Abstract
The study assesses the effectiveness of reversible head-only and back-of-the-head electrical stunning of chickens using 130–950 mA per bird at 50 Hz AC.
Three trials were conducted to compare both stunning systems: (a) behavioural assessment of return of consciousness, (b) insensibility to thermal pain, and (c) assessment of return of brain activity with visually evoked potentials (VEPs).
Assessment of behaviour suggested that the period of unconsciousness following head-only electrical stunning was shorter in hens compared to broilers.
Stunning across the back-of-the-head delayed the time to return of brainstem function compared to stunning with standard head-only electrodes. Additionally, back-of-the-head stunning produced a more prolonged period of electroanalgesia compared to head-only.
Based on examination of return of brain function with VEPs in hens, back-of-the-head stunning produced a shorter-lasting stun than standard head-only. However, even for standard head-only, the stun was notably shorter than previously reported. In some birds, brain function had returned within 9 s after the end of stunning.
The results suggest that some birds may recover consciousness prior to or during the neck cut. Based on these findings, back-of-the-head stunning and standard head-only stunning of hens should not be recommended without further development.
INTRODUCTION
Waterbath stunning of poultry is used in a number of countries. There have been calls for this system to be phased out in the United Kingdom (UK) and Europe, because of the risk of pre-stun shocks, suboptimum stuns, suspension of conscious birds upside down (which is stressful and, in some cases, painful) and variations in electrical current delivered to birds in multi-bird waterbath stunners (FAWC, 2009; Hindle et al., 2010; EFSA, 2014). In large-scale poultry processing plants, this system is being replaced by controlled atmosphere stunning (CAS) systems, which do not involve shackling before stunning. Presently, CAS systems are not feasible for small-scale poultry processing plants because of their relatively high capital and running costs. Instead, there is a need to develop cost-effective alternatives for stunning chickens in small-scale plants. Potential options include systems based on head-only stunning. Head-only stunning is already used for small-scale and seasonal poultry production and there has been recent development of cone restraint/head-only (Lambooij et al., 2010, 2014) and head-only waterbath (Lines et al., 2011) stunning systems.
Head-only electrical stunning, like some forms of high-frequency head-to-body and head-to-shackle stunning, produces a reversible stun. Consequently, it is important with head-only electrical stunning that the duration of induced unconsciousness should last longer than the time it would take for neck cutting to be performed and brain death to occur through bleeding. In mammals, electrical stunning can induce analgesia (electroanalgesia), which lasts longer than the period of unconsciousness. This is mediated by the neurotransmitter GABA (Cook et al., 1992, 1993), but has not been demonstrated in poultry. In other words, after delivering an electric current the animal is stunned and then it appears to regain consciousness but is still unresponsive to painful stimuli. This state can last for 10 or more min (Gregory and Wotton, 1988).
Commercially available head-only stunning equipment for poultry generally consists of either a pair of single tapered copper electrodes or more commonly two rosettes of pointed needle (pin) electrodes (up to 12 needles on each rosette) placed on either side of the head. During head-only stunning, often only two or three needles on each electrode actually contact the surface of the head. The reduced area of contact can result in localised heating effects and enhance the build-up of carbon, which increases the electrical impedance (Sparrey and Wotton, 1997). When this occurs, the operators are often required to press the electrodes with more force against the surface of the head to improve electrical contact. This can result in bending of the pins, which can further reduce electrical current flow and cause pain to the bird associated with increased pressure and tissue damage to the head prior to the onset of unconsciousness (T. J. Gibson, unpublished observation). An alternative novel system is making electrical contact across the back-of-head (corresponding to the occipital bone) with smooth-surfaced electrodes, which can provide a relatively large area of contact. Unpublished work by the authors suggests that stunning chickens in this position could be effective. The potential advantages of the back-of-the head position are that it is relativity simple to apply, and that a single operator can perform both manual restraint and the stun.
Consciousness/unconsciousness following head-only electrical stunning has previously been assessed indirectly with a number of indicators, including: coordinated behavioural responses (Gregory and Wotton, 1990a , 1994; Lines et al., 2011); response to noxious stimulus (Lambooij et al., 2010); induction of epileptic/polyspike waveform or a quiescent electroencephalogram (EEG) (Richards and Sykes, 1967; Wormuth et al., 1981; Gregory and Wotton, 1990a ; Schütt-Abraham, 1998; Raj and O’Callaghan, 2004; Lambooij et al., 2010, 2014; Lines et al., 2011), spectral analysis of the EEG (including correlation dimension analysis) (Raj and O’Callaghan, 2004; Lambooij et al., 2010, 2014; Lines et al., 2011), and recovery/absence of evoked potentials (Gregory and Wotton, 1990a ).
The aim of the study was to evaluate the effectiveness of back-of-the-head compared to head-only electrical stunning, in terms of peak current delivered, duration of induced unconsciousness (behaviour evoked responses and return of brain function) and insensibility to noxious thermal laser stimulation.
MATERIALS AND METHODS
All birds were sourced from either commercial indoor or free-range units. Birds were kept in accordance with normal husbandry practices. All procedures were carried out under the provisions of the Animals (Scientific Procedures) Act 1986 and with the approval of the institute’s Ethical Review Panel.
In all experiments, birds were stunned with a 50 Hz sine wave alternating current (AC) delivered using a constant voltage stunner (Whitehead Engineering Ltd, Bath, UK). Voltage and current in all experiments were recorded with the 199C Fluke Scope meter and 179 Fluke multimeter (Fluke Corporation, Everett, WA, USA). All current and voltage recordings in the paper are root mean squared (RMS) values.
Birds were stunned in the head-only or back-of-the-head positions. Standard commercially available needle pin electrodes were used for head-only stunning. These consisted of 12, pointed copper pins (10 mm high × 1 mm diameter) spaced apart in a circular rosette arrangement with an overall diameter of 15 mm. The electrodes were placed on either side of the head spanning the brain between the eyes and the ears. This corresponded to the lateral aspects of the frontal bone and extended to the squamosal bone. The back-of-the-head electrodes were custom built (Solutions for Research Ltd., Silsoe, Bedford, UK), consisting of two adjustable stainless steel plate electrodes (190 mm high × 100 mm wide × 5 mm thick) arranged in a V shape. The bird’s neck was placed in the V, with backward pressure applied so the caudal aspect of the cranium contacted the electrodes on either side of the head. This was caudal to the ears and corresponded to the occipital bone.
Experiment 1. Behavioural assessment of return of consciousness
The duration of unconsciousness following electrical stunning was examined in broilers and ex-layer hens from the return of: rhythmic breathing, neck tension and balance (Gregory and Wotton, 1990a ). Apnoea has been associated with induced epileptiform activity in the brain, while the termination of this activity has been shown to coincide with recovery of spontaneous breathing. The recovery and maintenance of neck tension relates to CNS control of muscle tone, which is absent during electrical stunning-induced unconsciousness. The recovery of and maintenance of balance indicates recovery of the vestibular system and cerebellar motor control. This reflects recovery of higher order brain function, which could be associated with consciousness.
Birds were stunned in either the head-only (broilers n = 24, hens n = 23) or back-of-the-head (hens n = 25) positions for 7 s; this duration was selected based on previous studies and government guidelines. As soon as rhythmic breathing returned, birds were repeatedly assessed in sequence for the time to return of neck tension and balance. Return of breathing was assessed by rhythmic movement of the vent and body related to respiration. While each bird was lying on its side, fingers were positioned under the head and used to repeatedly lift up the head and upper neck. Neck tension was recorded as returning when the birds were able to hold their head/neck up away from the supporting hand with obvious tension in the neck muscles. Immediately after testing for neck tension, birds were placed on their feet and shanks and gently pushed from the left or right sides of the body to assess for return of balance. This was conducted for a maximum of 5 s before retesting neck tension; if the bird fell onto its side it was said to not have control over its balance. Once breathing, neck tension, and balance had returned, all birds were immediately restunned and slaughtered with a ventral neck incision.
Experiment 2. Insensibility to thermal pain
Insensibility to pain (IP) was assessed using physical reactions to stimulation of the comb of hens with laser heat from a CO2 laser (48-1S Synrad, Inc., Mukilteo, WA, USA) before and after electrical stunning with head-only (n = 28) and back-of-the-head electrodes (n = 9). This technique has been used previously for nociceptive threshold research in pigs (Herskin et al., 2009), cattle (Veissier et al., 2000), and lambs (Guesgen et al., 2011). The same trained operator individually restrained each bird. A hood was placed over the head of the birds with an opening for exposure of the comb. This was to minimise distress and stress associated with handling and to protect the eyes and the rest of the head from the laser.
Hens were placed 380 mm in front of the laser, a visible low power cold laser beam (1.25 diode pointer with ZnSe beam combiner, Synrad, Inc., Mukilteo, WA, USA) was introduced into the path of the CO2 laser to allow for targeting of specific areas of the comb. The wavelength of the CO2 laser was 10.57–10.63 μm, with the power set to 4.5%, this equated to 0.3 watts as measured with a power meter (Flash-500–55, Gentec Electro-optics, Inc., Quebec, Canada). Laser beam diameter was 3 mm and was focused with a 5.0” focal lens (LaserMech, Inc., Novi, MI, USA). Surface temperature of the comb was measured in a sample of birds using a thermal imaging camera (P620, FLIR Systems, West Malling, United Kingdom) or a remote infrared thermometer (LaserSight, Optris GmbH, Berlin, Germany). Prior to stimulation the comb was 31.8 ± 1.0°C. During laser stimulation, the mean temperature on the target area was 42.4 ± 0.7°C.
Prior to electrical stunning, the time to physical response (latency) to a laser beam focused on the bird’s comb was assessed. This formed a baseline for time to physical response. The baseline laser stimulus was repeated no more than three times. Prior to electrical stunning, the hood was repositioned to allow placement of the stunning electrodes. Birds were then stunned with either a back-of-the-head or head-only electrical stunner with a minimum current of 240 mA. Immediately after the completion of convulsive wing flapping, the birds received a thermal laser stimulus on a fresh section of the comb. The stimulus was repeated every 30 s for up to 5 min after stunning. For each stimulus, the laser was directed to a fresh section of the comb (Figure 1). When there was no physical reaction, the laser stimulation lasted no longer than 10 s. The physical reaction consisted of recoil of the head and neck, with or without head shaking. Stimulation was discontinued as soon as a physical response occurred. The latency in the time to return of physical responses to the laser stimulus before and after stunning was assessed. Immediately on completion of experimentation, the birds were killed by electrical stunning followed by ventral neck incision.
Figure 1.
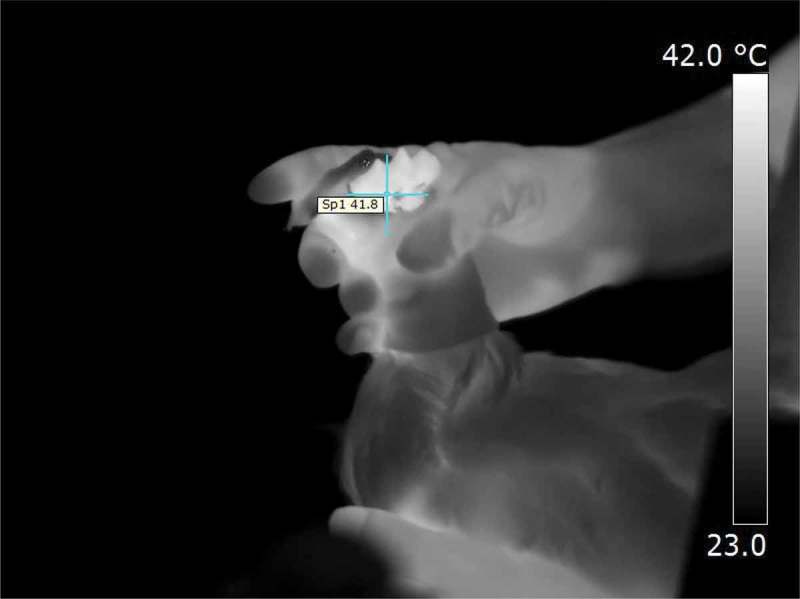
Experiment 2: thermal camera image of the site of laser stimulation of the comb.
Experiment 3. Assessment of return of brain activity with visually evoked potentials (VEPs)
Thirty end-of-lay hens were randomly allocated into two stunning treatment groups. Birds either received a head-only (n = 14) or a back-of-the-head (n = 16) stun with a 50 Hz constant voltage stunner. Prior to placement of the electrodes, the birds were restrained in a cat restraint bag (Four Flags Over Aspen, Inc., St. Clair, MN, USA), with the wings, body, and legs restrained and the head and neck exposed. The birds were restrained to minimise movement artefact during VEP recording. One channel of VEPs was recorded from a three-electrode montage using three 28 gauge stainless steel subdermal electrodes (F-E3-48, Grass Technologies, Natus Neurology Inc., Warwick, RI, USA). The tips of each electrode were placed as follows: active (non-inverting) 4 mm right of midline, ≈3 mm rostral of bregma over the right optic lobe; reference (inverting), over the right rostral aspect of the forebrain 4 mm right of midline, ≈16 mm rostral of bregma; and ground electrode caudal to the back-of-the-head, respectively. Interelectrode impedance ranged between 1.7 and 3.1 kΩ (MkIII Checktrode, UFI, Morro Bay, CA, USA). VEPs were amplified, digitalised (1 kHz), and recorded with a 4/20 PowerLab (ADInstruments Ltd, Sydney, Australia) digital to analogue converter. All signals were filtered with an analogue filter (Bio Amp, ADInstruments Ltd., Sydney, Australia) with low and high pass filters of 100 and 0.1 Hz, respectively. A Grass photic stimulator (PS33-PLUS, Grass Technologies, Natus Neurology Inc., Warwick, RI, USA) was positioned 200 mm from the bird’s head and was triggered and synchronised with the VEP recordings by the data acquisition software package Chart Pro 7 (ADInstruments Ltd., Sydney, Australia). All recordings were performed in a darkened room with the birds manually restrained. A 100 ms pre-stimulus (light) followed by a 200 ms post-stimulus period was recorded (flash every 300 ms). VEPs were assessed for a 1-min pretreatment period and for the first 5 min following the end of the stun for both treatments. After stunning, birds were monitored for return of rhythmic breathing and head shaking/movement that could be associated with movement artefact in the recorded waveforms. These waveforms were excluded from further analysis. On completion of the recording of VEPs, all birds were immediately killed with head-only electrical stunning followed by ventral neck incision.
Analysis of VEPs was conducted offline after the completion of experimentation. Initial averages were calculated from 15 consecutive sweeps (4.5 s). Where possible, the number of sweeps averaged was reduced to refine the time to return of VEP activity. Individual sweeps contaminated by noise were removed from analysis. The presence of genuine evoked potentials was assessed by comparison of positive and negative potential latencies with baseline (pre-stunning) and subsequent averaged evoked potentials. Waveforms with a signal to noise ratio < 2, based on baseline recordings were excluded from further analysis. All birds acted as their own controls. Signal averaging was performed with the Chart software, with subsequent analysis in Excel 2011 (Mac, Microsoft Corporation, Redmond, Washington, USA).
Statistical analysis
All analysis was performed using Prism 6.0e (GraphPad Software Inc., San Diego, CA, USA) and SPSS 20.0 (IBM Corporation, Chicago, IL, USA). The D’Agostino & Pearson omnibus normality test was used to determine the distribution of the data. Comparisons between head-only and back-of-the-head stunning were analysed with either an unpaired t-test with Welch’s correction or Mann–Whitney test where appropriate. The relationships between peak current, return of VEPs, and stunning electrode impedance were analysed with linear regression. Return of VEPs and rhythmic breathing were analysed with a Spearman correlation. Mean values are displayed ± standard error of the mean (SE). The level of significance for all tests was P < 0.05.
RESULTS
Experiment 1. Behavioural assessment of return of consciousness
In broilers that received a conventional head-only stun of 618 ± 43 mA (range 130–950) and 133 ± 1 V (range 123–142), breathing, neck tension, and balance returned after 33 ± 2, 113 ± 14, and 182 ± 22 s, respectively (Table 1). Hens that received a conventional head-only stun of 469 ± 28 mA (range 240–780) and 139 ± 1 V (range 125–142), had a shorter time to return of breathing (24 ± 1 vs. 33 ± 2 s, P < 0.05), neck tension (76 ± 4 vs. 113 ± 14 s, P < 0.05), and balance (111 ± 6 s vs. 182 ± 22 s, P < 0.05) compared to the broilers. Hens that received a back-of-the-head stun of 476 ± 23 mA (range 240–680) with 132 ± 1 V (range 125–140), had a longer time to recovery of breathing (34 ± 2 s) compared to conventionally head-only stunned hens (P < 0.05) (Table 1). The time to return of neck tension was similar between back-of-the-head stunned hens and conventionally head-only stunned hens. Time to return of balance was longer with the back-of-the-head compared to conventionally head-only stunned hens (P < 0.05). One bird after back-of-the-head stunning did not recover and died.
Table 1.
Experiment 1: effect of stunning method (mean ± SE (range)) on time to return of rhythmic breathing, neck tension, and balance in broilers and laying hens.
| Stunning method | Bird type | Mean ± SE (range) time to return of rhythmic breathing (s) | Mean ± SE (range) time to return of neck tension (s) | Mean ± SE (range) time to return of balance (s) |
|---|---|---|---|---|
| Head-only | Broilers (n = 24) | 33 ± 2 (17–59)a | 113 ± 14 (39–306) | 182 ± 22 (66–512)a |
| Head-only | End of lay hens (n = 23) | 24 ± 1 (19–39)b | 76 ± 4 (45–138) | 111 ± 6 (75–176)b |
| Back-of-the-head | End of lay hens (n = 25) | 34 ± 2 (20–53)a | 76 ± 4 (52–115) | 260 ± 36 (80–974)a |
Means in a column with no common superscript letter differ significantly at P < 0.05.
Experiment 2. Insensibility to thermal pain
Prior to electrical stunning, the latency in time to physical response to laser stimulation ranged between 1 and 5 (mean 1.5 ± 0.3) s. Mean peak current in the head-only and back-of-the-head groups were 502 ± 48 mA (range 250–920) and 589 ± 50 mA (range 420–910), respectively (P = 0.167). The mean peak voltage was 132 ± 2 V (range 110–143) and 128 ± 1 V (range 121–130) for head-only and back-of-the-head groups, respectively (P = 0.091) (Table 2). In the head-only electrical stunning group, 36% (n = 10) of birds responded to the laser after stunning, with the mean time for return of physical response in those birds of 83 ± 30 s. During the 5-min recording period, no birds in the back-of-the-head group had a physical response to the laser stimulus. There was an association between stunning methods and return of physical response that approached significance (P = 0.079). The mean time to return of breathing, neck tension, and balance was recorded in 19 birds (Table 2). There was no significant difference in return of breathing or neck tension between the two treatment groups. However, there was a significant difference in the time of return of balance (P = 0.002), with animals in the back-of-the-head group having a mean time of return of balance of 279 ± 13 s compared to head-only with 153 ± 16 s.
Table 2.
Experiment 2: effect of stunning method (head-only or back-of-the-head electrodes) on insensibility to thermal laser pain, behavioural indices, current and voltage.
| Stun method |
|||
|---|---|---|---|
| Head-only | Back-of-the-head | Significance | |
| Number of birds | 28 | 9 | - |
| Birds with physical response within 5 min, % | 36% (n = 10) | 0% (n = 0) | P = 0.079 |
| Mean (± SE) time to return of physical response (range), s | 83 ± 30 (9–32) | 0 | - |
| Mean (± SE) time to return of rhythmic breathing (range)1, s | 33 ± 4 (20–70) | 40 ± 4 (26–58) | P = 0.107 |
| Mean (± SE) time of return of neck tension (range)1, s | 79 ± 10 (45–132) | 100 ± 10 (69–156) | P = 0.098 |
| Mean (± SE) time of return of balance (range)1, s | 153 ± 16 (77–226) | 279 ± 13 (210–306) | P = 0.002 |
| Mean (± SE) peak current (range), mA | 502 ± 48 (250–920) | 589 ± 50 (420–910) | P = 0.167 |
| Mean (± SE) peak voltage (range), V | 132 ± 2 (110–143) | 128 ± 1 (121–130) | P = 0.091 |
1 Behavioural data only available for 19 birds (10 head-only; 9 back-of-the-head).
Experiment 3. Assessment of return of brain activity with visually evoked potentials (VEPs)
There was no significant difference in the mean peak current delivered to hens in the head-only (453 ± 34 mA (range 280–650)) or back-of-the-head (520 ± 16 mA (range 360–620)) stunning groups (P = 0.086) (Table 3). The mean peak voltage was 138 ± 1 V (range 131–146) and 138 ± 1 V (range 130–142) for head-only and back-of-the-head groups, respectively (P = 0.754). Initially, following electrical stunning, VEP was abolished in all hens from both treatment groups. However, VEP returned within 13 ± 1 s (range 7–26) in the back-of-the-head group, compared to 20 ± 2 s (range 9–32) for birds in the head-only group (P < 0.01) after the end of the electrical stun. Examples of VEPs from two hens that received either the head-only or back-of-the-head stuns are shown in Figure 2. The bird that received the head-only stun recovered VEP activity within 17.5–21.0 s, whereas the one that received the back-of-the-head stun was showing signs of VEP activity starting to return at 5.0–9.5 s, with full recovery within 10.5–14.0 s. There were no significant differences in the mean time to return of breathing (P = 0.262), or peak voltage (P = 0.754) between the treatment groups. In addition, there was no significant relationship between current and time to return of VEPs (head-only R2 = 0.008, P = 0.755; back-of-the-head R2 = 0.051, P = 0.397) (Figure 3).
Table 3.
Experiment 3: effect of stunning method (head-only or back-of-the-head electrodes) on brain function, rhythmic breathing, peak current, and voltage in hens assessed for visually evoked potentials (VEPs).
| Stun method |
|||
|---|---|---|---|
| Head-only | Back-of-the-head | Significance | |
| Number of birds | 14 | 16 | - |
| Mean (± SE) time to return of VEPs (range), s | 20 ± 2 (9–32) | 13 ± 1 (7–26) | P < 0.01 |
| Mean (± SE) time to return of rhythmic breathing (range), s | 26 ± 1 (20–36)1 | 25 ± 1 (19–36) | P = 0.262 |
| Mean (± SE) peak current (range), mA | 453 ± 34 (280–650) | 520 ± 16 (360–620) | P = 0.086 |
| Mean (± SE) peak voltage (range), V | 138 ± 1 (131–146) | 138 ± 1 (130–142) | P = 0.754 |
| Mean (± SE) weight (range), kg | 4.0 ± 0.1 (3.2–4.7) | 4.2 ± 0.1 (3.6–4.8) | P = 0.310 |
1 No data from one animal, which died after recovery of visually evoked potentials (VEPs).
Figure 2.
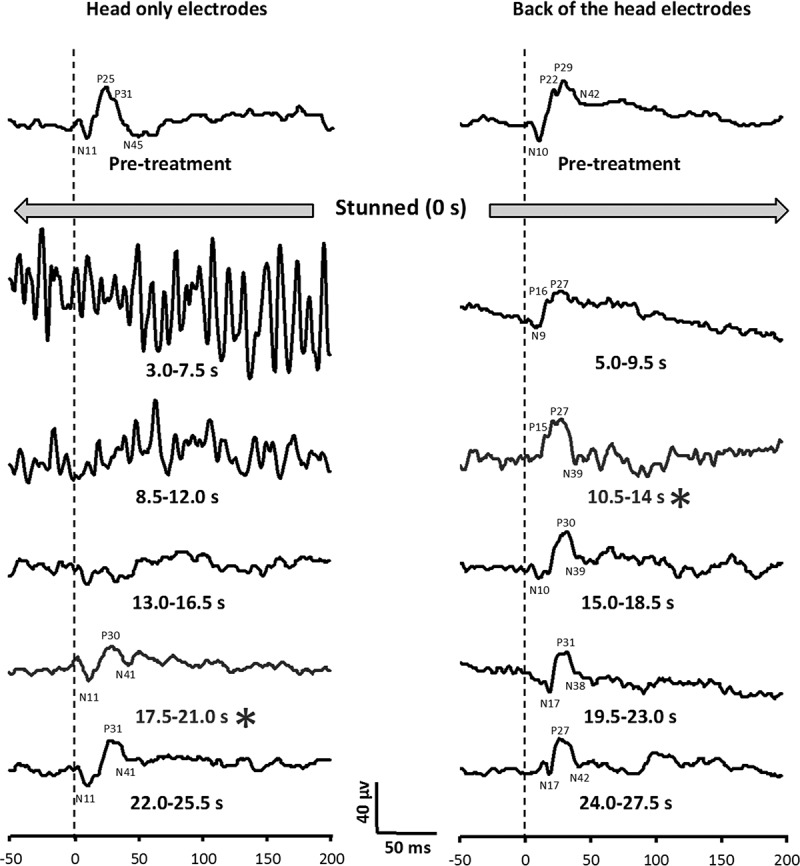
Experiment 3: examples of visual evoked potentials (VEP) from two hens after stunning with either standard head-only or back-of-the-head electrodes. The * (grey) waveforms are where VEP activity first returned. Each waveform is the average of 15 traces (4.5 s). There was a stimulus delay of 100 ms (50 ms shown) with the stimulus delivered at 0 ms. Time after treatment is from the end of the stun. The dashed line indicates the time of the visual stimulus.
Figure 3.
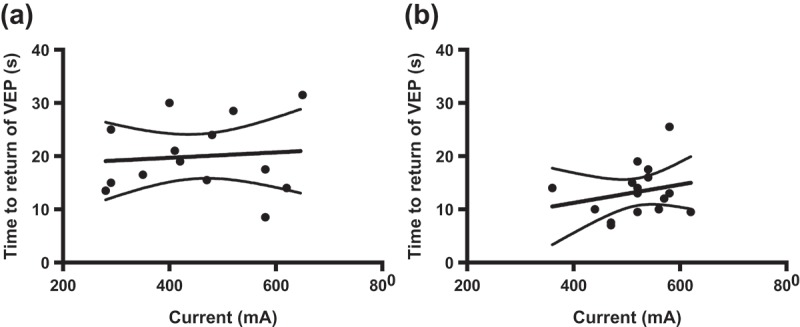
Experiment 3: linear regression with 95% CI for current (mA) compared to time to return of visual evoked potentials (VEPs) in hens stunned with (a) head-only (R 2 = 0.008, P = 0.755) and (b) back-of-the-head (R2 = 0.051, P = 0.397) electrodes.
The return of VEPs preceded the return of rhythmic breathing. There was no correlation between time to return of breathing and VEPs in birds stunned with the head-only electrodes (P = 0.449). However, in birds stunned with the back-of-the-head electrodes, there was a significant negative correlation between time to return of rhythmic breathing and time to return of VEP (r = −0.504, P = 0.046). There was a significant relationship between stunning current and impedance in both head-only (R2 = 0.943, P < 0.001) and back-of-the-head (R2 = 0.987, P < 0.001) stunning groups (Figure 4). There was a significant difference in the slopes of the regression lines between the two treatments (P = 0.013).
Figure 4.
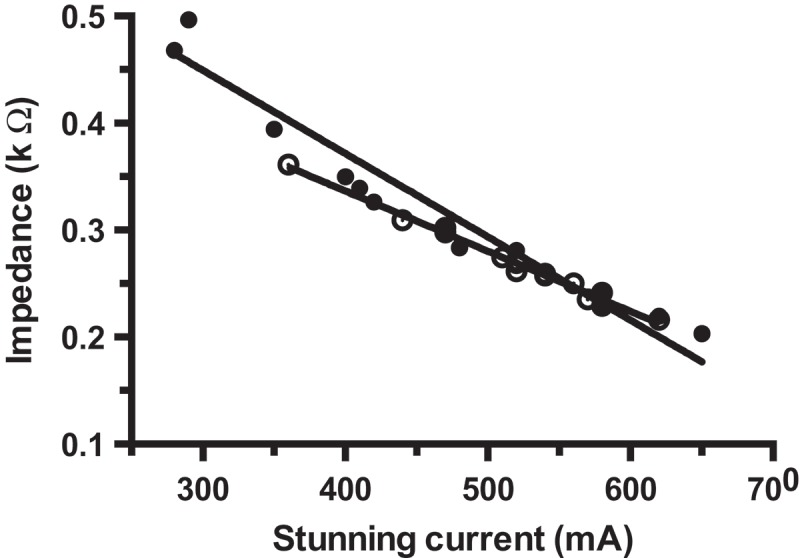
Experiment 3: linear regression for stunning current compared to impedance in hens stunned with (•) head-only (R 2 = 0.943, P < 0.001) or (◯) back-of-the-head electrodes (R 2 = 0.987, P < 0.001) in the visual evoked potentials (VEP) experiment.
DISCUSSION
Indicators of effectiveness of electrical stunning
Head-only electrical stunning involves the application of an electrical current across the head sufficient to induce brain dysfunction resulting in recoverable unconsciousness. This is in terms of generalised grand mal epileptic activity or an unresponsive isoelectric or quiescent EEG waveform (Raj and O’Callaghan, 2004; EFSA, 2014). Unlike stun to kill methods, the stun is reversible and does not induce cardiac arrest. In terms of animal welfare, the stun must induce near immediate unconsciousness and the duration of unconsciousness should last longer than the time it would take for neck cutting to be performed and brain death to occur through bleeding.
In broilers that received a conventional head-only stun, the time to return of breathing was similar to that previously reported by Gregory and Wotton (1990a ), however the time to return of neck tension was greater in the current study. This could be due to the higher current levels delivered to broilers in the present study (618 vs. 336 mA). Meanwhile, hens stunned with the needle head-only electrodes, had a shorter time to return of breathing, neck tension and balance compared to broilers. These findings suggest that the duration of unconsciousness following electrical stunning is shorter in hens than in broilers, and it shows that this applies also to head-only stunning, whereas previously this was only evaluated for waterbath stunning (Gregory and Wotton, 1994). The reported differences between broilers and hens may be due to: (a) the increased feather coverage in end-of-lay hens, which could have increased the impedance of the head, (b) broilers compared to hens have increased soft tissue over the skull, which could have improved the electrical contact with the pin electrodes, reducing the impedance of the head, (c) hens are generally more reactive, agile, and coordinated than broilers, this may allow them to more readily express behavioural signs of recovery than broilers, (d) there may be differences in skull bone density between broilers and hens, affecting the electrical resistance of the head, and (e) there may be differences between broilers and hens in the duration and severity of bradycardia induced during head-only electrical stunning. This may have an impact on cardiac performance and the time to return of consciousness.
To distinguish between the effects of current on brain function independent of the effects on spinal cord and brainstem function, VEPs were recorded from chickens prior to and after head-only and back-of-the-head stunning. VEPs are time-locked responses of the brain (optic lobe) to a light stimulus delivered in front of the eyes. They do not represent conscious awareness of the stimulus since they can occur in both the conscious and unconscious state. Rather they represent the rudimentary brain processing of the external stimulus below the level of conscious perception (Daly et al., 1988). In terms of stunning and slaughter, the absence of VEPs represents a degree of brain dysfunction that is inconsistent with the maintenance of consciousness.
From assessment of VEPs, the study found that brain dysfunction following head-only stunning on average only lasted 20 ± 2 s and ranged between 9–32 s. Compared to previous studies with head-only electrical stunning, the duration of brain dysfunction which was incompatible with consciousness is notably shorter, but it must be noted that the present finding was in hens rather than broilers. Richards and Sykes (1967) previously reported a period of unconsciousness that lasted between 30 and 60 s (ECoG) in chickens. Gregory and Wotton (1990a ) reported 26–108 s in broilers (behaviour), and Lambooij et al., (2010) reported a range of 30–65 s (EEG) in broilers. A shorter period of induced unconsciousness could result in some birds recovering from the stun prior to or during exsanguination. Based on the current results, it is suggested that head-only electrical stunning (with the minimum recommended stunning parameters of 240 mA) of hens should not be recommended as a reliable stunning method.
Previous work with broilers has shown that increasing current delivered to the head increased the duration of the stun (Gregory and Wotton, 1990b ; Raj and O’Callaghan, 2004). This relationship was not present in the current experiment and could be due to the low sample size and small current ranges in the study. However, work by Gregory and Wotton (1994) also found no effect of stunning current on the duration of unconsciousness in hens.
Electrical stunning and electroanalgesia
The results for noxious thermal laser stimulation demonstrate that in hens after electrical stunning there is a period of electroanalgesia that outlasts the period of induced unconsciousness. This was longest in hens stunned with the back-of-the-head electrodes (> 5 min). Previously electroanaglesia has been reported in mammals after electrical stunning; however this is the first report of it in birds and the first use of the thermal laser methodology in chickens. Potentially, the localised heating during electrical stunning could have produced desensitisation of the comb. However, this is considered unlikely as: (a) the stunning electrodes did not contact the comb, (b) they were sufficiently distant to avoid direct stimulation, (c) direct burning/cauterisation may cause localised deafferentation (however heating is not thought to cause widespread desensitisation, (d) the comb was still sensitive to non-noxious pressure in the form of touch, suggesting there was no localised numbing of the sensory nerves, and finally (e) the laser was used in multiple locations never stimulating the same area twice, reducing the potential for repeatedly stimulating desensitising regions or producing laser-induced desensitisation. The advantages of using lasers compared with physical tests such as a comb pinch are that: (a) the delivery of a laser stimulus is more reproducible because the stimulus is more uniform, (b) administering the laser does not involve a visual threat to the bird, whereas moving the hand to the comb does, (c) it is suspected that the bird would be less prone to becoming habituated to a laser stimulus compared with a pinch, (d) there are marked differences between regions of the comb in sensitivity to a pinch, and (e) an acute noxious laser stimulus does not involve other non-pain sensory modalities, which could complicate assessment of return of sensibility to pain. When doing repeated comb pinches, it is possible to run out of testable sensitive regions on the comb. This is less of an issue with laser stimulation as the area of the comb that is stimulated by the laser for each stimulus is relatively small (3 mm in diameter), leaving sufficient space for further measures.
Prior to experimentation on hens, the thermal laser stimulus was tested on the medial aspect of the forearm of the researchers. This confirmed that in humans the sensations provoked by thermal stimulation using this type of laser were comparable to those described by Willer et al., (1979) and Bromm and Treede (1987). Initially, there was a short-lasting pain, which was comparable to a localised pinprick. This was followed by a burning wave, which was a less severe pain, less localised, longer lasting and instilled was a sense of tissue injury. The initial pain is probably due to Aδ plus C-fibre activation, and the later pain to C-fibre activation alone (Bromm and Treede, 1987; Bromm and Lorenz, 1998; Ringkamp et al., 2013). In the chicken trials during baseline recordings the birds responded within 1.5 s, which would have corresponded to the initial pricking pain experienced by humans.
There are several regions in the brain, which are thought to mediate the analgesic effect produced by electrical stimulation of the brain (Mayer, 1984; White et al., 2001). Three important regions are the periaqueductal grey matter in the midbrain, the raphe nuclei of the midbrain, and raphe nuclei of the medulla of the brainstem (Liebeskind et al., 1973; Mayer and Liebeskind, 1974). Cook et al., (1992, 1993) reported that electroanalgesia following electrical stunning is mediated by the neurotransmitter GABA in sheep. Furthermore, it is well recognised from experience in rats, cats, sheep, and humans that under specific circumstances when analgesia is produced by electrical stimulation of deep brain structures, the subject can be conscious (Urca et al., 1981; Mayer, 1984; Hosobuchi, 1986; Young and Brechner, 1986; Gregory and Wotton, 1988; Kumar et al., 1997). The analgesia can apply to ongoing intractable pain associated with diseases and disorders, or pain provoked by noxious heat applied to the tail, pinching the ear, foot or tail, or electrical stimulation of a tooth.
Although a period of protracted electroanalgesia that outlasts the duration of unconsciousness provided by the stun is beneficial in terms of welfare, the purpose of electrical stunning should be to induce complete unconsciousness, not just insensibility to pain. Furthermore, this period of induced electroanalgesia could inhibit some brainstem/spinal indices that are used to assess stun performance (e.g. response to noxious stimulus: comb pinch).
Advantages and disadvantages of back-of-the-head and head-only electrical stunning
The findings from the behavioural and laser studies indicated that back-of-the-head compared to standard head-only electrical stunning of chickens can be effective in inducing unconsciousness based on time to return of behavioural responses. Furthermore, the advantages of back-of-the-head compared to other electrode positions is that it is relativity simple to apply, birds are not shackled before stunning, and the stun is reversible, potentially allowing its use for halal slaughter. However, based on examination of brain function in hens using VEPs, it was found that back-of-head stunning produced a shorter-lasting period of brain dysfunction than the standard head-only electrodes and that VEP activity returned sooner than behavioural indices. This suggests that the brainstem/cerebellum-mediated behavioural responses of return of breathing, neck tension, and balance under-represent the true time to return of consciousness following head-only electrical stunning of hens. Similarly, Lines et al. (2011) reported in broilers following head-only waterbath stunning that the suppression of breathing (22 s) and neck muscle (42 s) tone was longer than the period of induced EEG suppression (12 s).
Based on the time of return of VEPs, the back-of-the-head stun produces a shorter-lasting stun than the standard head-only electrodes. It is possible that higher current levels could result in a longer lasting stun in this position. When sheep (Gilbert et al., 1991) and pigs (Hoenderken, 1978: Anil and Mckinstry, 1998) are stunned in the high neck region, an effective stun can be produced, depending on the current level and the proximity of the electrodes to the atlanto-occipital axis. Until further work is conducted, which establishes a longer lasting stun, it is suggested that back-of-the-head stunning of chickens should not be used.
Head-only and back-of-the-head electrical stunning are reversible methods, where if the animals are not bled consciousness will return. When the duration of induced unconsciousness is short, or there are delays in the stun/cut interval, some animals could start recovering consciousness during the bleeding process prior to the onset of cerebral hypoxaemia from exsanguination. In the EU regulation 1099/2009, the requirement is that the loss of consciousness and sensibility from the stun shall be maintained until the death of the animal (Anon, 2009). Therefore, the duration of unconsciousness must be longer than the sum of the time from end of stunning and onset of death, including any delays in the stun-to-stick interval (Raj, 2006). To ensure this, the maximum stun/cut interval (SCmax) is used to calculate the interval in which the neck cut needs to be conducted to prevent recovery of consciousness prior to death. This is calculated by subtraction of the longest time to onset of unconsciousness following neck cutting without stunning (26 s based on Barnett et al. (2007)) from the shortest period of stun-induced unconsciousness.
Using return of neck tension (head-only stunned broilers: 39 s) as an indicator of return of consciousness, the SCmax to prevent recovery of consciousness during bleeding would be 13 s. This means that provided both carotid arteries are cut within the SCmax interval, birds will not resume consciousness during the bleeding period. However, in reality there can be considerable delays between stunning and the neck cut (transferring between cones and shackles, line speed, breakdowns etc.). Gregory and Wotton (1990b ) reported that the average interval between stunning and the neck cut in UK broiler plants was 21 s (range 14–37). Therefore, if there are any delays that are longer in duration than the SCmax, then there is a risk that some birds will start to regain some level of consciousness and may suffer pain and distress before the onset of cerebral hypoxaemia from exsanguination. If the time to return of VEP activity (head-only stunned hens: 9 s) is used, it is probable that some birds would have recovered from the stun prior to or during the bleeding process and could be conscious for up to 18 s prior to the loss of consciousness from cerebral hypoxia. This would result in significant suffering from the pain and distress associated with recovery from the stun, the neck cut, and during the bleeding process.
Conclusions
In conclusion, back-of-the-head stunning delayed the time to return of brainstem function in broilers and hens, and induced a period of electroanalgesia that exceeded that of stunning with head-only electrodes. However, based on examination of brain function with VEPs in hens, it was found that back-of-the-head stunning produced a shorter-lasting stun than the head-only electrodes. This could result in birds recovering consciousness prior to or during the neck cut. Furthermore, the study found that the duration of unconsciousness with standard head-only stunning was notably shorter than previously reported. Based on these findings, back-of-the-head stunning should not be recommended without further development. The results also suggest that head-only electrical stunning of hens with a minimum current of 240 mA should not be recommended as a reliable stunning method.
ACKNOWLEDGEMENTS
The authors would like to thank the assistance of Christopher Davies, Frances Benstead, Nikolas Dadios, Natalie Chancellor, and Emily Bill in experimental work and initial data analysis.
Funding Statement
This work was supported by the Department for Environment, Food and Rural Affairs (DEFRA) UK
disclosure statement
No potential conflict of interest was reported by the authors.
REFERENCES
- Anil M.H., Mckinstry J.L. Variations in electrical stunning tong placements and relative consequences in slaughter pigs. TheVeterinary Journal. 1998;155:85–90. doi: 10.1016/S1090-0233(98)80042-7. [DOI] [PubMed] [Google Scholar]
- Anon Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Offical Journal of the European Union. L 303/10. 2009
- Barnett J.L., Cronin G.M., Scott P.C. Behavioural responses of poultry during kosher slaughter and their implications for the birds’ welfare. Veterinary Record. 2007;160:45–49. doi: 10.1136/vr.160.2.45. [DOI] [PubMed] [Google Scholar]
- Bromm B., Lorenz J. Neurophysiological evaluation of pain. Electroencephalography and Clinical Neurophysiology. 1998;107:227–253. doi: 10.1016/S0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Bromm B., Treede R.D. Human cerebral potentials-evoked by Co2-laser stimuli causing pain. Experimental Brain Research. 1987;67:153–162. doi: 10.1007/BF00269463. [DOI] [PubMed] [Google Scholar]
- Cook C.J., Devine C.E., Maasland S.A., Gilbert K.V. Humane slaughter: an achievable goal? Proceedings of the New Zealand Society of Animal Production. 1993;53:197–199. [Google Scholar]
- Cook C.J., Devine C.E., Tavener A., Gilbert K.V. Contribution of amino acid transmitters to epileptiform activity and reflex suppression in electrically head stunned sheep. Research in Veterinary Science. 1992;52:48–56. doi: 10.1016/0034-5288(92)90057-9. [DOI] [PubMed] [Google Scholar]
- Daly C.C., Kallweit E., Ellendorf F. Cortical function in cattle during slaughter: conventional captive bolt stunning followed by exsanguination compared with shechita slaughter. Veterinary Record. 1988;122:325–329. doi: 10.1136/vr.122.14.325. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific opinion on electrical requirements for poultry waterbath stunning equipment. EFSA Journal. 2014;12:3745. [Google Scholar]
- FAWC . Report on the Welfare of Farmed Animals at Slaughter or Killing: Part 2. White Meat Animals. London: Farm Animal Welfare Council; 2009. [Google Scholar]
- Gilbert K.V., Cook C.J., Devine C.E. Electrical stunning in cattle and sheep: electrode placement and effectiveness. Proceedings of the International Congress of Meat Science Technology; Kulmback, Germany: 1991. pp. 245–248. [Google Scholar]
- Gregory N.G., Wotton S.B. Sheep slaughtering procedures V. Responsiveness to potentially painful stimuli following electrical stunning. British Veterinary Journal. 1988;144:573–580. doi: 10.1016/0007-1935(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Gregory N.G., Wotton S.B. An evaluation of the effectiveness of hand-held stunners for stunning chickens. Veterinary Record. 1990a;126:290–291. [PubMed] [Google Scholar]
- Gregory N.G., Wotton S.B. Effect of stunning on spontaneous physical activity and evoked activity in the brain. British Poultry Science. 1990b;31:215–220. doi: 10.1080/00071669008417248. [DOI] [PubMed] [Google Scholar]
- Gregory N.G., Wotton S.B. Effect of electrical stunning current on the duration of insensibility in hens. British Poultry Science. 1994;35:463–465. doi: 10.1080/00071669408417711. [DOI] [PubMed] [Google Scholar]
- Guesgen M.J., Beausoleil N.J., Minot E.O., Stewart M., Jones G., Stafford K.J. The effects of age and sex on pain sensitivity in young lambs. Applied Animal Behaviour Science. 2011;135:51–56. doi: 10.1016/j.applanim.2011.09.008. [DOI] [Google Scholar]
- Herskin M.S., Ladewig J., Arendt-Nielsen L. Measuring cutaneous thermal nociception in group-housed pigs using laser technique—effects of laser power output. Applied Animal Behaviour Science. 2009;118:144–151. doi: 10.1016/j.applanim.2009.02.016. [DOI] [Google Scholar]
- Hindle V.A., Lambooij E., Reimert H.G., Workel L.D., Gerritzen M.A. Animal welfare concerns during the use of the water bath for stunning broilers, hens, and ducks. Poultry Science. 2010;89:401–412. doi: 10.3382/ps.2009-00297. [DOI] [PubMed] [Google Scholar]
- Hoenderken R. Elektrische Bedwelming Van Eslachvarkens. Utrecht, The Netherlands: State University; 1978. [Google Scholar]
- Hosobuchi Y. Subcortical electrical-stimulation for control of intractable pain in humans – report of 122 Cases (1970-1984) Journal of Neurosurgery. 1986;64:543–553. doi: 10.3171/jns.1986.64.4.0543. [DOI] [PubMed] [Google Scholar]
- Kumar K., Toth C., Nath R.K. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736–747. doi: 10.1097/00006123-199704000-00015. [DOI] [PubMed] [Google Scholar]
- Lambooij E., Reimert H.G.M., Hindle V.A. Evaluation of head-only electrical stunning for practical application: assessment of neural and meat quality parameters. Poultry Science. 2010;89:2551–2558. doi: 10.3382/ps.2010-00815. [DOI] [PubMed] [Google Scholar]
- Lambooij E., Reimert H.G.M., Verhoeven M.T.W., Hindle V.A. Cone restraining and head-only electrical stunning in broilers: effects on physiological responses and meat quality. Poultry Science. 2014;93:512–518. doi: 10.3382/ps.2013-03318. [DOI] [PubMed] [Google Scholar]
- Liebeskind J.C., Guilbaud G., Besson J.-M., Oliveras J.-L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Research. 1973;50:441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Lines J.A., Raj A.B.M., Wotton S.B., O’callaghan M., Knowles T.G. Head-only electrical stunning of poultry using a waterbath: a feasibility study. British Poultry Science. 2011;52:432–438. doi: 10.1080/00071668.2011.587180. [DOI] [PubMed] [Google Scholar]
- Mayer D.J. Analgesia produced by electrical-stimulation of the brain. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1984;8:557–564. doi: 10.1016/0278-5846(84)90015-0. [DOI] [PubMed] [Google Scholar]
- Mayer D.J., Liebeskind J.C. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Research. 1974;68:73–93. doi: 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- Raj A.B.M. Recent developments in stunning and slaughter of poultry. Worlds Poultry Science Journal. 2006;62:467–484. doi: 10.1079/WPS2005109. [DOI] [Google Scholar]
- Raj A.B.M., O’Callaghan M. Effect of amount and frequency of head-only stunning currents on the electroencephalogram and somatosensory evoked potentials in broilers. Animal Welfare. 2004;13:159–170. [Google Scholar]
- Richards S.A., Sykes A.H. Physiological effects of electrical stunning and venesection in the fowl. Research in Veterinary Science. 1967;8:361–368. [PubMed] [Google Scholar]
- Ringkamp M., Raja S.N., Campbell J.N., Meyer R.A. Peripheral mechanisms of cutaneous nociception. In: McMahon S.B., Koltzenbury M., Tracey I., Turk D.C., editors. Wall and Melzack’s Textbook of Pain. Philadelphia: Elsevier; 2013. pp. 1–11. [Google Scholar]
- Schütt-Abraham I. Stunning methods for poultry: influence on birds’ welfare and prospects for future EC regulation. Proceedings of the COST Action 97, 2. Poultry Productions Microbiology, European Regulations and Quality Assurance Systems; Germany: Berlin; 1998. pp. 333–344. [Google Scholar]
- Sparrey J.M., Wotton S.B. The design of pig stunning tong electrodes - a review. Meat Science. 1997;47:125–133. doi: 10.1016/S0309-1740(97)00047-8. [DOI] [PubMed] [Google Scholar]
- Urca G., Yitzhaky J., Frenk H. Different opioid systems may participate in post-electroconvulsive shock (Ecs) analgesia and catalepsy. Brain Research. 1981;219:385–396. doi: 10.1016/0006-8993(81)90301-2. [DOI] [PubMed] [Google Scholar]
- Veissier I., Rushen J., Colwell D., de Passillé A.M. A laser-based method for measuring thermal nociception of cattle. Applied Animal Behaviour Science. 2000;66:289–304. doi: 10.1016/S0168-1591(99)00099-4. [DOI] [PubMed] [Google Scholar]
- White P.F., Li S.T., Chiu J.W. Electroanalgesia: its role in acute and chronic pain management. Anesthesia and Analgesia. 2001;92:505–513. doi: 10.1213/00000539-200102000-00042. [DOI] [PubMed] [Google Scholar]
- Willer J.C., Boureau F., Berny J. Nociceptive flexion reflexes elicited by noxious laser radiant-heat in man. Pain. 1979;7:15–20. doi: 10.1016/0304-3959(79)90103-9. [DOI] [PubMed] [Google Scholar]
- Wormuth H.J., Schutt I., Fessel J. Tierschutzgerechte Elektrische Betäubung Von Schlachtgeflügel. Berlin: Dietrich Reimer Verlag; 1981. VetMed Berichte 2/1981. [Google Scholar]
- Young R.F., Brechner T. Electrical-stimulation of the brain for relief of intractable pain due to cancer. Cancer. 1986;57:1266–1272. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]


