Abstract
Unlike other visceral organs, myocardial weight is maintained in relation to fetal body weight in intrauterine growth restriction (IUGR) fetal sheep despite hypoinsulinemia and global nutrient restriction. We designed experiments in fetal sheep with placental insufficiency and restricted growth to determine basal and insulin-stimulated myocardial glucose and oxygen metabolism and test the hypothesis that myocardial insulin sensitivity would be increased in the IUGR heart. IUGR was induced by maternal hyperthermia during gestation. Control (C) and IUGR fetal myocardial metabolism were measured at baseline and under acute hyperinsulinemic/euglycemic clamp conditions at 128–132 days gestation using fluorescent microspheres to determine myocardial blood flow. Fetal body and heart weights were reduced by 33% (P = 0.008) and 30% (P = 0.027), respectively. Heart weight to body weight ratios were not different. Basal left ventricular (LV) myocardial blood flow per gram of LV tissue was maintained in IUGR fetuses compared to controls. Insulin increased LV myocardial blood flow by ∼38% (P < 0.01), but insulin-stimulated LV myocardial blood flow in IUGR fetuses was 73% greater than controls. Similar to previous reports testing acute hypoxia, LV blood flow was inversely related to arterial oxygen concentration (r2 = 0.71) in both control and IUGR animals. Basal LV myocardial glucose delivery and uptake rates were not different between IUGR and control fetuses. Insulin increased LV myocardial glucose delivery (by 40%) and uptake (by 78%) (P < 0.01), but to a greater extent in the IUGR fetuses compared to controls. During basal and hyperinsulinemic–euglycemic clamp conditions LV myocardial oxygen delivery, oxygen uptake, and oxygen extraction efficiency were not different between groups. These novel results demonstrate that the fetal heart exposed to nutrient and oxygen deprivation from placental insufficiency appears to maintain myocardial energy supply in the IUGR condition via increased glucose uptake and metabolic response to insulin, which support myocardial function and growth.
Keywords: Glucose, insulin, heart, fetus, myocardial, sheep
Introduction
Intrauterine growth restriction (IUGR) is a common complication of pregnancy, occurring in up to 10% of pregnancies. Most commonly IUGR is the result of an insufficient placental supply of oxygen and nutrients to the developing fetus,1 both of which produce significant adaptations in fetal cardiac metabolism, growth, and development. For example, as an adaptation to acutely limited fetal oxygen supply during IUGR, cardiac output is redistributed to vital organs, resulting in asymmetric fetal growth restriction with relative sparing of brain and heart growth.2–5 Such changes in cardiac output redistribution may persist, or resolve to the level of normal rates of blood flow per fetal and cardiac size,6 but in either case, cardiac growth is maintained or even increased.
Placental insufficiency severe enough to produce fetal growth restriction also limits fetal glucose supply,7–10 which in turn produces relative fetal hypoglycemia that up-regulates insulin action and enhances the capacity for glucose uptake and metabolism by fetal cells in non-hepatic organs and tissues.10,11 Other models of ovine fetal IUGR from very different causes of placental insufficiency and fetal oxygen and nutrient restriction, such as the adolescent pregnant ewe model12,13 and the uterine carunculectomy model,14 also produce similar late gestation increases in fetal glucose and insulin sensitivity despite low circulating fetal glucose and insulin concentrations and relatively low fetal blood oxygen contents. The IUGR fetal phenotype produced by placental insufficiency, therefore, is a common response to nutrient and oxygen deficiency, regardless of how the placental insufficiency occurs.15 These adaptations also have been seen in fetal skeletal muscle,16 but there have been only limited studies of the fetal myocardium. One study, for example, showed that myocardial growth of the fetus is maintained in relation to its slowed body growth during placental insufficiency even as fetal plasma concentrations of glucose, insulin, and oxygen are decreased and lactate concentrations are increased.17
The fetal myocardium is an insulin-sensitive organ,18 which uses the carbohydrates, lactate, and glucose, as its primary energy substrates for oxidative metabolism.19 Previous studies in our ovine model of placental insufficiency and IUGR have demonstrated increased myocardial plasma membrane Glut 4 transporter and insulin receptor protein concentrations and maintained or normal concentrations of plasma membrane Glut 1 transporter protein, despite lower arterial plasma insulin and glucose concentrations and blood oxygen content.20 Such adaptations should act to maintain normal cardiac weight-specific glucose metabolism, normal or even increased rates of lactate production and glycogen content, and myocardial growth commensurate with fetal body growth, which together would support fetal survival in response to nutrient and oxygen deprivation that characterize placental insufficiency and IUGR. Whether these adaptations actually allow for normal glucose and oxygen metabolism during placental insufficiency and IUGR, however, is unknown. To date, no study has defined the adaptations in blood flow, glucose and oxygen metabolism, and insulin signaling in the myocardium of the IUGR fetus. Furthermore, despite such adaptations, IUGR fetuses with relatively spared heart growth still have increased rates of perinatal mortality and evidence of myocardial injury and defects in cardiac metabolism and cellular maturation.21–25 Understanding the physiological adaptations in the IUGR fetal heart is important, because the redistribution of fetal cardiac output, altered metabolism and myocellular development, and the associated asymmetrical growth pattern portend cardiovascular disease in later life.26
Therefore, we designed experiments in fetal sheep with placental insufficiency and restricted growth to determine basal and insulin-stimulated myocardial glucose and oxygen metabolism. We sought to test the hypothesis that basal- and insulin-stimulated myocardial glucose and oxygen metabolism would be maintained or even augmented in the IUGR fetus via increased insulin sensitivity, even while faced with lower concentrations of arterial plasma insulin and glucose concentrations and blood oxygen content, and that myocardial blood flow would increase to support cardiac metabolic function.
Materials and methods
Environmental conditions and surgical procedures
Institutional Animal Care and Use Committee (IACUC) approved studies were conducted in pregnant, 2 - to 3-year-old Columbia-Rambouillet ewes, each carrying a single fetus and were performed at the Perinatal Research Center at the University of Colorado Denver. All studies were in compliance with guidelines of the USDA, NIH, and the American Association for the Accreditation of Laboratory Animal Care.
Placental insufficiency-induced IUGR and control (C) ewes were generated as previously described.2,27 Surgery was performed at 124–126 days of gestational age (dGA, term = 148 days) with anesthesia and analgesia provided as previously described.20,28 Following laparotomy and hysterotomy, fetal polyvinyl catheters were placed into the ascending aorta via the right brachial artery, the right and left brachial vein, the left brachial artery, and the inferior vena cava and abdominal aorta via the femoral vein and artery. Through a left-sided thoracotomy fetal catheters were placed into the left atrium and coronary sinus.20 The pericardium remained open and the chest was closed in layers. An amniotic catheter also was placed for infusion of antibiotics and as a reference for fetal aortic blood pressure. Maternal catheters were placed in the femoral artery and vein via a small groin incision. All catheters were subcutaneously tunneled to a nylon mesh bag sutured onto the ewe’s flank and maintained as previously described.20,28 Ampicillin (500 mg) was infused into the amniotic cavity before the uterus was closed and procaine penicillin (6,000,000 U) was administered intramuscularly to the ewe at the time of surgery. The animals were allowed to recover for several days post-operatively prior to in vivo studies.
Blood pressure and heart rate measurement
On the day prior to metabolic study, fetal arterial blood pressure and heart rate were measured. Fetal abdominal aortic amniotic cavity pressure determinations were made using a computerized BioPac System (MP100A, Biopac Systems Inc., Santa Barbara, CA). Amniotic pressure readings were used as a reference for abdominal aortic pressures. Aortic systolic, diastolic, and mean pressures, as well as heart rate were measured every 10 min for 1 h.
Myocardial metabolic study
At 128–132 dGA basal and insulin-stimulated myocardial metabolism were measured. Insulin-stimulated myocardial metabolism was measured during an acute fetal hyperinsulinemic/euglycemic (HI/EG) clamp maintained over 2 h.10,29 Two baseline fetal right brachial and coronary sinus blood samples were obtained prior to tracer infusion. Fetal right brachial arterial and coronary sinus baseline concentrations of glucose, lactate, and blood gas values were determined in four consecutive samples during a 60 min basal metabolic period. Fetal right brachial arterial plasma insulin concentrations also were measured in the four samples. Following the basal period, the fetus received an infusion of human insulin (Humulin R; Eli Lilly, Indianapolis, IN) prepared in 0.9% wt/vol sodium chloride to provide a bolus of 45 mU/kg followed by a constant pharmacologic infusion of 3 mU/kg/min. An intravenous maternal dextrose infusion (50% wt/vol dextrose; Abbott Laboratories, North Chicago, IL) was adjusted every 10–20 min to maintain fetal euglycemia (±1–2 mg% of baseline).30,31 The HI/EG clamp was maintained for at least 60 min prior to steady-state blood draws. Four samples of fetal right brachial arterial and coronary sinus blood were analyzed as described for the basal period. An isovolumetric maternal blood transfusion was used to maintain baseline fetal hemoglobin concentration and hematocrit.
Left ventricular (LV) myocardial blood flow was measured using 15 µm diameter fluorescent-labeled, polystyrene microspheres (Triton Technologies, San Diego, CA).32 For each measurement 1.58 million microspheres were injected into the left fetal brachial vein catheter ensuring two-chamber cardiac mixing. Blood samples were withdrawn at 2 mL/min over 3 min from the fetal right brachial arterial catheter (tip in ascending aorta) at baseline and during the HI/EG clamp. Different color microspheres were used for basal and HI/EG clamp conditions. LV myocardium obtained at necropsy and blood were digested and filtration recovery of microspheres was performed using previously published methods (“Manual for Using Fluorescent Microspheres to Measure Regional Organ Perfusion,” Fluorescent Microsphere Resource Center, Univ. of Washington; http://fmrc.pulmcc.washington.edu/frmc/frmc.html).33 Sample fluorescence was determined using a Gemini XPS fluorometer (Molecular Devices, Sunnyvale, CA) at specified excitation and emission wavelengths. LV myocardial blood flow was calculated as:
where Qsample and Qref represent blood flows in mL/min of the LV and the reference blood sample withdrawal rate. Fsample and Fref are the specific fluorescent intensities measured for the LV and reference sample, respectively.
Myocardial glucose and oxygen calculations
All fetal blood samples were collected in EDTA-coated syringes and centrifuged (14,000 g) for 3 min at 4℃ and plasma glucose and lactate concentrations were determined immediately with a YSI Model 2700 Analyzer (Yellow Springs Instruments; Yellow Springs, OH). The remainder of the plasma was stored at −80℃. Blood gas, oxygen content, pH, and hematocrit were determined for two samples during the basal and HI/EG clamp periods each with an ABL 520 Hemoximeter (Copenhagen, Denmark). Fetal arterial plasma was analyzed for insulin concentration using an ELISA (ALPCO ovine insulin ELISA; Windham, NJ; intra- and inter-assay coefficients of variation <5%.34
The following equations were used:
Organ isolation and in vitro analysis
At the end of the metabolic study, the ewe received an intravenous infusion of ketamine (500 mg) and diazepam (5 mg). The ewe and fetus were euthanized by rapid intravenous infusion of Sleepaway pentobarbital solution (Fort Dodge Laboratories, Fort Dodge, IA) into the ewe. Fetal measurements and organ isolation were performed immediately. The heart was dissected into right ventricular free wall and LV plus septum. LV plus septum was snap frozen in liquid nitrogen and stored at −80℃.
Statistical analysis
Results are expressed as mean ± standard error of the mean. For measurements made only once, control and IUGR fetuses were compared with a two-tailed, unpaired Student’s t-test or a Mann-Whitney test for non-parametric data as appropriate. When comparing control and IUGR measurements at baseline and during the HI/EG clamp a two-way mixed models ANOVA was used which included terms for group (control or IUGR), period (basal or HI/EG clamp), group × period interaction, and a term to account for repeated measurements made within the same animal. Individual means were compared with Fisher’s least squares difference. Results were considered significant at P ≤ 0.05. Trends are noted when P < 0.1.
Results
Fetal growth, heart rate, and blood pressure measurements
Fetal age, heart rate, blood pressure, lengths, and weights are presented in Table 1. IUGR fetuses were 67% lighter than C fetuses (P < 0.05), as were IUGR heart weights (P < 0.05). As a percentage of total fetal weight, however, IUGR fetal heart weights were not different from C fetal heart weights.
Table 1.
Fetal growth and cardiac characteristics
| Control | IUGR | P value | |
|---|---|---|---|
| Gestational age (days) | 130.5 ± 1.3 | 129.2 ± 0.7 | 0.377 |
| Sex (% men) | 50 | 67 | |
| Crown rump length (cm) | 47.7 ± 2.0 | 40.8 ± 2.2* | 0.041 |
| Lower limb length (cm) | 35.7 ± 1.4 | 30.6 ± 1.9 | 0.058 |
| Fetal weight (g) | 3263.8 ± 189.1 | 2188.2 ± 262.7* | 0.008 |
| Heart (g) | 27.35 ± 1.89 | 18.95 ± 2.64* | 0.027 |
| Left ventricle + septum (g) | 10.12 ± 0.82 | 7.17 ± 1.01* | 0.047 |
| Right ventricle | 4.97 ± 0.48 | 3.37 ± 0.48* | 0.042 |
| Heart/fetal (%) | 0.84 ± 0.03 | 0.86 ± 0.05 | 0.694 |
| LVS/heart (%) | 37.13 ± 2.30 | 37.88 ± 1.02 | 0.772 |
| RV/heart (%) | 18.04 ± 0.94 | 18.17 ± 1.29 | 0.938 |
| LV + S/fetal weight (%) | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.635 |
| RV/fetal weight (%) | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.804 |
| Brain (g) | 47.0 ± 2.3 | 41.8 ± 2.3 | 0.144 |
| Brain/fetal (%) | 1.46 ± 0.10 | 2.01 ± 0.18* | 0.025 |
| Liver (g) | 105.8 ± 12.5 | 57.0 ± 6.1* | 0.005 |
| Liver/fetal (%) | 3.19 ± 0.17 | 2.63 ± 0.14* | 0.034 |
| Brain/liver (ratio) | 0.47 ± 0.07 | 0.78 ± 0.09* | 0.037 |
| Fetal heart rate | 171.2 ± 4.9 | 179.3 ± 5.4 | 0.289 |
| Systolic blood pressure (mmHg) | 61.6 ± 3.7 | 58.3 ± 2.6 | 0.477 |
| Diastolic blood pressure (mmHg) | 38.0 ± 1.5 | 35.3 ± 3.0 | 0.446 |
| Mean blood pressure (mmHg) | 47.8 ± 1.9 | 45.0 ± 3.0 | 0.463 |
Data are presented as mean ± SE and are analyzed with the Student's t-test or the Mann-Whitney test as appropriate.
Fetal metabolic measurements
Fetal arterial pH, blood gasses, hematocrit, plasma glucose, plasma lactate, and plasma insulin concentrations during the basal and HI/EG clamp periods are presented in Table 2. Although fetal arterial oxygen contents and plasma glucose and insulin concentrations tended to be lower in the IUGR fetuses, this trend did not reach statistical significance. Fetal arterial PaO2 and hemoglobin oxygen saturations were lower in the IUGR group (P < 0.05). During the HI/EG clamp period arterial plasma insulin concentrations increased to similar extents in both groups (P < 0.001) and the fetuses remained euglycemic.
Table 2.
Basal and hyperinsulinemic–euglyemic clamp fetal arterial and left ventricle characteristics
| Basal |
Hyperinsulinemic–euglycemic clamp |
ANOVA |
||||
|---|---|---|---|---|---|---|
| Control (n = 6) | IUGR (n = 6) | Control (n = 5) | IUGR (n = 5) | IUGR | Clamp | |
| Glucose (mmol/L) | 1.27 ± 0.10 | 0.94 ± 0.14 | 1.19 ± 0.15 | 1.09 ± 0.11 | ||
| Lactate (mmol/L) | 2.32 ± 0.24 | 4.89 ± 1.36 | 3.41 ± 0.75 | 7.87 ± 2.54# | P < 0.05 | |
| Insulin (ng/mL) | 0.16 ± 0.03 | 0.11 ± 0.03 | 7.46 ± 2.85# | 6.10 ± 1.71# | P < 0.001 | |
| pH (ratio) | 7.37 ± 0.01 | 7.36 ± 0.03 | 7.31 ± 0.01 | 7.23 ± 0.07# | P < 0.01 | |
| PaCO2 (mmHg) | 47.7 ± 1.7 | 48.4 ± 0.8 | 49.4 ± 1.5 | 53.2 ± 1.7# | P < 0.005 | |
| PaO2 (mmHg) | 20.0 ± 1.1 | 15.9 ± 1.2* | 19.7 ± 2.0 | 16.3 ± 0.7 | P < 0.05 | |
| Hemoglobin–oxygen saturation (%) | 56 ± 4 | 39 ± 5* | 45 ± 6# | 27 ± 4*# | P < 0.05 | P < 0.0001 |
| Arterial O2 content (mmol/L) | 3.40 ± 0.18 | 2.65 ± 0.40 | 2.71 ± 0.43# | 1.72 ± 0.28# | P < 0.001 | |
| Hematocrit (%) | 32 ± 1 | 35 ± 2 | 31 ± 1 | 32 ± 2# | P < 0.05 | |
Data are presented as mean ± SE and are analyzed with a mixed models ANOVA. * refers to significant differences between control and IUGR groups within a study period; # refers to significant differences between study periods within control or IUGR groups.
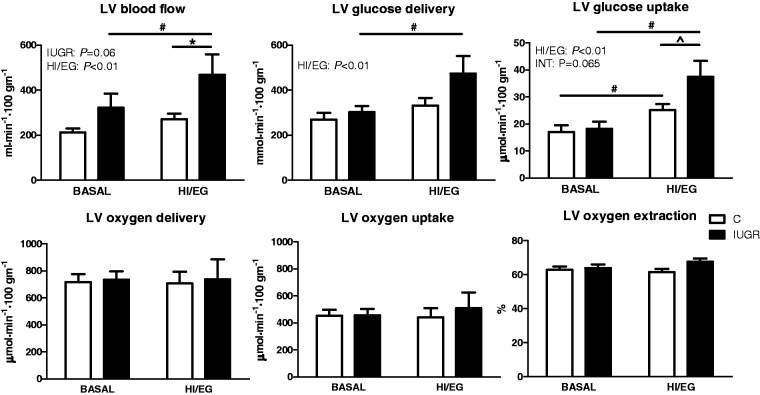
Basal LV myocardial blood flow was maintained in IUGR fetuses compared to controls (Figure 1a). Insulin increased LV myocardial blood flow (P < 0.01), but insulin-stimulated LV myocardial blood flow in IUGR fetuses was 73% greater than controls (Figure 1a). Similar to previous reports testing acute hypoxia, LV blood flow was inversely related to arterial oxygen concentration in both control and IUGR animals (Figure 2).35 Basal LV myocardial glucose delivery and uptake were not different between IUGR and control fetuses (Figure 1b,c). Insulin increased LV myocardial glucose delivery and uptake (P < 0.01), but to a greater extent in the IUGR fetuses compared to controls (Figure 1b,c). During basal and HI/EG clamp conditions LV myocardial oxygen delivery, oxygen uptake, and oxygen extraction efficiency were not different between groups (Figure 1d,e,f).
Figure 1.
Left ventricular (LV) myocardial blood flow and metabolic rates. LV myocardial blood flow (a), glucose delivery (b), glucose uptake rate (c), oxygen delivery (d), oxygen uptake rate (e), and oxygen extraction (f) were measured under basal conditions and during a hyperinsulinemic, euglycemic clamp (HI/EC) in control (C, white bar) and IUGR (black bar) fetal lambs during late gestation. Data are presented as mean ± SE. Significant effects from mixed models ANOVA on group (IUGR vs. C) and period (basal vs. HI/EC) with interaction between group and period (INT) are indicated. Differences in individual means are signified by an * refers to individual means comparisons between C and IUGR within a study period; # refers to individual means comparisons between study periods within C and IUGR, and ^ refers to a P = 0.06 for a difference between C and IUGR during the HI/EC period
Figure 2.
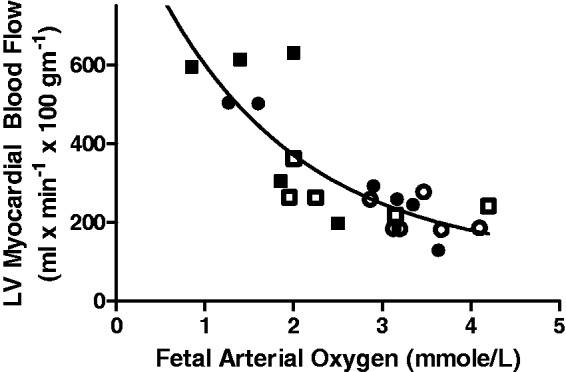
Inverse relationship between fetal arterial oxygen concentration and left ventricle (LV) myocardial blood flow. Open points are controls and closed are IUGR. Circles are during the basal period and squares during the hyperinsulinemic–euglycemic clamp. Data were fit to a second-order polynomial equation (r2 = 0.71)
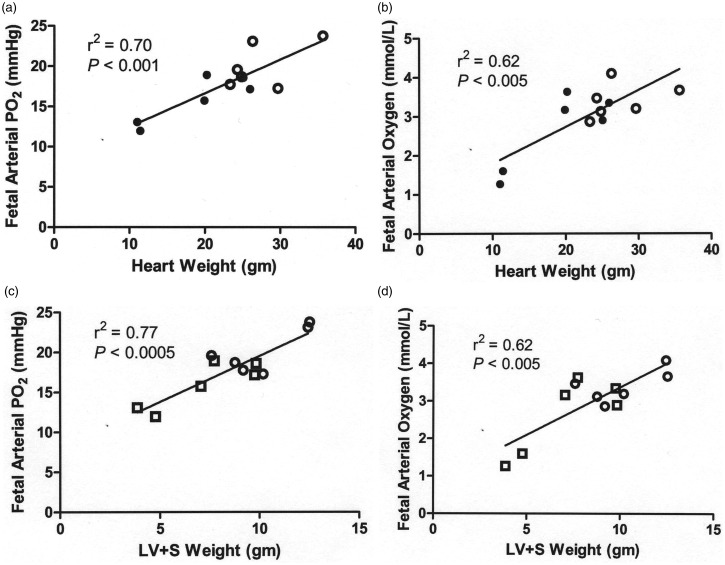
A positive correlation was demonstrated between fetal arterial oxygen values and cardiac weights, as shown in Figure 3 for fetal arterial partial pressure of oxygen (PO2, panels a and c) and fetal arterial oxygen concentrations (panels b and d) vs. heart weight (panels a and b) and the combined left ventricle and ventricular septum (panels c and d).
Figure 3.
Positive correlation between fetal arterial oxygen and cardiac weights. Open circles are controls and closed circles are IUGR. Fetal arterial partial pressure of oxygen (PO2) (a,c) and fetal arterial oxygen concentrations (b,d) were measured during the basal period and are plotted against heart weight (a,b) and the combined left ventricle and ventricular septum (LV + S) weight (c,d)
Discussion
We compared insulin-stimulated fetal LV myocardial blood flow, oxygen metabolism, and glucose metabolism between control and IUGR fetal sheep. We demonstrated increased myocardial blood flow as blood oxygen content declined and a direct correlation between fetal oxygenation and heart weight, similar to results from previous studies.24,36 We also noted a lack of observed differences in LV myocardial oxygen delivery, uptake, and extraction efficiency (under basal and clamp conditions), most likely because fetal heart weight to body weight ratios were similar between control and IUGR animals, with similarly increased LV myocardial blood flow for lower blood oxygen content in both. We also have shown maintained rates of umbilical oxygen uptake in the IUGR fetus when normalized to body weight, suggesting that substrate oxidation rates are preserved to maintain energy balance for survival.16 This is the first report, however, of increased myocardial glucose uptake in placental insufficiency-induced IUGR fetuses that exceeds that of normally grown control fetuses on a cardiac weight-specific basis during acute insulin stimulation in late gestation. Furthermore, we demonstrated that basal fetal myocardial weight-specific glucose metabolism is maintained during IUGR despite significant deficiencies in circulating concentrations of glucose, oxygen, and insulin, all critical regulators of myocardial metabolism and growth. Together these in vivo observations validate and extend our previous in vitro data that demonstrated up-regulated fetal cardiomyocyte glucose transporter and insulin receptor expression in IUGR fetuses, as well as higher myocardial glycogen content.20
The novel results in our current studies demonstrate that the fetal heart exposed to nutrient and oxygen deprivation from placental insufficiency appears to maintain myocardial energy supply in the IUGR condition via increased glucose uptake and metabolic response to insulin. These metabolic developments in the IUGR fetus are supportive of conditions conducive to myocardial function and growth. They are similar to whole fetal up-regulation of insulin sensitivity, which we have documented before,10–13 indicating a common response to reduced glucose supply that would sustain tissue and organ growth despite nutrient deprivation from placental insufficiency. It is important to note, however, that in contrast to maintained insulin sensitivity in the heart (data herein) and the whole fetus,10–13 our past investigations have shown evidence for increased hepatic glucose production that is relatively insulin resistant.37 The non-glucose sources of such hepatic glucose production are under investigation in our ongoing studies. Such hepatic insulin resistance and glucose production would prevent severe hypoglycemia from developing if placental insufficiency should be too severe, thereby maintaining glucose concentrations in the fetal plasma sufficient to support glucose supply to essential organs such as the heart. Such differences in insulin sensitivity in the PI-IUGR fetus, therefore, act in concert to promote critical fetal metabolism and function.
Other studies using similar in vivo methodology have documented the importance of glucose and lactate produced from glucose as the principal energy substrates for the fetal myocardium. Previous studies indicated that fetal cardiac glucose metabolism produces ATP production largely via glycolysis, while lactate produced from glucose produces ATP in the fetal heart largely by oxidation.38 This pattern of energy metabolism differs from the mature postnatal myocardium that under normal conditions primarily uses fatty acids and very little lactate or glucose for oxidative metabolism.19,39,40 Rates of myocardial glucose consumption in these studies were reported to be on the order of 17–22 µmol/min/100 g in the late gestation sheep fetus. We found that basal myocardial glucose uptake rates in both control (17.0 µmol/min/100 g) and IUGR animals (18.2 µmol/min/100 g) were similar to each other and to the previously reported rates. Our results show that glucose remains as a significant substrate for the fetal myocardium during IUGR despite significant fetal hypoglycemia and hypoinsulinemia, supporting our previous observations of maintained to up-regulated myocardial glucose transporter concentrations.20
The effect of insulin on fetal myocardial metabolism has been inadequately described. Earlier studies indicated that the fetal myocardium is resistant to insulin until the transition to extra-uterine life when developmental increases in glycolytic enzymes and glucose transporters allow for increased myocardial insulin sensitivity.41 More recently studies in late gestation fetal piglets (88% of gestation) have demonstrated an insulin responsive myocardium.42 In these studies, using an isolated fetal heart, a myocardial infusion of glucose and insulin was associated with increased myocardial glycogen deposition, comparable to our model.20
Our studies expand further the understanding of the effects of insulin on fetal myocardial glucose metabolism. We found that acute insulin stimulation increases glucose uptake from baseline by 48% in control animals. Importantly, insulin-stimulated myocardial glucose uptake increased more dramatically in IUGR fetuses, by 105%. We previously reported increased myocardial insulin receptor protein and plasma membrane associated glucose transporter isoform 4 (GLUT4) concentrations in IUGR LV myocardium.20 Coupled with our present data, these results show that the fetal ovine myocardium adapts to IUGR by increasing myocardial plasma membrane insulin receptor and insulin sensitive GLUT4 concentrations and maintains normal concentrations of GLUT1 concentrations, allowing for maintained basal glucose uptake rates and a greater response to acute insulin stimulation compared with control animals, despite decreased basal glucose and insulin concentrations. Increased whole body sensitivity to insulin for glucose metabolism has been demonstrated in many animal models of IUGR and is consistent with human IUGR newborns.17,43–48 This is the first study to report increased fetal myocardial insulin sensitivity in the IUGR fetal myocardium, consistent with the data for whole animal insulin sensitivity.10–13
In our model of fetal growth restriction, circulating fetal arterial concentrations of insulin and glucose are lower, while those of lactate, the primary myocardial substrate for oxidative metabolism, are higher than in normally grown fetuses of the same gestational age and in fetuses from IUGR models that are less severe in terms of placental insufficiency and degree and duration of fetal oxygen supply and relative fetal hypoxemia.6 Increased lactate concentrations and metabolic acidosis increase in IUGR fetuses in our model of placental insufficiency as the fetal oxygen and nutrient supplies decrease and blood flow is shunted away from the placenta and peripheral fetal tissues to supply the brain, heart, and adrenals; such conditions increase in later gestation as the fetus becomes progressively more hypoxic.16,27 Our model is relatively severe in this regard16 vs. other chronic models.6,14,27,32 Cardiac lactate uptake and metabolism are directly related to plasma lactate concentrations.19 It is possible, therefore, that the increased capacity for the IUGR fetal myocardium to transport glucose intracellularly to maintain glycolysis, glycogen synthesis, and glycogenolysis, also produces the increased amounts of lactate noted in our study, which then would be available for preferential oxidation for energy production.19,28,39,49 Such mechanisms also might support the unique myocardial response to fasting in which the myocardium increases glycogen content, unlike other organs such as skeletal muscle and the liver where glycogen levels, normally unchanged in the IUGR fetus from normal levels,11,50 decrease under fasting conditions.51
Our studies also highlight adaptive increases in coronary blood flow during late gestation IUGR, a period of chronic fetal hypoxemia and higher umbilical artery Doppler pulsatility and resistance indices. A previous study using our IUGR model showed that increased umbilical arterial pulsatility and resistance indices in the IUGR ovine fetus correlate with significantly higher fetal systemic blood pressures and placental vascular resistance.52 Such increases in systemic and umbilical arterial resistance also could lead to a compensatory increase in myocardial contractility, which could induce GLUT4 translocation and increase cardiac glucose metabolism. We found control fetal LV myocardial blood flows and oxygen extraction were similar to the values that have been reported in previous studies of fetal sheep at similar gestational ages.53–57 Basal LV myocardial blood flow and insulin-stimulated LV myocardial blood flow, however, were increased to a greater extent in the IUGR fetuses than in the controls. Increased coronary blood flow during placental insufficiency-induced IUGR is an adaptation that allows for maintained fetal cardiac nutrient and oxygen supply, as well as subsequent energy metabolism and growth.
Arterial oxygen concentrations are critical determinants of coronary blood flow in both mature and fetal animals. During episodes of acute hypoxemia in fetal sheep, cardiac output is redistributed to critical organs, including the heart, which maintains myocardial oxygen delivery and consumption.12,58–60 This observation was corroborated in our studies (Figure 1), showing an exponentially increasing rate of myocardial blood flow as blood oxygen concentrations decreased. Studies using fetal anemia in late gestation fetal sheep show that the coronary blood flow increase in response to low oxygen concentrations was secondary to an increased coronary conductance, an increased myocardial vascular diameter, and a maintained vascularity and coronary flow reserve.61 Furthermore, with fetal anemic hypoxia, cardiac enlargement relative to the body is produced by cardiomyocyte proliferation (but not hypertrophy, which can be partially reversed by transfusion), indicating that a key regulator of cardiac growth is blood oxygen content.23,62 Because fetal hypoxia tends to increase blood pressure, however, relative hypertension also could promote cardiac cellular proliferation and hypertrophy.63 In contrast, in the absence of fetal arterial hypertension, placental insufficiency from placental embolization in late gestation is associated with substantially depressed growth of the heart through suppressed proliferation and maturation of cardiomyocytes. This observation indicates that other characteristics of placental insufficiency, such as cardiomyocyte insulin sensitivity, glucose and amino acid supply and metabolism, oxygen availability to individual cardiomyocytes, and IGF-1 production and function, might have a prominent role in regulating myocardial metabolism, growth, and function independent of hypertension.24
The present studies also provide insight into how the IUGR fetal myocardium could maintain function despite reduced oxygen and nutrient supplies. This is important as previous studies in other animal models have shown that in utero undernutrition is associated with impaired cardiac muscle energetics, including decreased fatty acid oxidative capacity, decreased maximum oxidative phosphorylation rate, and decreased proton leak respiration.64 IUGR fetuses also show signs of cardiac dysfunction from early stages and a significant decline in cardiac systolic function together with the appearance of biochemical signs of cell damage as gestation proceeds and their IUGR condition worsens.65,66
In summary, we have identified mechanisms of fetal myocardial adaptations that allow for normal or even increased glucose uptake during late gestation IUGR, which maintain cardiac metabolism and thus might help maintain cardiac growth and function, despite significant limitations in fetal arterial glucose, oxygen, and insulin availability. The results also demonstrate that there are adaptations in myocardial vascular development and function in response to hypoxemia that allow for increased basal coronary blood flow during IUGR when placental oxygen delivery is reduced. Thus, while current evidence indicates that relative sparing of heart growth and function might have survival value for the fetus, the resulting differential fetal cardiac growth rates serve as a marker for compromised myocardial metabolism that portend cardiovascular diseases in later life.
Acknowlegment
WWH was supported by NIH T32 HD007186 (PI and PD) and NIH RO1 DK52138 (PI); JSB was supported by NIH T32 HD007186 (Trainee); PJR was supported by NIH R01 DK088139 and K08 HD060688 (PI); LDB was supported by NIH Building Interdisciplinary Careers in Women's Health K12 HD057022 (Scholar), the University of Colorado Center for Women’s Health Research, and NIH R01HD079404 (PI); RVA was supported by NIH HD043089 (PI); KLT was supported by NICHD P01 HD034430, NHLBI grant R01 HL102763, and the M. Lowell Edwards Endowment at OHSU.
Author contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review and editing of the manuscript. JSB, PJR, LDB, and WWH conducted the experiments. JSB, PJR, and WWH wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Creasy RK, Resnick R. Intrauterine growth restriction. In: Creasy RK, Resnick R. (eds). Maternal–fetal medicine, Philadelphia: WB Saunders, 1999, pp. 569–84. [Google Scholar]
- 2.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl 2003; 61: 183–94. [PubMed] [Google Scholar]
- 3.Hoffman C, Galan H. Assessing the “at risk” fetus: Doppler ultrasound. Curr Opin Obstet Gynecol 2009; 121: 161–66. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt MR, Kristian Sen SB, White P, Smerup M, Botker HE, Vogel H, Hjortdal V, Sorensen K, Redington A. Glucose-insulin infusion improves cardiac function during fetal tachycardia. J Am Coll Cardiol 2004; 43: 445–52. [DOI] [PubMed] [Google Scholar]
- 5.Baschaat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet Gynecol Surv 2004; 59: 617–27. [DOI] [PubMed] [Google Scholar]
- 6.Poudel R, McMillen IC, Dunn SL, Zhang S, Morrison JL. Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late-gestation IUGR sheep fetus. Am Physiol Regul Integr Comp Physiol 2015; 308: R151–62. [DOI] [PubMed] [Google Scholar]
- 7.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1297–305. [DOI] [PubMed] [Google Scholar]
- 8.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 2005; 147: 1488–97. [DOI] [PubMed] [Google Scholar]
- 9.Aldoretta PW, Hay WW., Jr Effect of glucose supply on ovine uteroplacental glucose metabolism. Am J Physiol 1999; 277: R947–58. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MS, Flowers-Ziegler J, Das UG, Hay WW., Jr Glucose transporter protein responses to selective hyperglycemia or hyperinsulinemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1545–52. [DOI] [PubMed] [Google Scholar]
- 11.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 2007; 293: E1716–25. [DOI] [PubMed] [Google Scholar]
- 12.Wallace J, Aitken RP, Milne JS, Hay WW., Jr Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol Reprod 2004; 71: 1055–62. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol 2002; 282: R1027–36. [DOI] [PubMed] [Google Scholar]
- 14.Owens JA, Falconer J, Robinson JS. Glucose metabolism in pregnant sheep when placental growth is restricted. Am J Physiol 1989; 26: R350–57. [DOI] [PubMed] [Google Scholar]
- 15.Wallace JM, Regnault TRH, Limesand SW, Hay WW, Jr, Anthony RV. Investigating the causes of low birth weight in contrasting ovine paradigms. J Physiol 2005; 565: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 2012; 303: E352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 2007; 148: 1350–8. [DOI] [PubMed] [Google Scholar]
- 18.Brownsey RW, Boone AN, Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res 1997; 34: 3–24. [DOI] [PubMed] [Google Scholar]
- 19.Bartelds B, Knoester H, Smid GB, Takens J, Visser GH, Penninga L, van der Leij FR, Beaufort-Krol GC, Zijlstra WG, Heymans HS, Kuipers JR. Perinatal changes in myocardial metabolism in lambs. Circulation 2000; 102: 926–31. [DOI] [PubMed] [Google Scholar]
- 20.Barry JS, Davidsen ML, Limesand SW, Galan HL, Friedman JE, Regnault TRH, Hay WW., Jr Developmental changes in ovine myocardial glucose transporters and insulin signaling following hyperthermia-induced intrauterine fetal growth restriction. Exp Biol Med 2006; 231: 566–75. [DOI] [PubMed] [Google Scholar]
- 21.Chaoui R. Coronary arteries in fetal life: physiology, malformations and the “heart-sparing effect”. Acta Paediatr Suppl 2004; 93: 6–12. [DOI] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kalache K, Edwin S, Blackwell S, Yoon BH, Tolosa JE, Silve M, Behnke E, Gomez R, Romero R. Subclinical myocardial injury in small-for-gestational-age neonates. J Matern–Fetal Neonatal Med 2002; 11: 385–90. [DOI] [PubMed] [Google Scholar]
- 23.Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louoey S, Davis LE, Faber JJ, Thornburg KL. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol 2010; 95: 131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louey S, Jonker SS, Giraud G, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 2007; 580: 639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation 2002; 105: 2058–63. [DOI] [PubMed] [Google Scholar]
- 26.Thornburg KL. The programming of cardiovascular disease. J Dev Orig Health Dis 2015; 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV. Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol 1999; 162: 433–42. [DOI] [PubMed] [Google Scholar]
- 28.Hay WW, Jr, Myers SA, Sparks JW, Wilkening RB, Meschia G, Battaglia FC. Glucose and lactate oxidation rates in the fetal lamb. Proc Soc Exp Biol Med 1983; 173: 553–63. [DOI] [PubMed] [Google Scholar]
- 29.Anderson MS, He J, Flowers-Ziegler J, Devaskar SU, Hay WW., Jr Effects of selective hyperglycemia and hyperinsulinemia on glucose transporters in fetal ovine skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1256–63. [DOI] [PubMed] [Google Scholar]
- 30.Hay WW, Jr, Meznarich HK. The effect of hyperinsulinaemia on glucose utilization and oxidation and on oxygen consumption in the fetal lamb. Q J Exp Physiol 1986; 71: 689–98. [DOI] [PubMed] [Google Scholar]
- 31.Hay WW, Jr, Meznarich HK, Sparks JW, Battaglia FC, Meschia G. The effect of insulin on glucose uptake in near-term fetal lambs. Proc Soc Exp Biol Med 1985; 178: 557–64. [DOI] [PubMed] [Google Scholar]
- 32.Dalshaug GB, Scholz TD, Smith OM, Bedell KA, Caldarone CA, Segar JL. Effects of gestational age on myocardial blood flow and coronary flow reserve in pressure-loaded ovine fetal hearts. Am J Physiol 2002; 282: H1359–69. [DOI] [PubMed] [Google Scholar]
- 33.Olson Ak, Protheroe KN, Segar JL, Scholz TD. Mitogen-activated protein kinase activation and regulation in the pressure-loaded fetal ovine heart. Am J Physiol Heart Circ Physiol 2006; 290: H1587–95. [DOI] [PubMed] [Google Scholar]
- 34.de Vries WB, Davidsen ML, Wilkening RB, Anthony RV, Regnault TR. Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy. Pediatr Res 2006; 60: 507–12. [DOI] [PubMed] [Google Scholar]
- 35.Ardehali A, Ports TA. Myocardial oxygen supply and demand. Chest 1990; 98: 699–705. [DOI] [PubMed] [Google Scholar]
- 36.Reller MD, Burson MA, Lohr JL, Morton MJ, Thornburg KL. Nitric oxide is an important determinant of coronary flow at rest and during hypoxemic stress in fetal lambs. Am J Physiol (Heart Circ Physiol 38) 1995; 269: H2074–81. [DOI] [PubMed] [Google Scholar]
- 37.Thorn SR, Brown LD, Rozance PJ, Hay WW, Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 2013; 62: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopashuck GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 2010; 56: 130–40. [DOI] [PubMed] [Google Scholar]
- 39.Fisher DJ, Heymann MA, Rudolph AM. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol 1980; 238: H399–405. [DOI] [PubMed] [Google Scholar]
- 40.Kodde IF, van der SJ, Smolenski RT, de Jong JW. Metabolic and genetic regulation of cardiac energy substrate preference. Comp Biochem Physiol A Mol Integr Physiol 2007; 146: 26–39. [DOI] [PubMed] [Google Scholar]
- 41.Ascuitto RJ, Ross-Ascuitto NT. Substrate metabolism in the developing heart. Semin Perinatol 1996; 20: 542–63. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt MR, Kristiansen SB, White P, Smerup M, Botker HE, Vogel M, Hjortdal V, Sorensen K, Redington A. Glucose-insulin infusion improves cardiac function during fetal tachycardia. J Am Coll Cardiol 2004; 43: 445–52. [DOI] [PubMed] [Google Scholar]
- 43.Aldoretta PW, Carver TD, Hay WW., Jr Ovine uteroplacental glucose and oxygen metabolism in relationship to chronic changes in maternal and fetal glucose concentrations. Placenta 1994; 15: 753–64. [DOI] [PubMed] [Google Scholar]
- 44.Carver TD, Hay WW., Jr Uteroplacental carbon substrate metabolism and O2 consumption after long-term hypoglycemia in pregnant sheep. Am J Physiol 1995; 269: E299–308. [DOI] [PubMed] [Google Scholar]
- 45.Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans 1996; 24: 341–50. [DOI] [PubMed] [Google Scholar]
- 46.Setia S, Sridhar MG, Bhat V, Chaturvedula L, Vinayagamoorti R, John M. Insulin sensitivity and insulin secretion at birth in intrauterine growth retarded infants. Pathology 2006; 38: 236–8. [DOI] [PubMed] [Google Scholar]
- 47.Setia S, Sridhar MG, Koner BC, Bobby Z, Bhat V, Chaturvedula L. Increased insulin sensitivity in intrauterine growth retarded newborns—do thyroid hormones play a role? Clin Chim Acta 2007; 376: 37–40. [DOI] [PubMed] [Google Scholar]
- 48.Bazaes RA, Salzar TE, Pittaluga E, Pena V, Alegria A, Iniguez G, Ong KK, Dunger DB, Mericq MV. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics 2003; 111: 804–9. [DOI] [PubMed] [Google Scholar]
- 49.Werner JC, Sicard RE. Lactate metabolism of isolated, perfused fetal and newborn pig hearts. Pediatr Res 1987; 22: 552–6. [DOI] [PubMed] [Google Scholar]
- 50.Brown L, Rozance PJ, Bruce JL, Friedman JE, Hay WW, Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 2015; 309: R920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider CA, Nguyen VT, Taegtmeyer H. Feeding and fasting determine postischemic glucose utilization in isolated working rat hearts. Am J Physiol 1991; 260: H442–8. [DOI] [PubMed] [Google Scholar]
- 52.Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB, Regnault TR. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 2005; 192: 272–9. [DOI] [PubMed] [Google Scholar]
- 53.Ralphe JC, Nau PN, Mascio CE, Segar JL, Scholz TD. Regulation of myocardial glucose transporters GLUT1 and GLUT4 in chronically anemic fetal lambs. Pediatr Res 2005; 58: 713–8. [DOI] [PubMed] [Google Scholar]
- 54.Bartelds B, Knoester H, Beaufort-Krol GC, Smid GB, Takens J, Zijlstra WG, Heymans HS, Kuipers JR. Myocardial lactate metabolism in fetal and newborn lambs. Circulation 1999; 99: 1892–7. [DOI] [PubMed] [Google Scholar]
- 55.Davis LE, Hohimer AR, Morton MJ. Myocardial blood flow and coronary reserve in chronically anemic fetal lambs. Am J Physiol 1999; 277: R306–13. [DOI] [PubMed] [Google Scholar]
- 56.Fisher DJ, Heymann MA, Rudolph AM. Fetal myocardial oxygen and carbohydrate metabolism in sustained hypoxemia in utero. Am J Physiol 1982; 243: H959–63. [DOI] [PubMed] [Google Scholar]
- 57.Fisher DJ, Heymann MA, Rudolph AM. Regional myocardial blood flow and oxygen delivery in fetal, newborn and adult sheep. Am J Physiol 1982; 243: H729–31. [DOI] [PubMed] [Google Scholar]
- 58.Cohn HE, Sacks EJ, Heymann, Rudolph AM. Cardiovascular responses to hypoxemia and academia in fetal lambs. Am J Obstet Gynecol 1974; 120: 817–24. [DOI] [PubMed] [Google Scholar]
- 59.Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 1998; 119: 717–23. [DOI] [PubMed] [Google Scholar]
- 60.Fisher DJ, Heymann MA, Rudolph AM. Fetal myocardial oxygen and carbohydrate consumption during acutely induced hypoxemia. Am J Physiol 1982; 242: H657–61. [DOI] [PubMed] [Google Scholar]
- 61.Davis L, Thornburg KL, Giraud GD. The effects of anaemia as a programming agent in the fetal heart. J Physiol 2005; 565: 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jonker SS, Scholz TD, Segar JL. Transfusion effects on cardiomyocyte growth and proliferation in fetal sheep after chronic anemia. Pediatr Res 2011; 69: 485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol 2007; 292: R913–9. [DOI] [PubMed] [Google Scholar]
- 64.Beauchamp B, Thrush AB, Quizi J, Antoun G, McIntosh N, Al-Dirbashi OY, Patti ME, Harper ME. Undernutrition during pregnancy in mice leads to dysfunctional cardiac muscle respiration in adult offspring. Biosci Rep 2015; 35: 00200–00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crispi F, Hernandez-Andrade E, Pelsers MM, Plasencia W, Benavides-Serralde JA, Eixarch E, Le Noble F, Ahmed A, Glatz JF, Nicolaides KH, Gratacos E. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol 2008; 199: 254 e-e41–e251 e258. [DOI] [PubMed] [Google Scholar]
- 66.Bahtiyar MO, Copel JA. Cardiac changes in the intrauterine growth-restricted fetus. Semin Perinatal 2008; 32: 190–3. [DOI] [PubMed] [Google Scholar]