Abstract
Generalized pustular psoriasis of pregnancy is a rare dermatosis with potential serious consequences for both the mother and fetus. Treatment is difficult and historically steroids were the mainstay of treatment. Cyclosporin has been used for a few cases resistant to steroids. We report our own experience of two cases of generalized pustular psoriasis of pregnancy. Cases of generalized pustular psoriasis of pregnancy need review by a dermatologist with experience of skin disorders in pregnancy. Both the fetus and mother need to be monitored closely when systemic illness occurs, as there is a risk of stillbirth. Maternal sepsis is a known complication of generalized pustular psoriasis of pregnancy. Cyclosporin, when used appropriately is effective and relatively safe.
Keywords: Generalized pustular psoriasis, cyclosporin, sepsis, pregnancy
Background
Skin changes in pregnancy can be classified as either physiological, specific dermatoses of pregnancy or other common skin conditions of pregnancy.1 The dermatoses of pregnancy are a group of skin disorders that only occur in pregnancy and the puerperium. They include pemphigoid gestationis, polymorphic eruption of pregnancy (PEP, previously known as PUPPS), and atopic eruption of pregnancy (Table 1). Pemphigoid gestationis usually presents in the third trimester with intense pruritis followed by urticarial papules and plaques progressing to bullae. PEP classically starts in the striae is intensely pruritic and is more common in late pregnancy and post-partum. Atopic eruptions of pregnancy include eczema, prurigo, and pruritic folliculitis.1
Table 1.
Dermatoses of pregnancy.
| Diagnosis | Trimester | Description of rash | Histopathology | Fetal risk | Treatment |
|---|---|---|---|---|---|
| Pemphigoid gestationis | 3rd | Vesicobullous eruption arising on urticated erythematous papules and plaques, periumbilical involvment | Subepidermal blister, positive immunofloresence | Prematurity, small for dates | Steroids, antihistamines |
| Polymorphic eruption of pregnancy | 3rd | Pruritic urticarial papules arising within striae, periumbilical sparing | Non-specific | No fetal risk | Steroids, antihistamines |
| Atopic eruption of pregnancy | 1st–2nd | Eczematous or papular lesions, background history of atopic dermatitis | Non-specific | No fetal risk | Steroids, antihistamines, UVB |
UVB: ultraviolet B.
Generalized pustular psoriasis is a severe inflammatory skin disease characterized by generalized erythematous plaques with sheaths of pustules usually sparing the hands, feet, and face. When this rare and dramatic rash occurs during pregnancy, it is called generalized pustular psoriasis of pregnancy (GPPP), previously known as impetigo herpetiformis. It differs from other dermatoses of pregnancy because frequently there are systemic symptoms of malaise and fever with elevated C-reactive protein and leucocytosis. Skin cultures should be performed to rule out secondary viral or bacterial infection and a skin biopsy for histopathology may be required. In severe cases, the rash may progress causing the skin to become erythrodermic, resulting in extensive loss of fluids and electrolytes, an inability to thermoregulate, with complications including maternal secondary infection and sepsis.2 The fetus needs to be closely monitored when systemic illness occurs as there is an increased risk of fetal anomalies, placental insufficiency, and still birth.3,4
In many of cases of GPPP, a pre-existing history or family history of psoriasis is well documented;2,5 however, some cases arise de novo.6–9 A literature review in 1995 found less than 350 cases reported in the literature, and10 thus experience in managing and treating this condition is limited. Typical onset is during the third trimester and the rash usually resolves in the post-partum period but may reoccur more severely in subsequent pregnancies. Associations with hypocalcaemia and hypoparathyroidism have been suggested, but with such a small number of cases reported, a clear association has yet to be proven.3,9
Treatment is difficult; historically oral and topical steroids were the mainstay of treatment. Other treatments have been described for cases resistant to steroids. These include narrow band ultraviolet B (UVB) therapy,11 psoralen ultraviolet A,12 clofazimine,13 and infliximab.14 Methotrexate and oral retinoids remain treatment options only in the post-natal period.15 Cyclopsorin can be used for patients refractory to steroids; however, there have been concerns regarding the safety of cyclosporin in pregnancy. Most of the experience in prescribing cyclosporin during pregnancy has been in patients with solid-organ transplants. A previous meta-analysis of cyclosporin use in pregnancy did not find the drug to be a human teratogen but suggested it may be associated with increased rates of prematurity, with a trend towards increased risk of congenital malformations in infants born to transplant recipients on cyclosporin.16 A recent review classified cyclosporin as compatible for use in pregnancy.1 It continues to be used extensively in organ-transplant recipients with good outcomes. There have been a few reports in the literature of cyclosporin use in GPPP.2,5,17–22
Case 1
In October 2014, a 34-year-old woman at 28 weeks’ gestation in her third pregnancy was transferred by the obstetric team from her local community hospital to St. James’s Hospital (a tertiary referral adult hospital) with GPPP. She had a background history of hypothyroidism. She had fairly extensive plaque psoriasis since childhood, but she had never attended a dermatologist and was using potent topical steroids over the years. Her psoriasis had improved during her two previous pregnancies. When her psoriasis worsened the year prior to her third pregnancy, she intensified her use of topical 0.5% clobetasol to 100 g/month. When she discovered she was pregnant, she stopped using her topical steroids.
During this pregnancy, pustules appeared at 24 weeks’ gestation and she intensified her use of topical steroids further. The flare in her psoriasis caused her significant distress. Her general practitioner prescribed oral flucloxacillin and arranged review at her local obstetric unit where she was admitted for further treatment with antibiotics on the assumption that the rash was “septic.” Testing at 28 weeks’ gestation for fetal wellbeing with ultrasound and cardiotocography (CTG) was reassuring. After several courses of antibiotics, she was referred to our Dermatology clinic.
Clinical examination revealed large erythematous plaques with sheaths of pustules studded peripherally on her lower limbs and anterior abdominal wall (Figure 1). A clinical diagnosis of GPPP on a background of longstanding plaque psoriasis was made. It had been aggravated by prolonged and intensified use of topical steroids. Skin cultures were negative and laboratory studies were significant for a mild lymphopenia 0.5 × 109/L (1.5–3.5), corrected calcium of 2.12 mmol/L (2.20–2.60), and elevated CRP 85 mg/L (<5). Thyroid function tests were normal. Histopathology of skin biopsies confirmed a diagnosis of GPPP (Figure 2). A decision was made not to commence oral steroids and to reduce the potency of her topical steroid. Antibiotics were discontinued. She declined admission and was instructed to rest, use copious amounts of emollient, and apply betamethasone diluted 1:8 once daily. Treatment with cyclosporin was discussed; however, when reviewed on a weekly basis, she remained stable and her rash gradually improved.
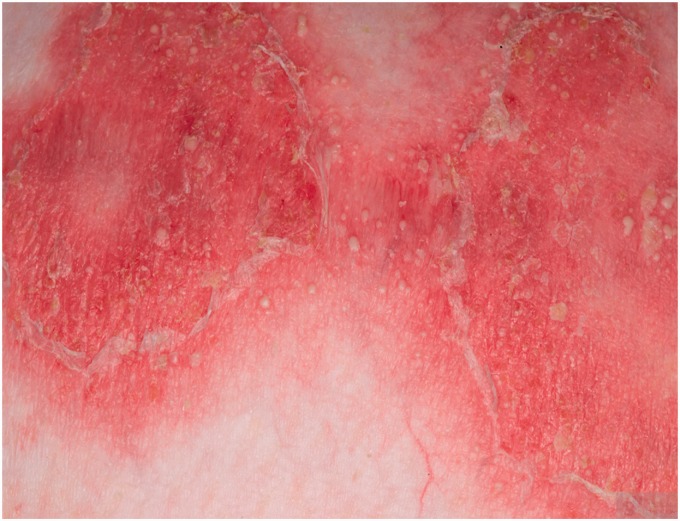
Figure 1.
Erythematous plaques studded with sterile pustules peripherally.
Figure 2.
Histopathology of a skin biopsy shows subcorneal pustules with neutrophilic infiltration.
At 31 + 4 weeks’ gestation, she was admitted to St James’s Hospital because her rash had deteriorated rapidly. Her skin was tender with intense erythema and pustulation involving most of her trunk, buttocks, and limbs. On admission, she was afebrile and hemodynamically stable. She was commenced on intravenous fluids, antibiotics, emollients, and topical steroids. Laboratory studies revealed a rise in CRP 187 mg/L (<5), lymphopenia 0.2 × 109/L (1.5–3.5), and an acute drop in albumin 23 g/L (35–50). A decision was made to commence cyclosporin within 24–48 h if there were no signs of improvement in her skin. That night she became systemically unwell developing a pyrexia of 38.3℃ (36.5–37.5), tachycardia 114 bpm, hypotension BP 77/41, and respiratory rate 20. Her serum lactate was raised at 2.58 mmol/L (0.36–1.39). A repeat septic screen was performed including blood cultures, skin cultures, high-vaginal swab, mid-stream urine, and throat swab. Fetal ultrasound scan showed that the fetus was appropriately grown for gestation with normal fetal heart rate and liquor volume. She was hemodynamically supported with intravenous fluids and broader antimicrobial cover with consultation from microbiology, gynecology, and intensive care. With her sudden clinical deterioration and rise in inflammatory markers, there was a grave concern of maternal sepsis. Skin cultures were initially reported to be suggestive for betahemolytic streptococcus; however, the final result from all cultures was negative. The fragility and tenderness of her abdominal skin precluded cardiotocograph tracing. The biophysical profile by ultrasound was assessed twice daily and was reassuring. Following 24 h of intravenous antibiotics, she was given steroids to assist fetal lung maturation (two doses of dexamethasone 6 mg intramuscularly 12 hourly) and magnesium sulfate was administered for fetal neuroprotection. Her skin continued to deteriorate with new sheaths of pustules, erythema, skin tenderness, and desquamation across her shoulders, breasts, and abdomen (Figure 3) and she continued to be pyrexial, tachycardic, and hypotensive. It was deemed inappropriate to commence cyclosporin at that time, and an emergency caesarean section was performed at 31 + 6 weeks’ gestation.
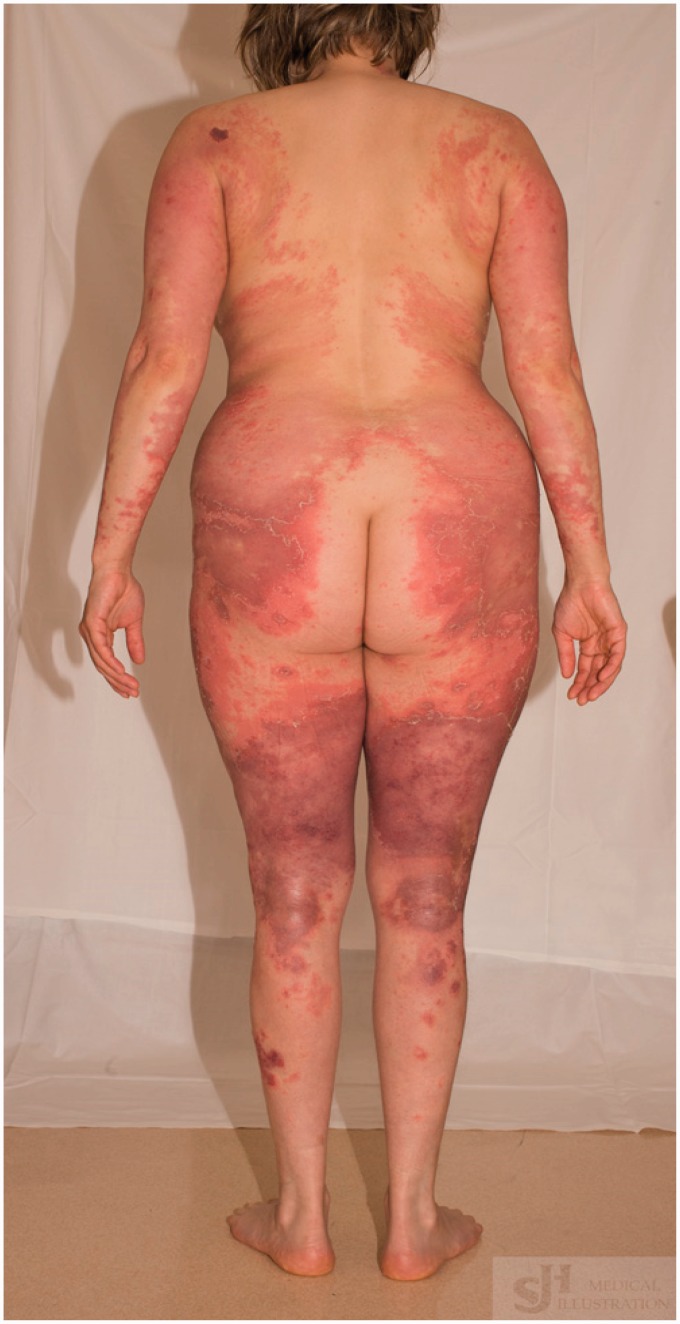
Figure 3.
Sheaths of pustules and erythema across abdomen and upper thighs.
A healthy baby boy weighing 1.9 kg was delivered in good condition and transferred immediately to the neonatal intensive care unit at the Coombe Women and Infants University Hospital (CWIUH). Our patient was transferred back to intensive care following delivery and commenced on oral cyclosporin 3 mg/kg. Once she was clinically more stable, she was transferred to the CWIUH to be near her baby. Her skin showed no further evidence of pustulation, but she remained erythrodermic. The dose of cyclosporin was increased to 3.5 mg/kg. She slowly recovered and was discharged home 10 days post-partum. The baby was discharged well four weeks post-natally. Since discharge, her psoriasis has almost totally cleared and her cyclosporin dose has been gradually reduced to 1.5 mg/kg.
Case 2
In September 2007, a 32-year-old woman with a background history of hypothyroidism and a 10-year history of intertriginous psoriasis presented with a 1-month history of generalized pustular psoriasis. Clinical examination revealed large annular non-tender erythematous plaques with sheaths of pustules and desquamation involving 70% body surface area. Skin biopsy was consistent with pustular psoriasis. She was admitted to hospital and treated with emollients, intravenous fluids, and commenced on oral cyclosporin 3 mg/kg. Laboratory studies revealed a mild rise in CRP 40 mg/L (<5) and normal thyroid function tests. Her rash improved and she was discharged home a few days later. Over the following eight months, the dose of cyclosporin was gradually reduced. Despite advice to avoid pregnancy while taking cyclosporine, she became pregnant and stopped the medication at six weeks’ gestation. Fetal anomaly scan was normal at 19 weeks’ gestation. She developed a flare of her pustular psoriasis involving her trunk and limbs (approximately 25% body surface area) at 21 weeks. She was treated with emollients, topical steroids, and narrow band UVB but flared after one exposure. Two weeks later, her GPPP flared causing a significant deterioration in her skin (Figure 4). At this point, she was recommenced on cyclosporin 3 mg/kg and her rash improved. Throughout her third trimester, she continued to have flares of her GPPP and her dose of cyclosporin was increased to 4 mg/kg. Serial ultrasounds showed normal fetal growth.
Figure 4.
Erythematous plaques with sheaths of pustules.
She had a spontaneous vaginal delivery of a healthy baby girl at full term with no maternal or neonatal complications. Her pustular psoriasis persisted post-partum, and two months post-delivery, 10% of her body surface area was affected. Eight months post-partum, she developed nephrotoxicity and hypertension secondary to cyclosporin. Cyclosporin was discontinued and adalimumab 40 mg fortnightly was introduced. The following year she developed psoriatic arthritis and methotrexate 10 mg weekly was added to her adalimumab.
To date, her psoriasis and psoriatic arthritis have been reasonably well controlled on adalimumab 40 mg weekly and methotrexate 10 mg weekly.
Discussion
Our two cases demonstrate different outcomes for these women with GPPP and their babies. Both young women had a background history of psoriasis and primary hypothyroidism.
Case 1 manifested as GPPP in the second trimester on a background of plaque psoriasis, improvement of psoriasis in her two previous pregnancies, and probable aggravation of her GPPP by withdrawal of potent topical steroids during her pregnancy. Since generalized pustular psoriasis is such a rare disorder and to the non-dermatologist it looks like a bacterial dermatitis with pustules and desquamation, she was treated with oral or intravenous antibiotics on four different occasions. By the time she was assessed in our dermatology unit, she was distressed and exhausted. She was reluctant to commence cyclosporin and she initially responded reasonably well to topical steroid treatment with some improvement in her skin and inflammatory markers. She was managing at home with good family support, reluctant to be admitted to hospital at any point, and not eager to commence on cyclosporin despite appropriate counselling. Her skin deteriorated suddenly in the third trimester at 31/40 weeks’ gestation. Given how systemically unwell she was with a high suspicion of maternal sepsis, we were reluctant to use systemic steroids and cyclosporin at this time. She underwent emergency caesarean section at 31 + 6 weeks’ gestation. Maternal bacteremia was never confirmed. Reflecting upon her treatment, perhaps if cyclosporin had been commenced earlier in the pregnancy, better control of her GPPP could have allowed her pregnancy to proceed to term.
In Case 2, the mother was known to have generalized pustular psoriasis prior to pregnancy and had been treated successfully with cyclosporin. When a generalized flare of her pustular psoriasis developed in her first trimester of pregnancy, she was closely monitored by the dermatology team throughout her pregnancy and re-commenced on cyclosporin during her second trimester when her rash flared. Her GPPP responded reasonably well to cyclosporin. Her pregnancy continued to term and she delivered a healthy baby girl by spontaneous vaginal delivery at full term. Her pustular psoriasis persisted post-partum requiring continuation of cyclosporin. She developed nephrotoxicity on cyclosporin and was subsequently changed to adalimumab along with methotrexate because she developed psoriatic arthritis. She remains well six years later.
Treatment with topical and oral steroids has been the standard treatment in the past for GPPP. However, such treatment is counter intuitive as steroids, both topical and oral, are known to precipitate pustular psoriasis. As GPPP is very rare, there are not enough clinical cases to demonstrate statistical significance for different treatment options. To date, cyclosporin has been used in a number of cases with good outcomes.2,5,17–22 Despite placental transfer which is thought to be dose related, cyclosporin appears to be safe.1 Most of the studies on cyclosporin in pregnancy have been in patients with renal transplants. There have not been any specific birth defects attributed to cyclosporin, but it has been noted that there is a risk of premature labor and low birth weight.23–25 It is difficult to determine whether these risks are due to the drug or due to the fact that patients receiving cyclosporin in pregnancy have complicated health conditions. Cyclosporin is not recommended while breastfeeding, as there are varying results of infant exposure to the drug and there is no long-term data regarding the effects to infants.15 All patients on cyclosporin must be monitored closely for hypertension and renal impairment. Adalimumab is classified as category B during pregnancy.15 The experience with adalimumab in pregnancy is limited to a few case reports which did not show it to be harmful.26,27
These two cases perfectly illustrate the challenges posed by severe GPPP. In Case 1, the GPPP flared to such an extent that the mother was pyrexial, hypotensive and tachycardic, and sepsis could not be ruled out. Maternal sepsis is a serious medical emergency that carries a substantial risk of maternal and fetal mortality. Sepsis was the leading direct cause of maternal mortality in the most recent MBRRACE report28 and Case 1 was appropriately investigated and treated for suspected severe sepsis. No infective organisms were subsequently cultured. The decision to deliver was made by a multidisciplinary team with careful consideration of the risks and benefit to both mother and fetus. In retrospect, earlier commencement of cyclosporin may have prevented such a severe flare. In Case 2, the patient was already known to the dermatology service and cyclosporin was recommenced early when a flare occurred, allowing the disease to stablized sufficiently so the pregnancy could progress to term.
There are a number of variants of pustular psoriasis. These can be classified into localized or generalized, depending on the location of the pustules.29,30 Localized pustular psoriasis can be palmoplantar, where sterile pustules develop on the palms and soles. The other localized form is acrodermatitis continua of hallopeau, where pustules are located on the tips of fingers and toes. There is a risk of progression to generalized pustular psoriasis, where sheets of sterile pustules develop across the body in various configurations. They can be studded on the peripheries of psoriatic plaques, as isolated pustules, or on a background of generalized erythema.29,30
When approaching a patient with a generalized pustular rash, the differential diagnosis includes acute generalized exanthemous pustulosis (AGEP), pemphigoid gestationis, and subcorneal pustular dermatosis.20 It is important to take a thorough history and ask about any family history of psoriasis. Examination of the skin should include the hair and nails, looking for scalp psoriasis, or psoriatic nail changes such as pitting or onycholysis. Swabs of the pustules should be taken for culture and sensitivity to exclude an infectious cause. AGEP is a drug-induced pustular rash and is usually associated with an infiltration of eosinophils on histology. Pemphigoid gestationis usually occurs late in pregnancy and consists of an intense pruritis followed by urticarial papules and plaques progressing to bullae. Pemphigus vulgaris consists of bullae which are often flaccid and burst easily leaving erosions. There may be associated oral mucosal involvement. Subcorneal pustular dermatosis is another important differential; however, this rash usually develops over several months and patients are not systemically unwell. Dermatitis herpetiformis consists of grouped pruritic vesicular lesions on the lateral aspects of the arms and buttocks, but patients are generally well.20 Histopathology can help confirm the clinical diagnosis of a pustular rash and rule out other differential diagnosis. On histopathology in pustular psoriasis, there is evidence of spongiform subcorneal sterile pustules filled with neutrophils. Often there is parakeratosis with neutrophils located between keratinocytes.30
Important triggering factors for pustular psoriasis include withdrawal of potent topical steroids, pregnancy, infection, and medications such as lithium, aspirin, and betablockers. IL36RN is a recessive mutation which has been reported to occur in a number of patients with pustular psoriasis.31,32 We tested our two patients for this mutation and both were found to be negative.
Factors triggering GPPP such as hypoparathyroidism and hypocalcaemia have been suggested.2 Both of our patients had a background history of primary hypothyroidism. They were adequately treated with levothyroxine, demonstrating normal range thyroid function during their flares of GPPP. We are not aware of any reports suggesting an association between hypothyroidism and GPPP.
In summary, GPPP is a rare dermatosis with potential serious consequences for mother and child. Maternal sepsis is a known complication and must be recognized and managed appropriately. Erythrodermic patients must be supported with good nursing care, fluid and electrolyte replacement, adequate analgesia, antibiotics for secondary infections, and close fetal monitoring. Treatment of GPPP is difficult, and historically steroids were the mainstay of treatment, with a few case reports suggesting steroid-sparing agents. There is a an increased risk of cleft palate when steroids are used in the first trimester.33 Both oral and high potency topical steroids during pregnancy are associated with low birth weight.34,35 Cyclosporin has been used for cases resistant to steroids and when used appropriately it is effective and relatively safe in treating GPPP.
Acknowledgements
The authors would like to acknowledge the medical team and nursing staff in St James’s Hospital as well as the nursing staff in The Coombe Women and Infants Maternity Hospital.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Written informed consent for patient information and images for publication was obtained.
Guarantor
AF
Contributorship
All authors have consented to the publication and made a substantial contribution to the care of the patients discussed.
References
- 1.Vaughan-Jones SA, et al. Skin disease in pregnancy. Br Med J 2014; 348: 3489–3489. [DOI] [PubMed] [Google Scholar]
- 2.Shaw CJ, Wu P and Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep 2011: bcr0220113915. [DOI] [PMC free article] [PubMed]
- 3.Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol 2006; 24: 101–104. [DOI] [PubMed] [Google Scholar]
- 4.Gambling D, Douglas MJ, McKay RSF (eds). Obstetric anesthesia and uncommon disorders. 2nd ed. Cambridge: Cambridge University Press, 2008, p.349.
- 5.Patsatsi A, Theodoridis TD, et al. Cyclosporine in the management of impetigo herpetiformis: a case report and review of the literature. Case Rep Dermatol 2013; 5: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol 2014; 53: 676–684. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths CEM, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007; 156: 258–262. [DOI] [PubMed] [Google Scholar]
- 8.Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermtol 1968; 80: 771–793. [DOI] [PubMed] [Google Scholar]
- 9.Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin 2013; 31: 405–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf Y, Groutz A, Walman I, et al. Impetigo herpetiformis during pregnancy: case report and review of the literature. Acta Obstet Gynecol Scand 1995; 74: 229–232. [DOI] [PubMed] [Google Scholar]
- 11.Vun YY, Jones B, et al. Generalized pustular psoriasis of pregnancy treated with narrowband UVB and topical steroids. J Am Acad Dermatol 2006; 54(2 Suppl): S28–S30. [DOI] [PubMed] [Google Scholar]
- 12.El-Din SMM, Rehak A, Abdel-Hafez K, et al. Impetigo herpetiformis. Report of a case treated with photochemotherapy (PUVA). Dermatol Monatsschr 1982; 168: 44–48. [PubMed] [Google Scholar]
- 13.Zabel J, Erenski P. Clofazimine in the treatment of impetigo herpetiformis. Przegl Dermatol 1984; 71: 161–163. [PubMed] [Google Scholar]
- 14.Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology 2010; 220: 71–76. [DOI] [PubMed] [Google Scholar]
- 15.Bae YS, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2012; 67: 459–477. [DOI] [PubMed] [Google Scholar]
- 16.Oz BB, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation 2001; 71: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 17.Imai N, Watanabe R, Fujiwara H, et al. Successful treatment of impetigo herpetiformis with oral cyclosporine during pregnancy. Arch Dermatol 2002; 138: 128–129. [DOI] [PubMed] [Google Scholar]
- 18.Luan L, Hans S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther 2014; 27: 174–177. [DOI] [PubMed] [Google Scholar]
- 19.Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol 2009; 75: 638–638. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor R, Kapoor JR. Cyclosporine resolves generalized pustular psoriasis of pregnancy. Arch Dermatol 2006; 142: 1361–1375. [DOI] [PubMed] [Google Scholar]
- 21.Brightman L, et al. Third-trimester impetigo herpetiformis treated with cyclosporine. J Am Acad Dermatol 2007; 56: S62–S64. [DOI] [PubMed] [Google Scholar]
- 22.Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporine A. Br J Dermatol 2000; 142: 582–584. [DOI] [PubMed] [Google Scholar]
- 23.Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in renal transplant recipients with Sandimmune. Transplant Proc 1997; 29: 2480–2480. [DOI] [PubMed] [Google Scholar]
- 24.Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation 2001; 71: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 25.Armenti VT, McGrory CH, Cater JR, et al. Pregnancy outcomes in female transplant recipients. Transplanation Proc 1998; 30: 1732–1734. [DOI] [PubMed] [Google Scholar]
- 26.Berthelot JM, et al. Exposition to anti-TNF drugs during pregnancy: outcome of 15 cases and review of literature. Joint Bone Spine 2009; 76: 28–34. [DOI] [PubMed] [Google Scholar]
- 27.Mishkin DS, Van Deinse W, Becker JM, et al. Successful use of adalimumab (Humira) for Crohn’s disease in pregnancy. Inflamm Bowel Dis 2006; 12: 827–828. [DOI] [PubMed] [Google Scholar]
- 28.Knight M, Kenyon S, et al. MBRRACE-UK report, Oxford: NPEU, University of Oxford, 2014. [Google Scholar]
- 29.A du Vivier. Atlas of clinical dermatology. 4th ed. Philadelphia, USA: Elsevier Saunders, 2013, pp.82–85.
- 30.Bolognia JL, et al. Dermatology. 3rd ed. Philadelphia, USA: Elsevier Saunders, 2012, pp.140–142.
- 31.Onoufriadis A, Simpson MA, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet 2011; 89: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain S, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol 2015; 135: 1067–1070. [DOI] [PubMed] [Google Scholar]
- 33.Park-Wyllie L, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000; 62: 385–392. [DOI] [PubMed] [Google Scholar]
- 34.Crowther CA, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Eng J Med 2007; 357: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 35.Chi CC, Wang SH, Kirtschig G, et al. Systematic review of the safety of topical corticosteroids in pregnancy. J Am Acad Dermatol 2010; 62: 694–705. [DOI] [PubMed] [Google Scholar]