference, BMI, and serum levels of AST were significantly different between the vitamin D and placebo groups at baseline (Tables 2 and 3). In addition, the median age and baseline serum concentrations of TG, LDL-C, and fasting insulin were significantly different between the vitamin D and placebo in women (Tables 1–3; indicated with *).
Table 1.
Comparisons of the baseline characteristics between the vitamin D and placebo groups in men and women with NAFLD.a
| Men |
Women |
|||
|---|---|---|---|---|
| Characteristics | Vitamin D n = 13 | Placebo n = 13 | Vitamin D n = 14 | Placebo n = 13 |
| Smoker (n)b | 2 | 1 | 0 | 0 |
| Age | 39 (31, 48.5) | 35 (32, 50) | 41 (35, 46.7) | 47 (43.5, 52.5)* |
| Grade of fatty liver (%)b based on US | ||||
| Grade I | 77 | 54 | 64 | 46.5 |
| Grade II | 23 | 46 | 29 | 46.5 |
| Grade III | 0 | 0 | 7 | 7 |
| Energy (kcal/d) | 1885 (1500, 2373) | 1945 (1696, 2232) | 2020 (1737, 2325) | 1973(1541, 2204) |
| Dietary vitamin D intake (µg/d) | 0.00 (0.00, 1.94) | 0.00 (0.00, 1.50) | 0.00 (0.00, 0.55) | 0.00 (0.00, 0.17) |
| Dietary calcium intake (mg/d) | 531 (328, 744) | 541 (342, 844) | 632 (474, 795) | 472 (266, 763) |
| Dietary saturated fatty acids intake (g/d) | 13.8 (8.7, 21.2) | 14.9 (9.5, 21.9) | 17.9 (12.9, 18.5) | 11.1 (9.6, 18.7) |
| Dietary cholesterol intake (mg/d) | 176 (93, 281) | 173 (99, 259) | 197 (151, 377) | 117 (83, 231) |
| Physical activity (MET-min/week)c | 231 (99, 643.5) | 297 (49.5, 594) | 110 (0, 594) | 198 (0, 544.5) |
NAFLD: non-alcoholic fatty liver disease; US: ultrasonography; MET: metabolic equivalent of task.
Data is expressed as median (25th, 75th percentiles) and tested by Mann–Whitney U test.
Data were analyzed by chi-square or Fisher’s exact tests.
Total MET-minutes/week = walking (3.3 METs*min*days) + moderate intensity (4 METs*min*days) + vigorous intensity (8 METs*min*days), based on the short form of International Physical Activity Questionnaire (IPAQ).
P value < 0.05 between the vitamin D and placebo groups.
Table 2.
Within- and between-group comparisons of the changes from baseline to endpoint measures for anthropometric variables in vitamin D and placebo groups in NAFLD patients.a
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Variables | Vitamin D n = 13 | Placebo n = 13 | P value (time × group)d | Vitamin D n = 14 | Placebo n = 13 | P value (time × group)d |
| Weight (kg) | 0.20 | 0.41 | ||||
| Baseline | 87.5 (84.5, 97) | 78 (72.5, 91.2)* | 75 (71.5, 89) | 75 (71.5, 83.7) | ||
| 4 mo | 86 (82.2, 98) | 78 (70.5, 85) | 71.5 (70,83.7) | 73 (70, 82) | ||
| Changeb | −2.5 (−3.5, −1) | −1 (−4, 1.2) | −1.7 (−3.8, 0) | −1 (−4, 0) | ||
| P (within)c | 0.02 | 0.14 | 0.02 | 0.05 | ||
| Waist circumference (cm) | 0.10 | 0.22 | ||||
| Baseline | 105 (110, 112) | 100 (96, 108.5)* | 106.7 (102.2, 109.2) | 101 (107, 112.5) | ||
| 4 mo | 109 (105.5, 110.5) | 100 (96.5, 106) | 105.5 (100, 107.7) | 100 (100.4, 113.5) | ||
| Change | −1 (−2, 0.44) | 0.0 (−1.5, 0.5) | −1 (−2.2, 0.67) | −1 (−1.7, 1) | ||
| P (within) | 0.052 | 0.25 | 0.11 | 0.31 | ||
| Waist to hip ratio | 0.74 | 0.65 | ||||
| Baseline | 0.98 (0.95, 0.99) | 0.97 (0.93, 1.0) | 0.92 (0.89,0.96) | 0.93 (0.90, 0.95) | ||
| 4 mo | 0.98 (0.94, 1.01) | 0.96 (0.93,1.0) | 0.92 (0.89, 0.96) | 0.93 (0.91, 0.95) | ||
| Change | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.01) | 0.001 (−0.008, 0.01) | −0.01 (0.00, 0.01) | ||
| P (within) | 0.65 | 0.59 | 0.42 | 0.31 | ||
| Body fat percentage | 0.72 | 0.78 | ||||
| Baseline | 21.6 (19.6, 25.8) | 23.4 (20.2, 30.6) | 40.7 (36.8, 45.1) | 40.7 (35.5, 45.6) | ||
| 4 mo | 20.1 (18.2, 23.1) | 20.6 (18.9, 24.3) | 39.5 (36.6,43.3) | 39.5 (32, 44.9) | ||
| Change | −1.5 (−3.9, −0.6) | −3.6 (−5.3, −0.4) | −3 (−3.9, 0.17) | −1.5 (−3.8, −0.7) | ||
| P (within) | 0.06 | 0.06 | 0.08 | 0.05 | ||
| BMI (kg/m2) | 0.20 | 0.42 | ||||
| Baseline | 30.9 (28.6, 32.0) | 27.4 (25.7, 29.9)* | 31.3 (29.1, 35.9) | 30.4 (28.6, 34.1) | ||
| 4 mo | 30.4 (27.5,32.2) | 27.0 (25.4, 28.9) | 30.4 (27.7, 33.5) | 31.0 (27.1, 33.1) | ||
| Change | −0.9 (−1.1, −0.4) | −0.4 (−1.4, 0.4) | −0.7 (−1.7, 0.0) | −0.4 (−1.5, 0.0) | ||
| P (within) | 0.02 | 0.11 | 0.02 | 0.05 | ||
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index.
Data are expressed as median (25th, 75th percentiles).
End values minus baseline.
P value for comparing baseline with end point values within each group. Wilcoxon Paired Rank test was used.
Repeated measure ANOVA was used to analyze the time × group (vitamin D or placebo) interaction adjusted for the baseline covariates.
*P value < 0.05, **P value < 0.01 between the vitamin D and placebo groups comparing the baseline values.
Table 3.
Within-group and between-group comparisons of the changes from baseline to endpoint measures for cardiometabolic biomarkers in vitamin D and placebo groups in NAFLD patients.a
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Variables | Vitamin D n = 13 | Placebo n = 13 | P value (time × group)d | Vitamin D n = 14 | Placebo n = 13 | P value (time × group)d |
| 25 (OH) D3 (ng/mL) | <0.001 | <0.001 | ||||
| Baseline | 15.7 (10.6, 29.2) | 15.4 (23.5, 11.7) | 10 (7.6, 18.1) | 18.3 (10.6, 43.1) | ||
| 4 mo | 30 (25.4, 40.3) | 17.5 (14.5, 25.1) | 33.6 (25.6, 53.27) | 24.4 (13, 33.2) | ||
| changeb | 13.3 (8.7, 23.5) | 1.8 (−3.7, 4.6) | 20.5 (11.7, 38.5) | 1 (−6.6, 9.4) | ||
| P (within)c | 0.002 | 0.70 | 0.001 | 0.86 | ||
| Calcium (mg/dL) | 0.88 | 0.85 | ||||
| Baseline | 9 (8.7, 9.4) | 9.2 (8.9, 9.6) | 9.3 (8.9, 9.4) | 9 (8.9, 9.4) | ||
| 4 mo | 9.1 (9.1, 9.4) | 9.6 (8.9, 9.7) | 9.2 (8.7, 9.6) | 9 (8.7, 9.3) | ||
| Change | 0.4 (0.0, 0.6) | 0.3 (−0.7, 0.5) | −0.2 (−0.5, 0.3) | −0.2 (−0.4, 0.1) | ||
| P (within) | 0.04 | 0.75 | 0.57 | 0.30 | ||
| ALT (IU/L) | 0.18 | 0.10 | ||||
| Baseline | 32 (31, 35) | 48 (32.5, 54) | 23.5 (20, 44.7) | 20 (19, 26) | ||
| 4 mo | 28 (23.5, 42) | 39 (22.5, 57.5) | 20 (14.5, 28.5) | 16 (12.5, 21.5) | ||
| Change | −4 (−9, 7.5) | −3 (−15, 12) | −3.5 (−9.2, −0.5) | −5 (−8.5, −0.5) | ||
| P (within) | 0.57 | 0.55 | 0.03 | 0.16 | ||
| AST (IU/L) | 0.96 | 0.18 | ||||
| Baseline | 24 (21, 27.5) | 31 (25.5, 39)* | 24 (17.7, 34) | 22 (17, 25) | ||
| 4 mo | 22 (19.5, 27.5) | 26 (20, 31) | 18.5 (15, 26.2) | 17 (15, 22) | ||
| Change | 0.0 (−6.5, 2) | −4 (−7, 3) | 0.0 (−10, 2) | −4 (−7.5, 1) | ||
| P (within) | 0.60 | 0.25 | 0.41 | 0.18 | ||
| HOMA-IR | 0.91 | 0.60 | ||||
| Baseline | 3.2 (2.4, 4.8) | 2.6 (1.8, 4.2) | 4.1 (3, 5.2) | 2.4 (1.9, 3) | ||
| 4 mo | 3.6 (2.4, 5.2) | 3.1 (2.1, 4) | 3.4 (2.3, 4) | 2.2 (1.6, 3.7) | ||
| Change | 0.6 (−1, 1.2) | 0.3 (0.9, 1.8) | −0.4 (−1.6, 1.0) | −0.02 (−0.9, 1.0) | ||
| P (within) | 0.55 | 0.65 | 0.30 | 0.70 | ||
| Total cholesterol (mg/dL) | 0.02 | 0.04 | ||||
| Baseline | 173 (146, 191) | 189 (153, 203) | 197 (166, 235) | 183 (155, 203) | ||
| 4 mo | 189 (162, 223) | 161 (132, 168) | 174 (148, 204) | 170 (153, 209) | ||
| Change | 16 (−0.5, 32.5) | −21 (−44, −9) | −13 (−50, 11.5) | 1 (−11.5, 13.5) | ||
| P (within) | 0.02 | 0.004 | 0.19 | 0.67 | ||
| Triglyceride (mg/dL) | 0.24 | 0.50 | ||||
| Baseline | 143 (115, 241) | 136 (106, 241) | 177 (99.5, 210) | 94 (82, 117)* | ||
| 4 mo | 186 (167, 244) | 117 (97, 235) | 126 (101, 204) | 130 (104, 172) | ||
| Change | 35 (−12, 71) | −5 (−55.5, 35) | 7 (−58.5, 38.5) | 30 (1.5, 71.5) | ||
| P (within) | 0.09 | 0.42 | 0.73 | 0.07 | ||
| LDL cholesterol (mg/dL) | 0.14 | 0.006 | ||||
| Baseline | 96 (80, 103) | 105 (81.5, 119.5) | 107.5 (89.5, 127.7) | 92 (80, 101.5)* | ||
| 4 mo | 95 (80.5, 117) | 87 (65.5, 94) | 90.5 (77.7, 118.2) | 88 (75.5, 119) | ||
| Change | 4 (−3, 19) | −13 (−42, 2.5) | −9.5 (41, 6.7) | −3 (−13, 20.5) | ||
| P (within) | 0.12 | 0.02 | 0.13 | 0.87 | ||
| HDL cholesterol (mg/dL) | 0.78 | 0.21 | ||||
| Baseline | 39 (32, 43.5) | 38 (33, 42) | 42 (37, 50.2) | 43 (36, 49) | ||
| 4 mo | 41 (34, 45.5) | 38 (31.5, 42) | 40 (35.2, 47.7) | 45 (38, 48) | ||
| Change | 1 (−1.5, 5) | −1 (−2.5, 2) | −2.5 (−12.7, 4.7) | −3 (−5.5, 0.5) | ||
| P (within) | 0.57 | 0.62 | 0.21 | 0.15 | ||
| hs-CRP (mg/L) | 0.30 | 0.04 | ||||
| Baseline | 1.2 (0.7, 1.8) | 0.8 (0.6, 1.4) | 2.6 (1.2, 7.5) | 1.5 (0.6, 2.6) | ||
| 4 mo | 1.1 (0.6, 1.6) | 1.3 (0.6, 1.9) | 2.3 (1.3, 5.5) | 2.5 (1.1, 3.0) | ||
| Change | −0.3 (−0.6, 0.5) | −0.1 (−0.3, 0.7) | −0.1 (−1.2, 1.5) | 0.5 (0.03, 1.2) | ||
| P (within) | 0.38 | 0.66 | 0.87 | 0.04 | ||
| Adiponectin (µg/mL) | 0.63 | 0.64 | ||||
| Baseline | 1.2 (0.7, 1.4) | 1.1 (0.8, 1.6) | 1.1 (0.9, 2.5) | 1.1 (0.8, 1.6) | ||
| 4 mo | 1.0 (0.8, 1.2) | 1.3 (1.0, 1.5) | 1.4 (1.1, 3.4) | 1.8 (1.5, 3.3) | ||
| Change | 0.0 (−0.6, 0.2) | 0.3 (−0.6, 0.7) | 0.3 (−0.1, 1.4) | 0.4 (−0.05, 1.3) | ||
| P (within) | 0.33 | 0.47 | 0.02 | 0.11 | ||
Data are expressed as median (25th, 75th percentiles).
End values minus baseline.
P value for comparing the baseline with end point values within each group. Wilcoxon Paired Rank test was used.
Repeated measure ANOVA was used to analyze the time × group (vitamin D or placebo) interaction adjusted for the baseline covariates and season.
*P value < 0.05, **P value < 0.01 between the vitamin D and placebo groups comparing the baseline values.
Within- and between-group comparisons for each subgroup of men and women are shown in Tables 2 and 3. The changes of the anthropometric variables between the study groups were not significant during the intervention in both genders (Table 2). In both men and women, weight, body fat percentage, and BMI decreased nearly significant in each of the vitamin D and placebo groups. However, the decrease in weight, WC, and BMI are more pronounced in the vitamin D group of men (Table 2). Energy and nutrient intakes and physical activity levels showed no significant changes between the groups post-intervention in both men and women (data not shown).
Both men and women showed near two- to three-fold increments in serum 25(OH) D3 in the vitamin D group post-intervention (Table 3), which indicates subjects' satisfactory compliance with supplementation in both subgroups.
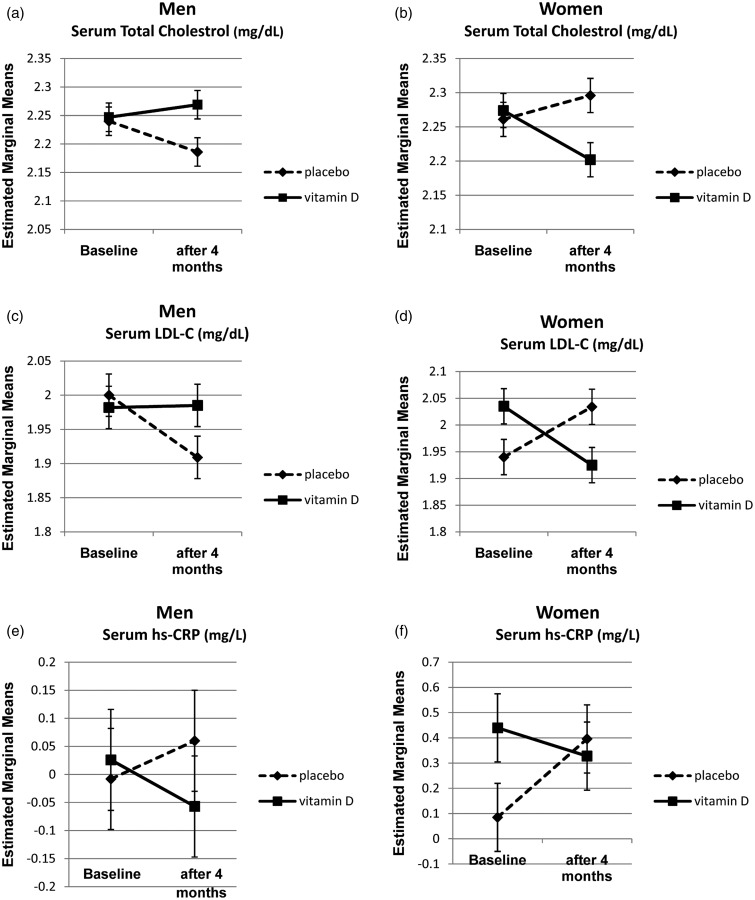
In men, the results of the repeated measure ANOVA test adjusted for the baseline variables such as weight, waist circumference, BMI, season, and serum levels of AST showed significant differences in serum TC levels between the vitamin D and placebo groups after intervention (Table 3). The serum levels of TC increased in the vitamin D group, although it significantly decreased in the placebo group. However, this pattern was quite different in women. Vitamin D supplementation decreased serum TC and LDL-C in the vitamin D group after adjusting for baseline variables such as season, age, serum levels of TG, LDL-C, and fasting insulin. We represented adjusted marginal means of logarithmic transformed data, derived from repeated measure ANOVA test for TC, and LDL-C to illustrate this different pattern between men and women (Figure 1).
Figure 1.
Log10 transformed estimated marginal means of serum total cholesterol, LDL-C and hs-CRP in vitamin D and placebo at baseline and post-intervention in men and women with NAFLD*: a: total cholesterol in men, b: total cholesterol in women, c: LDL-c in men, d: LDL-c in women, e: hs-CRP in men, f: hs-CRP in women. *Results are based on the repeated measure ANOVA test and estimated marginal means adjusted for baseline values of BMI, waist circumference, serum ALT and AST in men and age, serum vitamin D, TG, LDL and insulin in women. Moreover, season is considered for adjustment in both genders
In both men and women with NAFLD, serum hs-CRP decreased in the vitamin D wing, although the between group comparison of the changes reached the significant level only in women (Table 3 and Figure 1).
Vitamin D supplementation made no changes in serum levels of ALT, AST, calcium, TG, HDL, adiponectin, and HOMA-IR compared to the placebo in both gender subgroups (Table 3).
Discussion
In this gender-specific subgroup analysis study, supplementation with one oral pearl consisting 50,000 IU vitamin D3 every 14 days for 120 days could significantly decrease serum levels of TC and LDL-C in women diagnosed with NAFLD. However, this intervention increased the levels of serum TC and made no effects on LDL-C in men with NAFLD. Dyslipidemia in NAFLD leads to accelerated atherosclerosis and CVDs.21 The results of the observational studies have shown that low serum levels of vitamin D have been associated with dyslipidemia.9,22 Nevertheless, interventional studies revealed inconsistent results about the effects of vitamin D on lipid profiles.23,24 A meta-analysis study of the qualified randomized clinical trials by Wang et al. revealed that supplementation with vitamin D led to a statistically significant increase in LDL-C and non-significant decrease in TG and HDL-C in patients with CVDs.25 However, the reasons for increasing LDL-C were not explained in that study. One of the trials that was included in the above-mentioned meta-analysis was the study by Zittermann et al.24 Their results revealed that supplementation with 3320 IU/d vitamin D could increase LDL-C concentrations in overweight subjects who were participating in a weight loss diet when compared with the placebo group.24 One possible explanation proposed by them is that vitamin D increases the intestinal calcium absorption and consequently reduces the formation of insoluble calcium-fatty acid soaps.24 Increased absorption of saturated fatty acids, would, therefore, raise the serum LDL-C levels. They also concluded that a higher calcium intake might prevent the vitamin-D induced elevation in LDL-C.24 Similarly, we found an increment in serum TC only in men subjects in the vitamin D group. Conversely, the corresponding values of TC and LDL-C were significantly decreased in women who were in the treatment wing. The increase in men’s serum TC concentrations can be explained based on the hypothesis suggested by Zittermann et al.24 Although the median daily calcium intake was comparable between men and women in our study; however, it was about 500–700 mg/day in both genders that is well below the dietary reference intake for adults. This might be a possible reason for the increase in TC concentrations after supplementation with vitamin D in men based on the mentioned hypothesis. However, in women, with the same intake of dietary calcium, the trend is opposite. One possible assumption for explaining this discrepancy between men and women in our trial is a gender-related difference in intestinal calcium transport mediated by vitamin D.26 The intestinal calcium absorption might be more vitamin D-dependent in men than in women.26 The results of previous animal studies showed that in a vitamin D sufficient condition, the intestinal calcium transport was significantly greater in the male rats than in the females.27 On the other hand, vitamin D deficiency makes a lower intestinal absorption of calcium in the male rats but not in the females.27
The sex hormones could be responsible for the different calcium absorption under the same vitamin D condition.28 A recent study by Dong et al. aimed to determine the effect of estrogen and vitamin D interaction on epithelial calcium transport proteins.29 They compared female sham-operated with ovariectomized rats that fed with either low calcium or high calcium diet for 12 weeks.29 Their results showed that apart from active form of vitamin D, estrogen also increased the mRNA expression of intestinal transient receptor potential cation channel (TRPV6) and modulated the parathyroid hormone (PTH) levels in low calcium diet condition.29 The results of the other animal studies also revealed some evidence about the differences in intestinal calcium absorption mediated by vitamin D and estrogen between males and females.30,31 This hypothesis needs more evidence from future human and cellular studies.
Preventing the rise in serum levels of hs-CRP would be an important strategy to decrease CVD risks in patients with NAFLD.3 In our study, serum hs-CRP levels decreased in both men and women in the vitamin D group while it showed increasing trend in placebo group of both genders. However, the reduction of serum hs-CRP reached a significant level only in women after four months of vitamin D supplementation. Women had higher levels of serum hs-CRP compared with men in the vitamin D group (the median: 2.6 vs. 1.5 mg/L, P = 0.03). This could be regarded as the reason for more pronounced reduction in hs-CRP observed in women after vitamin D supplementation. The mechanisms by which vitamin D can influence hs-CRP levels in patients with NAFLD have been discussed elsewhere.18
Serum total adiponectin levels have been reported to be lower in patients with NAFLD than healthy controls.32 Decreased adiponectin concentrations may be another underlying factor linking NAFLD and CVDs.33 Scarce evidence exists about the effects of vitamin D on serum adiponectin levels especially in patients with NAFLD. Nakano et al. found that phototherapy through increased serum vitamin D levels could significantly increase serum adiponectin in rats with NAFLD.34 In our study, however, vitamin D supplementation had no effect on serum adiponectin levels in both subgroups of men and women. In agreement with our results, supplementation with high doses of vitamin D could not change serum adiponectin in two clinical trials that were performed in patients with type 2 diabetes and obese adolescents for four and six months, respectively.35,36 Nevertheless, a number of cross sectional studies have shown an independent and strong positive relationship between serum vitamin D and adiponectin levels in obese patients.37,38 Interestingly, the active form of vitamin D has reduced adiponectin secretions from human cultured adipocytes in a cellular study.39 It seems that many factors may influence the effects of vitamin D on serum adiponectin such as doses of vitamin D, duration of intervention, VDR polymorphisms, and using calcium supplements in addition to vitamin D.40 More mechanisms remain unknown in terms of the relationship between vitamin D and adiponectin that are waiting to be investigated in further studies.
Vitamin D supplementation made no changes in serum levels of ALT, AST, and HOMA-IR compared to the placebo in both genders. The possible reasons for finding no effects of vitamin D on mentioned biomarkers have been discussed elsewhere.18
In our study, supplementation with vitamin D had no effect on anthropometric indices in both subgroups of men and women. Similarly, Zittermann et al. indicated that supplementation with 3320 IU/d vitamin D3 did not change the anthropometric indices when compared with the placebo among subjects who were on a weight loss diet.24 It seems that because of dietary recommendations in our study, in both genders, some of the anthropometric measures decreased nearly significant in each vitamin D and placebo groups. Men in the vitamin D group had greater reductions in weight, waist circumference, and BMI when compared with the other subgroups of the study. At baseline, the values for weight, WC, and BMI of men in the vitamin D group were significantly higher than men in the placebo group. This might be a possible reason for greater reductions in these variables in the vitamin D group of men. The results of previous studies have shown that initial body weight could be a significant predictor of weight loss in subjects.41,42
One of the limitations of present study was the small number of subjects in each subgroup. However, the obtained results may suggest new hypothesis in terms of the gender difference in vitamin D-induced intestinal calcium absorption that can be investigated in larger randomized control trials and/or cellular studies.
To the best of our knowledge, this is the first research that assessed the response of men and women with NAFLD separately to vitamin D supplementation in terms of serum lipid profile, hs-CRP, adiponectin, and HOMA-IR. The other strength of our study is that we adjusted the obtained results for the baseline variables that were not comparable between vitamin D and placebo in each subgroup. In addition, we evaluated the effects of body fat percentage, season; daily dietary intakes of energy, vitamin D and calcium; and physical activity levels at baseline and at the end of study.
In conclusion, supplementation with one oral pearl consisting of 50,000 IU vitamin D3 every 14 days for four months might decrease serum TC and LDL-C levels as well as hs-CRP levels in women with NAFLD. However, it might lead to increase in serum TC in men due to a low dietary calcium intake. Further studies with larger sample sizes are needed to investigate the effects of joint vitamin D and calcium supplementation on CVD risk biomarkers in men and women with NAFLD.
Acknowledgments
This study would have not been possible without the great co-operation of our patients. This work has been carried out as a part of Nasrin Sharifi's PhD thesis and was financially supported by a Grant (No. RDC-9105) from Vice-Chancellor for Research Affairs of Jundishapur University of Medical Sciences and approval of the Research Institute for Infectious Diseases of the Digestive System, Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. All of them drafted the article and revised it critically for important intellectual content and approved the version to be published. RA designed and supervised the study, NS designed the study and collected the data, EH involved in clinical evaluation and diagnosis of the participants, and BC performed statistical analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006; 40: S5–10. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Behling C and Lavine JE. Fatty liver as a determinant of atherosclerosis. Hepatology 2005; 42(suppl 1): 610A.
- 3.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007; 191: 235–40. [DOI] [PubMed] [Google Scholar]
- 4.Sheriff D, Manopriya T. Obesity, non alcoholic fatty liver disease (NAFLD) and coronary artery disease (CAD). Endocrinol Metabol Syndr 2011. Doi: 10.4172/2161-1017.S1-007. [Google Scholar]
- 5.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 2008; 51: 1947–53. [DOI] [PubMed] [Google Scholar]
- 6.Pekkanen MP, Ukkola O, Hedberg P, Piira OP, Lepojärvi S, Lumme J, Tulppo MP, Huikuri HV. Serum 25-hydroxyvitamin D is associated with major cardiovascular risk factors and cardiac structure and function in patients with coronary artery disease. Nutr Metab Cardiovasc Dis 2015; 25: 471–8. [DOI] [PubMed] [Google Scholar]
- 7.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci 2010; 55: 2624–8. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2007; 17: 517–24. [DOI] [PubMed] [Google Scholar]
- 9.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr 2010; 64: 1457–64. [DOI] [PubMed] [Google Scholar]
- 10.Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Kalayi A, Shariatzadeh N, Khalaji N. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 2012; 28: 424–30. [DOI] [PubMed] [Google Scholar]
- 11.Santoro D, Gitto L, Ferraro A, Satta E, Savica V, Bellinghieri G. Vitamin D status and mortality risk in patients with chronic kidney disease. Ren Fail 2011; 33: 184–91. [DOI] [PubMed] [Google Scholar]
- 12.Santoro D, Lucisano S, Gagliostro G, Alibrandi A, Benvenga S, Ientile R, Bellinghieri G, Buemi M, Caccamo D. Vitamin D receptor polymorphism in chronic kidney disease patients with complicated cardiovascular disease. J Ren Nutr 2015; 25: 187–93. [DOI] [PubMed] [Google Scholar]
- 13.Santoro D, Gagliostro G, Alibrandi A, Ientile R, Bellinghieri G, Savica V, Buemi M, Caccamo D. Vitamin D receptor gene polymorphism and left ventricular hypertrophy in chronic kidney disease. Nutrients 2014; 6: 1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testa A, Mallamaci F, Benedetto FA, Pisano A, Tripepi G, Malatino L, Thadhani R, Zoccali C. Vitamin D receptor (VDR) gene polymorphism is associated with left ventricular (LV) mass and predicts left ventricular hypertrophy (LVH) progression in end-stage renal disease (ESRD) patients. J Bone Miner Res 2010; 25: 313–9. [DOI] [PubMed] [Google Scholar]
- 15.El-Shehaby AM, El-Khatib MM, Marzouk S, Battah AA. Relationship of BsmI polymorphism of vitamin D receptor gene with left ventricular hypertrophy and atherosclerosis in hemodialysis patients. Scand J Clin Lab Invest 2013; 73: 75–81. [DOI] [PubMed] [Google Scholar]
- 16.Santoro D, Caccamo D, Gagliostro G, Ientile R, Benvenga S, Bellinghieri G, Savica V. Vitamin D metabolism and activity as well as genetic variants of the vitamin D receptor (VDR) in chronic kidney disease patients. J Nephrol 2013; 26: 636–44. [DOI] [PubMed] [Google Scholar]
- 17.Xhyheri B, Bugiardini R. Diagnosis and treatment of heart disease: are women different from men? Prog Cardiovasc Dis 2010; 53: 227–36. [DOI] [PubMed] [Google Scholar]
- 18.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014; 47: 70–80. [DOI] [PubMed] [Google Scholar]
- 19.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137: 1–10. [DOI] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1381. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Lu H-Y. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol 2014; 20: 8407–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, Niemelä O, Kuusisto J. Diverse associations of 25-hydroxyvitamin D and 1, 25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med 2010; 268: 604–10. [DOI] [PubMed] [Google Scholar]
- 23.Ponda MP, Huang XX, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. Epub ahead of print 2012. DOI: 10.1161/CIRCULATIONAHA. 111.077875. [DOI] [PMC free article] [PubMed]
- 24.Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009; 89: 1321–7. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis 2012; 11: 42–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris ME, Lee H-J, Predko LM. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol Rev 2003; 55: 229–40. [DOI] [PubMed] [Google Scholar]
- 27.Uhland-Smith A, DeLuca HF. 1, 25-dihydroxycholecalciferol analogs cannot replace vitamin D in normocalcemic male rats. J Nutr 1993; 123: 1777–85. [DOI] [PubMed] [Google Scholar]
- 28.Brommage R, Binacua C, Carrie A-l. Ovulation-associated increase in intestinal calcium absorption during the rat estrous cycle is blunted by ovariectomy. Biol Reprod 1993; 49: 544–8. [DOI] [PubMed] [Google Scholar]
- 29.Dong XL, Zhang Y, Wong MS. Estrogen deficiency-induced Ca balance impairment is associated with decrease in expression of epithelial Ca transport proteins in aged female rats. Life Sci 2014; 96: 26–32. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Lai WP, Wu CF, Favus MJ, Leung PC, Wong MS. Ovariectomy worsens secondary hyperparathyroidism in mature rats during low-Ca diet. Am J Physiol Endocrinol Metab 2007; 292: E723–31. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Fleet JC. 1,25 dihydroxycholecalciferol-mediated calcium absorption and gene expression are higher in female than in male mice. J Nutr 2004; 134: 1857–61. [DOI] [PubMed] [Google Scholar]
- 32.Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, Falezza G. Associations between plasma adiponectin concentrations and liver histology in patients with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2006; 64: 679–83. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291: 1730–7. [DOI] [PubMed] [Google Scholar]
- 34.Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, Huang YZ, Honda H, Chen KD, Wang CC. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol 2011; 55: 415–25. [DOI] [PubMed] [Google Scholar]
- 35.Patel P, Poretsky L, Liao E. Lack of effect of subtherapeutic vitamin D treatment on glycemic and lipid parameters in type 2 diabetes: a pilot prospective randomized trial. J Diabetes 2010; 2: 36–40. [DOI] [PubMed] [Google Scholar]
- 36.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 2013; 97: 774–81. [DOI] [PubMed] [Google Scholar]
- 37.Gannagé-Yared M-H, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol 2009; 160: 965–71. [DOI] [PubMed] [Google Scholar]
- 38.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine 2009; 36: 205–10. [DOI] [PubMed] [Google Scholar]
- 39.Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Åström G, Sjölin E, Wåhlén K, Carlberg C, Laurencikiene J. Differential effects of 1α, 25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr 2012; 51: 335–42. [DOI] [PubMed] [Google Scholar]
- 40.Wright OR, Hickman IJ, Petchey WG, Sullivan CM, Ong C, Rose FJ, Ng C, Prins JB, Whitehead JP, O'Moore-Sullivan TM. The effect of 25-hydroxyvitamin D on insulin sensitivity in obesity: is it mediated via adiponectin? Can J Physiol Pharmacol 2013; 91: 496–501. [DOI] [PubMed] [Google Scholar]
- 41.Tseng M-C, Lee Y-J, Chen S-Y, Lee M-B, Lin K-H, Chen P-R, Lai J-S. Psychobehavioral response and weight loss prediction in a hospital-based weight reduction program. J Formos Med Assoc 2002; 101: 705–11. [PubMed] [Google Scholar]
- 42.Teixeira P, Going S, Sardinha L, Lohman T. A review of psychosocial pre-treatment predictors of weight control. Obes Rev 2005; 6: 43–65. [DOI] [PubMed] [Google Scholar]