Abstract
There is no standard method by which to establish a right one-lung ventilation (OLV) model in rabbits. In the present study, a novel method is proposed to compare with two other methods. After 0.5 h of baseline two-lung ventilation (TLV), 40 rabbits were randomly divided into sham group (TLV for 3 h as a contrast) and three right-OLV groups (right OLV for 3 h with different methods): Deep intubation group, clamp group and blocker group (deeply intubate the self-made bronchial blocker into the left main bronchus, the novel method). These three methods were compared using a number of variables: Circulation by heart rate (HR), mean arterial pressure (MAP); oxygenation by arterial blood gas analysis; airway pressure; lung injury by histopathology; and time, blood loss, success rate of modeling. Following OLV, compared with the sham group, arterial partial pressure of oxygen and arterial hemoglobin oxygen saturation decreased, peak pressure increased and lung injury scores were higher in three OLV groups at 3 h of OLV. All these indexes showed no differences between the three OLV groups. During right-OLV modeling, less time was spent in the blocker group (6±2 min), compared with the other two OLV groups (13±4 min in deep intubation group, P<0.05; 33±9 min in clamp group, P<0.001); more blood loss was observed in clamp group (11.7±2.8 ml), compared with the other two OLV groups (2.3±0.5 ml in deep intubation group, P<0.001; 2.1±0.6 ml in blocker group, P<0.001). The first-time and final success rate of modeling showed no differences among the three OLV groups. Deep intubation of the self-made bronchial blocker into the left main bronchus is an easy, effective and reliable method to establish a right-OLV model in rabbits.
Keywords: one-lung ventilation, rabbits, methods
Introduction
One-lung ventilation (OLV) has become a standard procedure for many interventions in thoracic surgery (1), and is particularly mandatory in thoracoscopic surgery. Since it is not physiological, OLV may lead to lung injury in both lungs (2–4). This is one of the causes of postoperative pulmonary complications which contribute substantially to the risks associated with surgery and anesthesia, with major impact on health outcomes and are a financial burden to health systems (5). Therefore, there are numerous animal and clinical studies investigating OLV.
Rabbits, pigs, dogs and rats are commonly used animals in this area of study (6–9). Due to their relatively low cost and convenience in raising and operation, rabbits are very popular among researchers. Rabbits are used in OLV modeling in vivo and in vitro (isolated or perfused lung model) (10,11). As the right lung is larger than the left lung, oxygenation is better during right-OLV than left-OLV (12), therefore right-OLV is often selected when oxygenation is concerned. In our pre-experimental work, it was relatively easy to build a left-OLV model based on the present method of deeply intubating the endotracheal tube into the left main bronchus. Therefore, in the present study we specifically focused on right-OLV.
Several different methods are used to build in vivo right-OLV model in rabbits, including deeply intubating the endotracheal tube into the right main bronchus (13), clamping the left main bronchus with a clip (14) and other methods, such as inserting an artificial double-lumen endotracheal tube (DLT) into the trachea, and inserting a bronchial blocker through the ventilation tube into the left primary bronchus (6,15). However, these methods each have disadvantages. The objective of the present study was to compare a novel method with two other methods in a number of variables: Circulation, oxygenation, airway pressure, lung injury caused by OLV and time, blood loss and success rate of modeling.
Materials and methods
Experimental protocol
Forty healthy New Zealand white rabbits (Jinling Shuto Field, Nanjing, China) were studied following approval by the Ethics Review Committee for Animal Experimentation of the Affiliated Hospital of Nanjing Medical University (Nanjing, China).
Induction of anesthesia was by intravenous injections (4 ml/kg, i.v.) of 30% urethane (Shanghai Green Analysis of Chemical Technology Co., Shanghai, China), after which animals were placed on an operating table (DWV-II; On Haishao Feng Laboratory Animal Equipment Co., Ltd, Shanghai, China) in the supine position. Following initial anesthesia, urethane was maintained at 5–8 ml/kg/h and tracheotomy was performed aseptically at the level of 2–3 tracheal cartilage using a midline incision of the neck. After infiltration with 1 ml lidocaine (2%; China Otsuka Pharmaceutical Co., Ltd., Tianjin, China) and intravenous injection of cisatracurium (2 mg/kg; Jiangsu Hengrui Medical Co., Ltd., Lianyungang, China), the trachea was intubated with a 3.0-mm uncuffed endotracheal tube (Mallinckrodt Medical, Athlone, Ireland), to 4 cm deep. Breathing sound and peak pressure (Ppeak) were checked and the left lung was observed through a hole (0.5 cm) between the 7–8 ribs of the left thoracic wall using a fiberscope (FI-9RBS Portable Intubation Fiberscope; Pentax Canada, Inc., ON, Canada). Then the trachea was tied to prevent air leak and 0.5 h of baseline TLV (DW3000B small animal ventilation machine; ZS Dichuang Science and Technology Development Co., Ltd., Beijing, China) was started in all animals. Rabbits received volume controlled ventilation with the following setting: Tidal volume (VT) of 10 ml/kg, respiratory rate (RR) of 40 breaths/min, inspiratory:expiratory ratio (I:E) of 1:2, inspired oxygen fraction (FiO2) of 0.6 and no PEEP. The right femoral artery was cannulated (22, 24 G, BD arterial cannula; BD Biosciences, Franklin Lakes, NJ, USA) for arterial blood pressure and arterial blood gas analysis.
The room temperature was set at 25°C. Maintenance anesthesia consisted of 30% urethane 1 ml/kg/h after tracheotomy and cisatracurium 2 mg/kg/h was administered to achieve muscle relaxation. Ringer's solution was infused at 10 ml/kg/h continuously throughout the procedure. Depth of anesthesia was assessed according to changes in vital signs (PM-9000 Express Patient Monitor; Mindray Medical International Limited, Shenzen, China) as primary criteria.
After 30 min of baseline TLV, rabbits were randomly divided into four groups: Sham group (TLV for 3 h to be a contrast), deep intubation group (right-OLV for 3 h by deeply intubating the tube into the right main bronchus), clamp group (right-OLV for 3 h by clamping the left main bronchus) and blocker group (right-OLV for 3 h by deeply intubating the self-made bronchial blocker into the left main bronchus). Breathing sound and Ppeak were checked and the collapsed left lung was observed before and after rabbits were turned to the right-lateral position, which was used to simulate surgical position. Subsequently, breath sound, Ppeak and the collapsed left lung were checked every 30 min.
After arterial blood gas analysis at the end point (3 h of OLV), anesthesia was deepened with boluses of urethane (30%; 3 ml), the animal was euthanized by exsanguination. After the position of the deeply intubated endotracheal tube, the clamp, self-made bronchial blocker, collapsed left lung and ventilated right lung were checked, specimens were surgically obtained immediately which includes trimmed lung-tissue block concerning ~1.5×1×0.3 cm3 at the same location of different lung individually. The tissue block was fixed in 10% neutral-buffered formaldehyde and embedded in paraffin.
Data collection
Arterial blood samples were measured (i-STAT portable clinical analyzer; Abbott Laboratories, Inc., Lake Bluff, IL, USA) at 30 min of TLV (T=0); 10 min (T=10 min), 30 min (T=30 min), 1 h (T=1 h), 2 h (T=2 h), 3 h (T=3 h) of OLV (arterial blood gas analysis was measured at same time after changing to the right-lateral position for the sham group).
The length of the left and right main bronchus and the trachea from the carina to the tracheal cut were recorded. During the right-OLV modeling, time, blood loss, first-time and final success rate of right-OLV modeling were all recorded.
The paraffin-embedded tissue blocks were sliced to 5 µm sections. Routine hematoxylin and eosin staining was performed on each section for histopathological examination, and were evaluated by two individual pathologists being ignored by experimental protocols. Random fields from each slide were digitally imaged for qualitative analysis (BX40; Olympus Corporation, Tokyo, Japan) and photographed using a Panasonic WV-CP450 camera (Panasonic Corporation, Osaka, Japan). Lung injury was scored on the basis of the following four morphological features: i) Alveolar congestion; ii) hemorrhage; iii) infiltrations or aggregations of neutrophils in airspace or the vessel wall; and iv) thickness of the alveolar wall/hyaline membrane formation. Each item was graded according to a five-point scale, as described previously: 0=Minimal (little) damage, 1=mild damage, 2=moderate damage, 3=severe damage and 4=maximal damage (16).
Right-OLV modeling
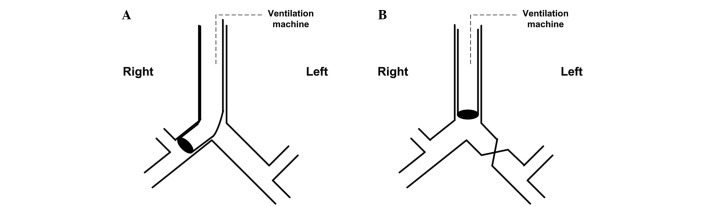
Deep intubation group (Fig. 1A): Loosen the tie of the trachea, turn the rabbit's head to the left, deeply intubate the endotracheal tube to the right main bronchus until feeling resistance, then slightly regulate the tube position by checking breathing sound, Ppeak and observing the collapsed left lung. Finally, tie the trachea again to prevent air leak and tube movement.
Figure 1.
Methods used in (A) deep intubation group: Deeply intubate the tube into the right main bronchus; (B) clamp group: Clamp the left main bronchus.
Clamp group (Fig. 1B): Perform thoracotomy by cutting between the fourth and fifth ribs, separate the left main bronchus, then completely clamp the left main bronchus with a clip, determine the effect of the clamp through checking breathing sound, Ppeak and observing the collapsed left lung.
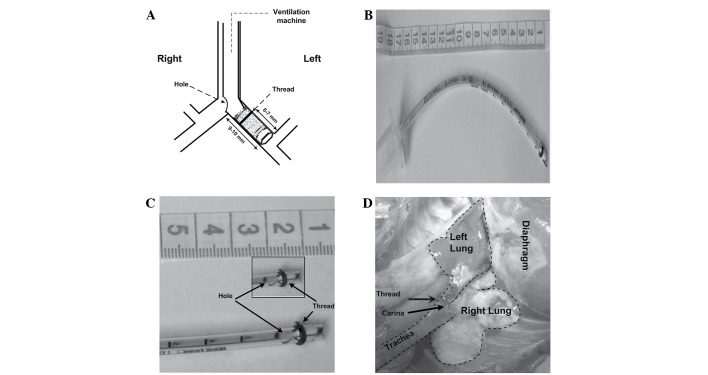
Blocker group (Fig. 2): Loosen the tie of the trachea, turn the rabbit head to the right, deeply intubate the self-made bronchial blocker to the left main bronchus until resistance is felt, then slightly regulate the tube position by checking breathing sound, Ppeak and observing the collapsed left lung. Finally, tie the trachea again to prevent air leak and tube movement. The bronchial blocker was made as follows: First, an uncuffed endotracheal tube (3.0 mm, endotracheal tube; Mallinckrodt Medical, Athlone, Ireland) was blocked at the tip with a 5-mm endotracheal tube core wrapped up with several levels of water-repellent film; Second, a leaking test was conducted. Connect the blocked tube to the ventilation machine with the following settings: VT of 10 ml/kg, RR of 40 breaths/min, I:E of 1:2, FIO2 of 0.6 and no PEEP. No bubble was found when the blocked tube was put in the water while the small animal ventilation machine showed the Ppeak of 4.2 cm H2O (~2x of the highest Ppeak observed in the experiment); Third, thread (3–0, 2 metric, SA84G, Mersilk, silk braided non-absorbable suture; Johnson & Johnson Medical China Ltd., Shanghai, China) was wound around the blocker at 6–7 mm from the tip to make the blocker attach tightly to the left bronchial wall and an elliptical hole (4×3 mm) was made (9–10 mm from the head) to be adjacent to the opening of the right main bronchus.
Figure 2.
Representative photographs of the self-made bronchial blocker. (A) Dimensions of the self-made bronchial blocker: Uncuffed endotracheal tube (3.0-mm) was blocked at the tip using a 5-mm endotracheal tube core wrapped up with several layers of water-repellent film, 3–0 silk thread was wound around the blocker at 6–7 mm from the tip and an elliptical hole (4×3 mm) was made at 9–10 mm from the head to face the opening of the right main bronchus. (B) Image of the self-made bronchial blocker. (C) Details of the self-made bronchial blocker. (D) Right one-lung ventilation model built by self-made bronchial blocker in vivo, the thread can be seen in the left main bronchus beyond the carina, the left lung was collapsed and the right lung was ventilated.
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation for quantitative variables (all the results except ALI scores and success rate), and ALI scores were presented as the mean rank. Quantitative variables were analyzed by general linear model followed by multivariate test. Lung injury scores were analyzed by general linear model followed by univariate test after rank transform. First-time and final success rate of modeling were analyzed by crosstabs with χ2. P<0.05 was considered to indicate a statistically significant difference.
Results
Rabbit treatment and anatomy index
The rabbits in the four groups had similar weight, total dosage of urethane, cisatracurium and Ringer's solution (Table I). Based on anatomy, the rabbits in four groups had similar length from the carina to the tracheal cut (2–3 tracheal cartilage), and similar length of the left and right main bronchus (Table II).
Table I.
Weight, total usage of urethane, cisatracurium and Ringer's solution.
| Group | Weight (kg) | Urethane (g) | Cisatracurium (mg) | Ringer's solution (ml) |
|---|---|---|---|---|
| Sham | 2.07±0.17 | 20±3 | 12.9±4.3 | 117±15 |
| Deep intubation | 1.97±0.10 | 20±5 | 12.6±4.1 | 127±9 |
| Clamp | 2.00±0.14 | 23±4 | 16.0±3.9 | 116±9 |
| Blocker | 2.06±0.14 | 19±3 | 15.4±4.1 | 125±15 |
Data are expressed as mean ± standard deviation.
Table II.
Anatomy index of rabbit airway.
| Group | Length from the carina to the tracheal cut (cm) | Length of the left main bronchus (cm) | Length of the right main bronchus (cm) |
|---|---|---|---|
| Sham | 6.10±0.42 | 0.97±0.14 | 0.53±0.08 |
| Deep intubation | 6.04±0.57 | 1.00±0.10 | 0.55±0.11 |
| Clamp | 6.37±0.55 | 0.95±0.12 | 0.55±0.08 |
| Blocker | 5.97±0.51 | 1.00±0.11 | 0.52±0.06 |
Data are expressed as mean ± standard deviation.
Circulation, oxygenation and airway pressure
HR and MAP showed no differences at all time points among the three OLV groups. Ppeak increased in three OLV groups after OLV, compared with sham group, and it showed no differences among three OLV groups (Fig. 3). PaO2 and SaO2 were lower in three OLV groups at 10 min, 30 min, 1 h, 2 h and 3 h of OLV, compared with the sham group, and it showed no differences among three OLV groups (Fig. 4). PH and PaCO2 showed no differences among the three OLV groups.
Figure 3.
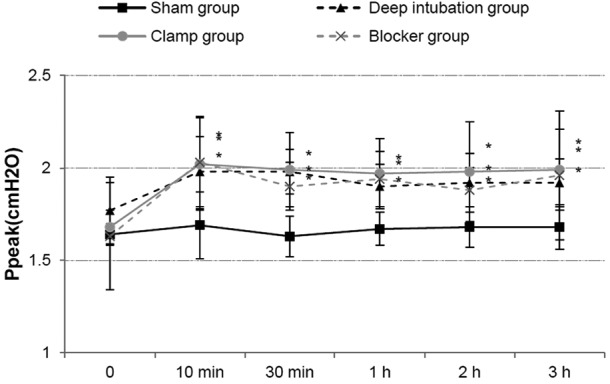
Ppeak increased at 10 min, 30 min, 1 h, 2 h, 3 h of one-lung ventilation (OLV) in the three OLV groups compared with sham group, and it showed no differences among the three OLV groups. *P<0.05 vs. sham group. Ppeak, peak pressure.
Figure 4.
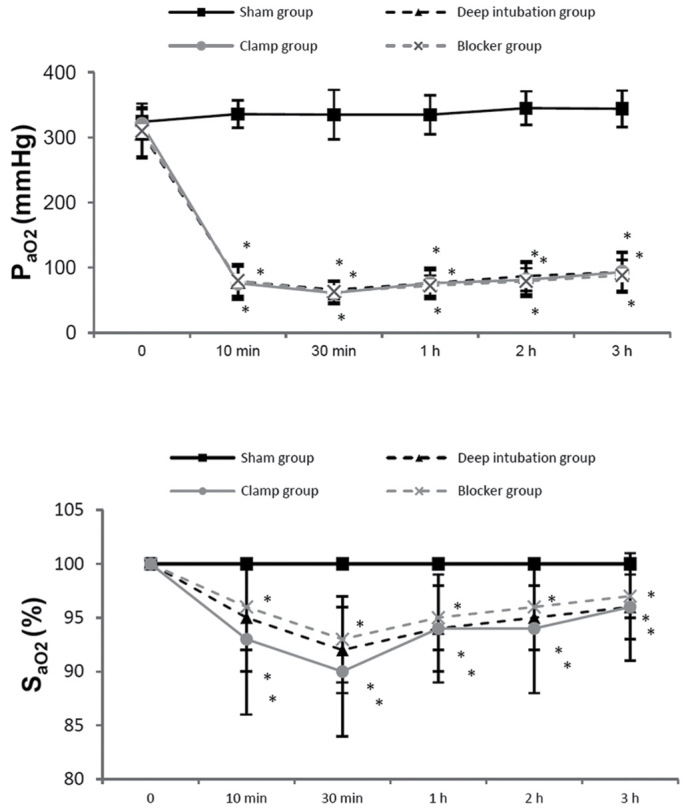
PaO2 and SaO2 were lower in three one-lung ventilation (OLV) groups at 10 min, 30 min, 1 h, 2 h, 3 h of OLV, compared with sham group, and no differences were detected among the three OLV groups. *P<0.05 vs. sham group. PaO2, arterial partial pressure of oxygen; SaO2, arterial hemoglobin oxygen saturation.
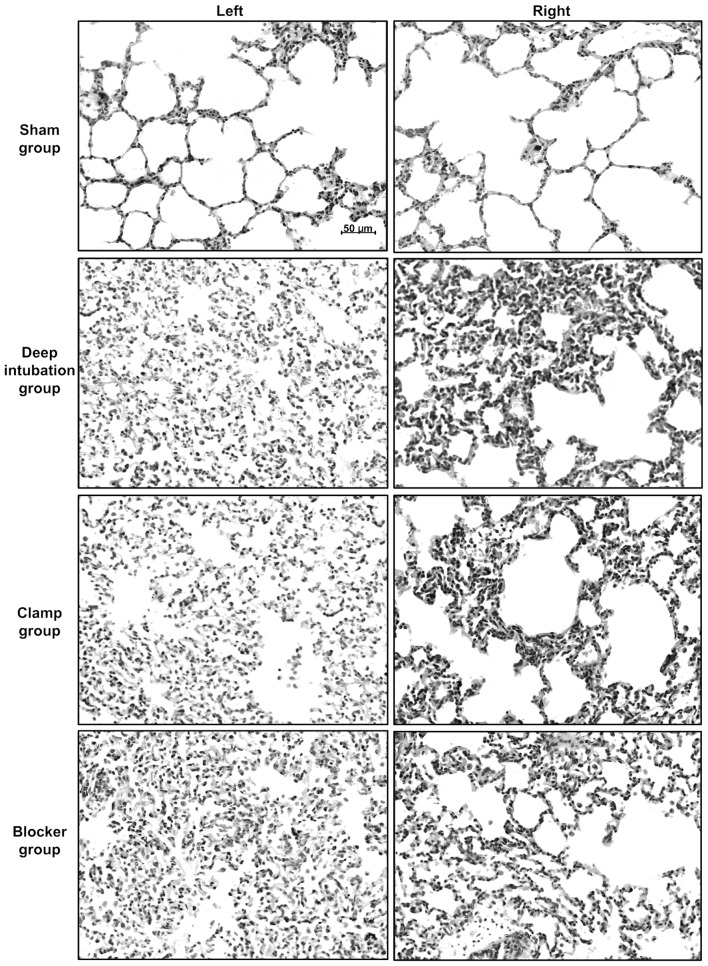
Lung injury caused by OLV
Sham group showed nearly normal lung histology. The three OLV groups showed edema, thickening of the alveolar wall, infiltration of inflammatory cells into alveolar spaces and interstitial spaces at 3 h of OLV (Fig. 5). Lung injury score was higher at 3 h of OLV in three OLV groups (Deep intubation group/left: 2.4±1.1, P<0.05, right: 2.4±1.1, P<0.01; Clamp group/left: 2.7±1.1, P<0.01, right: 2.3±1.5, P<0.05; Blocker group/left: 2.1±1.4, P<0.05, right: 2.4±1.1, P<0.01) compared with sham group (left: 1.0±0.7, right: 0.9±0.7), and no differences were detected among three OLV groups.
Figure 5.
Representative photographs of histological sections of rabbit lungs at 3 h of one-lung ventilation (OLV). Lung histology specimens from the left/right lungs of the sham, deep intubation, clamp and blocker groups. Three OLV groups showed edema, thickening of the alveolar wall, infiltration of inflammatory cells into alveolar spaces and interstitial spaces, compared with sham group. All images are at ×200 magnification. Lung injury score was higher at 3 h of OLV in three OLV groups (deep intubation group/left: 2.4±1.1, P<0.05, right: 2.4±1.1, P<0.01; clamp group/left: 2.7±1.1, P<0.01, right: 2.3±1.5, P<0.05; blocker group/left: 2.1±1.4, P<0.05, right: 2.4±1.1, P<0.01) compared with sham group (left: 1.0±0.7, right: 0.9±0.7), and it showed no differences among three OLV groups.
Time of modeling
(build right-OLV model after 30 min of TLV, and check breathing sound, Ppeak and the collapsed left lung immediately after changed to lateral position). Less time was spent in blocker group (6±2 min), compared with the other two OLV groups (13±4 min in the deep intubation group, P<0.05; 33±9 min in the clamp group, P<0.001) (Fig. 6).
Figure 6.
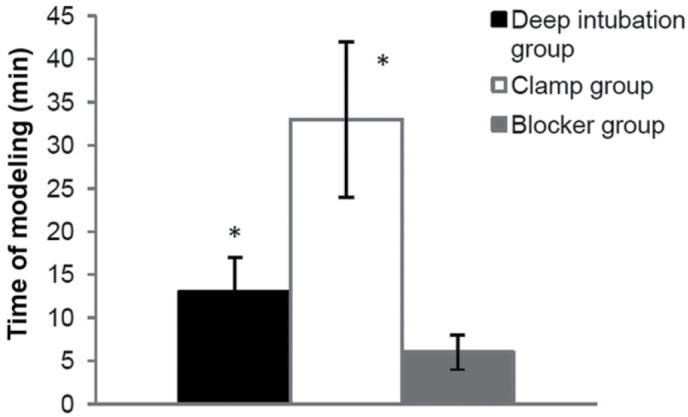
Time of modeling. Less time was required in the blocker group (6±2 min), compared with the other two OLV groups (13±4 min in deep intubation group, P<0.05; 33±9 min in clamp group, P<0.001). *P<0.05 vs. blocker group.
Blood loss during modeling
During right-OLV modeling, more blood loss was observed in the clamp group (11.7±2.8 ml), compared with the other two OLV groups (2.3±0.5 ml in deep intubation group, P<0.001; 2.1±0.6 ml in blocker group P<0.001) (Fig. 7).
Figure 7.
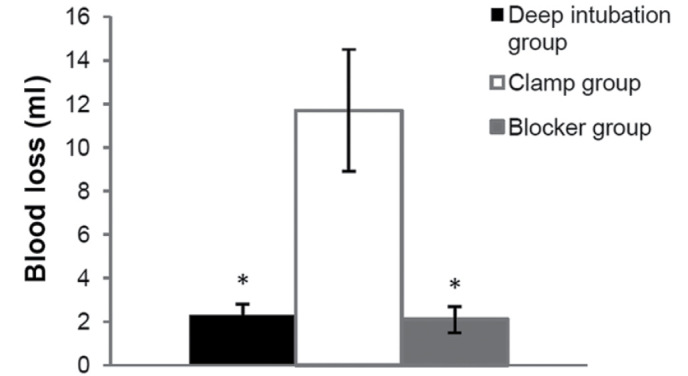
Blood loss during modeling. More blood loss was observed in the clamp group (11.7±2.8 ml), compared with the other two one-lung ventilation groups (2.3±0.5 ml in deep intubation group, P<0.001; 2.1±0.6 ml in blocker group P<0.001). *P<0.05 vs. clamp group.
Success rate
The first-time success rate of right-OLV modeling showed no differences among three OLV groups (50% in deep intubation group, 70% in clamp group and 70% in blocker group). The final success rate of right-OLV modeling also showed no differences among three OLV groups (70% in deep intubation group, 70% in clamp group and 80% in blocker group).
Discussion
The present study presents a novel method for establishing a right-OLV model in rabbits. Rabbits, dogs, pigs and rats are the most commonly used animals in an building OLV model. Dogs and pigs have relatively bigger airway size, which makes it easy and convenient to build both left and right OLV models using a DLT or endobronchial blocker (7,8,17). However, they are expensive and difficult to raise, so they are less commonly selected once research funding and lab qualification are taken into consideration. By contrast, rabbits are cheap and easy to raise, and compared with rats, rabbits have a median airway size and are convenient for building OLV models.
As the right lung is larger than the left lung, it is not surprising that oxygenation during right-OLV is better than during left-OLV (12). In Schwarzkopf et al (18), they found that while ventilating with 1.0 FiO2, PaO2 during OLV was ~280 mmHg during left-sided thoracic surgery as compared with ~170 mmHg during right-sided operations. Slinger et al (19) using regression analysis, found the side of operation to be one of the important factors in predicting hypoxemia during OLV. Therefore, right-OLV is safer than left-OLV when oxygenation is concerned, numerous researchers select right-OLV in their studies (20,21).
In our pre-experimental work, it was easier to build left-OLV model in rabbits by deeply intubating the endotracheal tube into the left main bronchus which is ~1 cm long. However, the right main bronchus is ~0.5-cm long according to our anatomical examination, and the right upper pulmonary lobe is easily blocked by deeply intubated tube. Based on these reasons, we particularly focused on right-OLV rabbit model in the present study. Furthermore, there are several methods to build right-OLV in rabbits, which each have disadvantages.
One method to build right-OLV is deeply intubating the tracheal tube into the right main bronchus (13). In the present study, the right main bronchus of rabbit (~0.5 cm long) was found to be markedly shorter than the left main bronchus. Thus, it was difficult to fix the deeply intubated endotracheal tube in the right place and required a longer time (13±4 min), compared with the blocker group (6±2 min, P<0.05). Moreover, the endotracheal tube is easier to slide to the wrong position after the rabbits turned from supine to right lateral position. We failed to establish a right-OLV model in half of the rabbits (five out of ten rabbits in the deep intubation group: part of the left lung was ventilated in three rabbits, part of the right upper lung was collapsed in two rabbits, failure rate: 50%) in the first time. Following regulation, the final failure rate decreased to 30% (two cases with partial ventilated left lung were corrected, the other three failure cases were certified until final anatomy), so the final success rate was 70%.
Another method is to clamp the left main bronchus. After a thoracotomy was performed, the left main bronchus was separated and completely clamped with a clip (14). Since thoractomy and bronchus separation should be performed, this method was accompanied by further trauma and surgical interference. In our pre-experimental work, it took us a relatively long time to learn this method under a surgeon's guidance, compared with the other two OLV group methods. More blood loss was observed in the clamp group (11.7±2.8 ml), compared with the other two OLV groups (2.3±0.5 ml in deep intubation group, P<0.001; 2.1±0.6 ml in blocker group, P<0.001), and bleeding was the predominant cause of failure (failure rate: 30%). Also it took longer time (33±9 min) to complete the whole procedure, compared with the other two OLV groups (13±4 min in deep intubation group, P<0.001; 6±2 min in blocker group, P<0.001).
Our self-made bronchial blocker may be easily constructed, with no special materials or technique required. The material includes a 3.0 mm endotracheal tube, 3–0 silk, small scissors, common tube core which is used clinically during adult intubation, water-repellent film and wire cutters, which are common materials purchasable in hardware stores. A single person may construct our bronchial blocker in ~10 min.
Technically, the present novel method relies on two key factors: Blocking and ventilation. The tube core was wrapped up with several layers of water-repellent film before blocking the tube, then leaking test was performed. These guaranteed effective air-block from inside the blocker. The 3.0-mm uncuffed endotracheal tube was selected based on pre-experimental work and measurement of the diameter of the rabbit trachea and left/right main bronchus. The reason we gave up the 3.0-mm cuffed endotracheal tube is that the length from the tip to the cuff is >1 cm even part of the cuff is tied; thus, the cuff is up the level of carina instead of completely inside the left main bronchus. The experimental results indicate that the performance of 3.0-mm uncuffed endotracheal tube was adequate and only one of the rabbits had a thinner left bronchus, in which a 2.5-mm endotracheal tube was used. Besides the appropriate size of the blocker, thread was wound around the blocker at 6–7 mm from the tip to attach the blocker tightly to the left bronchial wall, and the thread was in the left main bronchus beyond the carina in those successful cases shown by anatomy. Meanwhile, immediately after endpoint, the left main bronchus was cut beyond the blocker and put in water with ventilation machine still running, and no air bubbles were found. These all guaranteed effective air-block from between the blocker and the left main bronchus. Thus, from inside to outside the blocker, the blocking effect is certified.
The hole was made 9–10 mm from the tip to face the opening of the right main bronchus. The hole was up the carina shown by anatomy after endpoint. These ensured effective ventilation of the right lung. In conclusion, all the sizes were selected on the basis of anatomy, and little difference was found between the rabbits which did not influence our modeling so much. During OLV, breathing sound, Ppeak and the collapsed left lung were examined. Ultimately, the position of the blocker, the ventilated right lung and collapsed left lung were checked through anatomy, these all guaranteed the reliability of our modeling.
In the present study, Ppeak increased, PaO2, SaO2 decreased in three OLV groups at 10 min, 30 min, 1 h, 2 h, 3 h of OLV, compared with sham group. The three OLV groups showed more lung injury with higher lung injury score at 3 h of OLV, compared with sham group, which was consistent with previous studies (22–24), indicating the reliability of the OLV model in all three OLV groups. Meanwhile, the three OLV groups had similar oxygenation, circulation, airway pressure and acute lung injury score, which meant there were no differences among the three OLV models built by three different methods. Furthermore, our novel method (self-made bronchial blocker) required less time and resulted in reduced blood loss during modeling.
There are other methods used in OLV modeling in rabbits. One method recently proposed by You et al involves inserting a self-made artificial DLT into the trachea (6). A specific technique is required to construct the artificial DLT, which has limited its use. Meanwhile, DLT has two thinner individual lumens, the airway pressure is higher and the lumen is more easily blocked by sputum. Another method previously advanced is a 4F bronchial blocker through the ventilation tube into the left main bronchus with the blocker cuff placed precisely distal to the carina (15). The disadvantage of this method is similar to that of the artificial DLT. The outer diameter of 4F bronchial blocker is 1.33 mm, the inner diameter of 3.0 mm endotracheal tube is 3.0 mm. After the 4F bronchial blocker is inserted through the 3.0 mm endotracheal tube, the airway pressure will be increased and the tube is more easily blocked by airway secretion. As these methods are infrequently used, they were not included in the present study.
In conclusion, the present study suggests that the use of our self-made bronchial blocker to block the left main lung may be an effective, convenient and reliable method for establishing a right-OLV model in rabbits.
Acknowledgements
The present study was subsidized by grants from the Department of Anesthesiology, Jiangsu Cancer Hospital. The authors thank the epidemiologist Mr. Suping Li for assistance with the statistical work in the present study and thoracic surgeon Dr Dongjie Feng for assistance with rabbit modeling.
Glossary
Abbreviations
- OLV
one-lung ventilation
- TLV
two lung ventilation
- HR
heart rate
- MAP
mean arterial pressure
- PaO2
arterial partial pressure of oxygen
- SaO2
arterial hemoglobin oxygen saturation
- Ppeak
peak pressure
- VT
tidal volume
- RR
respiratory rate
- DLT
double-lumen endotracheal tube
References
- 1.Della Rocca G, Coccia C. Ventilatory management of one-lung ventilation. Minerva Anestesiol. 2011;77:534–536. [PubMed] [Google Scholar]
- 2.Eguchi T, Hamanaka K, Kondo R, Saito G, Shiina T, Koizumi T, Yoshida K. Lung re-expansion following one-lung ventilation induces neutrophil cytoskeletal rearrangements in rats. Ann Thorac Cardiovasc Surg. 2014;20:276–283. doi: 10.5761/atcs.oa.13.02247. [DOI] [PubMed] [Google Scholar]
- 3.Kozian A, Schilling T, Fredén F, Maripuu E, Röcken C, Strang C, Hachenberg T, Hedenstierna G. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: An experimental study. Br J Anaesth. 2008;100:549–559. doi: 10.1093/bja/aen021. [DOI] [PubMed] [Google Scholar]
- 4.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Abreu MG, Pelosi P. How can we prevent postoperative pulmonary complications? Curr Opin Anaesthesiol. 2013;26:105–106. doi: 10.1097/ACO.0b013e32835e80d8. [DOI] [PubMed] [Google Scholar]
- 6.You Z, Feng D, Xu H, Cheng M, Li Z, Kan M, Yao S. Nuclear factor-kappa B mediates one-lung ventilation-induced acute lung injury in rabbits. J Invest Surg. 2012;25:78–85. doi: 10.3109/08941939.2011.603817. [DOI] [PubMed] [Google Scholar]
- 7.Schilling T, Kretzschmar M, Hachenberg T, Hedenstier-Na G, Kozian A. The immune response to one-lung-ventilation is not affected by repeated alveolar recruitment manoeuvres in pigs. Minerva Anestesiol. 2013;79:590–603. [PubMed] [Google Scholar]
- 8.Adami C, Axiak S, Rytz U, Spadavecchia C. Alternating one lung ventilation using a double lumen endobronchial tube and providing CPAP to the non-ventilated lung in a dog. Vet Anaesth Analg. 2011;38:70–76. doi: 10.1111/j.1467-2995.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Siegl S, Uhlig S. Using the one-lung method to link p38 to pro-inflammatory gene expression during overventilation in C57BL/6 and BALB/c mice. PLoS One. 2012;7:e41464. doi: 10.1371/journal.pone.0041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siempos II, Maniatis NA, Kopterides P, Magkou C, Glynos C, Roussos C, Armaganidis A. Pretreatment with atorvastatin attenuates lung injury caused by high-stretch mechanical ventilation in an isolated rabbit lung model. Crit Care Med. 2010;38:1321–1328. doi: 10.1097/CCM.0b013e3181d9dad6. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Yang Y, Li Y, Li J, Ma Q, Zhao Y, Wang D. Effects of sevoflurane on pulmonary cytosolic phospholipase A2 and clara cell secretory protein expressions inrabbits with one-lung ventilation-induced lung injury. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:469–473. [PubMed] [Google Scholar]
- 12.Katz Y, Zisman E, Isserles SA, Rozenberg B. Left, but not right, one-lung ventilation causes hypoxemia during endoscopic transthoracic sympathectomy. J Cardiothorac Vasc Anesth. 1996;10:207–209. doi: 10.1016/S1053-0770(96)80238-2. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber T, Niemann C, Schmidt B, Karzai W. A novel model of selective lung ventilation to investigate the long-term effects of ventilation-induced lung injury. Shock. 2006;26:50–54. doi: 10.1097/01.shk.0000215318.99241.25. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M, Fujishima S, Sawafuji M, Ishizaka A, Oguma T, Soejima K, Matsubara H, Tasaka S, Kikuchi K, Kobayashi K, et al. Importance of interleukin-8 in the development of reexpansion lung injury in rabbits. Am J Respir Crit Care Med. 2000;161:1030–1036. doi: 10.1164/ajrccm.161.3.9906039. [DOI] [PubMed] [Google Scholar]
- 15.Groh J, Kuhnle GE, Sckell A, Ney L, Goetz AE. Isoflurane inhibits hypoxic pulmonary vasoconstriction. An in vivo fluorescence microscopic study in rabbits. Anesthesiology. 1994;81:1436–1444. doi: 10.1097/00000542-199412000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291–299. doi: 10.33549/physiolres.930822. [DOI] [PubMed] [Google Scholar]
- 17.Mayhew PD, Culp WT, Pascoe PJ, Kass PH, Johnson LR. Evaluation of blind thoracoscopic-assisted placement of three double-lumen endobronchial tube designs for one-lung ventilation in dogs. Vet Surg. 2012;41:664–670. doi: 10.1111/j.1532-950X.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzkopf K, Klein U, Schreiber T, Preussler NP, Bloos F, Helfritsch H, Sauer F, Karzai W. Oxygenation during one-lung ventilation: The effects of inhaled nitric oxide and increasing levels of inspired fraction of oxygen. Anesth Analg. 2001;92:842–847. doi: 10.1097/00000539-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Slinger P, Suissa S, Triolet W. Predicting arterial oxygenation during one-lung anaesthesia. Can J Anaesth. 1992;39:1030–1035. doi: 10.1007/BF03008370. [DOI] [PubMed] [Google Scholar]
- 20.Kozian A, Schilling T, Fredén F, Maripuu E, Röcken C, Strang C, Hachenberg T, Hedenstierna G. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: An experimental study. Br J Anaesth. 2008;100:549–559. doi: 10.1093/bja/aen021. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia R, Shaffer TH, Hossain J, Fisher AO, Horner LM, Rodriguez ME, Penfil S, Theroux MC. Surfactant administration prior to one lung ventilation: Physiological and inflammatory correlates in a piglet model. Pediatr Pulmonol. 2011;46:1069–1078. doi: 10.1002/ppul.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Noguchi E, Yamada S, Hamada N, Kano T. Sequential changes of arterial oxygen tension in the supine position during one-lung ventilation. Anesth Analg. 2000;90:28–34. doi: 10.1097/00000539-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Yin K, Gribbin E, Emanuel S, Orndorff R, Walker J, Weese J, Fallahnejad M. Histochemical alterations in one lung ventilation. J Surg Res. 2007;137:16–20. doi: 10.1016/j.jss.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol. 2013;26:40–46. doi: 10.1097/ACO.0b013e32835c4ea2. [DOI] [PubMed] [Google Scholar]